Initial Experience with Single-Session Resin-Based Transarterial Radioembolization Mapping and Treatment of Small Hepatocellular Carcinomas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
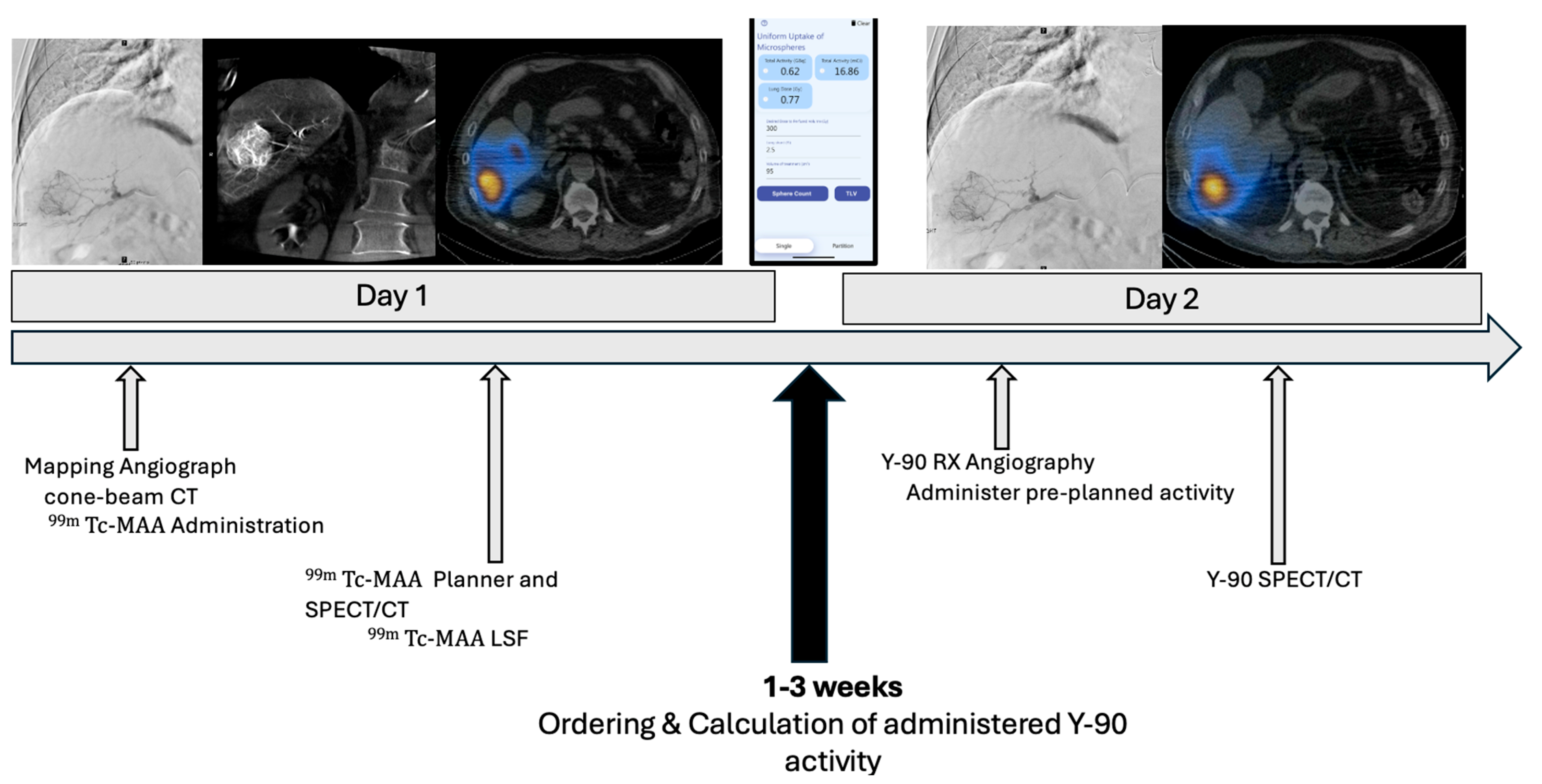
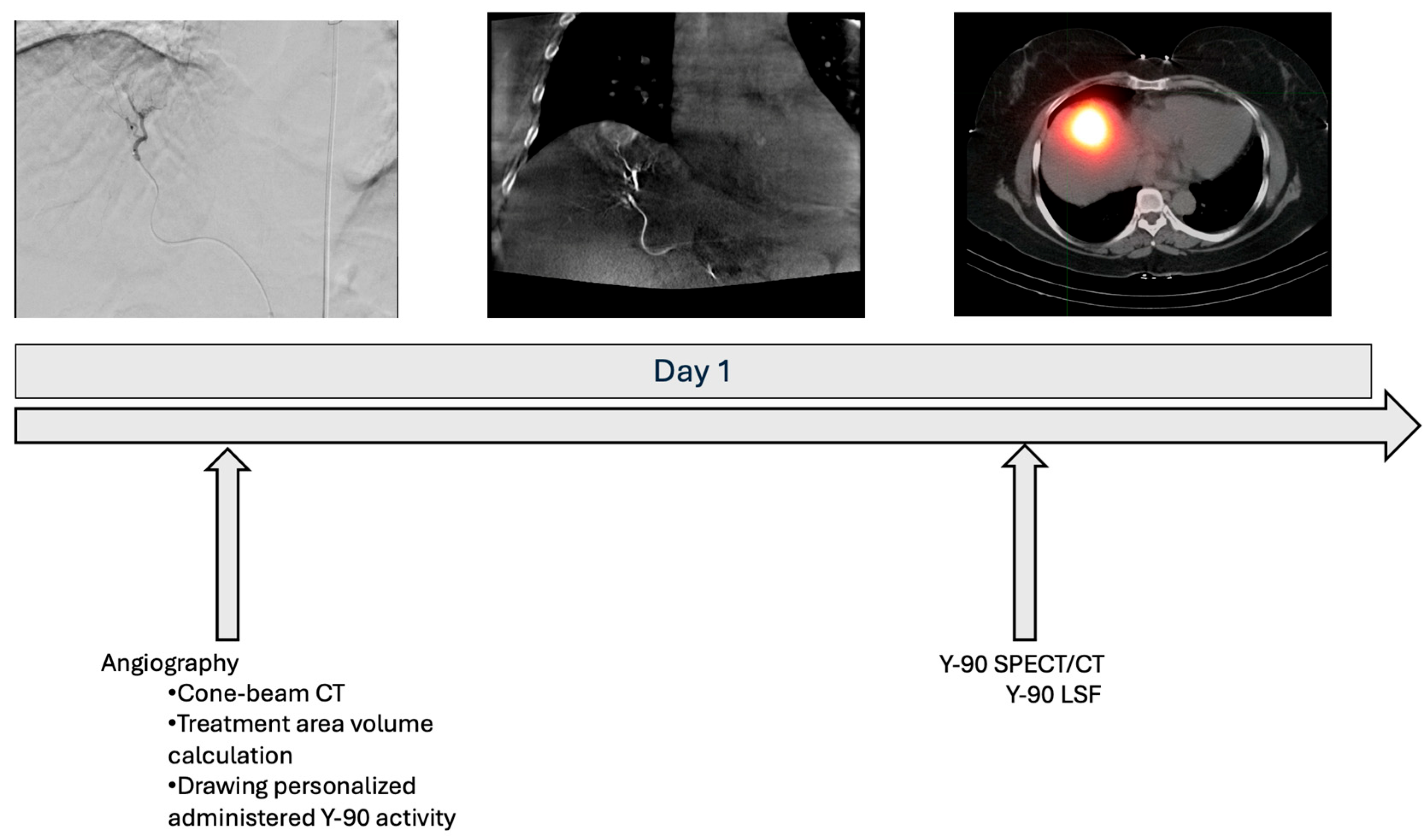
2.2. Treatment Approach
2.3. Outcomes
2.4. Comparison Group
2.5. Cost Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| BCLC | Barcelona Clinic Liver Cancer |
| Y90 | Yttrium 90 |
| TARE | Transarterial radioembolization |
| 99mTc-MAA | Technetium-99m macroaggregated albumin |
| SPECT | Single-photon emission computed tomography |
| LSF | Lung shunt fraction |
| GI | Gastrointestinal |
| OPTN | Organ Procurement and Transplant Network |
| MVI | Macrovascular invasion |
| TIPS | Transjugular intrahepatic portosystemic shunt |
| SSMT | Single-session mapping and treatment |
| CBCT | Cone-beam computed tomography |
| MIRD | Medical internal radiation dosimetry |
| IR | Interventional radiology |
| AE | Adverse event |
| CTCAEs | Common terminology for adverse events |
| IQR | Interquartile ratio |
References
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatol. Baltim. Md. 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrar-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Siddharth, A.P. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatol. Baltim. Md. 2021, 74, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Kallini, J.R.; Gabr, A.; Salem, R.; Lewandowski, R.J. Transarterial Radioembolization with Yttrium-90 for the Treatment of Hepatocellular Carcinoma. Adv. Ther. 2016, 33, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Kis, B.; Gyano, M. Radiation Pneumonitis after Yttrium-90 Radioembolization: A Systematic Review. J. Vasc. Interv. Radiol. JVIR 2024, 36, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Levillain, H.; Bagni, O.; Deroose, C.M.; Dieudonne, A.; Gnesin, S.; Grosser, O.S.; Kappadath, S.C.; Kennedy, A.; Kokabi, N.; Liu, D.M.; et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Padia, S.A.; Lam, M.; Chiesa, C.; Haste, P.; Sangro, B.; Toskich, B.; Fowers, K.; Herman, J.M.; Kappadath, S.C.; et al. Clinical, dosimetric, and reporting considerations for Y-90 glass microspheres in hepatocellular carcinoma: Updated 2022 recommendations from an international multidisciplinary working group. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Gabr, A.; Ranganathan, S.; Mouli, S.K.; Riaz, A.; Gates, V.L.; Kulik, L.; Ganger, D.; Maddur, H.; Moore, C.; Hohlastos, E.; et al. Streamlining radioembolization in UNOS T1/T2 hepatocellular carcinoma by eliminating lung shunt estimation. J. Hepatol. 2020, 72, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Kokabi, N.; Arndt-Webster, L.; Chen, B.; Brandon, D.; Sethi, I.; Davarpanahfakhr, A.; Galt, J.; Elsayed, M.; Bercu, Z.; Cristecu, M.; et al. Voxel-based dosimetry predicting treatment response and related toxicity in HCC patients treated with resin-based Y90 radioembolization: A prospective, single-arm study. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Berman, Z.T.; Pianka, K.; Qaseem, Y.; Redmond, J.; Minocha, J. Single-Session Ablative Transarterial Radioembolization for Patients with Hepatocellular Carcinoma to Streamline Care: An Initial Experience. Cardiovasc. Intervent. Radiol. 2024, 47, 1239–1245. [Google Scholar] [CrossRef]
- Kim, H.; Suh, M.; Paeng, J.C.; Lee, J.H.; Lee, M.; Chung, J.W.; Choi, J.W. Streamlining Radioembolization without Lung Shunt Estimation versus Regular Radioembolization in Patients with Hepatocellular Carcinoma within Milan Criteria. J. Vasc. Interv. Radiol. JVIR 2024, 36, 78–87. [Google Scholar] [CrossRef]
