Sex-Related Differences in Immunotherapy Toxicities: Insights into Dimorphic Responses
Simple Summary
Abstract
1. Introduction
2. Methods
3. Biological Factors Underpinning Gender-Related Differences in Drug Toxicities: The Role of Sex Hormones and Pharmacokinetics
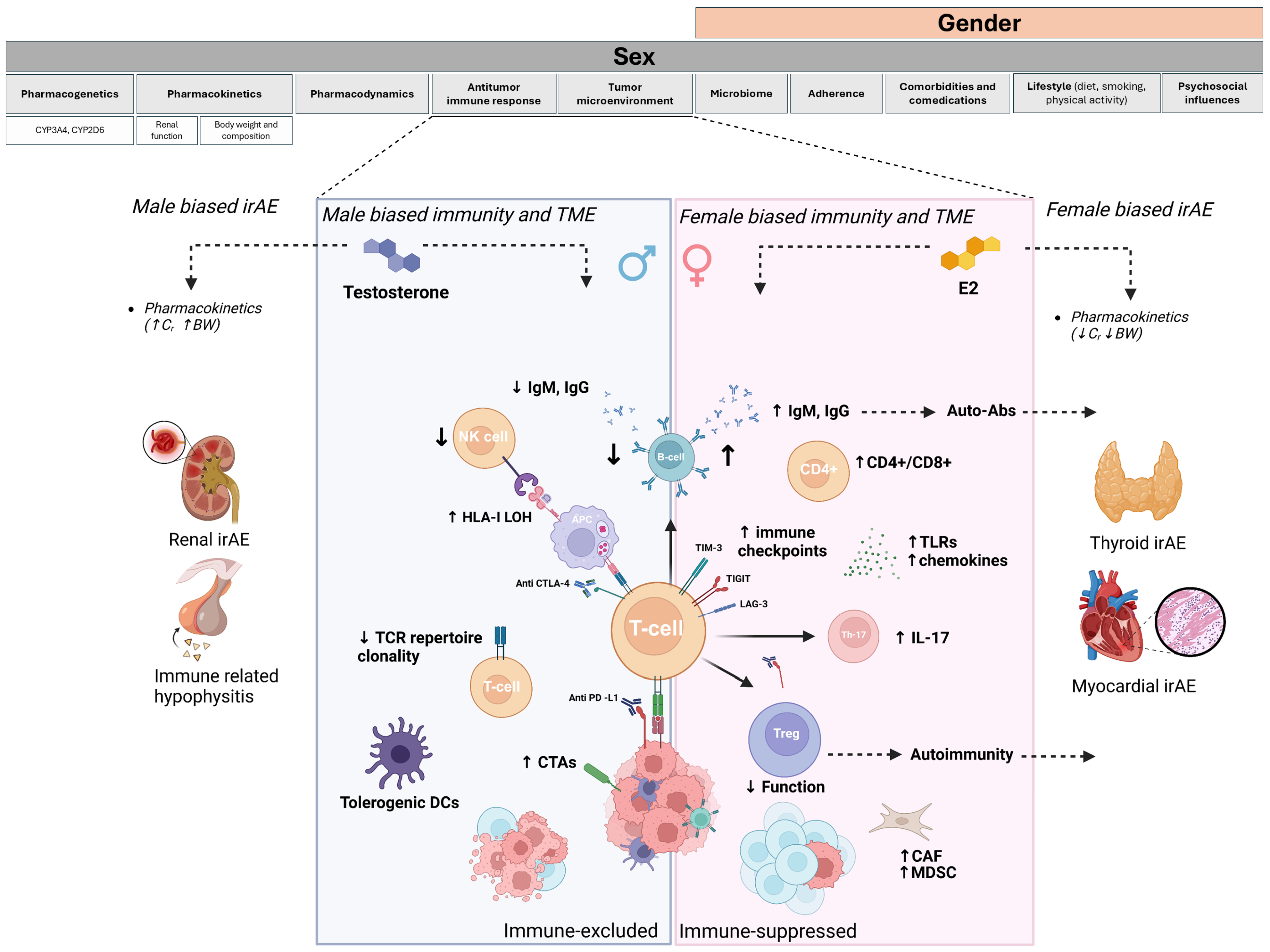
4. Sex Differences in Immunity and Antitumor Response
4.1. Sex Differences and Immunity
4.2. Sex Differences and Antitumor Response
5. Sex-Based Differences in Efficacy and Immune-Related Toxicities of Immune Checkpoint Inhibitors: Evidence and Insights
5.1. Sex Differences and ICIs Efficacy
5.2. Sex Difference and irAE Incidence
| Study | Pts | Type of Cancer | ICIs | irAE Type | Incidence (Female vs. Male) | OR (Female vs. Male) 95% CI [p = Value] |
|---|---|---|---|---|---|---|
| Unger et al. (2022) [59] | 2319 | Multiple cancer types | Anti-PD-1/PD-L1 +/− Anti-CTLA-4 | Hematologic; Cardiotoxicity; Skin; Endocrine; GI; Neurological | 56.6% vs. 48.8% (severe) | 1.49 (1.24–1.78) p < 0.001 |
| Duma et al. (2021) [66] | 476 | Melanoma; NSCLC | Anti-PD-1 | Endocrine; Arthralgia; Pneumonitis | 67% vs. 60% vs. 46% | 1.12 (1.08–1.20) p < 0.04 |
| Kudura et al. (2022) [69] | 103 | Melanoma | Anti-PD-1; Anti-PD-1 + Anti-CTLA-4 | Skin; Thyroid, | 45% vs. 32% | / |
| Bui et al. (2022) [67] | 235 | Melanoma | Anti-PD-1, PD-L1, CTLA4, PD-1 + CTLA-4 | Dermatological | 62.4% vs. 48.6% | 2.1 (1.2–3.8) p = 0.01 |
| Valpione et al. (2018) [71] | 140 | Melanoma | Anti-CTLA-4 | Dermatological, GI, Endocrine | / | 1.5 (1.06–2.16) p = 0.22 |
| Jing et al. (2021) [65] | 2982 | Melanoma, NSCLC, RCC | Anti-PD-1, PD-L1 | / | NSCLC 38.7% vs. 43.8%; Melanoma 39% vs. 45% | Pooled OR 1.19 (0.91–1.54); [p = 0.21] NSCLC: 0.97 (0.58–1.62); [p = 0.92] Melanoma 1.28 (1.01–1.63) [p = 0.04] |
| Miceli et al. (2023) [73] | 204 | Multiple cancer types | Anti-PD-1/PD-L1 +/− Anti-CTLA-4 | Endocrine, hepatic | 27% vs. 11% | / |
| Wahli et al. (2022) [74] | 689 | Multiple cancer types | Anti-PD-1, PD-L1, CTLA-4, Anti-PD-1 + Anti-CTLA-4 | 38.4% vs. 28.1% | / | |
| Muir et al. (2021) [63] | 1246 | Melanoma | Anti-PD-1, Anti-PD-1 + Anti-CTLA-4 | Thyroid | / | 1.62 (1.27–2.08) p < 0.001 |
6. Mechanisms Underlying Sex Dimorphism in irAEs
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| CAF | cancer-associated fibroblast |
| CTAs | cancer–testis antigens |
| CYP2C19 | Cytochrome P450 2C19 |
| CYP2D6 | Cytochrome P450 2D6 |
| CYP3A4 | Cytochrome P450 3A4 |
| DCs | Dendritic cells |
| E2 | 17-β-estradiol |
| GFR | Glomerular filtration rate |
| HLA | Human leukocyte antigen |
| HSPA5 | Heat shock protein family A (Hsp70) member 5 |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IL-17 | Interleukin 17 |
| LAG3 | Lymphocyte-activation gene 3 |
| LAGE-1 | L antigen family member 1 |
| MAGE-A1 | Melanoma-associated antigen A1 |
| MAGE-A3 | Melanoma-associated antigen A3 |
| MAGE-A4 | Melanoma-associated antigen A4 |
| MANF | Mesencephalic astrocyte-derived neurotrophic factor |
| NY-ESO-1 | New York esophageal squamous cell carcinoma 1 |
| RCTs | Randomized controlled trials |
| SNPs | Single nucleotide polymorphisms |
| TCR | T-cell receptor |
| TIGIT | T-cell immunoreceptor with Ig and ITIM domains |
| TIM-3 | T-cell immunoglobulin and mucin-domain containing-3 |
| TLR | Toll-like receptor |
| TME | Tumor microenvironment |
| TMZ | Temozolomide |
References
- Rubin, J.B. The Spectrum of Sex Differences in Cancer. Trends Cancer 2022, 8, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Rakshith, H.T.; Lohita, S.; Rebello, A.P.; Goudanavar, P.S.; Raghavendra Naveen, N. Sex Differences in Drug Effects and/or Toxicity in Oncology. Curr. Res. Pharmacol. Drug Discov. 2023, 4, 100152. [Google Scholar] [CrossRef] [PubMed]
