Beyond Telomeres: Unveiling the Extratelomeric Functions of TERT in B-Cell Malignancies
Simple Summary
Abstract
1. Introduction
2. Telomere Erosion—A Driving Force in Oncogenesis
3. Telomerase
Regulation of TERT Expression and Telomerase Activity
4. Telomeres and Telomerase in Normal and Neoplastic B Cells
5. TERT’s Non-Canonical Functions: Focus on B-Cell Malignancies
6. Telomerase Inhibition Strategies
Importance of TERT Inhibition in B-Cell Malignancies
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TERT | telomerase reverse transcriptase |
| TERC | telomerase RNA component |
| DDR | DNA damage response |
| ATM | ATM serine/threonine kinase |
| ATR | ATR serine/threonine kinase |
| TP53 | tumor protein p53 |
| RB1 | RB transcriptional corepressor 1 |
| p16 | cyclin-dependent kinase inhibitor 2A |
| ALT | Alternative Lengthening of Telomeres |
| SP1 | Sp1 transcription factor |
| MYC | MYC proto-oncogene, bHLH transcription factor |
| HIF1A | hypoxia-inducible factor 1 subunit alpha |
| AP-2 | transcription factor AP-2 alpha |
| ETS | E-twenty-six |
| TCF | ternary complex factors |
| NF-κB | RELA proto-oncogene |
| β-catenin | catenin beta 1 |
| WT1 | Wilms’ Tumor 1 |
| NFX-1 | Nuclear Transcription Factor X-Box Binding |
| MAD1 | MAX dimerization protein 1 |
| CTCF | CCCTC Binding Factor |
| PI3K | phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| AKT1 | serine/threonine kinase 1 |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| KSHV | Kaposi’s sarcoma-associated herpes virus |
| EBV | Epstein–Barr virus |
| CMV | cytomegalovirus |
| HTLV-1 | human T-cell leukemia virus-1 |
| LCL | lymphoblastoid cell line |
| LMP1 | latent membrane protein 1 |
| MAPK | mitogen-activated protein kinase 1 |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| GC | germinal center |
| DLBCL | diffuse large B-cell lymphoma |
| BL | Burkitt lymphoma |
| CLL | chronic lymphocytic leukemia |
| WNT | wingless-type MMTV integration site family |
| BCL2 | BCL2 apoptosis regulator |
| P21 | cyclin-dependent kinase inhibitor 1A |
| FOXO3 | forkhead box O3 |
| NOXA | phorbol-12-myristate-13-acetate-induced protein 1 |
| BAD | BCL2-associated agonist of cell death |
| NOTCH2 | notch receptor 2 |
| BATF | basic leucine zipper ATF-like transcription factor |
| BZLF1 | BamHI Z fragment leftward open reading frame 1 |
| ZIF-8 | zeolitic imidazole framework-8 |
| ROS | reactive oxygen species |
| BRG1 | SWI/SNF-related BAF chromatin remodeling complex subunit ATPase 4 |
| IL6 | interleukin 6 |
| TNFα | tumor necrosis factor |
| NME2 | NME/NM23 nucleoside diphosphate kinase 2 |
| TOM 20 | translocase of outer mitochondrial membrane 20 |
| TOM 40 | translocase of outer mitochondrial membrane 40 |
| TIM 23 | translocase of inner mitochondrial membrane 23 |
| COX | cyclooxygenase-1 |
| ACSL4 | acyl-CoA synthetase long-chain family member 4 |
| SLC7A11 | solute carrier family 7 member 11 |
References
- Blackburn, E.H.; Greider, C.W.; Szostak, J.W. Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 2006, 12, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Role of telomeres and telomerase in cancer. Semin. Cancer Biol. 2011, 21, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Dolcetti, R.; De Rossi, A. Telomere/telomerase interplay in virus-driven and virus-independent lymphomagenesis: Pathogenic and clinical implications. Med. Res. Rev. 2012, 32, 233–253. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef]
- Ségal-Bendirdjian, E.; Geli, V. Non-canonical Roles of Telomerase: Unraveling the Imbroglio. Front. Cell Dev. Biol. 2019, 7, 332. [Google Scholar] [CrossRef]
- Kumar, N.; Sethi, G. Telomerase and hallmarks of cancer: An intricate interplay governing cancer cell evolution. Cancer Lett. 2023, 578, 216459. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini-Denchi, E.; Sfeir, A. Stop pulling my strings—What telomeres taught us about the DNA damage response. Nat. Rev. Mol. Cell Biol. 2016, 17, 364–378. [Google Scholar] [CrossRef]
- Palm, W.; de Lange, T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008, 42, 301–334. [Google Scholar] [CrossRef]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef]
