Mimicking the Complexity of Solid Tumors: How Spheroids Could Advance Cancer Preclinical Transformative Approaches
Simple Summary
Abstract
1. Introduction
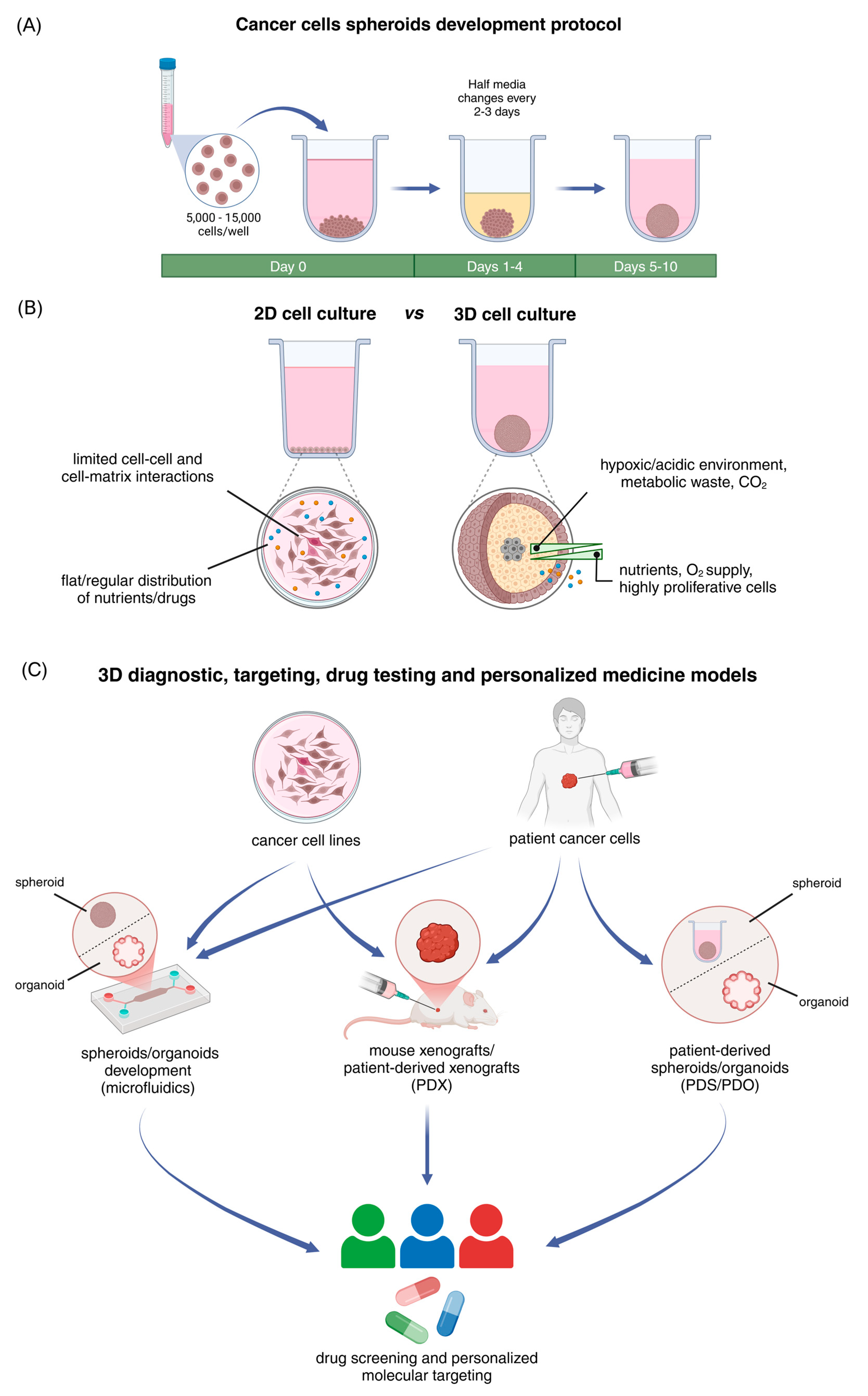
2. Spheroids as Effective Models That Replicate the Structural and Functional Characteristics of Solid Tumors
| Cancer Properties/ECM Composition | Differential Gene/Protein Expression in 2D vs. 3D Cell Models | Cancer Type | References |
|---|---|---|---|
| Proliferation/growth | AURKA, AURKB, CDK1, CDK2, CDK4, CDK5, CDK8, CDK16 | Breast cancer | [42] |
| c-Myc, Rac1 | Lymphoma | [53] | |
| PIM2 | Neuroblastoma cancer | [55] | |
| MYC | Colorectal cancer | [56] | |
| MYC | Lung cancer | [44] | |
| EMT/MET | CDH1 *, CDH2 *, VIM *, FN1, TWIST1 | HNSCC | [46] |
| TWIST1, CDH2, VIM, FN1 | Lung cancer (NSCLC) | [49] | |
| CDH1 *, VIM *, Claudin, N-cadherin | Breast cancer | [57,58] | |
| EPCAM, VIM | Lung cancer | [44] | |
| FN1 | Ovarian/Cervical cancer | [48,59] | |
| Vimentin, P-Cadherin, N-Cadherin, E-Cadherin, β-catenin, Snail | Ovarian cancer | [60,61] | |
| E-cadherin, β-catenin, Vimentin, N-Cadherin, Fibronectin | Gastric cancer | [45] | |
| CDH1 *, CDH2 *, VIM *, SNAI1 *, SNAI2 *, TWIST1 *, ZEB1 * | Prostate cancer | [62] | |
| Migration/invasion | MMP2, MMP9 | Lung cancer (NSCLC) | [49] |
| MMP1 | Ovarian/Cervical cancer | [48,59] | |
| MMP-2, MMP-9, Tiam1 | Lymphoma | [53] | |
| MMP-9 | Colorectal cancer | [63] | |
| MMP9, MMP14 | Lung cancer | [44] | |
| Receptors | CD44 | Colorectal cancer | [1] |
| CD44 isoforms | Prostate cancer | [62] | |
| EGFR | HNSCC | [46] | |
| EGFR, HER2, HER3, HER4 | Breast cancer | [52] | |
| EGFR | Pancreas cancer | [47] | |
| ESR1, ESR2 | Thyroid cancer | [64] | |
| Stemness | OCT4, SOX2 | Colorectal cancer | [1] |
| NANOG, SOX2 * | HNSCC | [46] | |
| NANOG, SOX2, CD133, POU5F1, ALDH1A3 | Lung cancer (NSCLC) | [49] | |
| ALDH1A1, SERPINB3, SERPINB5, CDH3, KRT19 | Breast cancer | [42] | |
| c-Kit, Sca-1 | Lymphoma | [53] | |
| NANOG, SOX2, POU5F1 | Thyroid cancer | [64] | |
| ALDH3A1 | Melanoma, renal cancer | [51] | |
| CD117, CD133 | Prostate cancer | [65] | |
| ECM composition/deposition | P3H3, P3H4, PLOD1, PLOD3, TIMP2, ITGB1, LAMA3, LAMA4, COL4A1, COL4A2 | Breast cancer | [42] |
| LAMC2 | Ovarian cancer | [59] | |
| COL1A1 | Lung cancer | [44] | |
| Drug response/Drug resistance | MDR1, ABCG2 | Lung cancer (NSCLC) | [49] |
| MRP1, LRP | Prostate cancer | [50] | |
| MDR1, MRP1, BCRP | Lymphoma | [53] | |
| MDR1 * | Melanoma, renal cancer | [51] | |
| BCRP | Breast cancer | [52] | |
| TP53 | Hepatocellular carcinoma | [54] |
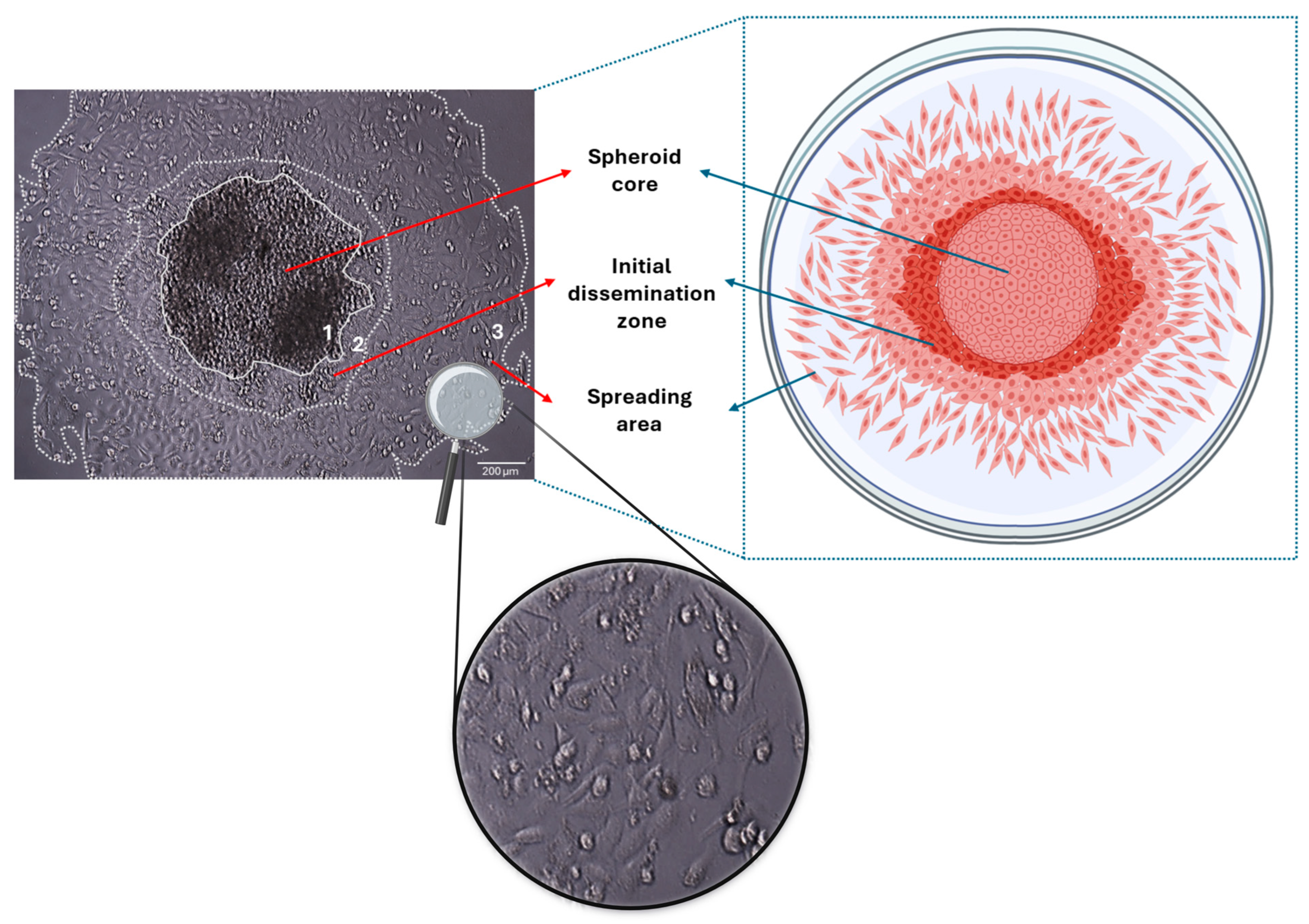
3. Three-Dimensional Spheroids—Models in Studying Tumor Development and the Initial Stages of Tumor Spreading/Cancer Cell Dissemination