- Mendis, S.; Anand, S.; Karasinska, J.M.; Dasari, A.; Unger, J.M.; Gothwal, A.; Ellis, L.M.; Varadhachary, G.; Kopetz, S.; Overman, M.J.; et al. Sex Representation in Clinical Trials Associated with FDA Cancer Drug Approvals Differs Between Solid and Hematologic Malignancies. Oncologist 2020, 26, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Zucker, I.; Prendergast, B.J. Sex Differences in Pharmacokinetics Predict Adverse Drug Reactions in Women. Biol. Sex Differ. 2020, 11, 32. [Google Scholar] [CrossRef]
- Özdemir, B.C.; Csajka, C.; Dotto, G.-P.; Wagner, A.D. Sex Differences in Efficacy and Toxicity of Systemic Treatments: An Undervalued Issue in the Era of Precision Oncology. J. Clin. Oncol. 2018, 36, 2680–2683. [Google Scholar] [CrossRef]
- Soldin, O.P.; Chung, S.H.; Mattison, D.R. Sex Differences in Drug Disposition. Biomed Res. Int. 2011, 2011, 187103. [Google Scholar] [CrossRef]
- Van Pelt, R.E.; Gavin, K.M.; Kohrt, W.M. Regulation of Body Composition and Bioenergetics by Estrogens. Endocrinol. Metab. Clin. N. Am. 2015, 44, 663–676. [Google Scholar] [CrossRef]
- Cotreau, M.M.; Von Moltke, L.L.; Greenblatt, D.J. The Influence of Age and Sex on the Clearance of Cytochrome P450 3A Substrates. Clin. Pharmacokinet. 2005, 44, 33–60. [Google Scholar]
- Bebawy, M.; Chetty, M. Gender Differences in P-Glycoprotein Expression and Function: Effects on Drug Disposition and Outcome. Curr. Drug Metab. 2009, 10, 322–328. [Google Scholar] [CrossRef]
- Milano, G.; Etienne, M.C.; Cassuto-Viguier, E.; Thyss, A.; Santini, J.; Frenay, M.; Renee, N.; Schneider, M.; Demard, F. Influence of Sex and Age on Fluorouracil Clearance. J. Clin. Oncol. 1992, 10, 1171–1175. [Google Scholar] [CrossRef]
- Haupt, S.; Caramia, F.; Klein, S.L.; Rubin, J.B.; Haupt, Y. Sex Disparities Matter in Cancer Development and Therapy. Nat. Rev. Cancer 2021, 21, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Souyris, M.; Cenac, C.; Azar, P.; Daviaud, D.; Canivet, A.; Grunenwald, S.; Pienkowski, C.; Chaumeil, J.; Mejía, J.E.; Guéry, J.-C. TLR7 Escapes X Chromosome Inactivation in Immune Cells. Sci. Immunol. 2018, 3, eaap8855. [Google Scholar] [CrossRef]
- Bianchi, I.; Lleo, A.; Gershwin, M.E.; Invernizzi, P. The X Chromosome and Immune Associated Genes. J. Autoimmun. 2012, 38, J187–J192. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E. Why Autoimmunity Is Most Common in Women. Nature 2021, 595, S51–S53. [Google Scholar] [CrossRef]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Chakraborty, B.; Byemerwa, J.; Krebs, T.; Lim, F.; Chang, C.Y.; McDonnell, D.P. Estrogen Receptor Signaling in the Immune System. Endocr. Rev. 2023, 44, 117–141. [Google Scholar] [CrossRef]
- Tan, I.J.; Peeva, E.; Zandman-Goddard, G. Hormonal Modulation of the Immune System—A Spotlight on the Role of Progestogens. Autoimmun. Rev. 2015, 14, 536–542. [Google Scholar] [CrossRef]
- Pujantell, M.; Altfeld, M. Consequences of Sex Differences in Type I IFN Responses for the Regulation of Antiviral Immunity. Front. Immunol. 2022, 13, 986840. [Google Scholar] [CrossRef]
- Dodd, K.C.; Menon, M. Sex Bias in Lymphocytes: Implications for Autoimmune Diseases. Front. Immunol. 2022, 13, 945762. [Google Scholar] [CrossRef]
