Significance of 5-ALA-Guided Fluorescence in Resection of Invasive Intracranial Meningiomas: Findings from a Prospective Clinical Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Outcomes
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-ALA | 5-aminolevulinic acid |
| MRI | magnetic resonance imaging |
| PpIX | protoporphyrin IX |
| WHO | World Health Organization |
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro-Oncology 2023, 25, iv1–iv99. [Google Scholar] [CrossRef]
- Aizer, A.A.; Bi, W.L.; Kandola, M.S.; Lee, E.Q.; Nayak, L.; Rinne, M.L.; Norden, A.D.; Beroukhim, R.; Reardon, D.A.; Wen, P.Y.; et al. Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer 2015, 121, 4376–4381. [Google Scholar] [CrossRef]
- Winther, T.L.; Torp, S.H. Significance of the Extent of Resection in Modern Neurosurgical Practice of World Health Organization Grade I Meningiomas. World Neurosurg. 2017, 99, 104–110. [Google Scholar] [CrossRef]
- Przybylowski, C.J.; Suki, D.; Raza, S.M.; DeMonte, F. Volumetric extent of resection and survival for recurrent atypical meningioma. J. Neurosurg. 2023, 139, 769–779. [Google Scholar] [CrossRef]
- Soni, P.; Davison, M.A.; Shao, J.; Momin, A.; Lopez, D.; Angelov, L.; Barnett, G.H.; Lee, J.H.; Mohammadi, A.M.; Kshettry, V.R.; et al. Extent of resection and survival outcomes in World Health Organization grade II meningiomas. J. Neuro-Oncol. 2020, 151, 173–179. [Google Scholar] [CrossRef]