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D/3D | Two-dimensional/three-dimensional |
| ECM | Extracellular matrix |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-to-mesenchymal transition |
| PDO | Patient-derived organoids |
| PDS | Patient-derived spheroids |
| PDX | Patient-derived xenografts |
| TME | Tumor microenvironment |
References
- Abbas, Z.N.; Al-Saffar, A.Z.; Jasim, S.M.; Sulaiman, G.M. Comparative analysis between 2D and 3D colorectal cancer culture models for insights into cellular morphological and transcriptomic variations. Sci. Rep. 2023, 13, 18380. [Google Scholar] [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2016, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Piperigkou, Z.; Kyriakopoulou, K.; Koutsakis, C.; Mastronikolis, S.; Karamanos, N.K. Key Matrix Remodeling Enzymes: Functions and Targeting in Cancer. Cancers 2021, 13, 1441. [Google Scholar] [CrossRef]
- Dogan, E.; Galifi, C.A.; Cecen, B.; Shukla, R.; Wood, T.L.; Miri, A.K. Extracellular matrix regulation of cell spheroid invasion in a 3D bioprinted solid tumor-on-a-chip. Acta Biomater. 2024, 186, 156–166. [Google Scholar] [CrossRef]
- Mai, Z.; Lin, Y.; Lin, P.; Zhao, X.; Cui, L. Modulating extracellular matrix stiffness: A strategic approach to boost cancer immunotherapy. Cell Death Dis. 2024, 15, 307. [Google Scholar] [CrossRef]
- Deng, B.; Zhao, Z.; Kong, W.; Han, C.; Shen, X.; Zhou, C. Biological role of matrix stiffness in tumor growth and treatment. J. Transl. Med. 2022, 20, 540. [Google Scholar] [CrossRef]
- Franchi, M.; Piperigkou, Z.; Karamanos, K.-A.; Franchi, L.; Masola, V. Extracellular Matrix-Mediated Breast Cancer Cells Morphological Alterations, Invasiveness, and Microvesicles/Exosomes Release. Cells 2020, 9, 2031. [Google Scholar] [CrossRef]
- Benelli, R.; Zocchi, M.R.; Poggi, A. Three-Dimensional (3D) Culture Models in Cancer Investigation, Drug Testing and Immune Response Evaluation. Int. J. Mol. Sci. 2020, 22, 150. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Koutsakis, C.; Piperigkou, Z.; Karamanos, N.K. Recreating the extracellular matrix: Novel 3D cell culture platforms in cancer research. FEBS J. 2023, 290, 5238–5247. [Google Scholar] [CrossRef] [PubMed]
- Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Scaffold-based 3D cell culture models in cancer research. J. Biomed. Sci. 2024, 31, 7. [Google Scholar] [CrossRef]
- Mangani, S.; Piperigkou, Z.; Koletsis, N.E.; Ioannou, P.; Karamanos, N.K. Estrogen receptors and extracellular matrix: The critical interplay in cancer development and progression. FEBS J. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.; Bentivoglio, V.; Varani, M.; Signore, A. Three-Dimensional In Vitro Tumor Spheroid Models for Evaluation of Anticancer Therapy: Recent Updates. Cancers 2023, 15, 4846. [Google Scholar] [CrossRef]