- von Zglinicki, T.; Saretzki, G.; Ladhoff, J.; d’Adda di Fagagna, F.; Jackson, S.P. Human cell senescence as a DNA damage response. Mech. Ageing. Dev. 2005, 126, 111–117. [Google Scholar] [CrossRef]
- Jacobs, J.J.; de Lange, T. Significant role for p16INK4a in p53-independent telomere-directed senescence. Curr. Biol. 2004, 14, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Maciejowski, J.; de Lange, T. Telomeres in cancer: Tumour suppression and genome instability. Nat. Rev. Mol. Cell Biol. 2017, 18, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.A.; Greider, C.W. Balancing instability: Dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene 2002, 21, 619–626. [Google Scholar] [CrossRef]
- Rampazzo, E.; Bonaldi, L.; Trentin, L.; Visco, C.; Keppel, S.; Giunco, S.; Frezzato, F.; Facco, M.; Novella, E.; Giaretta, I.; et al. Telomere length and telomerase levels delineate subgroups of B-cell chronic lymphocytic leukemia with different biological characteristics and clinical outcomes. Haematologica 2012, 97, 56–63. [Google Scholar] [CrossRef]
- Mansouri, L.; Grabowski, P.; Degerman, S.; Svenson, U.; Gunnarsson, R.; Cahill, N.; Smedby, K.E.; Geisler, C.; Juliusson, G.; Roos, G.; et al. Short telomere length is associated with NOTCH1/SF3B1/TP53 aberrations and poor outcome in newly diagnosed chronic lymphocytic leukemia patients. Am. J. Hematol. 2013, 88, 647–651. [Google Scholar] [CrossRef]
- Hoxha, M.; Fabris, S.; Agnelli, L.; Bollati, V.; Cutrona, G.; Matis, S.; Recchia, A.G.; Gentile, M.; Cortelezzi, A.; Morabito, F.; et al. Relevance of telomere/telomerase system impairment in early stage chronic lymphocytic leukemia. Genes Chromosomes Cancer 2014, 53, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Li, L.; Zhou, Y.; Wang, C.; Hou, S. The Association between Telomere Length and Cancer Prognosis: Evidence from a Meta-Analysis. PLoS ONE 2015, 10, e0133174. [Google Scholar] [CrossRef]
- Lin, T.T.; Letsolo, B.T.; Jones, R.E.; Rowson, J.; Pratt, G.; Hewamana, S.; Fegan, C.; Pepper, C.; Baird, D.M. Telomere dysfunction and fusion during the progression of chronic lymphocytic leukemia: Evidence for a telomere crisis. Blood 2010, 116, 1899–1907. [Google Scholar] [CrossRef]
- De Vitis, M.; Berardinelli, F.; Sgura, A. Telomere Length Maintenance in Cancer: At the Crossroad between Telomerase and Alternative Lengthening of Telomeres (ALT). Int. J. Mol. Sci. 2018, 19, 606. [Google Scholar] [CrossRef]
- Roake, C.M.; Artandi, S.E. Regulation of human telomerase in homeostasis and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 384–397. [Google Scholar] [CrossRef]
- Schmidt, J.C.; Cech, T.R. Human telomerase: Biogenesis, trafficking, recruitment, and activation. Genes Dev. 2015, 29, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, E.; Tatsumoto, N.; Kodama, T.; Hiyama, K.; Shay, J.; Yokoyama, T. Telomerase activity in human intestine. Int. J. Oncol. 1996, 9, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Sirma, H.; Kumar, M.; Meena, J.K.; Witt, B.; Weise, J.M.; Lechel, A.; Ande, S.; Sakk, V.; Guguen-Guillouzo, C.; Zender, L.; et al. The promoter of human telomerase reverse transcriptase is activated during liver regeneration and hepatocyte proliferation. Gastroenterology 2011, 141, 326–337.e3. [Google Scholar] [CrossRef]
- Igarashi, H.; Sakaguchi, N. Telomerase activity is induced in human peripheral B lymphocytes by the stimulation to antigen receptor. Blood 1997, 89, 1299–1307. [Google Scholar] [CrossRef]
- Weng, N.P.; Levine, B.L.; June, C.H.; Hodes, R.J. Regulated expression of telomerase activity in human T lymphocyte development and activation. J. Exp. Med. 1996, 183, 2471–2479. [Google Scholar] [CrossRef]
- Ozturk, S. Telomerase activity and telomere length in male germ cells. Biol. Reprod. 2015, 92, 53. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.C.; Counter, C.M.; Lundberg, A.S.; Beijersbergen, R.L.; Brooks, M.W.; Weinberg, R.A. Creation of human tumour cells with defined genetic elements. Nature 1999, 400, 464–468. [Google Scholar] [CrossRef]
- Akincilar, S.C.; Unal, B.; Tergaonkar, V. Reactivation of telomerase in cancer. Cell. Mol. Life. Sci. 2016, 73, 1659–1670. [Google Scholar] [CrossRef]