- Abdullah, M.; Chai, P.S.; Chong, M.Y.; Tohit, E.R.M.; Ramasamy, R.; Pei, C.P.; Vidyadaran, S. Gender Effect on in Vitro Lymphocyte Subset Levels of Healthy Individuals. Cell. Immunol. 2012, 272, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Oertelt-Prigione, S. The Influence of Sex and Gender on the Immune Response. Autoimmun. Rev. 2012, 11, A479–A485. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Accardi, G.; Virruso, C.; Candore, G. Sex, Gender and Immunosenescence: A Key to Understand the Different Lifespan between Men and Women? Immun. Ageing 2013, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender Differences in Autoimmune Disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [CrossRef]
- Dragin, N.; Bismuth, J.; Cizeron-Clairac, G.; Biferi, M.G.; Berthault, C.; Serraf, A.; Nottin, R.; Klatzmann, D.; Cumano, A.; Barkats, M.; et al. Estrogen-Mediated Downregulation of AIRE Influences Sexual Dimorphism in Autoimmune Diseases. J. Clin. Investig. 2016, 126, 1525. [Google Scholar] [CrossRef]
- Cunningham, M.; Gilkeson, G. Estrogen Receptors in Immunity and Autoimmunity. Clin. Rev. Allergy Immunol. 2011, 40, 66–73. [Google Scholar] [CrossRef]
- Fairweather, D.L.; Rose, N.R. Women and Autoimmune Diseases. Emerg. Infect. Dis. 2004, 10, 2005. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Wong, S.K.; Wan Hasan, W.N.; Jolly, J.J.; Nur-Farhana, M.F.; Ima-Nirwana, S.; Chin, K.Y. The Relationship between Circulating Testosterone and Inflammatory Cytokines in Men. Aging Male 2019, 22, 129–140. [Google Scholar] [CrossRef]
- Trigunaite, A.; Dimo, J.; Jørgensen, T.N. Suppressive Effects of Androgens on the Immune System. Cell. Immunol. 2015, 294, 87–94. [Google Scholar] [CrossRef]
- Page, S.T.; Plymate, S.R.; Bremner, W.J.; Matsumoto, A.M.; Hess, D.L.; Lin, D.W.; Amory, J.K.; Nelson, P.S.; Wu, J.D. Effect of Medical Castration on CD4+CD25+ T Cells, CD8+ T Cell IFN-γ Expression, and NK Cells: A Physiological Role for Testosterone and/or Its Metabolites. Am. J. Physiol. Endocrinol. Metab. 2006, 290, 856–863. [Google Scholar] [CrossRef]
- Pan, F.; Du, H.; Tian, W.; Xie, H.; Zhang, B.; Fu, W.; Li, Y.; Ling, Y.; Zhang, Y.; Fang, F.; et al. Effect of GnRH Immunocastration on Immune Function in Male Rats. Front. Immunol. 2023, 13, 1023104. [Google Scholar] [CrossRef]
- Tiwari, A.; Oravecz, T.; Dillon, L.A.; Italiano, A.; Audoly, L.; Fridman, W.H.; Clifton, G.T. Towards a Consensus Definition of Immune Exclusion in Cancer. Front. Immunol. 2023, 14, 1084887. [Google Scholar] [CrossRef]
- Laffont, S.; Seillet, C.; Guéry, J.C. Estrogen Receptor-Dependent Regulation of Dendritic Cell Development and Function. Front. Immunol. 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Ben-Batalla, I.; Vargas-Delgado, M.E.; von Amsberg, G.; Janning, M.; Loges, S. Influence of Androgens on Immunity to Self and Foreign: Effects on Immunity and Cancer. Front. Immunol. 2020, 11, 1184. [Google Scholar] [CrossRef]
- Pala, L.; Conforti, F. The Effect of Patient Sex on the Efficacy and Safety of Anticancer Immunotherapy. Expert Opin. Drug Saf. 2021, 20, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Pagan, E.; Bagnardi, V.; De Pas, T.; Queirolo, P.; Pennacchioli, E.; Catania, C.; Cocorocchio, E.; Ferrucci, P.F.; et al. Sex-Based Dimorphism of Anticancer Immune Response and Molecular Mechanisms of Immune Evasion. Clin. Cancer Res. 2021, 27, 4311–4324. [Google Scholar] [CrossRef]
- Castro, A.; Pyke, R.M.; Zhang, X.; Thompson, W.K.; Day, C.P.; Alexandrov, L.B.; Zanetti, M.; Carter, H. Strength of Immune Selection in Tumors Varies with Sex and Age. Nat. Commun. 2020, 11, 4128. [Google Scholar] [CrossRef]