- Ryu, N.-E.; Lee, S.-H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef]
- Vakhshiteh, F.; Bagheri, Z.; Soleimani, M.; Ahvaraki, A.; Pournemat, P.; Alavi, S.E.; Madjd, Z. Heterotypic tumor spheroids: A platform for nanomedicine evaluation. J. Nanobiotechnol. 2023, 21, 249. [Google Scholar] [CrossRef]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef]
- Salinas-Vera, Y.M.; Valdés, J.; Pérez-Navarro, Y.; Mandujano-Lazaro, G.; Marchat, L.A.; Ramos-Payán, R.; Nuñez-Olvera, S.I.; Pérez-Plascencia, C.; López-Camarillo, C. Three-Dimensional 3D Culture Models in Gynecological and Breast Cancer Research. Front. Oncol. 2022, 12, 826113. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.L.; McDevitt, T.C. Biofunctional Hydrogels for Three-Dimensional Stem Cell Culture. In Biology and Engineering of Stem Cell Niches; Elsevier: Amsterdam, The Netherlands, 2017; pp. 345–362. ISBN 978-0-12-802734-9. [Google Scholar]
- Karamanos, N.K.; Piperigkou, Z.; Gourdoupi, C.; Mangani, S.; Vivanco, M.d. Extracellular matrix matters: Matrix-based bioscaffolds in advancing translational cancer research and targeted therapy. Am. J. Physiol.-Cell Physiol. 2025. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.P.; Gaspar, V.M.; Mendes, L.; Duarte, I.F.; Mano, J.F. Organotypic 3D decellularized matrix tumor spheroids for high-throughput drug screening. Biomaterials 2021, 275, 120983. [Google Scholar] [CrossRef]
- Alsharabasy, A.M.; Pandit, A. Hyaluronan-Based Hydrogels for 3D Modeling of Tumor Tissues. Tissue Eng. Part C Methods 2024, 30, 452–499. [Google Scholar] [CrossRef]
- Redmond, J.; McCarthy, H.; Buchanan, P.; Levingstone, T.J.; Dunne, N.J. Advances in biofabrication techniques for collagen-based 3D in vitro culture models for breast cancer research. Mater. Sci. Eng. C 2021, 122, 111944. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Ibañez-Fonseca, A.; Orbanic, D.; Ximenes-Carballo, C.; Perez-Amodio, S.; Rodríguez-Cabello, J.C.; Engel, E. Elastin-like Recombinamer Hydrogels as Platforms for Breast Cancer Modeling. Biomacromolecules 2023, 24, 4408–4418. [Google Scholar] [CrossRef]
- El Harane, S.; Zidi, B.; El Harane, N.; Krause, K.-H.; Matthes, T.; Preynat-Seauve, O. Cancer Spheroids and Organoids as Novel Tools for Research and Therapy: State of the Art and Challenges to Guide Precision Medicine. Cells 2023, 12, 1001. [Google Scholar] [CrossRef]
- Kamatar, A.; Gunay, G.; Acar, H. Natural and Synthetic Biomaterials for Engineering Multicellular Tumor Spheroids. Polymers 2020, 12, 2506. [Google Scholar] [CrossRef]
- Teixeira Polez, R.; Huynh, N.; Pridgeon, C.S.; Valle-Delgado, J.J.; Harjumäki, R.; Österberg, M. Insights into spheroids formation in cellulose nanofibrils and Matrigel hydrogels using AFM-based techniques. Mater. Today Bio 2024, 26, 101065. [Google Scholar] [CrossRef]
