Retrospective Review of Intra-Cerebrospinal Fluid (CSF) Drug Delivery in CNS Malignancies: Safety, Clinical Efficacy and Pharmacokinetic Profiles of Intracerebroventricular (ICV), Lumbar Intrathecal (LIT), and Intra-Cisterna Magna (ICM) Injections
Simple Summary
Abstract
1. Introduction
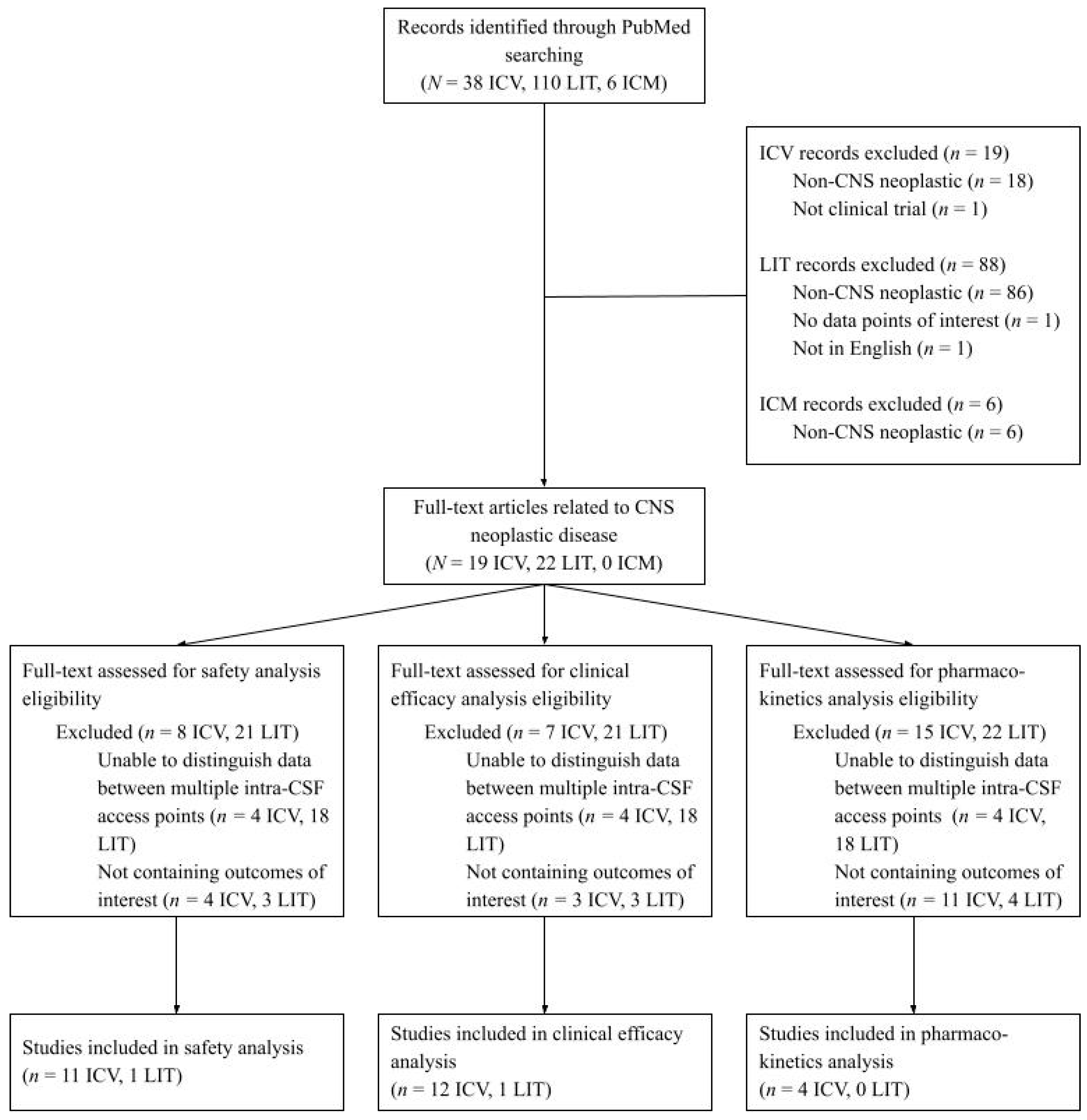
2. Materials and Methods
3. Results
3.1. Intracerebroventricular Findings
3.1.1. ICV Safety
3.1.2. ICV Clinical Efficacy
3.1.3. ICV Pharmacokinetics
3.2. Lumbar Intrathecal Findings
3.2.1. LIT Safety
3.2.2. LIT Clinical Efficacy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSF | Cerebrospinal fluid |
| CNS | Central nervous system |
| ICV | Intracerebroventricular |
| LIT | Lumbar intrathecal |
| ICM | Intra-cisterna magna |
| AE | Adverse event |
| NSCLC | Non-small cell lung cancer |
| PFS | Progression-free survival |
| OS | Overall survival |
| BBB | Blood–brain barrier |
| BCSFB | Blood–cerebrospinal fluid barrier |
| CR | Complete response |
| PD | Progressive disease |
| PR | Partial response |
| SD | Stable disease |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CED | Convection-enhanced delivery |
| VLP | Ventriculolumbar perfusion |
References
- Redzic, Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: Similarities and differences. Fluids Barriers CNS 2011, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Why is the global CNS pharmaceutical market so under-penetrated? Drug Discov. Today 2002, 7, 5–7. [Google Scholar] [CrossRef]
- Griffith, J.I.; Rathi, S.; Zhang, W.; Zhang, W.; Drewes, L.R.; Sarkaria, J.N.; Elmquist, W.F. Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors. Pharmaceutics 2020, 12, 1205. [Google Scholar] [CrossRef]
- Ilic, I.; Ilic, M. International patterns and trends in the brain cancer incidence and mortality: An observational study based on the global burden of disease. Heliyon 2023, 9, e18222. [Google Scholar] [CrossRef]
- Slavc, I.; Cohen-Pfeffer, J.L.; Gururangan, S.; Krauser, J.; Lim, D.A.; Maldaun, M.; Schwering, C.; Shaywitz, A.J.; Westphal, M. Best practices for the use of intracerebroventricular drug delivery devices. Mol. Genet. Metab. 2018, 124, 184–188. [Google Scholar] [CrossRef]
- Marchi, P.M.; Marrone, L.; Azzouz, M. Delivery of therapeutic AAV9 vectors via cisterna magna to treat neurological disorders. Trends Mol. Med. 2021, 28, 79–80. [Google Scholar] [CrossRef]
- Sadekar, S.S.; Bowen, M.; Cai, H.; Jamalian, S.; Rafidi, H.; Shatz-Binder, W.; Lafrance-Vanasse, J.; Chan, P.; Meilandt, W.J.; Oldendorp, A.; et al. Translational Approaches for Brain Delivery of Biologics via Cerebrospinal Fluid. Clin. Pharmacol. Ther. 2022, 111, 826–834. [Google Scholar] [CrossRef]