- Güdük, M.; Özduman, K.; Pamir, M.N. Sphenoid Wing Meningiomas: Surgical Outcomes in a Series of 141 Cases and Proposal of a Scoring System Predicting Extent of Resection. World Neurosurg. 2019, 125, e48–e59. [Google Scholar] [CrossRef]
- Hénaux, P.-L.; Bretonnier, M.; Le Reste, P.-J.; Morandi, X. Modern Management of Meningiomas Compressing the Optic Nerve: A Systematic Review. World Neurosurg. 2018, 118, E677–E686. [Google Scholar] [CrossRef]
- Eatz, T.A.; Eichberg, D.G.; Lu, V.M.; Di, L.; Komotar, R.J.; Ivan, M.E. Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: A systematic review. J. Neuro-Oncol. 2022, 156, 233–256. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- McCracken, D.J.; Schupper, A.J.; Lakomkin, N.; Malcolm, J.; Bray, D.P.; Hadjipanayis, C.G. Turning on the light for brain tumor surgery: A 5-aminolevulinic acid story. Neuro-Oncology 2022, 24, S52–S61. [Google Scholar] [CrossRef]
- Barbosa, B.J.A.P.; Mariano, E.D.; Batista, C.M.; Marie, S.K.N.; Teixeira, M.J.; Pereira, C.U.; Tatagiba, M.S.; Lepski, G.A. Intraoperative assistive technologies and extent of resection in glioma surgery: A systematic review of prospective controlled studies. Neurosurg. Rev. 2014, 38, 217–227. [Google Scholar] [CrossRef]
- Specchia, F.M.C.; Monticelli, M.; Zeppa, P.; Bianconi, A.; Zenga, F.; Altieri, R.; Pugliese, B.; Di Perna, G.; Cofano, F.; Tartara, F.; et al. Let Me See: Correlation between 5-ALA Fluorescence and Molecular Pathways in Glioblastoma: A Single Center Experience. Brain Sci. 2021, 11, 795. [Google Scholar] [CrossRef]
