4. Discussion
Several first-line combinations of platinum-based chemotherapy (ChT) plus an immune-checkpoint inhibitor successfully confirmed improved overall survival compared with ChT alone for mNSCLC patients, regardless of tumor PD-L1 status. In metastatic non-squamous and squamous non-small-cell carcinoma, RCTs for Pembrolizumab [
30,
31], Atezolizumab with or without bevacizumab (non-squamous only) [
32,
33], Cemiplimab [
34], and Nivolumab/Ipilimumab [
35] plus ChT unequivocally proved efficacy, and indications of use were approved by the Food and Drug Administration (FDA) and European Medicine Agency (EMA) [
36,
37,
38,
39,
40]. A Durvalumab–Tremelimumab–ChT regimen is approved only by the FDA [
41]. First-line monotherapy ICIs in tumor PD-L1 ≥ 50% is the standard of care according to clinical trials [
42,
43,
44]. Second-line therapy for previously ChT-treated, PD-L1 inhibitor-naïve mNSCLCs, irrespective of tumor PD-L1 expression, is monotherapy ICIs with Nivolumab [
45,
46], Atezolizumab [
47], and Pembrolizumab (only in PD-L1 ≥ 1%) [
48], as the OS benefit was irrefutably evidenced.
In Romania, non-oncogene mNSCLC patients are treated following European Society for Medical Oncology guidelines [
4], but not all of ICI indications are reimbursed [
49]. The vast majority of patients is treated with first-line Pembrolizumab and second-line nivolumab, as our retrospective study evidenced. There is small to absent clinical experience with the 9LA regimen (first-line Nivolumab plus Ipilimumab combined with two cycles of chemotherapy) or Cemiplimab in mNSCLC (recently approved in December 2024), and this situation may have an impact on dealing with special patient populations, treating rare or late-onset immune-related adverse events. Even if ICI clinical trials [
30,
31,
32,
33,
34,
35] included only mNSCLCs in good performance status (PS), such as ECOG PS ≤ 1, and international guidelines recommend immunotherapy to ECOG PS ≤ 2 patients [
3,
4], real-life medical practice in oncology may be a different challenge. In our retrospective study, we identified 21.4% of ECOG PS = 3 mNSCLC patients treated with just one or two cycles of immunotherapy. For a more homogeneous cohort and clearer statistical analysis, these patients should have been excluded from the study. Bearing in mind the small sample size of mNSCLC patients, and to preserve the challenges and limitations of real-practice in Romania, where salvage therapy still used, we kept these patients in our retrospective research. Almost 60% of the entire cohort presented more than two sites of metastatic disease before ICIs and brain or liver metastasis were present in 20% of patients. This reality is completely different from the patients included in the RCTs, RWD being critical in understanding how RCTs are translated into clinical practice. On the other hand, really good RWE is needed because, as Pellat published in 2023 [
50], only 8% of RWE sources were from more than one country; half of the studies were conducted in Asia, and 87% of the studies were retrospective. From our knowledge, this observational study on mNSCLC patients is the second one to report RWE from Romania, an Eastern European country. Furthermore, the mean time from diagnosis till the first therapeutic gesture was 3.7 months during the analyzed period, meaning a possible immortal time bias. Forty percent of mNSCLC patients were heavily pretreated before ICIs with surgery or radio-chemotherapy, and 38.6% of patients received palliative RT for brain or bone metastases or to control loco-regional disease. On top of the high metastatic burden of the mNSCLC patients’ cohort study, thirty-eight patients (17.6%) presented synchronous or metachronous neoplasia, as specified above, challenging further therapies for already profoundly pretreated patients or requiring alternative treatments for other neoplasia. In a nutshell, our entire cohort of mNSCLC patients was part of a significant share, unlike mNSCLC patients included in RCTs.