- D’angelo, S.P.; Russell, J.; Lebbé, C.; Chmielowski, B.; Gambichler, T.; Grob, J.-J.; Kiecker, F.; Rabinowits, G.; Terheyden, P.; Zwiener, I.; et al. Efficacy and Safety of First-Line Avelumab Treatment in Patients With Stage IV Metastatic Merkel Cell Carcinoma A Preplanned Interim Analysis of a Clinical Trial Supplemental Content. JAMA Oncol. 2018, 4, 180077. [Google Scholar] [CrossRef]
- Ramchandren, R.; Domingo-Domènech, E.; Rueda, A.; Trněný, M.; Feldman, T.A.; Lee, H.J.; Provencio, M.; Sillaber, C.; Cohen, J.B.; Savage, K.J.; et al. Nivolumab for Newly Diagnosed Advanced-Stage Classic Hodgkin Lymphoma: Safety and Efficacy in the Phase II CheckMate 205 Study. J. Clin. Oncol. 2019, 37, 1997–2007. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Maio, M.; Scherpereel, A.; Calabrò, L.; Aerts, J.; Perez, S.C.; Bearz, A.; Nackaerts, K.; Fennell, D.A.; Kowalski, D.; Tsao, A.S.; et al. Tremelimumab as Second-Line or Third-Line Treatment in Relapsed Malignant Mesothelioma (DETERMINE): A Multicentre, International, Randomised, Double-Blind, Placebo-Controlled Phase 2b Trial. Lancet Oncol. 2017, 18, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Murciano-Goroff, Y.R.; Betof Warner, A.; Wolchok, J.D. The Future of Cancer Immunotherapy: Microenvironment-Targeting Combinations. Cell Res. 2020, 30, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, S.Y. Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Immune Netw. 2020, 20, e9. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer Immunotherapy Efficacy and Patients’ Sex: A Systematic Review and Meta-Analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Kwon, H.; Schafer, J.M.; Song, N.-J.; Kaneko, S.; Li, A.; Xiao, T.; Ma, A.; Allen, C.; Das, K.; Zhou, L.; et al. Androgen Conspires with the CD8+ T Cell Exhaustion Program and Contributes to Sex Bias in Cancer. Sci. Immunol. 2022, 7, eabq2630. [Google Scholar] [CrossRef]
- Litchfield, K.; Reading, J.L.; Puttick, C.; Thakkar, K.; Abbosh, C.; Bentham, R.; Watkins, T.B.K.; Rosenthal, R.; Biswas, D.; Rowan, A.; et al. Meta-Analysis of Tumor- and T Cell-Intrinsic Mechanisms of Sensitization to Checkpoint Inhibition. Cell 2021, 184, 596–614.e14. [Google Scholar] [CrossRef]
- Pinto, J.A.; Vallejos, C.S.; Raez, L.E.; Mas, L.A.; Ruiz, R.; Torres-Roman, J.S.; Morante, Z.; Araujo, J.M.; Gómez, H.L.; Aguilar, A.; et al. Gender and Outcomes in Non-Small Cell Lung Cancer: An Old Prognostic Variable Comes Back for Targeted Therapy and Immunotherapy? ESMO Open 2018, 3, e000344. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Pagan, E.; Corti, C.; Bagnardi, V.; Queirolo, P.; Catania, C.; De Pas, T.; Giaccone, G. Sex-Based Differences in Response to Anti-PD-1 or PD-L1 Treatment in Patients with Non-Small-Cell Lung Cancer Expressing High PD-L1 Levels. A Systematic Review and Meta-Analysis of Randomized Clinical Trials. ESMO Open 2021, 6, 100251. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Butaney, M.; Satkunasivam, R.; Freedland, S.J.; Patel, S.P.; Hamid, O.; Pal, S.K.; Klaassen, Z. Association of Patient Sex With Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Advanced Cancers: A Systematic Review and Meta-Analysis. JAMA Oncol. 2019, 5, 529–536. [Google Scholar] [CrossRef]
- Wu, Y.; Ju, Q.; Jia, K.; Yu, J.; Shi, H.; Wu, H.; Jiang, M. Correlation between Sex and Efficacy of Immune Checkpoint Inhibitors (PD-1 and CTLA-4 Inhibitors). Int. J. Cancer 2018, 143, 45–51. [Google Scholar] [CrossRef]