- Leung, T.W.; Lau, W.Y.; Ho, S.K.; Ward, S.C.; Chow, J.H.; Chan, M.S.; Metrewli, C.; Johnson, P.J.; Li, A.K. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Parikh, P.; Atassi, B.; Lewandowski, R.J.; Ryu, R.K.; Sato, K.T.; Gates, V.L.; Ibrahim, S.; Mulcahy, M.F.; Kulik, L.; et al. Incidence of radiation pneumonitis after hepatic intra-arterial radiotherapy with yttrium-90 microspheres assuming uniform lung distribution. Am. J. Clin. Oncol. 2008, 31, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, A.; Arndt, L.; Cheng, B.; Dabbous, H.; Loya, M.; Majdalany, B.; Bercu, Z.; Kokabi, N. Yttrium-90 Radiation Segmentectomy of Hepatocellular Carcinoma: A Comparative Study of the Effectiveness, Safety, and Dosimetry of Glass-Based versus Resin-Based Microspheres. J. Vasc. Interv. Radiol. JVIR 2023, 34, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Ritaccio, G.; Barritt IV, A.S.; Conklin, J.L.; Richardson, D.R.; Evon, D.M.; Sanoff, H.K.; Basch, E.; Wheeler, S.B.; Moon, A.M. Scoping review of values elicitation tools for treatment decisions in hepatocellular carcinoma. BMC Gastroenterol. 2024, 24, 90. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Single-Session Mapping and Treatment (N = 10) | Traditional Treatment (N = 58) | p-Value |
|---|---|---|---|
| Median age (years), IQR | 64 (63, 77) | 69 (62, 74) | p < 0.001 |
| Male (%) | 6 (60%) | 51 (88%) | p = 0.085 |
| Race (%) | p = 0.178 | ||
| White | 8 (80%) | 46 (79%) | |
| Black or African American | 1 (10%) | 5 (9%) | |
| Asian | 1 (10%) | 0 (0%) | |
| Other | 0 (0%) | 7 (12%) | |
| Ethnicity (%) | p = <0.001 | ||
| Not Hispanic or Latino | 10 (100%) | 53 (91%) | |
| Hispanic or Latino | 0 (0%) | 5 (9%) | |
| Child-Pugh Classification (%) | p = 0.24 | ||
| A | 9 (90%) | 51 (89%) | |
| B | 1 (10%) | 7 (12%) | |
| Liver-disease etiology | p = 0.0023 | ||
| Alcohol | 1 (10%) | 10 (18%) | |
| HCV | 4 (40%) | 19 (32%) | |
| MASLD | 1 (10%) | 14 (25%) | |
| HCV/Alcohol-associated liver disease | 3 (30%) | 5 (9%) | |
| HCV/MASLD | 1 (10%) | 1 (2%) | |
| MASLD/Alcohol-associated liver disease | 0 (0%) | 1 (2%) | |
| HBV | 0 (0%) | 2 (4%) | |
| HCV/Hemochromatosis | 0 (0%) | 1 (2%) | |
| Unknown | 0 (0%) | 5 (9%) | |