Clinical characteristics at baseline acknowledged 53% of mNSCLC patients diagnosed with dyslipidemia and 20% with diabetes mellitus. Moreover, 46.5% of patients were overweight. Registered data for co-medication evaluated 74 patients (34.4%) with treated infections during ICIs, such as antibiotic, antifungal, and retroviral treatments, 120 patients (55.8%) treated with steroids, 131 patients (60.9%) with PPIs, and 72 patients (33.5%) with minor or major opioids. As already widely ascertained, the gut microbiota’s impressive diversity reflects dietary habits, environmental exposure, antibiotic usage, and lifestyle factors [
51,
52]. The human microbiota dynamically interacts with the host’s immune system, implying an essential role in immune maturation, maintenance of mucosal barriers, and regulation of inflammatory responses [
53,
54]. Chalabi M. previously published in 2020 the negative impact of cumulative use of multiple or prolonged antibiotics on treatment outcomes of immunotherapy [
55]. A similar retrospective study evaluating antibiotic use in advanced cancer patients treated with immunotherapy highlighted clear worse survival outcomes [
16]. In conclusion, the intestinal microbiome emerged as a factor that might modulate ICI efficacy and may be altered by antibiotic use [
56]. Similarly, long-term usage of proton-pump inhibitors (PPIs) before ICI therapy seems to have a dismal impact on overall survival [
57] because microbial alterations in gut microbiota are more significant than with antibiotics or other drug use [
58]. Chen B et al. reported in 2022 a meta-analysis and systematic review that confirmed PPIs’ alteration to the gut microbiome and influence on response to ICIs [
59]. In our research as well, PPI usage and treated infections during ICI therapy demonstrated a harmful effect on overall ICI outcome. Moreover, our retrospective study lines up with the literature data in proving the detrimental effect of steroid use on ICI efficacy [
15]. Considering that ICIs are designed to enhance the immune system’s inherent antitumor activity, and steroids have immunosuppressive properties, inevitably the association might have undesirable consequences. Available data suggest that 10 mg of prednisone, as substitution therapy, has no influence over ICI outcomes, and this dose is often used in treating hypophysitis, as Cortisone Acetate has limited availability in Romania. If in other irAEs the steroid or immunosuppressant’s need could have detrimental effects on the antitumor efficacy and this effect could be dose and time-exposure related, in endocrine irAEs the substitutive therapy is not expected to counteract or impact the antitumor ICI activity. Larger doses of steroids used before or soon after ICI initiation were correlated with poorer clinical outcomes [
15]. Our cohort study identified palliative usage of steroids and PPIs for brain metastasis, bone, or brain radiotherapy, meaning patients with severe disease during ICIs. Also, steroids and PPIs were used in chemoprevention emesis protocols. To specifically assess the cause–effect relationship between steroid and PPI usage and ICI efficacy, dedicated specific protocol studies should be implemented. Our cohort study was a retrospective, non-interventional in nature, with no pre-specified protocol. Consequently, we could not assess the causality effect of steroids and PPIs used in lung cancer patients treated with immunotherapy, and it was not a main point of the study. To finalize the assessment of co-medication used in our cohort, it is worth revealing opioid usage during ICI treatment. Alongside the central nervous system, opioid receptors are also expressed in the gastrointestinal tract, especially on neurons of the enteric nervous system, intestinal epithelial cells, and immune cells, indicating a possible effect on gut barrier function [
60,
61]. Therefore, opioid usage might lead to dysregulated immune response and dysbiosis [
62,
63,
64], and, not surprisingly, inferior outcomes in cancer patients treated with ICIs [
65]. To summarize, co-medication is crucial during ICIs in mNSCLC patients in need of steroids and PPIs alongside palliative bone or whole brain radiotherapy or chemotherapy emesis prevention or requiring opioids for pain management. Not only co-medication influences gut microbiota, but there is an evolving hypothesis of interactions between microbiota and the endocrine system [
66].
The incidence of endocrinopathies in the whole population has dramatically changed over the past decades, influenced by lifestyle factors. Golden S.H. et al. reported a comprehensive review including articles and cohorts of patients from the United States, indicating at least a 5% prevalence of endocrine disorders. The most prevalent disorders were diabetes mellitus and metabolic dysfunctions, with no published population-based data on hypothyroidism incidence [
67]. During immunotherapy in cancer patients, the incidence of endocrine disorders as immune-related adverse events was completely different, reported as high and increasing over years of getting more experience using ICIs. If in 2017 Barroso-Sousa R. et al. reported a 3.8% incidence of all grade hypothyroidism with anti-CTLA-4 antibodies, 7% with anti-PD-1, and 13.2% with a combination [
68], in 2023, Won S.Y. et al. published an increased incidence of immune-related hypothyroidism of 4.4% with CTLA-4 inhibitors, 13.7% with PD-1 inhibitors, and a decreased incidence of 9.7% with an anti-CTLA-4/PD-L1 antibody combination [
69]. To summarize, the incidence of immune-mediated endocrine disorders was increasing as more cancer patients and additional indications of usage in further diverse cancer sites were legally approved by regulatory authorities. Taking into consideration that patients excluded from RCTs, such as patients with active brain metastasis, active infections, steroid usage above 10 mg of daily prednisone or equivalent, were not investigated for endocrine irAEs, RWD were even more crucial to provide experience from clinical practice in such challenging populations of cancer patients.