- Grassadonia, A.; Sperduti, I.; Vici, P.; Iezzi, L.; Brocco, D.; Gamucci, T.; Pizzuti, L.; Maugeri-Saccà, M.; Marchetti, P.; Cognetti, G.; et al. Effect of Gender on the Outcome of Patients Receiving Immune Checkpoint Inhibitors for Advanced Cancer: A Systematic Review and Meta-Analysis of Phase III Randomized Clinical Trials. J. Clin. Med. 2018, 7, 542. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.R.; Nikita, N.; Banks, J.; Keith, S.W.; Johnson, J.M.; Wilson, M.; Lu-Yao, G. Association between Sex and Immune Checkpoint Inhibitor Outcomes for Patients with Melanoma. JAMA Netw. Open 2021, 4, e2136823. [Google Scholar] [CrossRef] [PubMed]
- Kugel, C.H.; Douglass, S.M.; Webster, M.R.; Kaur, A.; Liu, Q.; Yin, X.; Weiss, S.A.; Darvishian, F.; Al-Rohil, R.N.; Ndoye, A.; et al. Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. Clin. Cancer Res. 2018, 24, 5347–5356. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Khoja, L.; Day, D.; Wei-Wu Chen, T.; Siu, L.L.; Hansen, A.R. Tumour- and Class-Specific Patterns of Immune-Related Adverse Events of Immune Checkpoint Inhibitors: A Systematic Review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-Related Adverse Events and Anti-Tumor Efficacy of Immune Checkpoint Inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-Related Adverse Events of Checkpoint Inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef]
- Unger, J.M.; Vaidya, R.; Albain, K.S.; Leblanc, M.; Minasian, L.M.; Gotay, C.C.; Lynn Henry, N.; Fisch, M.J.; Lee, S.M.; Blanke, C.D.; et al. Sex Differences in Risk of Severe Adverse Events in Patients Receiving Immunotherapy, Targeted Therapy, or Chemotherapy in Cancer Clinical Trials. J. Clin. Oncol. 2022, 40, 1474–1486. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, C.; Jin, Z.; Wu, B.; Xu, T. Sex Differences in Immune-Related Adverse Events with Immune Checkpoint Inhibitors: Data Mining of the FDA Adverse Event Reporting System. Int. J. Clin. Pharm. 2022, 44, 689–697. [Google Scholar] [CrossRef]
- Qu, J.; Ding, Y.; Jiang, K.; Hao, J.; Li, Y.; Zhang, A.; Li, Z.; Qi, G.; Xu, Z.; Liu, X.; et al. Nephrotoxicity of Immune Checkpoint Inhibitors: A Disproportionality Analysis from 2013 to 2020. Tohoku J. Exp. Med. 2021, 254, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Yao, J.; Yuan, G.; Gao, Y.; Zhang, J.; Guo, X. Immune Checkpoint Inhibitor-Associated New-Onset Hypophysitis: A Retrospective Analysis Using the FAERS. Endocrine 2024, 86, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Muir, C.A.; Clifton-Bligh, R.J.; Long, G.V.; Scolyer, R.A.; Lo, S.N.; Carlino, M.S.; Tsang, V.H.M.; Menzies, A.M. Thyroid Immune-Related Adverse Events Following Immune Checkpoint Inhibitor Treatment. J. Clin. Endocrinol. Metab. 2021, 106, E3704–E3713. [Google Scholar] [CrossRef] [PubMed]
- Zamami, Y.; Niimura, T.; Okada, N.; Koyama, T.; Fukushima, K.; Izawa-Ishizawa, Y.; Ishizawa, K. Factors Associated with Immune Checkpoint Inhibitor-Related Myocarditis. JAMA Oncol. 2019, 5, 1635–1637. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Wang, J.; Li, K.; Chen, X.; Heng, J.; Gao, Q.; Ye, Y.; Zhang, Z.; Liu, Y.; et al. Association between Sex and Immune-Related Adverse Events during Immune Checkpoint Inhibitor Therapy. J. Natl. Cancer Inst. 2021, 113, 1396–1404. [Google Scholar] [CrossRef]
- Duma, N.; Abdel-Ghani, A.; Yadav, S.; Hoversten, K.P.; Reed, C.T.; Sitek, A.N.; Enninga, E.A.L.; Paludo, J.; Aguilera, J.V.; Leventakos, K.; et al. Sex Differences in Tolerability to Anti-Programmed Cell Death Protein 1 Therapy in Patients with Metastatic Melanoma and Non-Small Cell Lung Cancer: Are We All Equal? Oncologist 2019, 24, e1148–e1155. [Google Scholar] [CrossRef]