- Calias, P.; Papisov, M.; Pan, J.; Savioli, N.; Belov, V.; Huang, Y.; Lotterhand, J.; Alessandrini, M.; Liu, N.; Fischman, A.J.; et al. CNS Penetration of Intrathecal-Lumbar Idursulfase in the Monkey, Dog and Mouse: Implications for Neurological Outcomes of Lysosomal Storage Disorder. PLoS ONE 2012, 7, e30341. [Google Scholar] [CrossRef]
- Lutters, B.; Koehler, P.J. A road less travelled: The centenary of cisterna magna puncture. Brain 2020, 143, 2858–2862. [Google Scholar] [CrossRef]
- Cohen-Pfeffer, J.L.; Gururangan, S.; Lester, T.; Lim, D.A.; Shaywitz, A.J.; Westphal, M.; Slavc, I. Intracerebroventricular Delivery as a Safe, Long-Term Route of Drug Administration. Pediatr. Neurol. 2017, 67, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ommaya, A. Subcutaneous Reservoir and Pump for Sterile Access to Ventricular Cerebrospinal Fluid. Lancet 1963, 282, 983–984. [Google Scholar] [CrossRef]
- Bier, A. Versuche über Cocainisirung des Rückenmarkes. Dtsch. Z. Chir. 1899, 51, 361–369. [Google Scholar] [CrossRef]
- Ayer, J.B. Puncture of the Cisterna Magna. Arch. Neurol. Psychiatry 1920, 4, 529–541. [Google Scholar] [CrossRef]
- Simon, M.J.; Iliff, J.J. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1862, 442–451. [Google Scholar] [CrossRef]
- Kouzehgarani, G.N.; Feldsien, T.; Engelhard, H.H.; Mirakhur, K.K.; Phipps, C.; Nimmrich, V.; Clausznitzer, D.; Lefebvre, D.R. Harnessing cerebrospinal fluid circulation for drug delivery to brain tissues. Adv. Drug Deliv. Rev. 2021, 173, 20–59. [Google Scholar] [CrossRef] [PubMed]
- Oliva, I.C.G.; Ferguson, S.D.; Bassett, R.; Foster, A.P.; John, I.; Hennegan, T.D.; Rohlfs, M.; Richard, J.; Iqbal, M.; Dett, T.; et al. Concurrent intrathecal and intravenous nivolumab in leptomeningeal disease: Phase 1 trial interim results. Nat. Med. 2023, 29, 898–905. [Google Scholar] [CrossRef]
- Kumthekar, P.U.; Avram, M.J.; Lassman, A.B.; Lin, N.U.; Lee, E.; A Grimm, S.; Schwartz, M.; Burdett, K.L.B.; Lukas, R.V.; Dixit, K.; et al. A phase I/II study of intrathecal trastuzumab in human epidermal growth factor receptor 2-positive (HER2-positive) cancer with leptomeningeal metastases: Safety, efficacy, and cerebrospinal fluid pharmacokinetics. Neuro-Oncol. 2022, 25, 557–565. [Google Scholar] [CrossRef]
- Li, H.; Zheng, S.; Lin, Y.; Yu, T.; Xie, Y.; Jiang, C.; Liu, X.; Qian, X.; Yin, Z. Safety, Pharmacokinetic and Clinical Activity of Intrathecal Chemotherapy with Pemetrexed via the Ommaya Reservoir for Leptomeningeal Metastases From Lung Adenocarcinoma: A Prospective Phase I Study. Clin. Lung Cancer 2022, 24, e94–e104. [Google Scholar] [CrossRef]