The actual retrospective study reported endocrine irAEs that followed the incidence, severity grading, and well-known management from RCTs [
5,
6,
7,
8,
9,
10,
11]. Immune-related thyroid disorder is well established to be the most regular and recurrent autoimmune endocrinopathy in the course of immune check-point inhibitors, mainly associated with anti-PD-1 monotherapy [
8,
9,
43,
70]. Clinical presentation is challenging, as new onset hypothyroidism or a transient hyperthyroid state followed by an euthyroid state or hypothyroidism might occur. ICI protocols recommend TFTs each cycle to diagnose thyroid toxicity early, and oncology practice adheres to close surveillance. Also, in our cohort study, we evaluated TFTs each cycle and monitored thyroid function as developing toward immune-related adverse events or recovering to euthyroid status after subclinical alteration. Even if TFTs were normal each ICI cycle, we assessed thyroid function regularly, as guideline protocols recommended. We reported patients with transitional modified TFTs to better acknowledge the possibility of temporary effects of ICIs on thyroid function. Our retrospective analysis did not convey any case of Graves’ disease or thyroid storm or myxedema coma, as the literature rarely reports such [
71,
72,
73,
74]. Thyroid immune-related adverse events revealed an exciting research endpoint, associated with better overall survival in some studies [
19,
22,
75,
76] and conflicting results in others [
20,
21,
77]. ICI-induced hypophysitis is a thought-provoking clinical situation, because non-specific symptoms or adrenal crisis, a potential life-threatening emergency may occur in cancer patients treated with immunotherapy. When immune-related hypophysitis is suspected, the diagnose needs detection of low morning cortisol and low ACTH values. If thyroid gland is also affected, low TSH and low FT4 values are present. If gonadotroph lines are affected, low gonadal hormones plus low luteinizing hormone (LH) and follicle stimulating hormone (FSH) are also present. Additionally, electrolytes should be obtained. Posterior pituitary involvement may present with hyponatremia. Ir-hypophysitis is more related to and associated with improved response to anti-CTLA4 antibodies [
68,
78]. Primary adrenal insufficiency (PAI) stands also as a sporadic immune-related endocrinopathy that mimics symptoms of cancer progression, and a high suspicion should be well maintained during treatment to avoid adrenal crisis [
79,
80]. If low morning cortisol levels and high ACTH values are found inconclusive, a cosyntropin (Synacthene) test could be performed [
81]. For patients already on substitution therapy with prednisone, cortisol testing was performed after a 24 h pause of substitution therapy plus a cosyntropin test for reassurance. Laboratory testing often reveals hyponatremia, hyperkalemia, and hypoglycemia. Unlike secondary adrenal insufficiency, mineralocorticoid deficiency might be caused by PAI, and plasma renin and aldosterone levels could be useful for differential diagnosis [
82]. Our research identified just one moderate to severe endocrine irAE, and that was one case of PAI, associated with a Nivolumab/Ipilimumab combination and treated with Prednisone hormonal therapy (the only available therapy). Diabetes mellitus as a worsening preexisting DM or new onset of insulin-dependent DM is one of the most infrequent autoimmune endocrinopathies during ICI therapy in mNSCLC patients, with an incidence below 0.6%, as reported in RCTs [
9,
11,
30,
32,
33,
34,
43]. To help differentiate type 1 from type 2 DM, low levels of insulin and C peptide might be useful [
83,
84]. Despite the usual management of treatment with methylprednisolone in irAEs, immune-related-DM does not benefit from steroid therapy [
84]. In our study, type I DM occurred in three mNSCLC patients treated with ICIs, and insulin therapy was initiated by a specialist.