- Baniahmad, A. Tumor spheroids and organoids as preclinical model systems. Med. Genet. 2021, 33, 229–234. [Google Scholar] [CrossRef]
- Gilazieva, Z.; Ponomarev, A.; Rutland, C.; Rizvanov, A.; Solovyeva, V. Promising Applications of Tumor Spheroids and Organoids for Personalized Medicine. Cancers 2020, 12, 2727. [Google Scholar] [CrossRef]
- Zhai, J.; Liu, Y.; Ji, W.; Huang, X.; Wang, P.; Li, Y.; Li, H.; Wong, A.H.-H.; Zhou, X.; Chen, P.; et al. Drug screening on digital microfluidics for cancer precision medicine. Nat. Commun. 2024, 15, 4363. [Google Scholar] [CrossRef] [PubMed]
- Patra, B.; Chen, Y.-H.; Peng, C.-C.; Lin, S.-C.; Lee, C.-H.; Tung, Y.-C. A microfluidic device for uniform-sized cell spheroids formation, culture, harvesting and flow cytometry analysis. Biomicrofluidics 2013, 7, 54114. [Google Scholar] [CrossRef]
- Dadgar, N.; Gonzalez-Suarez, A.M.; Fattahi, P.; Hou, X.; Weroha, J.S.; Gaspar-Maia, A.; Stybayeva, G.; Revzin, A. A microfluidic platform for cultivating ovarian cancer spheroids and testing their responses to chemotherapies. Microsyst. Nanoeng. 2020, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Cong, L.; Cong, X. Patient-Derived Organoids in Precision Medicine: Drug Screening, Organoid-on-a-Chip and Living Organoid Biobank. Front. Oncol. 2021, 11, 762184. [Google Scholar] [CrossRef]
- Gayibov, E.; Sychra, T.; Spálenková, A.; Souček, P.; Oliverius, M. The use of patient-derived xenografts and patient-derived organoids in the search for new therapeutic regimens for pancreatic carcinoma. A review. Biomed. Pharmacother. 2025, 182, 117750. [Google Scholar] [CrossRef]
- Maliszewska-Olejniczak, K.; Brodaczewska, K.K.; Bielecka, Z.F.; Solarek, W.; Kornakiewicz, A.; Szczylik, C.; Porta, C.; Czarnecka, A.M. Development of extracellular matrix supported 3D culture of renal cancer cells and renal cancer stem cells. Cytotechnology 2019, 71, 149–163. [Google Scholar] [CrossRef]
- Choi, Y.; Jang, H.-S.; Shim, J.; Yeo, E.; Kim, M.-H.; Noh, H.; Oh, S.; Park, J.-H.; Lee, D.; Lee, J.H. 3D keloid spheroid model: Development and application for personalized drug response prediction. Commun. Biol. 2024, 7, 1470. [Google Scholar] [CrossRef]
- Arutyunyan, I.V.; Soboleva, A.G.; Kovtunov, E.A.; Kosyreva, A.M.; Kudelkina, V.V.; Alekseeva, A.I.; Elchaninov, A.V.; Jumaniyazova, E.D.; Goldshtein, D.V.; Bolshakova, G.B.; et al. Gene Expression Profile of 3D Spheroids in Comparison with 2D Cell Cultures and Tissue Strains of Diffuse High-Grade Gliomas. Bull. Exp. Biol. Med. 2023, 175, 576–584. [Google Scholar] [CrossRef]
- Fontoura, J.C.; Viezzer, C.; Dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C 2020, 107, 110264. [Google Scholar] [CrossRef]