- Spille, D.C.; Bunk, E.C.; Thomas, C.; Özdemir, Z.; Wagner, A.; Akkurt, B.H.; Mannil, M.; Paulus, W.; Grauer, O.M.; Stummer, W.; et al. Protoporphyrin IX (PpIX) Fluorescence during Meningioma Surgery: Correlations with Histological Findings and Expression of Heme Pathway Molecules. Cancers 2023, 15, 304. [Google Scholar] [CrossRef]
- Bunk, E.C.; Wagner, A.; Stummer, W.; Senner, V.; Brokinkel, B. 5-ALA kinetics in meningiomas: Analysis of tumor fluorescence and PpIX metabolism in vitro and comparative analyses with high-grade gliomas. J. Neuro-Oncol. 2021, 152, 37–46. [Google Scholar] [CrossRef]
- Della Puppa, A.; Rustemi, O.; Gioffrè, G.; Troncon, I.; Lombardi, G.; Rolma, G.; Sergi, M.; Munari, M.; Cecchin, D.; Gardiman, M.P.; et al. Predictive value of intraoperative 5-aminolevulinic acid–induced fluorescence for detecting bone invasion in meningioma surgery. J. Neurosurg. 2014, 120, 840–845. [Google Scholar] [CrossRef]
- Millesi, M.; Kiesel, B.; Mischkulnig, M.; Martínez-Moreno, M.; Wöhrer, A.; Wolfsberger, S.; Knosp, E.; Widhalm, G. Analysis of the surgical benefits of 5-ALA–induced fluorescence in intracranial meningiomas: Experience in 204 meningiomas. J. Neurosurg. 2016, 125, 1408–1419. [Google Scholar] [CrossRef]
- Cornelius, J.; Slotty, P.; Kamp, M.; Schneiderhan, T.; Steiger, H.; El-Khatib, M. Impact of 5-aminolevulinic acid fluorescence-guided surgery on the extent of resection of meningiomas–With special regard to high-grade tumors. Photodiagnosis Photodyn. Ther. 2014, 11, 481–490. [Google Scholar] [CrossRef]