- Bui, A.T.N.; Bougrine, A.; Buchbinder, E.I.; Giobbie-Hurder, A.; LeBoeuf, N.R. Female Sex Is Associated with Higher Rates of Dermatologic Adverse Events among Patients with Melanoma Receiving Immune Checkpoint Inhibitor Therapy: A Retrospective Cohort Study. J. Am. Acad. Dermatol. 2022, 87, 403–406. [Google Scholar] [CrossRef]
- Cortellini, A.; Buti, S.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Bersanelli, M.; Michiara, M.; Grassadonia, A.; Brocco, D.; et al. Clinical Outcomes of Patients with Advanced Cancer and Pre-Existing Autoimmune Diseases Treated with Anti-Programmed Death-1 Immunotherapy: A Real-World Transverse Study. Oncologist 2019, 24, e327–e337. [Google Scholar] [CrossRef]
- Kudura, K.; Basler, L.; Nussbaumer, L.; Foerster, R. Sex-Related Differences in Metastatic Melanoma Patients Treated with Immune Checkpoint Inhibition. Cancers 2022, 14, 5145. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Lin, H.; Wang, J.; Cao, B. Predictive Biomarkers for Immune-Related Adverse Events in Cancer Patients Treated with Immune-Checkpoint Inhibitors. BMC Immunol. 2024, 25, 8. [Google Scholar] [CrossRef]
- Valpione, S.; Pasquali, S.; Campana, L.G.; Piccin, L.; Mocellin, S.; Pigozzo, J.; Chiarion-Sileni, V. Sex and Interleukin-6 Are Prognostic Factors for Autoimmune Toxicity Following Treatment with Anti-CTLA4 Blockade. J. Transl. Med. 2018, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Eagan, L.E.; Chesney, C.A.; Mascone, S.E.; Shenouda, N.; Ranadive, S.M. Interleukin-6 Is Higher in Naturally Menstruating Women Compared with Oral Contraceptive Pill Users during the Low-Hormone Phase. J. Appl. Physiol. 2021, 131, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Miceli, R.; Eriksson, H.; Eustace, A.J.; Lo Russo, G.; Alfieri, S.; Bjaanæs, M.M.; Pietrantonio, F.; De Cecco, L.; Prelaj, A.; Proto, C.; et al. Sex Differences in Burden of Adverse Events in Patients Receiving Immunotherapy. J. Clin. Oncol. 2023, 41 (Suppl. S16), 2646. [Google Scholar] [CrossRef]
- Wahli, M.N.; Hayoz, S.; Hoch, D.; Ryser, C.O.; Hoffmann, M.; Scherz, A.; Schwacha-Eipper, B.; Häfliger, S.; Wampfler, J.; Berger, M.D.; et al. The Role of Immune Checkpoint Inhibitors in Clinical Practice: An Analysis of the Treatment Patterns, Survival and Toxicity Rates by Sex. J. Cancer Res. Clin. Oncol. 2023, 149, 3847–3858. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Trejo, T.; Luthra, H.; Griffiths, M.; David, C.S.; Taneja, V. Mechanism by Which HLA-DR4 Regulates Sex-Bias of Arthritis in Humanized Mice. J. Autoimmun. 2010, 35, 1–9. [Google Scholar] [CrossRef]
- Bluestone, J.A.; Anderson, M.; Herold, K.C.; Stamatouli, A.M.; Quandt, Z.; Perdigoto, A.L.; Clark, P.L.; Kluger, H.; Weiss, S.A.; Gettinger, S.; et al. Collateral Damage: Insulin-Dependent Diabetes Induced with Checkpoint Inhibitors. Diabetes 2018, 67, 1471–1480. [Google Scholar] [CrossRef]
- Bajaj, G.; Suryawanshi, S.; Roy, A.; Gupta, M. Evaluation of Covariate Effects on Pharmacokinetics of Monoclonal Antibodies in Oncology. Br. J. Clin. Pharmacol. 2019, 85, 2045–2058. [Google Scholar] [CrossRef]
- Melhem, M.; Hanze, E.; Lu, S.; Alskär, O.; Visser, S.; Gandhi, Y. Population Pharmacokinetics and Exposure–Response of Anti-Programmed Cell Death Protein-1 Monoclonal Antibody Dostarlimab in Advanced Solid Tumours. Br. J. Clin. Pharmacol. 2022, 88, 4142–4154. [Google Scholar] [CrossRef]
- Gill, K.L.; Machavaram, K.K.; Rose, R.H.; Chetty, M. Potential Sources of Inter-Subject Variability in Monoclonal Antibody Pharmacokinetics. Clin. Pharmacokinet. 2016, 55, 789–805. [Google Scholar] [CrossRef]