- Sandberg, D.I.; Yu, B.; Patel, R.; Hagan, J.; Miesner, E.; Sabin, J.; Smith, S.; Fletcher, S.; Shah, M.N.; Sirianni, R.W.; et al. Infusion of 5-Azacytidine (5-AZA) into the fourth ventricle or resection cavity in children with recurrent posterior Fossa Ependymoma: A pilot clinical trial. J. Neuro-Oncol. 2018, 141, 449–457. [Google Scholar] [CrossRef]
- Mrugala, M.M.; Kim, B.; Sharma, A.; Johnson, N.; Graham, C.; Kurland, B.F.; Gralow, J. Phase II Study of Systemic High-dose Methotrexate and Intrathecal Liposomal Cytarabine for Treatment of Leptomeningeal Carcinomatosis from Breast Cancer. Clin. Breast Cancer 2019, 19, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.; Pandit-Taskar, N.; Humm, J.L.; Zanzonico, P.B.; Haque, S.; Dunkel, I.J.; Wolden, S.L.; Donzelli, M.; Goldman, D.A.; Lewis, J.S.; et al. A Phase II Study of Radioimmunotherapy with Intraventricular 131I-3F8 for Medulloblastoma. Pediatr. Blood Cancer 2018, 65, e26754. [Google Scholar] [CrossRef]
- Rubenstein, J.L.; Li, J.; Chen, L.; Advani, R.; Drappatz, J.; Gerstner, E.; Batchelor, T.; Krouwer, H.; Hwang, J.; Auerback, G.; et al. Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood 2013, 121, 745–751. [Google Scholar] [CrossRef]
- Blaney, S.M.; Tagen, M.; Onar-Thomas, A.; Berg, S.L.; Gururangan, S.; Scorsone, K.; Su, J.; Goldman, S.; Kieran, M.W.; Kun, L.; et al. A phase-1 pharmacokinetic optimal dosing study of intraventricular topotecan for children with neoplastic meningitis: A pediatric brain tumor consortium study. Pediatr. Blood Cancer 2012, 60, 627–632. [Google Scholar] [CrossRef]
- Glantz, M.J.; Van Horn, A.; Fisher, R.; Chamberlain, M.C. Route of intracerebrospinal fluid chemotherapy administration and efficacy of therapy in neoplastic meningitis. Cancer 2010, 116, 1947–1952. [Google Scholar] [CrossRef]
- Groves, M.D.; Glantz, M.J.; Chamberlain, M.C.; Baumgartner, K.E.; Conrad, C.A.; Hsu, S.; Wefel, J.S.; Gilbert, M.R.; Ictech, S.; Hunter, K.U.; et al. A multicenter phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro-Oncol. 2008, 10, 208–215. [Google Scholar] [CrossRef]
- Slavc, I.; Schuller, E.; Falger, J.; Günes, M.; Pillwein, K.; Czech, T.; Dietrich, W.; Rössler, K.; Dieckmann, K.; Prayer, D.; et al. Feasibility of Long-Term Intraventricular Therapy with Mafosfamide (n = 26) and Etoposide (n = 11): Experience in 26 Children With Disseminated Malignant Brain Tumors. J. Neuro-Oncol. 2003, 64, 239–247. [Google Scholar] [CrossRef]