- Kajimoto, Y.; Kuroiwa, T.; Miyatake, S.-I.; Ichioka, T.; Miyashita, M.; Tanaka, H.; Tsuji, M. Use of 5-aminolevulinic acid in fluorescence-guided resection of meningioma with high risk of recurrence. J. Neurosurg. 2007, 106, 1070–1074. [Google Scholar] [CrossRef]
- Scheichel, F.; Popadic, B.; Kitzwoegerer, M.; Ungersboeck, K.; Marhold, F. Fluorescence-guided resection in bone and soft tissue infiltrating meningiomas. Acta Neurochir. 2019, 162, 605–611. [Google Scholar] [CrossRef]
- Coluccia, D.; Fandino, J.; Fujioka, M.; Cordovi, S.; Muroi, C.; Landolt, H. Intraoperative 5-aminolevulinic-acid-induced fluorescence in meningiomas. Acta Neurochir. 2010, 152, 1711–1719. [Google Scholar]
- Potapov, A.A.; Goryaynov, S.A.; Okhlopkov, V.A.; Shishkina, L.V.; Loschenov, V.B.; Savelieva, T.A.; Golbin, D.A.; Chumakova, A.P.; Goldberg, M.F.; Varyukhina, M.D.; et al. Laser biospectroscopy and 5-ALA fluorescence navigation as a helpful tool in the meningioma resection. Neurosurg. Rev. 2016, 39, 437–447. [Google Scholar] [CrossRef]
- Marbacher, S.; Klinger, E.; Schwyzer, L.; Fischer, I.; Nevzati, E.; Diepers, M.; Roelcke, U.; Fathi, A.-R.; Coluccia, D.; Fandino, J. Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg. Focus 2014, 36, E10. [Google Scholar] [CrossRef]
- Wilbers, E.; Hargus, G.; Wölfer, J.; Stummer, W. Usefulness of 5-ALA (Gliolan®)-derived PPX fluorescence for demonstrating the extent of infiltration in atypical meningiomas. Acta Neurochir. 2014, 156, 1853–1854. [Google Scholar] [CrossRef] [PubMed]