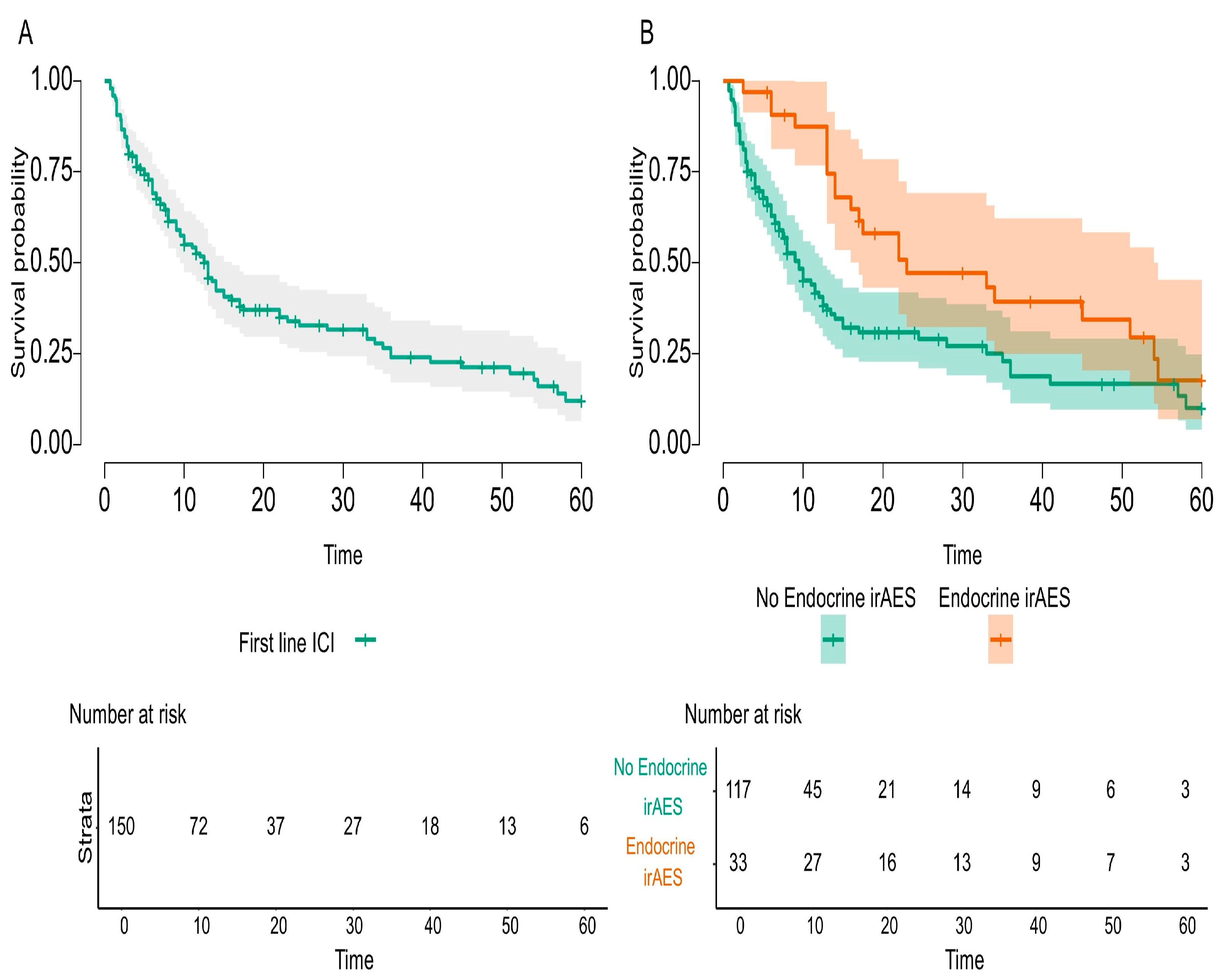
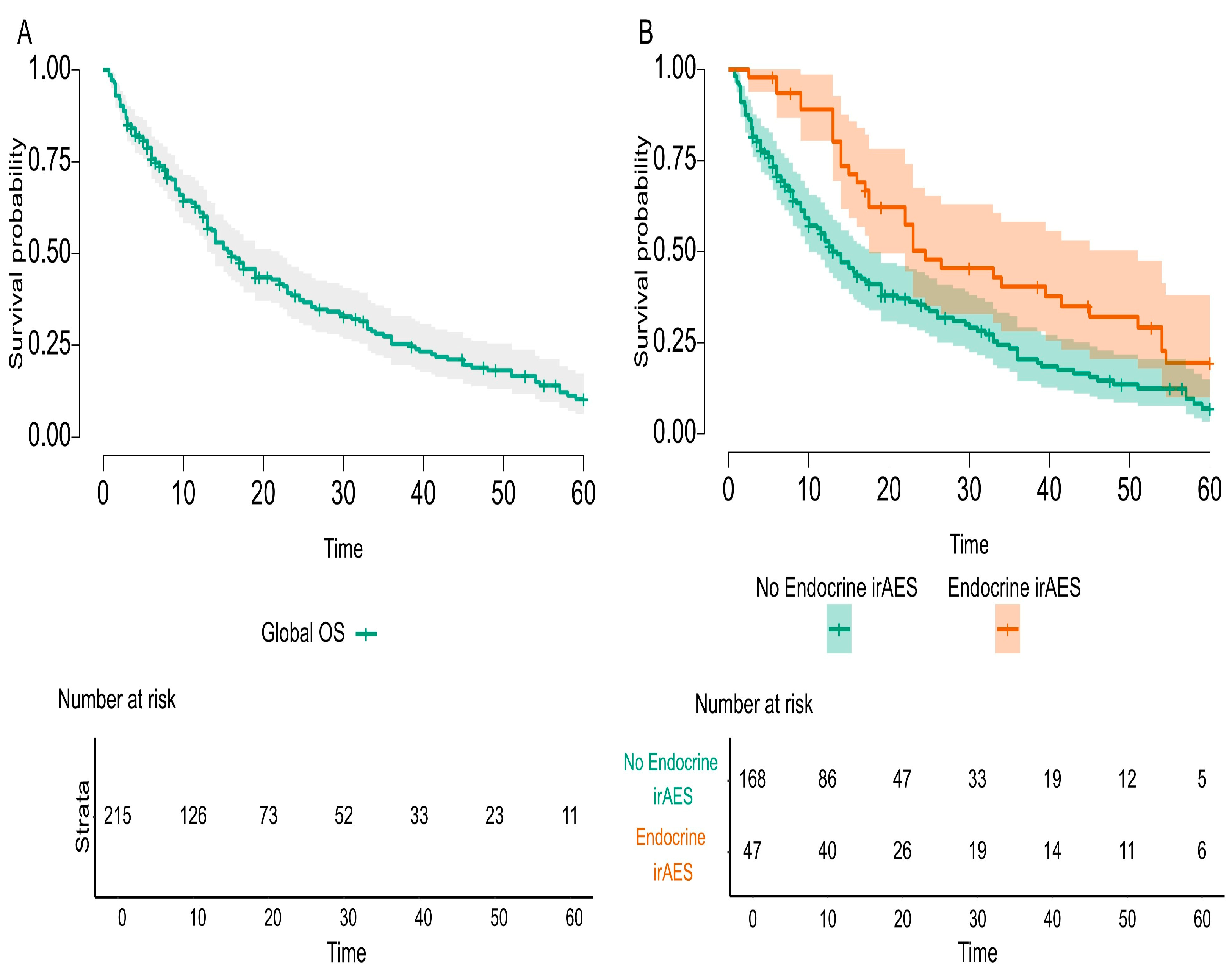
The main endpoint of the study was to investigate the possible relationship between endocrine irAEs and immunotherapy response, and our study fulfilled this imperative outcome. The overall survival was statistically significant and improved in mNSCLC patients that developed endocrine irAEs compared to the entire cohort of the study (
p-value = 0.002) and compared to first-line ICI-treated patients (
p-value = 0.004). Nevertheless, the literature acknowledges conflicting results. On the one hand, there are data that prove better overall survival for cancer patients treated with immune check-point inhibitors [
19,
22,
85,
86], and, conversely, inconsistent statistics that diminish the value of irAEs [
20,
21]. Enlightenment could arise from late-onset and long-lasting irAEs that are underreported but common events during ICI therapy. In our cohort research, we discovered an interesting and challenging clinical practice of mNSCLC patients treated in the first line with Pembrolizumab (plus ChT for the first 4–6 cycles, in PD-L1 < 50%, and according to the histological report) beyond 35 cycles, as studied in RCTs [
8,
9,
30,