- Fleischhack, G.; Reif, S.; Hasan, C.; Jaehde, U.; Hettmer, S.; Bode, U. Feasibility of intraventricular administration of etoposide in patients with metastatic brain tumours. Br. J. Cancer 2001, 84, 1453–1459. [Google Scholar] [CrossRef]
- Fan, C.; Zhao, Q.; Li, L.; Shen, W.; Du, Y.; Teng, C.; Gao, F.; Song, X.; Jiang, Q.; Huang, D.; et al. Efficacy and Safety of Intrathecal Pemetrexed Combined With Dexamethasone for Treating Tyrosine Kinase Inhibitor-Failed Leptomeningeal Metastases From EGFR-Mutant NSCLC—A Prospective, Open-Label, Single-Arm Phase 1/2 Clinical Trial (Unique Identifier: ChiCTR1800016615). J. Thorac. Oncol. 2021, 16, 1359–1368. [Google Scholar] [CrossRef]
- Ingrand, I.; Defossez, G.; Lafay-Chebassier, C.; Chavant, F.; Ferru, A.; Ingrand, P.; Pérault-Pochat, M. Serious adverse effects occurring after chemotherapy: A general cancer registry-based incidence survey. Br. J. Clin. Pharmacol. 2019, 86, 711–722. [Google Scholar] [CrossRef]
- Garavaglia, J.; Hardigan, T.; Turner, R.; Monachello, G.; Khan, M.B.; Hodge, J.O.; Brandmeir, N.J. Continuous Intrathecal Medication Delivery with the IRRAflow Catheter: Pearls and Early Experience. Neurosurgery 2023, 26, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Gwak, H.-S.; Joo, J.; Doh, Y.-S.; Shin, S.-H.; Yoo, H.; Wang, K.-C. The efficacy of slow-rate ventriculolumbar perfusion chemotherapy for leptomeningeal carcinomatosis: A phase II study. Acta Neurochir. 2024, 166, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, P.; Schwartz, M.; Nagpal, S.; Glitza, I.C.; Forsyth, P.A.J.; Gormley, W.; Patel, R.; Glicksman, M.; Sonabend, A.M. Novel non-invasive method for measuring intracranial pressure for drug delivery and other neurologic disorders. J. Clin. Oncol. 2024, 42, 2031. [Google Scholar] [CrossRef]
- Kumthekar, P.; Benatti, H.R.; Taghian, T.; Nagpal, S.; Gormley, W.; Baker, W.; Patel, R.; Brown, E.; Glicksman, M.; Gray-Edwards, H. BSLD-01 Dynamic Control of CSF for Personalized CNS Drug Delivery for Leptomingeal Disease. Neuro-Oncol. Adv. 2023, 5, iii5–iii6. [Google Scholar] [CrossRef]
| Delivery Route | Uses [10,11] | Advantages [7,12] | Limitations [9] | History |
|---|---|---|---|---|
| ICV | -Oncology -Pain management -Seizure/epilepsy -Neurodegenerative diseases -Infectious meningitis | -Widespread CNS delivery -Delivery at constant rate to minimize changes in intracranial pressure -Can be used for long-term administration -Minimizes systemic toxicity -Developed neurosurgery protocols | -Invasive surgery -Crosses parenchyma -Risk of neurosurgical complications -Risk of infectious complications | Ommaya 1963 [13] |