- Thomas, V.A.; Balthasar, J.P. Understanding Inter-Individual Variability in Monoclonal Antibody Disposition. Antibodies 2019, 8, 56. [Google Scholar] [CrossRef]
- Shang, J.; Huang, L.; Huang, J.; Ren, X.; Liu, Y.; Feng, Y. Population Pharmacokinetic Models of Anti-PD-1 MAbs in Patients with Multiple Tumor Types: A Systematic Review. Front. Immunol. 2022, 13, 871372. [Google Scholar] [CrossRef]
- Gu, Y.; Tang, Y.Y.; Wan, J.X.; Zou, J.Y.; Lu, C.G.; Zhu, H.S.; Sheng, S.Y.; Wang, Y.F.; Liu, H.C.; Yang, J.; et al. Sex Difference in the Expression of PD-1 of Non-Small Cell Lung Cancer. Front. Immunol. 2022, 13, 1026214. [Google Scholar] [CrossRef]
- Chennamadhavuni, A.; Abushahin, L.; Jin, N.; Presley, C.J.; Manne, A. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front. Immunol. 2022, 13, 779691. [Google Scholar] [CrossRef]
- Downey, S.G.; Klapper, J.A.; Smith, F.O.; Yang, J.C.; Sherry, R.M.; Royal, R.E.; Kammula, U.S.; Hughes, M.S.; Allen, T.E.; Levy, C.L.; et al. Prognostic Factors Related to Clinical Response in Patients with Metastatic Melanoma Treated by CTL-Associated Antigen-4 Blockade. Clin. Cancer Res. 2007, 13, 6681–6688. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Li, Y.; Qin, J.; Amancherla, K.; Jing, Y.; Hu, Q.; Liang, K.; Zhang, Z.; Ye, Y.; et al. Hormonal Therapies Up-Regulate MANF and Overcome Female Susceptibility to Immune Checkpoint Inhibitor Myocarditis. Sci. Transl. Med. 2022, 14, eabo1981. [Google Scholar] [CrossRef]
- Takahashi, M.; Kimura, A. HLA and CTLA4 Polymorphisms May Confer a Synergistic Risk in the Susceptibility to Graves Disease. J. Hum. Genet. 2010, 55, 323–326. [Google Scholar] [CrossRef]
- Pizarro, C.; García-Díaz, D.F.; Codner, E.; Salas-Pérez, F.; Carrasco, E.; Pérez-Bravo, F. PD-L1 Gene Polymorphisms and Low Serum Level of PD-L1 Protein Are Associated to Type 1 Diabetes in Chile. Diabetes Metab. Res. Rev. 2014, 30, 761–766. [Google Scholar] [CrossRef]
- Polanczyk, M.J.; Hopke, C.; Vandenbark, A.A.; Offner, H. Treg Suppressive Activity Involves Estrogen-Dependent Expression of Programmed Death-1 (PD-1). Int. Immunol. 2007, 19, 337–343. [Google Scholar] [CrossRef]
- Grigoriou, M.; Banos, A.; Hatzioannou, A.; Kloetgen, A.; Kouzis, P.; Aggouraki, D.; Zakopoulou, R.; Bamias, G.; Kassi, E.; Mavroudis, D.; et al. Regulatory T-Cell Transcriptomic Reprogramming Characterizes Adverse Events by Checkpoint Inhibitors in Solid Tumors. Cancer Immunol. Res. 2021, 9, 726–734. [Google Scholar] [CrossRef]
- Von Euw, E.; Chodon, T.; Attar, N.; Jalil, J.; Koya, R.C.; Comin-Anduix, B.; Ribas, A. CTLA4 Blockade Increases Th17 Cells in Patients with Metastatic Melanoma. J. Transl. Med. 2009, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, A.A.; Zahoor, H.; Lin, Y.; Malhotra, U.; Sander, C.; Butterfield, L.H.; Kirkwood, J.M. Baseline Circulating IL-17 Predicts Toxicity While TGF-Β1 and IL-10 Are Prognostic of Relapse in Ipilimumab Neoadjuvant Therapy of Melanoma. J. Immunother. Cancer 2015, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Stubelius, A.; Karlsson, M.N.; Engdahl, C.; Erlandsson, M.; Grahnemo, L.; Lagerquist, M.K.; Islander, U. Estrogen Regulates T Helper 17 Phenotype and Localization in Experimental Autoimmune Arthritis. Arthritis Res. Ther. 2015, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When Worlds Collide: Th17 and Treg Cells in Cancer and Autoimmunity. Cell. Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef]
- Iwama, S.; De Remigis, A.; Callahan, M.K.; Slovin, S.F.; Wolchok, J.D.; Caturegli, P. Pituitary Expression of CTLA-4 Mediates Hypophysitis Secondary to Administration of CTLA-4 Blocking Antibody. Sci. Transl. Med. 2014, 6, 230ra45. [Google Scholar] [CrossRef]