31]. Taking into consideration that this first-line cohort of patients treated with Pembrolizumab represented almost half of the entire cohort study, we could assess the incidence and prevalence of late-onset and long-lasting endocrine irAEs, as specified before. Additionally, this first-line cohort of patients received steroids and PPIs as co-medication for chemotherapy emesis prevention. On the one hand, longer exposure of responding mNSCLC patients to ICIs produced a higher incidence of endocrine irAEs; the question raised was how to differentiate between steroid-induced endocrinopathy and immune-related endocrine toxicity? On the other hand, endocrine irAEs are not treated with steroids as are all other irAEs, so the detrimental effect of steroids did not counteract this kind of toxicity. Finally, to better distinguish and identify the causality effect of co-medication and late-onset and long-lasting endocrine irAEs in responding to ICI mNSCLC patients, specific protocol studies should be implemented. Consequently, patients have an assurance of survival up to the onset of that toxicity, better explained by the immortal-time bias. Specific endocrine irAEs, such as PAI or hypophysitis or DM, usually appear after several months of immunotherapy or even years, and the short period of follow-up or incomplete reporting of duration and resolution of toxicities definitely underrates the clinical magnitude of endocrine irAEs. RWE should be a mandatory consecutive support to RCTs, as it delivers data on a broader spectrum of real-life cancer patients on long time scales.