| LIT | -Oncology -Pain management -Spasticity -Neurodegenerative diseases -Infectious meningitis | -Minimally invasive -Routine outpatient procedure | -Longer distance to the brain -Influenced by posture -Require repeated punctures | Bier 1898 [14] |
| ICM | -Oncology -Pain management -Neurodegenerative diseases -Infectious meningitis | -Delivery closer to brain -Does not cross parenchyma | -Less developed surgical protocols -Risk of surgical complications | Ayer 1920 [15] |
| Author, Year | Disease (Tumor Type) | Study Phase | Patient Population (N) |
|---|---|---|---|
| Glitza Olivia et al., 2023 [18] | Leptomeningeal metastases (melanoma) | I/Ib | 25 |
| Kumthekar et al., 2022 [19] | Leptomeningeal metastases (breast cancer) | I/II | 34 |
| Li et al., 2023 [20] | Leptomeningeal metastases (lung cancer) | I | 23 |
| Sandberg et al., 2019 [21] | Recurrent ependymoma (posterior fossa) | Pilot | 6 |
| Mrugala et al., 2019 [22] | Leptomeningeal metastases (breast cancer) | II | 3 |
| Kramer et al., 2018 [23] | Medulloblastoma | II | 43 |
| Rubenstein et al., 2013 [24] | Recurrent CNS lymphoma (non-Hodgkin lymphoma) | I | 14 |
| Blaney et al., 2013 [25] | Pediatric neoplastic meningitis (leukemia/lymphoma or solid CNS tumor) | I | 19 |
| Glantz et al., 2010 [26] | Neoplastic meningitis (solid tumors of different origin) | Retrospective of phase IV | 100 |
| Groves et al., 2008 [27] | Meningeal malignancies (leukemia/lymphoma and solid tumors) | II | 62 |
| Slavc et al., 2003 [28] | Disseminated brain malignant tumors | Retrospective | 26 |
| Fleischhack et al., 2001 [29] | Brain metastases (medulloblastoma, primitive neuroectodermal tumor, glioblastoma, ependymoma) | Pilot | 14 |
| Author, Year | Disease | Drug | Study Phase | Patient Population (N) |
|---|---|---|---|---|
| Fan et al., 2021 [30] | Leptomeningeal metastases (EGFR-mutant NSCLC) | Pemetrexed combined with dexamethasone | I/II | 30 |
| Glantz et al., 2010 [26] | Neoplastic meningitis (NSCLC, primary CNS tumor, breast cancer) | sustained-release cytarabine or methotrexate | Retrospective of phase IV | 100 |
| Author, Year | Grade | Possibly Related/Related AEs (Frequency, if Reported) |
|---|---|---|
| Glitza Olivia et al., 2023 [18] | 1 | Nausea (n = 7, 28%), dizziness (n = 4, 16%), vomiting (n = 3, 12%), paresthesia (n = 2, 8%), pruritis (n = 1, 4%), anorexia (n = 1, 4%), eye disorders (n = 1, 4%) |
| 2 | Neck pain (n = 2, 8%), transient aphasia (n = 1, 4%) | |
| Kumthekar et al., 2022 [19] | 1 | Headache (n = 3, 12%), noninfectious meningitis/arachnoiditis (n = 1, 4%), fatigue (n = 1, 4%), fever (n = 1, 4%), nausea (n = 1, 4%), malaise (n = 1, 4%), vertigo (n = 1, 4%), anorexia (n = 1, 4%) |