- Koedoot, E.; Wolters, L.; Smid, M.; Stoilov, P.; Burger, G.A.; Herpers, B.; Yan, K.; Price, L.S.; Martens, J.W.M.; Le Dévédec, S.E.; et al. Differential reprogramming of breast cancer subtypes in 3D cultures and implications for sensitivity to targeted therapy. Sci. Rep. 2021, 11, 7259. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.S.; Costa, E.C.; Barros, A.S.; De Melo-Diogo, D.; Correia, I.J. Establishment of 2D Cell Cultures Derived From 3D MCF-7 Spheroids Displaying a Doxorubicin Resistant Profile. Biotechnol. J. 2019, 14, 1800268. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, A.S.; McVicar, R.N.; Finlay, D.; Murad, R.; Vuori, K.; Grimmig, B.A.; Bush, A.; Smith, E.; Mandel-Clausen, T.; McGee, H.M.; et al. The effects of cell-cell orientation in modeling the hallmarks of lung cancer in vitro. bioRxiv 2023. [Google Scholar] [CrossRef]
- Jang, M.; Koh, I.; Lee, S.J.; Cheong, J.-H.; Kim, P. Droplet-based microtumor model to assess cell-ECM interactions and drug resistance of gastric cancer cells. Sci. Rep. 2017, 7, 41541. [Google Scholar] [CrossRef]
- Melissaridou, S.; Wiechec, E.; Magan, M.; Jain, M.V.; Chung, M.K.; Farnebo, L.; Roberg, K. The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int. 2019, 19, 16. [Google Scholar] [CrossRef]
- Betriu, N.; Andreeva, A.; Semino, C.E. Erlotinib Promotes Ligand-Induced EGFR Degradation in 3D but Not 2D Cultures of Pancreatic Ductal Adenocarcinoma Cells. Cancers 2021, 13, 4504. [Google Scholar] [CrossRef]
- Kumar, R.; Iden, M.; Tsaih, S.-W.; Schmidt, R.; Ojesina, A.I.; Rader, J.S. Deciphering the divergent transcriptomic landscapes of cervical cancer cells grown in 3D and 2D cell culture systems. Front. Cell Dev. Biol. 2024, 12, 1413882. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-J.; Hsu, S. Acquisition of epithelial–mesenchymal transition and cancer stem-like phenotypes within chitosan-hyaluronan membrane-derived 3D tumor spheroids. Biomaterials 2014, 35, 10070–10079. [Google Scholar] [CrossRef]
- Xu, X.; Sabanayagam, C.R.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. A hydrogel-based tumor model for the evaluation of nanoparticle-based cancer therapeutics. Biomaterials 2014, 35, 3319–3330. [Google Scholar] [CrossRef]
- Filipiak-Duliban, A.; Brodaczewska, K.; Kajdasz, A.; Kieda, C. Spheroid Culture Differentially Affects Cancer Cell Sensitivity to Drugs in Melanoma and RCC Models. Int. J. Mol. Sci. 2022, 23, 1166. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Lim, Y.; Baek, S.-Y.; Jin, S.; Jeong, Y.H.; Kwak, J.-Y.; Yoon, S. Co-targeting of Tiam1/Rac1 and Notch ameliorates chemoresistance against doxorubicin in a biomimetic 3D lymphoma model. Oncotarget 2018, 9, 2058–2075. [Google Scholar] [CrossRef] [PubMed]