- Wadiura, L.I.; Millesi, M.; Makolli, J.; Wais, J.; Kiesel, B.; Mischkulnig, M.; Mercea, P.A.; Roetzer, T.; Knosp, E.; Rössler, K.; et al. High Diagnostic Accuracy of Visible 5-ALA Fluorescence in Meningioma Surgery According to Histopathological Analysis of Tumor Bulk and Peritumoral Tissue. Lasers Surg. Med. 2020, 53, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Valdes, P.A.; Millesi, M.; Widhalm, G.; Roberts, D.W. 5-aminolevulinic acid induced protoporphyrin IX (ALA-PpIX) fluorescence guidance in meningioma surgery. J. Neuro-Oncol. 2019, 141, 555–565. [Google Scholar] [CrossRef]
- Foster, N.; Eljamel, S. ALA-induced fluorescence image guided surgery of meningiomas: A meta-analyses. Photodiagnosis Photodyn. Ther. 2016, 15, 73–78. [Google Scholar] [CrossRef]
- Dijkstra, B.M.; Jeltema, H.-R.; Kruijff, S.; Groen, R.J.M. The application of fluorescence techniques in meningioma surgery—A review. Neurosurg. Rev. 2018, 42, 799–809. [Google Scholar] [CrossRef]
- Chung, I.W.H.; Eljamel, S. Risk factors for developing oral 5-aminolevulenic acid-induced side effects in patients undergoing fluorescence guided resection. Photodiagnosis Photodyn. Ther. 2013, 10, 362–367. [Google Scholar] [CrossRef]
- Bianconi, A.; Bonada, M.; Zeppa, P.; Colonna, S.; Tartara, F.; Melcarne, A.; Garbossa, D.; Cofano, F. How Reliable Is Fluorescence-Guided Surgery in Low-Grade Gliomas? A Systematic Review Concerning Different Fluorophores. Cancers 2023, 15, 4130. [Google Scholar] [CrossRef]