| 2 | Noninfectious meningitis/arachnoiditis (n = 3, 12%), headache (n = 2, 8%), fatigue (n = 1, 4%), laryngitis (n = 1, 4%), vomiting (n = 1, 4%), back pain (n = 1, 4%), extremity pain (n = 1, 4%) | |
| 3 | Hydrocephalus (n = 1, 4%), nausea (n = 1, 4%) | |
| 4 | Noninfectious meningitis/arachnoiditis (n = 2, 8%) | |
| Li et al., 2023 [20] | 1 | Elevation of ALT/AST (n = 5, 22%), myelosuppression (n = 2, 9%), anemia (n = 1, 4%) |
| 2 | Anemia (n = 3, 13%), myelosuppression (n = 2, 9%), epilepsy (n = 1, 4%), scalp infection (n = 1, 4%) | |
| 3 | Myelosuppression (n = 3, 13%), epilepsy (n = 1, 4%), elevation of ALT/AST (n = 1, 4%) | |
| 4 | Myelosuppression (n = 1, 4%) | |
| Sandberg et al., 2019 [21] | 1 | Vomiting (n = 3, 50%), nausea (n = 2, 33%), headache (n = 1, 17%), stomach cramps (n = 1, 17%) |
| 3 | Reservoir infection (n = 1, 17%) | |
| Mrugala et al., 2019 [22] | 3 | Transaminitis (n = 3, 100%) |
| 4 | Lymphopenia (n = 1, 33%) | |
| Kramer et al., 2018 [23] | 2/3 | Fever, headache, nausea, vomiting |
| 3 | Transient acute bradycardia with somnolence (n = 2, 5%), headache, fatigue, pleocytosis, acute dystonic reaction | |
| Rubenstein et al., 2013 [24] | 1 | Paresthesias, chills, rigors |
| 3/4 | Lymphopenia (n = 2, 14%), fatigue, cataract, gait/CN III neuropathy, neutropenia, muscle weakness | |
| Blaney et al., 2013 [25] | 1 | Electrolyte imbalance (n = 15, 79%), vomiting (n = 5, 26%), fatigue (n = 5, 26%), fever (n = 2, 11%), diarrhea (n = 2, 11%). nausea (n = 1, 5%), anorexia (n = 1, 5%), headache (n = 1, 5%), hepatic test abnormalities (n = 1, 5%), vision-blurred (n = 1, 5%) |
| 2 | Arachnoiditis (n = 2, 11%), headache (n = 2, 11%), albumin abnormalities (n = 1, 5%), alopecia (n = 1, 5%), electrolyte imbalance (n = 1, 5%), hepatic test abnormalities (n = 1, 5%), vomiting (n = 1, 5%) | |
| 3 | Arachnoiditis (n = 2, 11%), electrolyte imbalance (n = 1, 5%), hepatic test abnormalities (n = 1, 5%), infection/febrile neutropenia (n = 1, 5%), nausea (n = 1, 5%) | |
| 4 | Headache (n = 1, 5%) | |
| Groves et al., 2008 [27] | 1/2 | Chemical meningitis (n = 17, 65%), fatigue (n = 2, 8%), nausea or vomiting (n = 1, 4%), dyspnea (n = 1, 4%) |
| 3/4 | CNS symptoms (n = 11, 42%), leukopenia (n = 4, 15%), constipation (n = 4, 15%), chemical meningitis (n = 3, 12%), anorexia (n = 3, 12%), nausea or vomiting (n = 3, 12%), dyspnea (n = 3, 12%), infection (n = 3, 12%), pain (n = 3, 12%), fatigue (n = 2, 8%), anemia (n = 2, 8%), hyponatremia (n = 2, 8%), thrombocytopenia (n = 1, 4%), chest pain (n = 1, 4%), diarrhea (n = 1, 4%), fever (n = 1, 4%), pruritus (n = 1, 4%), seizure (n = 1, 4%), upper GI bleed (n = 1, 4%), thrombosis (n = 1, 4%) | |