- Pomo, J.M.; Taylor, R.M.; Gullapalli, R.R. Influence of TP53 and CDH1 genes in hepatocellular cancer spheroid formation and culture: A model system to understand cancer cell growth mechanics. Cancer Cell Int. 2016, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Bingel, C.; Koeneke, E.; Ridinger, J.; Bittmann, A.; Sill, M.; Peterziel, H.; Wrobel, J.K.; Rettig, I.; Milde, T.; Fernekorn, U.; et al. Three-dimensional tumor cell growth stimulates autophagic flux and recapitulates chemotherapy resistance. Cell Death Dis. 2017, 8, e3013. [Google Scholar] [CrossRef]
- Zhang, X.; Mofers, A.; Hydbring, P.; Olofsson, M.H.; Guo, J.; Linder, S.; D’Arcy, P. MYC is downregulated by a mitochondrial checkpoint mechanism. Oncotarget 2017, 8, 90225–90237. [Google Scholar] [CrossRef]
- Dunne, L.W.; Huang, Z.; Meng, W.; Fan, X.; Zhang, N.; Zhang, Q.; An, Z. Human decellularized adipose tissue scaffold as a model for breast cancer cell growth and drug treatments. Biomaterials 2014, 35, 4940–4949. [Google Scholar] [CrossRef]
- Liverani, C.; Mercatali, L.; Cristofolini, L.; Giordano, E.; Minardi, S.; Porta, G.D.; De Vita, A.; Miserocchi, G.; Spadazzi, C.; Tasciotti, E.; et al. Investigating the Mechanobiology of Cancer Cell–ECM Interaction Through Collagen-Based 3D Scaffolds. Cell. Mol. Bioeng. 2017, 10, 223–234. [Google Scholar] [CrossRef]
- Kerslake, R.; Belay, B.; Panfilov, S.; Hall, M.; Kyrou, I.; Randeva, H.S.; Hyttinen, J.; Karteris, E.; Sisu, C. Transcriptional Landscape of 3D vs. 2D Ovarian Cancer Cell Models. Cancers 2023, 15, 3350. [Google Scholar] [CrossRef]
- Myungjin Lee, J.; Mhawech-Fauceglia, P.; Lee, N.; Cristina Parsanian, L.; Gail Lin, Y.; Andrew Gayther, S.; Lawrenson, K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Investig. 2013, 93, 528–542. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, T.; Shi, Y.; Feng, W.; Lyu, T. A SMYD3/ITGB6/TGFβ1 Positive Feedback Loop Promotes the Invasion and Adhesion of Ovarian Cancer Spheroids. Front. Oncol. 2021, 11, 690618. [Google Scholar] [CrossRef]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Sommariva, M.; Limonta, P.; Gagliano, N. Epithelial-To-Mesenchymal Transition Markers and CD44 Isoforms Are Differently Expressed in 2D and 3D Cell Cultures of Prostate Cancer Cells. Cells 2019, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Mohamadian Namaqi, M.; Moll, F.; Wiedemeier, S.; Grodrian, A.; Lemke, K. Dynamic cell culture modulates colon cancer cell migration in a novel 3D cell culture system. Sci. Rep. 2024, 14, 18851. [Google Scholar] [CrossRef]
- Li, M.; Chai, H.-F.; Peng, F.; Meng, Y.-T.; Zhang, L.-Z.; Zhang, L.; Zou, H.; Liang, Q.-L.; Li, M.-M.; Mao, K.-G.; et al. Estrogen receptor β upregulated by lncRNA-H19 to promote cancer stem-like properties in papillary thyroid carcinoma. Cell Death Dis. 2018, 9, 1120. [Google Scholar] [CrossRef]