In addition, our study revealed surgery before ICIs as another predictor, with a positive impact on lung cancer patients’ survival. Deeply investigating the medical trajectory of our cohort, surgery was identified as curative gesture in early stages of the disease, with a more favorable prognostic factor, allowing the patient a long time of progression free survival until relapse. Surgical removal of the primary lung tumor, if resectable, continues to stand as the cornerstone of lung cancer treatment [
87,
88]. Surgery itself does not impact ICI survival; it just grants alongside immunotherapy an extended survival.
In conclusion, this retrospective cohort research of mNSCLC patients investigated the possible correlation between immune-mediated endocrinopathies as predictive events for ICI response and overall survival, as a core endpoint outcome. The secondary endpoint of the study fulfilled its purpose by identifying clinical conditions with potential impacts on ICI efficacy. As thoroughly discussed above, the analysis acknowledged significant statistical correlations between endocrine irAEs and ICI responses and proved the negative impact of specific co-medications during ICIs, such as steroids, PPIs, and opioids. Nevertheless, being a retrospective cohort from real-life clinical practice, the patient population was different from RCTs, more heterogenous, frailer, and with more severe disease. Even if the sample size appeared to be rather small, thinking of one tertiary-level hospital in Romania, not the high-volume Lung Cancer Institute, the number of patients could be matched with RCTs that are multicentered, multinational, and delivered in specialized oncologic medical institutions [
43,
89]. A pooled analysis of KEYNOTE-021, -189 and -407 studies that investigated Pembrolizumab plus platinum-based chemotherapy for mNSCLC patients with stable brain metastasis showed a proportion of patients with brain metastasis of 13.17% [
90]. In addition, all patients were in good performance status, with an ECOG 0-1. In contrast, our study included 21.4% of patients with brain metastasis and patients in moderate performance status, with an ECOG 0-2, and 21.4% of patients with an ECOG-3, normally excluded from ICI RCTs. As RCTs were conducted in homogenous cancer populations, such as histopathological subtypes, positive and negative PD-L1 cohorts, ICI in monotherapy, or plus ChT, the current study included all treated mNSCLC patients in a specific period of time, leading to a more heterogenous patient population. To divide this population into specific subtypes would have increased homogeneity but prevented a fluent statistical analysis.
We assert the limitations of our retrospective research. Nonetheless, this study cohort was a transparent and honest piece of real-life data, unveiling the challenges, imperfections and limitations that oncologists faced in daily practice. Our research has several limitations to be listed. First of all, our retrospective-in-nature observational study did not a have an a priori-defined protocol and used registered data, resulting in potential selection bias of patients. Secondly, the retrospective eligibility criteria of mNSCLC treated with ICIs shaped a heterogeneous cohort of patients and pointed toward confusion bias. Multiple and varied types of treatment during the study might have induced an immortal-time bias. Finally, the frequency and methods of tumor assessment were not always standardized or recorded; some patients were lost to follow-up after one or two cycles of ICIs, implying missing data and information bias. Although RWD studies are more expected to present all these biases compared with clinical trials, RWD research might generate evidence from sub-populations under-represented in RCTs, building higher generalizability than clinical trials. Nonetheless, this study cohort was a transparent and honest piece of real-life data, unveiling the challenges, imperfections, and limitations that oncologists face in daily practice.