| Slavc et al., 2003 [28] | N/A | Headache, nausea, neck pain, vomiting |
| Fleischhack et al., 2001 [29] | N/A | Headache, infection (meningitis) (2 of 59 courses), reservoir malfunction (n = 1, 7%), vomiting, temporary confusion, transient coma, generalized seizure associated with hyponatremia |
| Author, Year | Patient Population (N) (Disease) | Drug Treatment | Response | Survival |
|---|---|---|---|---|
| Glitza Olivia et al., 2023 [18] | 25 (melanoma) | Nivolumab | - | Median OS: 4.9 mo |
| Kumthekar et al., 2022 [19] | 26 (breast cancer) | Trastuzumab | 13 SD, 5 PR, 8 PD | Median PFS: 2.2 mo Median OS: 8.3 mo |
| Li et al., 2023 [20] | 23 (lung cancer) | Pemetrexed | 9 SD, 10 PR, 4 SD | Median PFS: 6.3 mo Median OS: 9.5 mo |
| Sandberg et al., 2019 [21] | 6 (ependymoma) | 5-Azacytidine | 5 PD, 1 discontinued | - |
| Mrugala et al., 2019 [22] | 3 (breast cancer) | Methotrexate and Liposomal Cytarabine | 3 PD | Median PFS: 1.4 mo Median OS: 8.2 mo |
| Kramer et al., 2018 [23] | 42 (medulloblastoma) | Radioimmunotherapy 131I-3F8 | 9 SD, 1 PR, 12 PD 15 CR, 5 PD | Median PFS: 11 mo |
| Rubenstein et al., 2013 [24] | 14 (non-Hodgkin lymphoma) | Rituximab (1st treatment each week), rituximab + methotrexate (2nd treatment each week) | 6 CR, 1 PR, 1 SD, 6 PD | - |
| Blaney et al., 2013 [25] | 19 (leukemia/lymphoma or solid CNS tumor) | Topotecan | 0 CR, 3 SD | - |
| Glantz et al., 2010 [26] | 16 (NSCLC, primary CNS tumor, breast cancer) | liposomal cytarabine or methotrexate | - | PFS (ICV cytarabine): 43 days PFS (ICV methotrexate): 43 days |
| Groves et al., 2008 [27] | 62 (leukemia/lymphoma and solid tumors) | Topotecan | 18 SD, 10 PR, 12 PD | Median survival: 15 weeks |
| Slavc et al., 2003 [28] | 11 alive | Mafosfamide and Etoposide | 6 CR, 5 PR | - |
| Fleischhack et al., 2001 [29] | 14 (medulloblastoma, primitive neuroectodermal tumor, glioblastoma, ependymoma) | Etoposide | 5 PR, 3 PD, 6 SD | - |
| Author, Year | Drug Treatment | Dose | Patient Number | Results |
|---|---|---|---|---|
| Blaney et al., 2013 [22] | Topotecan | 0.1 and 0.2 mg | 18 | Therapeutic target concentration of 1 ng/mL was reached in all patients at the 0.2 mg dose level. |
| Fleischhack et al., 2001 [29] | Etoposide | 0.5 mg | 4 | The terminal half-life in the CSF was 7.4 ± 1.2 h, and the area under the curve was 25.0 ± 9.5 μg·h/mL. Volume of distribution at steady state averaging 0.16 L and total clearance averaging 0.46 mL/min. |