- Masubuchi, T.; Kajimoto, Y.; Kawabata, S.; Nonoguchi, N.; Fujishiro, T.; Miyatake, S.-I.; Kuroiwa, T. Experimental Study to Understand Nonspecific Protoporphyrin IX Fluorescence in Brain Tissues Near Tumors After 5-Aminolevulinic Acid Administration. Photomed. Laser Surg. 2013, 31, 428–433. [Google Scholar] [CrossRef]
- Knipps, J.; Beseoglu, K.; Kamp, M.; Fischer, I.; Felsberg, J.; Neumann, L.M.; Steiger, H.-J.; Cornelius, J.F. Fluorescence Behavior and Dural Infiltration of Meningioma Analyzed by 5-Aminolevulinic Acid–Based Fluorescence: Operating Microscope Versus Mini-Spectrometer. World Neurosurg. 2017, 108, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, J.; Placke, J.; Knipps, J.; Fischer, I.; Kamp, M.; Steiger, H. Minispectrometer with handheld probe for 5-ALA based fluorescence-guided surgery of brain tumors: Preliminary study for clinical applications. Photodiagnosis Photodyn. Ther. 2016, 17, 147–153. [Google Scholar] [CrossRef] [PubMed]
| n = 13 | % | |
|---|---|---|
| Age, years | ||
| median | 66 | |
| range | 42–76 | |
| Sex | ||
| male | 4 | 30.8 |
| female | 9 | 69.2 |
| Disease stage | ||
| newly diagnosed | 7 | 53.8 |
| recurrent | 6 | 46.2 |
| Location | ||
| convexity | 4 | 30.8 |
| parasagittal | 1 | 7.7 |
| olfactory groove | 1 | 7.7 |
| planum sphenoidale | 1 | 7.7 |
| sphenoid ridge | 2 | 15.4 |
| spheno-orbital | 2 | 15.4 |
| middle fossa | 1 | 7.7 |
| trigone of the lateral ventricle | 1 | 7.7 |
| Histological diagnosis | ||
| WHO Grade 1 | 10 | 76.9 |