- Song, W.H.; Lim, Y.S.; Kim, J.-E.; Kang, H.Y.; Lee, C.; Rajbongshi, L.; Hwang, S.Y.; Oh, S.-O.; Kim, B.S.; Lee, D.; et al. A Marine Collagen-Based 3D Scaffold for In Vitro Modeling of Human Prostate Cancer Niche and Anti-Cancer Therapeutic Discovery. Mar. Drugs 2024, 22, 295. [Google Scholar] [CrossRef]
- Murphy, R.J.; Browning, A.P.; Gunasingh, G.; Haass, N.K.; Simpson, M.J. Designing and interpreting 4D tumour spheroid experiments. Commun. Biol. 2022, 5, 91. [Google Scholar] [CrossRef]
- Thai, V.L.; Griffin, K.H.; Thorpe, S.W.; Randall, R.L.; Leach, J.K. Tissue engineered platforms for studying primary and metastatic neoplasm behavior in bone. J. Biomech. 2021, 115, 110189. [Google Scholar] [CrossRef] [PubMed]
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Ollivier, E.; Heymann, M.-F.; Vallette, F.M.; Oliver, L.; Heymann, D. Three-dimensional in vitro culture models in oncology research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef]
- Browning, A.P.; Sharp, J.A.; Murphy, R.J.; Gunasingh, G.; Lawson, B.; Burrage, K.; Haass, N.K.; Simpson, M. Quantitative analysis of tumour spheroid structure. eLife 2021, 10, e73020. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.; Zhang, J.; Chen, K.; Li, Y. Simulation of avascular tumor growth by agent-based game model involving phenotype-phenotype interactions. Sci. Rep. 2015, 5, 17992. [Google Scholar] [CrossRef]
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef]
- Parker, A.L.; Benguigui, M.; Fornetti, J.; Goddard, E.; Lucotti, S.; Insua-Rodríguez, J.; Wiegmans, A.P. Early Career Leadership Council of the Metastasis Research Society Current challenges in metastasis research and future innovation for clinical translation. Clin. Exp. Metastasis 2022, 39, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Lo, W.-C.; Majumder, P.; Roy, D.; Ghorai, M.; Shaikh, N.K.; Kant, N.; Shekhawat, M.S.; Gadekar, V.S.; Ghosh, S.; et al. Multiple roles for basement membrane proteins in cancer progression and EMT. Eur. J. Cell Biol. 2022, 101, 151220. [Google Scholar] [CrossRef]
- Novikov, N.M.; Zolotaryova, S.Y.; Gautreau, A.M.; Denisov, E.V. Mutational drivers of cancer cell migration and invasion. Br. J. Cancer 2021, 124, 102–114. [Google Scholar] [CrossRef]
- Coelho, L.L.; Vianna, M.M.; Da Silva, D.M.; Gonzaga, B.M.D.S.; Ferreira, R.R.; Monteiro, A.C.; Bonomo, A.C.; Manso, P.P.D.A.; De Carvalho, M.A.; Vargas, F.R.; et al. Spheroid Model of Mammary Tumor Cells: Epithelial–Mesenchymal Transition and Doxorubicin Response. Biology 2024, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Anseth, K.S. Recent advances in 3D models of tumor invasion. Curr. Opin. Biomed. Eng. 2021, 19, 100310. [Google Scholar] [CrossRef]