| Rubenstein et al., 2013 [24] | Rituximab and methotrexate | 10 mg or 25 mg Rituximab and 12 mg methotrexate | 14 | Biphasic decline in CSF rituximab concentrations, peak levels at 580 μg/mL at the 25 mg dose. The elimination rate of rituximab was slower when co-administered with methotrexate (0.36/h) compared to rituximab monotherapy (0.84/h). |
| Kumthekar et al., 2022 [19] | Trastuzumab | 80 mg | 10 | Mean volume of distribution of 73 ± 48 mL and a clearance rate of 14 ± 5 mL/h. The apparent CSF half-life was relatively short at 4.1 ± 3.0 h. |
| Author, Year | Grade | Possibly Related/Related AEs (Frequency, If Reported) |
|---|---|---|
| Fan et al., 2021 [30] | 1 | Vomiting (n = 6, 20%), nausea (n = 2, 7%), limb pain (n = 1, 3%), back pain (n = 1, 3%) |
| 2 | Myelosuppression (n = 6, 20%), limb pain (n = 2, 7%), paralysis (n = 2, 7%), headache (n = 2, 7%), back pain (n = 1, 3%) | |
| 3 | Myelosuppression (n = 4, 13%), limb pain (n = 2, 7%), headache (n = 1, 3%) | |
| 4 | Myelosuppression (n = 1, 3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.Y.; Glicksman, M.A.; Patel, R.; Malhotra, S.; Moelis, N.; Vanjani, N.N.; Kumthekar, P. Retrospective Review of Intra-Cerebrospinal Fluid (CSF) Drug Delivery in CNS Malignancies: Safety, Clinical Efficacy and Pharmacokinetic Profiles of Intracerebroventricular (ICV), Lumbar Intrathecal (LIT), and Intra-Cisterna Magna (ICM) Injections. Cancers 2025, 17, 1263. https://doi.org/10.3390/cancers17081263
Lee GY, Glicksman MA, Patel R, Malhotra S, Moelis N, Vanjani NN, Kumthekar P. Retrospective Review of Intra-Cerebrospinal Fluid (CSF) Drug Delivery in CNS Malignancies: Safety, Clinical Efficacy and Pharmacokinetic Profiles of Intracerebroventricular (ICV), Lumbar Intrathecal (LIT), and Intra-Cisterna Magna (ICM) Injections. Cancers. 2025; 17(8):1263. https://doi.org/10.3390/cancers17081263
Chicago/Turabian StyleLee, Grace Y., Marcie A. Glicksman, Rajan Patel, Saaz Malhotra, Nathan Moelis, Nisheka N. Vanjani, and Priya Kumthekar. 2025. "Retrospective Review of Intra-Cerebrospinal Fluid (CSF) Drug Delivery in CNS Malignancies: Safety, Clinical Efficacy and Pharmacokinetic Profiles of Intracerebroventricular (ICV), Lumbar Intrathecal (LIT), and Intra-Cisterna Magna (ICM) Injections" Cancers 17, no. 8: 1263. https://doi.org/10.3390/cancers17081263
APA StyleLee, G. Y., Glicksman, M. A., Patel, R., Malhotra, S., Moelis, N., Vanjani, N. N., & Kumthekar, P. (2025). Retrospective Review of Intra-Cerebrospinal Fluid (CSF) Drug Delivery in CNS Malignancies: Safety, Clinical Efficacy and Pharmacokinetic Profiles of Intracerebroventricular (ICV), Lumbar Intrathecal (LIT), and Intra-Cisterna Magna (ICM) Injections. Cancers, 17(8), 1263. https://doi.org/10.3390/cancers17081263