| meningothelial | 6 | 46.2 |
| transitional | 3 | 23.1 |
| angiomatous | 1 | 7.7 |
| WHO Grade 2 | 2 | 15.4 |
| atypical | 2 | 15.4 |
| WHO Grade 3 | 1 | 7.7 |
| anaplastic | 1 | 7.7 |
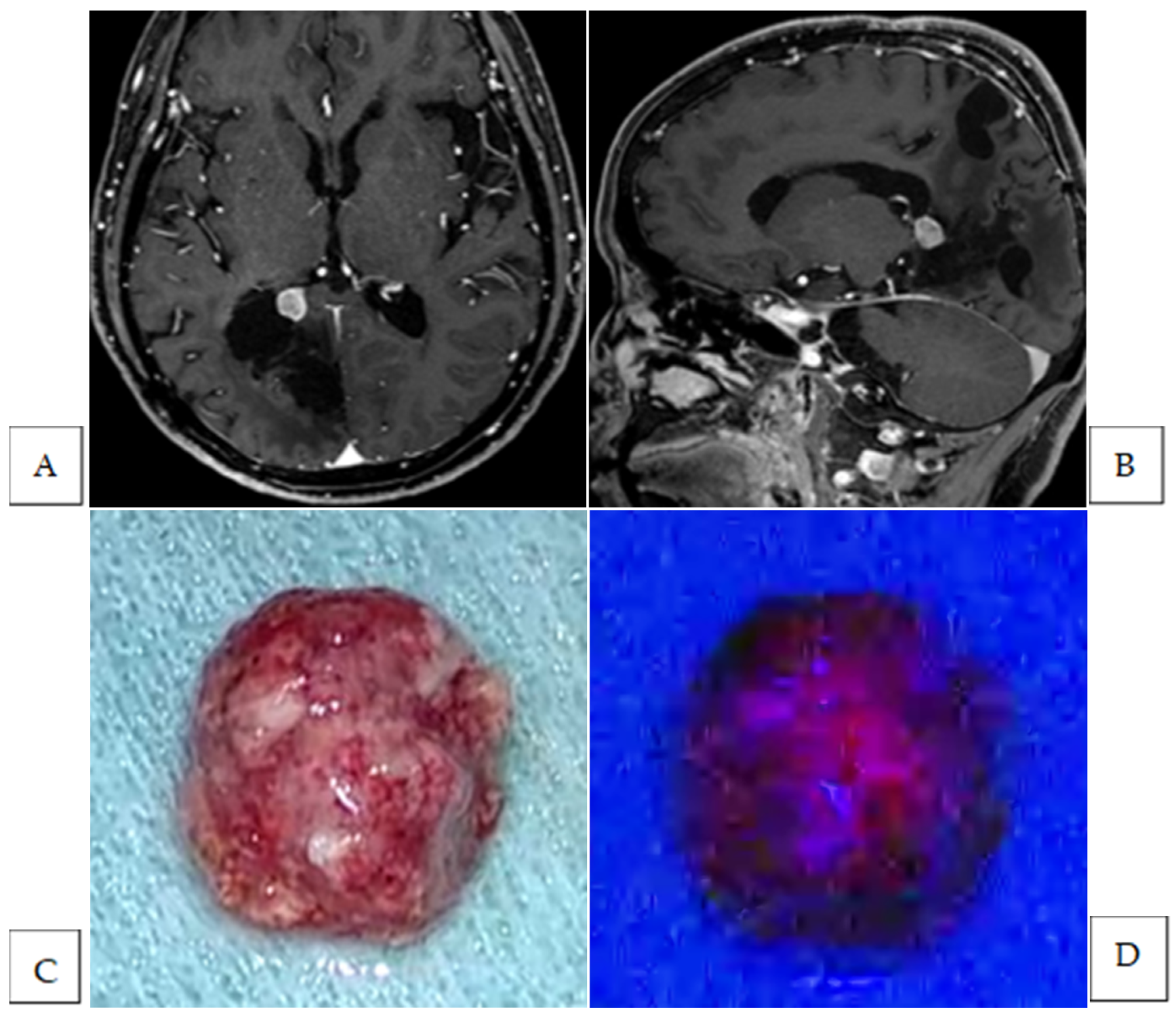
| Case No. | Location | Histological Diagnosis | Tumor Fluorescence | Residual Tumor First Identified by Fluorescence Diagnosis | Additional Resection Following Fluorescence Diagnosis | Site and Number of Additional Resection | Tumor Cells in Specimens from Additional Resection | |
|---|---|---|---|---|---|---|---|---|
| 1 | olfactory groove | meningothelial | None | |||||
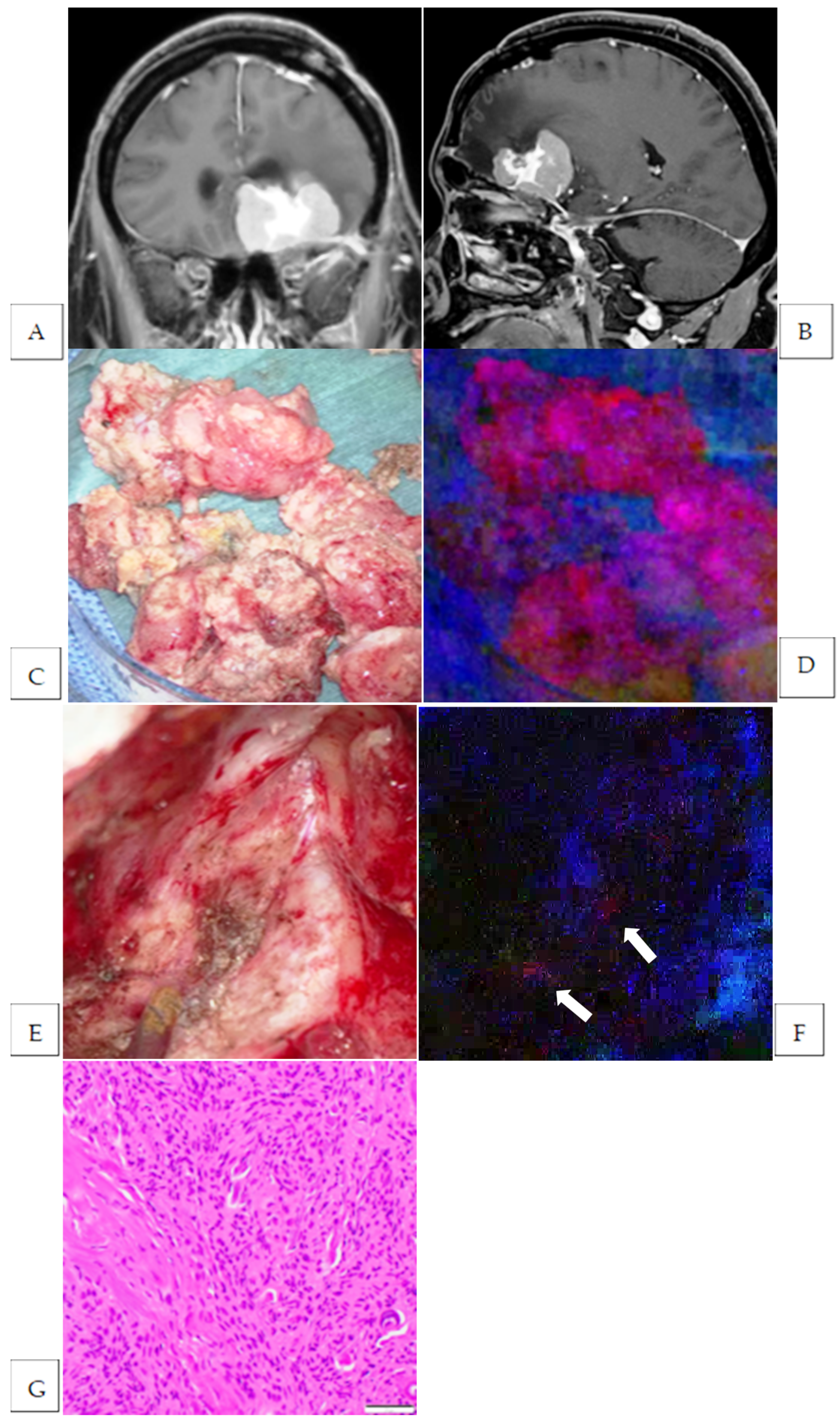
| 2 | parasagittal | atypical | Strong | yes | yes | brain | 2 | no |
| 3 | planum sphenoidale | meningothelial | Strong | yes | yes | brain | 1 | no |
| dura | 1 | yes | ||||||
| 4 | spheno-orbital | atypical | Vague | no | no | |||
| 5 | convexity | angiomatous | None | |||||
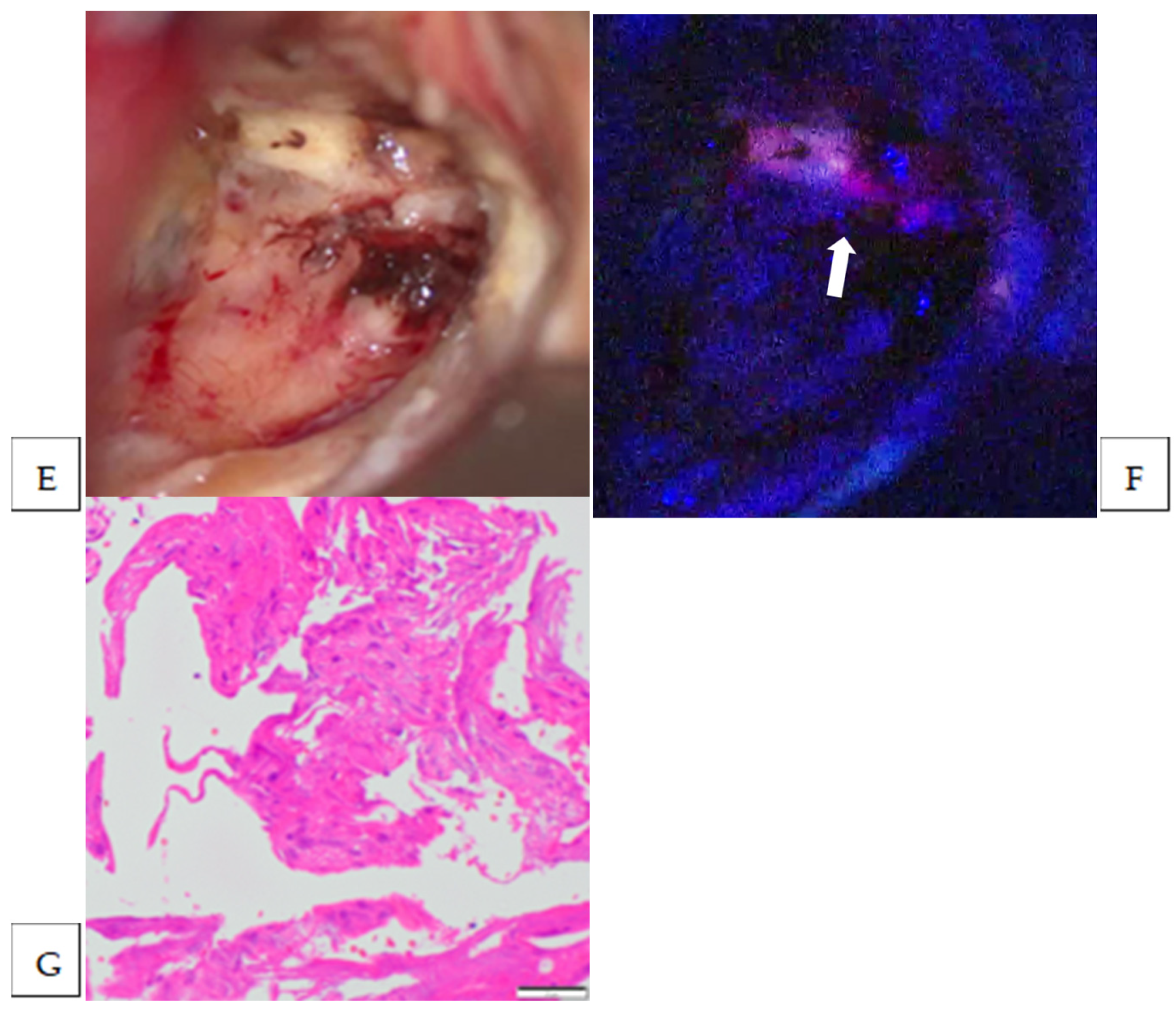
| 6 | trigone of the lateral ventricle | meningothelial | Strong | yes | yes | brain | 1 | yes |
| 7 | middle fossa | transitional | Strong | yes | yes | brain | 1 | no |
| dura | 1 | yes | ||||||
| bone | 2 | yes | ||||||
| 8 | convexity | meningothelial | Strong | no | no | |||
| 9 | convexity | meningothelial | Strong | yes | yes | brain | 1 | no |
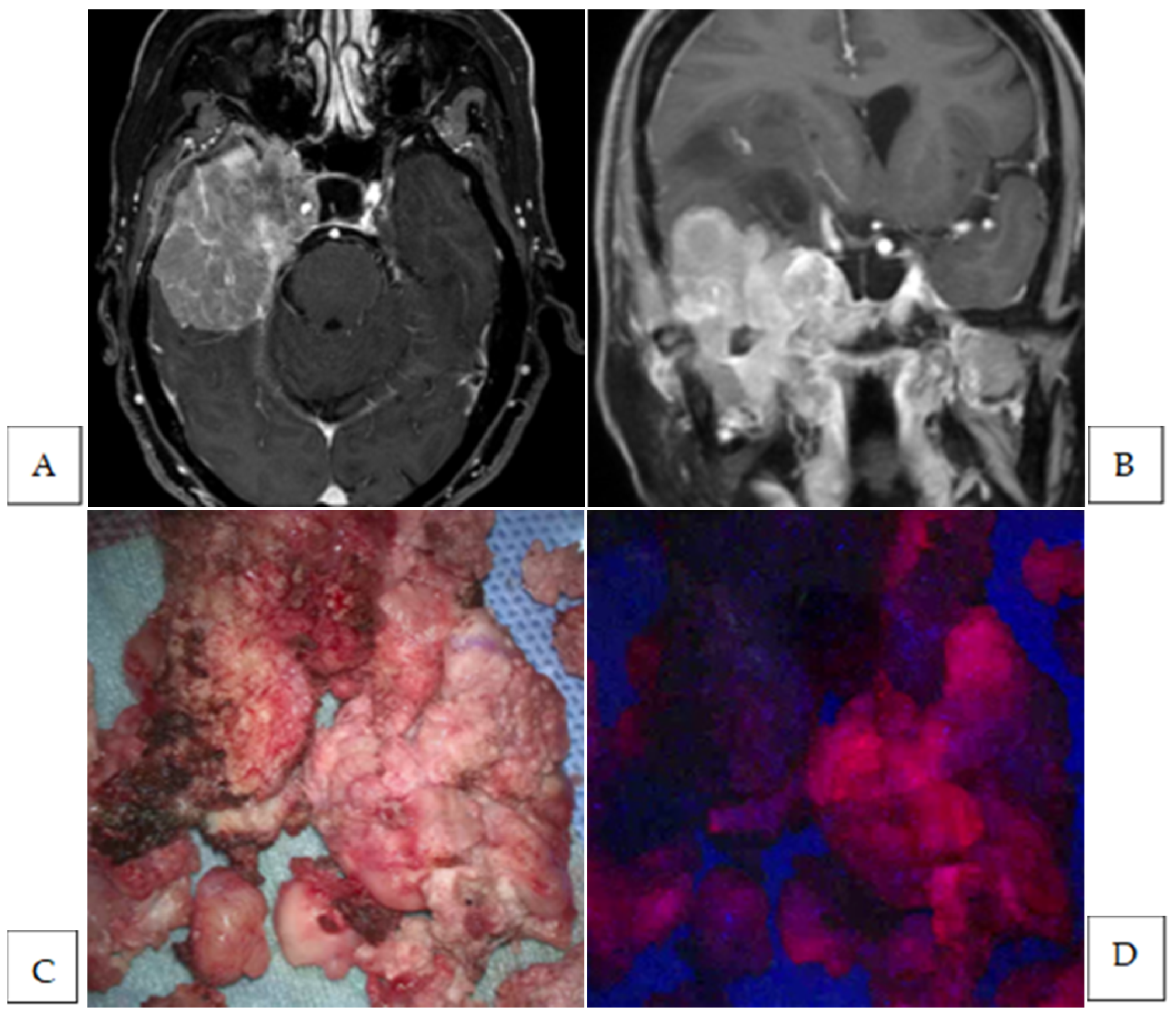
| 10 | sphenoid ridge | anaplastic | Strong | yes | yes | dura | 2 | NA |
| 11 | sphen-oorbital | meningothelial | Strong | yes | yes | bone | 1 | yes |