- Kunjithapatham, R.; Karthikeyan, S.; Geschwind, J.-F.; Kieserman, E.; Lin, M.; Fu, D.-X.; Ganapathy-Kanniappan, S. Reversal of Anchorage-Independent Multicellular Spheroid into a Monolayer Mimics a Metastatic Model. Sci. Rep. 2014, 4, 6816. [Google Scholar] [CrossRef] [PubMed]
- Roper, S.J.; Coyle, B. Establishing an In Vitro 3D Spheroid Model to Study Medulloblastoma Drug Response and Tumor Dissemination. Curr. Protoc. 2022, 2, e357. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.S. Generation of 3D Tumor Spheroids with Encapsulating Basement Membranes for Invasion Studies. Curr. Protoc. Cell Biol. 2020, 87, e105. [Google Scholar] [CrossRef]
- Arora, S.; Singh, S.; Mittal, A.; Desai, N.; Khatri, D.K.; Gugulothu, D.; Lather, V.; Pandita, D.; Vora, L.K. Spheroids in cancer research: Recent advances and opportunities. J. Drug Deliv. Sci. Technol. 2024, 100, 106033. [Google Scholar] [CrossRef]
- Paris, F.; Marrazzo, P.; Pizzuti, V.; Marchionni, C.; Rossi, M.; Michelotti, M.; Petrovic, B.; Ciani, E.; Simonazzi, G.; Pession, A.; et al. Characterization of Perinatal Stem Cell Spheroids for the Development of Cell Therapy Strategy. Bioengineering 2023, 10, 189. [Google Scholar] [CrossRef]
- Chiang, C.-C.; Anne, R.; Chawla, P.; Shaw, R.M.; He, S.; Rock, E.C.; Zhou, M.; Cheng, J.; Gong, Y.-N.; Chen, Y.-C. Deep learning unlocks label-free viability assessment of cancer spheroids in microfluidics. Lab. Chip 2024, 24, 3169–3182. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Kato, H.; Imai, Y.; Shibuta, M.; Kanie, K.; Kato, R. The importance of scoring recognition fitness in spheroid morphological analysis for robust label-free quality evaluation. Regen. Ther. 2020, 14, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangani, S.; Kremmydas, S.; Karamanos, N.K. Mimicking the Complexity of Solid Tumors: How Spheroids Could Advance Cancer Preclinical Transformative Approaches. Cancers 2025, 17, 1161. https://doi.org/10.3390/cancers17071161
Mangani S, Kremmydas S, Karamanos NK. Mimicking the Complexity of Solid Tumors: How Spheroids Could Advance Cancer Preclinical Transformative Approaches. Cancers. 2025; 17(7):1161. https://doi.org/10.3390/cancers17071161
Chicago/Turabian StyleMangani, Sylvia, Spyros Kremmydas, and Nikos K. Karamanos. 2025. "Mimicking the Complexity of Solid Tumors: How Spheroids Could Advance Cancer Preclinical Transformative Approaches" Cancers 17, no. 7: 1161. https://doi.org/10.3390/cancers17071161
APA StyleMangani, S., Kremmydas, S., & Karamanos, N. K. (2025). Mimicking the Complexity of Solid Tumors: How Spheroids Could Advance Cancer Preclinical Transformative Approaches. Cancers, 17(7), 1161. https://doi.org/10.3390/cancers17071161