- Jirillo, E.; Rink, L.; Fransen, F.; Van Beek, A.A.; Borghuis, T.; Meijer, B.; Van Der Gaast-De Jongh, C.; Savelkoul, H.F.; De Jonge, M.I.; Faas, M.M.; et al. The Impact of Gut Microbiota on Gender-Specific Differences in Immunity. Front. Immunol. 2017, 8, 30. [Google Scholar] [CrossRef]
- Elderman, M.; de Vos, P.; Faas, M. Role of Microbiota in Sexually Dimorphic Immunity. Front. Immunol. 2018, 9, 1018. [Google Scholar] [CrossRef]
- Hu, M.; Lin, X.; Sun, T.; Shao, X.; Huang, X.; Du, W.; Guo, M.; Zhu, X.; Zhou, Y.; Tong, T.; et al. Gut Microbiome for Predicting Immune Checkpoint Blockade-Associated Adverse Events. Genome Med. 2024, 16, 16. [Google Scholar] [CrossRef]
- Pala, L.; Nezi, L.; De Pas, T.; Pennacchioli, E.; Cocorocchio, E.; Ferrucci, P.; Conforti, F.; Goldhirsch, A. Sex Differences in Efficacy and Toxicity of Systemic Cancer Treatments: Role of the Microbiome. J. Clin. Oncol. 2019, 37, 439. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal Microbiome Analyses Identify Melanoma Patients at Risk for Checkpoint-Blockade-Induced Colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Zou, H.; Han, Z.; Xie, T.; Zhang, B.; Dai, D.; Yin, X.; Liang, Y.; Kou, Y.; et al. Correlation of the Gut Microbiome and Immune-Related Adverse Events in Gastrointestinal Cancer Patients Treated with Immune Checkpoint Inhibitors. Front. Cell. Infect. Microbiol. 2023, 13, 1099063. [Google Scholar] [CrossRef] [PubMed]
- Pace, F.; Watnick, P.I. The Interplay of Sex Steroids, the Immune Response, and the Intestinal Microbiota. Trends Microbiol. 2021, 29, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Gürgöze, M.T.; van der Galiën, O.P.; Limpens, M.A.M.; Roest, S.; Hoekstra, R.C.; IJpma, A.S.; Brugts, J.J.; Manintveld, O.C.; Boersma, E. Impact of Sex Differences in Co-Morbidities and Medication Adherence on Outcome in 25 776 Heart Failure Patients. ESC Heart Fail. 2021, 8, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Miceli, R.; Eriksson, H.; Lo Russo, G.; Alfieri, S.; Bjaanæs, M.M.; Pietrantonio, F.; De Cecco, L.; Prelaj, A.; Proto, C.; Franzén, J.; et al. Gender Difference in SidE EFfects of ImmuNotherapy: A Possible Clue to Optimize CancEr TReatment (G-DEFINER): Study Protocol of an Observational Prospective Multicenter Study. Acta Oncol. 2024, 63, 213–219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canzian, J.; Conforti, F.; Jacobs, F.; Benvenuti, C.; Gaudio, M.; Gerosa, R.; De Sanctis, R.; Zambelli, A. Sex-Related Differences in Immunotherapy Toxicities: Insights into Dimorphic Responses. Cancers 2025, 17, 1054. https://doi.org/10.3390/cancers17071054
Canzian J, Conforti F, Jacobs F, Benvenuti C, Gaudio M, Gerosa R, De Sanctis R, Zambelli A. Sex-Related Differences in Immunotherapy Toxicities: Insights into Dimorphic Responses. Cancers. 2025; 17(7):1054. https://doi.org/10.3390/cancers17071054
Chicago/Turabian StyleCanzian, Jacopo, Fabio Conforti, Flavia Jacobs, Chiara Benvenuti, Mariangela Gaudio, Riccardo Gerosa, Rita De Sanctis, and Alberto Zambelli. 2025. "Sex-Related Differences in Immunotherapy Toxicities: Insights into Dimorphic Responses" Cancers 17, no. 7: 1054. https://doi.org/10.3390/cancers17071054
APA StyleCanzian, J., Conforti, F., Jacobs, F., Benvenuti, C., Gaudio, M., Gerosa, R., De Sanctis, R., & Zambelli, A. (2025). Sex-Related Differences in Immunotherapy Toxicities: Insights into Dimorphic Responses. Cancers, 17(7), 1054. https://doi.org/10.3390/cancers17071054