| OPTN Stage | p = 0.49 | ||
| T1 | 1 (10%) | 0 (%) | |
| T2 | 9 (90%) | 38 (65%) | |
| T3 | 0 (0%) | 18 (31%) | |
| T4 | 0 (0%) | 2 (4%) | |
| BCLC Stage (%) | p = 0.0054 | ||
| 0 | 1 (10%) | 4 (7%) | |
| A | 9 (90%) | 33 (56%) | |
| B | 0 (0%) | 18 (31%) | |
| C | 0 (0%) | 3 (5%) | |
| Median tumor size, IQR (cm) | 2.6 (2.1, 2.9) | 3.8 (2.7, 6.1) | p < 0.0001 |
| Outcome | n = 12 |
|---|---|
| Median tumor dose (Gy), IQR | 377.7 (273.5, 570.1) |
| ≥Grade 3 AEs | 0 (0%) |
| Median imaging follow-up (days), IQR | 79 (58, 86) |
| Initial imaging response (n= 10) | |
| Complete response | 9 (90%) |
| Partial response | 1 (10%) |
| SSMT Cohort (n = 10) | Traditional Cohort (n = 58) | p-Value (SSMT vs. Traditional Cohort) | |
|---|---|---|---|
| Median time from IR clinic visit to treatment (days), IQR | 26.5 (15.3, 39.0) | 61.0 (48.0, 88.8) | p < 0.001 |
| Median procedure time (minutes), IQR | 142 (123, 152) | 151 (130, 199) | p = 0.12 |
| Median fluoroscopy time (minutes), IQR | 25.1 (22.0, 29.0) | 22.9 (15.6, 31.8) | p = 0.28 |
| Hospital Outpatient (OPPS) (USD) | Physician Services (MFFS) (USD) | Total Cost (USD) | |
|---|---|---|---|
| Mapping Total Cost | 25,400.86 | 1231.25 | 26,632.11 |
| 75,726: Angiography, visceral, RS&I | 5405.70 | 90.57 | |
| 37,242: Arterial emb or occ, RS&I; arterial other than hem or tumor (includes catheter placement, 3D post-scan, 99mTC-MAA dose, radiopharmaceutical quantification measurement) | 17,956.72 | 1042.99 | |
| 74,170: CT, abdomen; w and w/o contrast | 178.02 | 63.40 | |
| 78,801: Planar imaging of multiple areas | 401.83 | 32.35 | |
| 78,832: SPECT/CT imaging of multiple areas | 1458.59 | 92.51 | |
| Y90 Treatment Total Cost | 36,685.53 | 1283.67 | 37,969.20 |
| 75,726: Angiography, visceral, RS&I | 5405.70 | 90.57 | |
| C2616: Brachytherapy source (yttrium-90 non-stranded–Medicare) | 17,412.53 | - | |
| 37,243: Vascular emb or occ, inclusive of all RS&I, intraprocedural road mapping, and imaging guidance necessary to complete intervention; for tumors | 11,340.57 | 858.64 | |