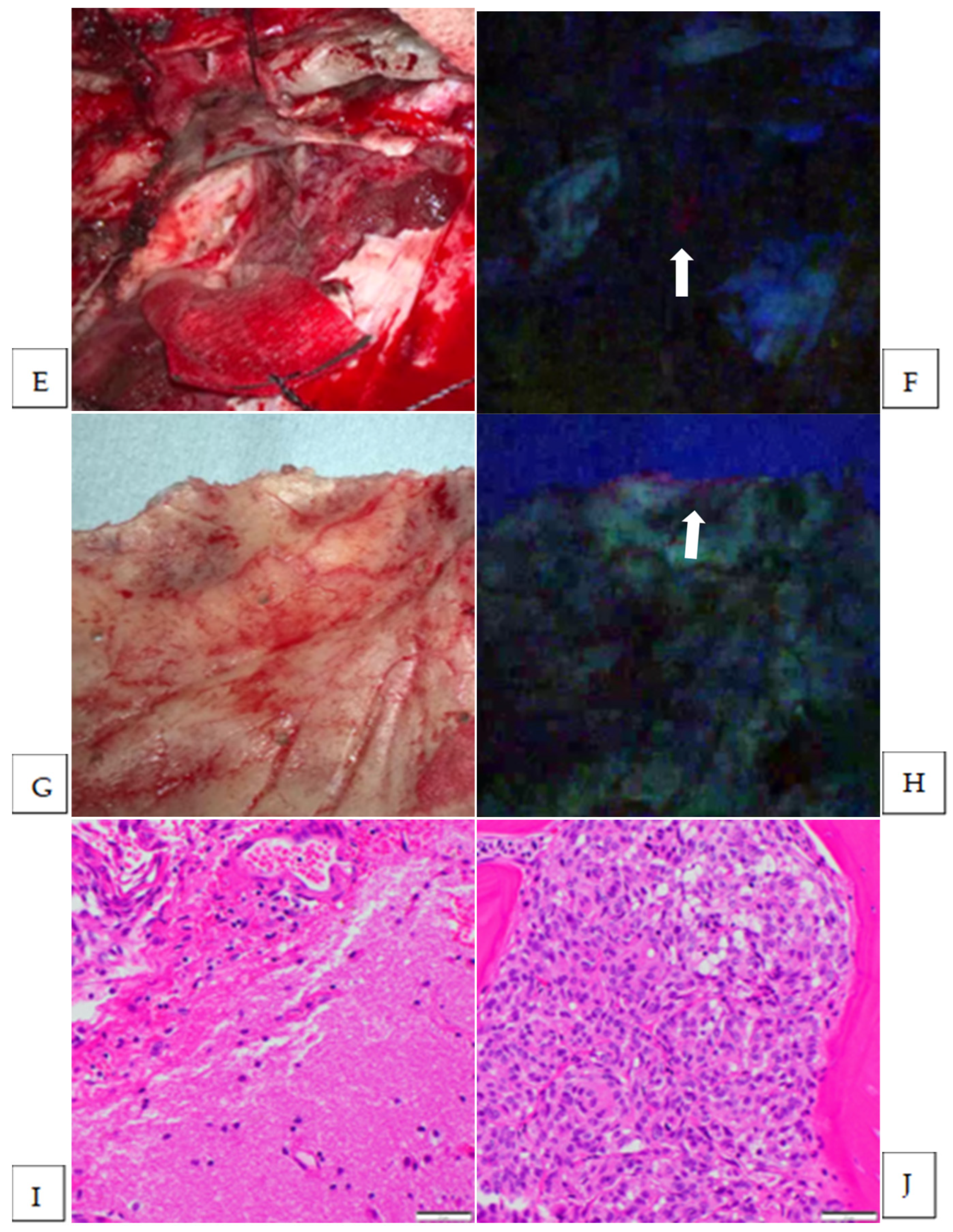
| 12 | convexity | transitional | Vague | yes | yes | brain | 1 | no |
| 13 | sphenoid ridge | transitional | Strong | yes | yes | brain | 2 | yes |
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 3–4 (%) |
|---|---|---|---|---|---|
| Appetite loss | 1 | 0.0 | |||
| Hypotension | 1 | 7.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, M.; Sugii, N.; Sakamoto, N.; Yamano, A.; Ishikawa, E. Significance of 5-ALA-Guided Fluorescence in Resection of Invasive Intracranial Meningiomas: Findings from a Prospective Clinical Study. Cancers 2025, 17, 1191. https://doi.org/10.3390/cancers17071191
Matsuda M, Sugii N, Sakamoto N, Yamano A, Ishikawa E. Significance of 5-ALA-Guided Fluorescence in Resection of Invasive Intracranial Meningiomas: Findings from a Prospective Clinical Study. Cancers. 2025; 17(7):1191. https://doi.org/10.3390/cancers17071191
Chicago/Turabian StyleMatsuda, Masahide, Narushi Sugii, Noriaki Sakamoto, Akinari Yamano, and Eiichi Ishikawa. 2025. "Significance of 5-ALA-Guided Fluorescence in Resection of Invasive Intracranial Meningiomas: Findings from a Prospective Clinical Study" Cancers 17, no. 7: 1191. https://doi.org/10.3390/cancers17071191
APA StyleMatsuda, M., Sugii, N., Sakamoto, N., Yamano, A., & Ishikawa, E. (2025). Significance of 5-ALA-Guided Fluorescence in Resection of Invasive Intracranial Meningiomas: Findings from a Prospective Clinical Study. Cancers, 17(7), 1191. https://doi.org/10.3390/cancers17071191