| 77,370: Special Medical Radiation Physics Consultation | 132.77 | - | |
| 77,470: Special Treatment Procedure | 578.47 | 104.48 | |
| 77,300: Basic Dosimetry Calculation | 132.77 | 32.02 | |
| 79,445: Radiopharmaceutical therapy, intra-arterial particulate admin (1 doctor model (IR/AU)) | 224.13 | 105.45 | |
| 78,832: SPECT/CT imaging of multiple areas | 1458.59 | 92.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohnasky, M.; Gad, S.; Fanous, M.; Du Pisanie, J.L.; Ivanovic, M.; Mauro, D.M.; Yu, H.; Villalobos, A.; Moon, A.M.; Sanoff, H.K.; et al. Initial Experience with Single-Session Resin-Based Transarterial Radioembolization Mapping and Treatment of Small Hepatocellular Carcinomas. Cancers 2025, 17, 1265. https://doi.org/10.3390/cancers17081265
Mohnasky M, Gad S, Fanous M, Du Pisanie JL, Ivanovic M, Mauro DM, Yu H, Villalobos A, Moon AM, Sanoff HK, et al. Initial Experience with Single-Session Resin-Based Transarterial Radioembolization Mapping and Treatment of Small Hepatocellular Carcinomas. Cancers. 2025; 17(8):1265. https://doi.org/10.3390/cancers17081265
Chicago/Turabian StyleMohnasky, Michael, Sandra Gad, Marco Fanous, Johannes L. Du Pisanie, Marija Ivanovic, David M. Mauro, Hyeon Yu, Alex Villalobos, Andrew M. Moon, Hanna K. Sanoff, and et al. 2025. "Initial Experience with Single-Session Resin-Based Transarterial Radioembolization Mapping and Treatment of Small Hepatocellular Carcinomas" Cancers 17, no. 8: 1265. https://doi.org/10.3390/cancers17081265
APA StyleMohnasky, M., Gad, S., Fanous, M., Du Pisanie, J. L., Ivanovic, M., Mauro, D. M., Yu, H., Villalobos, A., Moon, A. M., Sanoff, H. K., Jia, J., & Kokabi, N. (2025). Initial Experience with Single-Session Resin-Based Transarterial Radioembolization Mapping and Treatment of Small Hepatocellular Carcinomas. Cancers, 17(8), 1265. https://doi.org/10.3390/cancers17081265