- Gizard, F.; Heywood, E.B.; Findeisen, H.M.; Zhao, Y.; Jones, K.L.; Cudejko, C.; Post, G.R.; Staels, B.; Bruemmer, D. Telomerase activation in atherosclerosis and induction of telomerase reverse transcriptase expression by inflammatory stimuli in macrophages. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 245–252. [Google Scholar] [CrossRef]
- Zhang, Y.; Toh, L.; Lau, P.; Wang, X. Human telomerase reverse transcriptase (hTERT) is a novel target of the Wnt/β-catenin pathway in human cancer. J. Biol. Chem. 2012, 287, 32494–32511. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.W.; Hodis, E.; Xu, M.J.; Kryukov, G.V.; Chin, L.; Garraway, L.A. Highly recurrent TERT promoter mutations in human melanoma. Science 2013, 339, 957–959. [Google Scholar] [CrossRef]
- Lorbeer, F.K.; Hockemeyer, D. TERT promoter mutations and telomeres during tumorigenesis. Curr. Opin. Genet. Dev. 2020, 60, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Lam, G.; Xian, R.R.; Li, Y.; Burns, K.H.; Beemon, K.L. Lack of TERT Promoter Mutations in Human B-Cell Non-Hodgkin Lymphoma. Genes 2016, 7, 93. [Google Scholar] [CrossRef]
- Hafezi, F.; Perez Bercoff, D. The Solo Play of TERT Promoter Mutations. Cells 2020, 9, 749. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Chang, G.; Wang, F.; Wang, F.; Geng, X. Alternative Splicing of hTERT Pre-mRNA: A Potential Strategy for the Regulation of Telomerase Activity. Int. J. Mol. Sci. 2017, 18, 567. [Google Scholar] [CrossRef]
- Bortoletto, S.; Nunes-Souza, E.; Marchi, R.; Ruthes, M.O.; Okano, L.M.; Tofolo, M.V.; Centa, A.; Fonseca, A.S.; Rosolen, D.; Cavalli, L.R. MicroRNAs role in telomere length maintenance and telomerase activity in tumor cells. J. Mol Med. 2024, 102, 1089–1100. [Google Scholar] [CrossRef]
- Rampazzo, E.; Bojnik, E.; Trentin, L.; Bonaldi, L.; Del Bianco, P.; Frezzato, F.; Visentin, A.; Facco, M.; Semenzato, G.; De Rossi, A. Role of miR-15a/miR-16-1 and the TP53 axis in regulating telomerase expression in chronic lymphocytic leukemia. Haematologica 2017, 102, e253–e256. [Google Scholar] [CrossRef]
- Bhatia, S.; Kaul, D.; Varma, N. Potential tumor suppressive function of miR-196b in B-cell lineage acute lymphoblastic leukemia. Mol. Cell. Biochem. 2010, 340, 97–106. [Google Scholar] [CrossRef]
- Sfeir, A. Telomeres at a glance. J. Cell Sci. 2012, 125, 4173–4178. [Google Scholar] [CrossRef][Green Version]
- Kang, S.S.; Kwon, T.; Kwon, D.Y.; Do, S. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J. Biol. Chem. 1999, 274, 13085–13090. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Khadka, P.; Chung, I.K. Nuclear import of hTERT requires a bipartite nuclear localization signal and Akt-mediated phosphorylation. J. Cell Sci. 2012, 125, 2684–2697. [Google Scholar] [CrossRef]
- Haendeler, J.; Dröse, S.; Büchner, N.; Jakob, S.; Altschmied, J.; Goy, C.; Spyridopoulos, I.; Zeiher, A.; Brandt, U.; Dimmeler, S. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Singhapol, C.; Pal, D.; Czapiewski, R.; Porika, M.; Nelson, G.; Saretzki, G.C. Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PLoS ONE 2013, 8, e52989. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Nicot, C. Regulation of telomerase and telomeres: Human tumor viruses take control. J. Natl. Cancer Inst. 2008, 100, 98–108. [Google Scholar] [CrossRef]
- Tornesello, M.L.; Cerasuolo, A.; Starita, N.; Tornesello, A.L.; Bonelli, P.; Tuccillo, F.M.; Buonaguro, L.; Isaguliants, M.G.; Buonaguro, F.M. The Molecular Interplay between Human Oncoviruses and Telomerase in Cancer Development. Cancers 2022, 14, 5257. [Google Scholar] [CrossRef]
- Salimi-Jeda, A.; Badrzadeh, F.; Esghaei, M.; Abdoli, A. The role of telomerase and viruses interaction in cancer development, and telomerase-dependent therapeutic approaches. Cancer Treat. Res. Commun. 2021, 27, 100323. [Google Scholar] [CrossRef]
- Giunco, S.; Del Mistro, A.; Morello, M.; Lidonnici, J.; Frayle, H.; Gori, S.; De Rossi, A.; Boscolo-Rizzo, P. From infection to immortality: The role of HPV and telomerase in head and neck cancer. Oral. Oncol. 2025, 161, 107169. [Google Scholar] [CrossRef]
- Faumont, N.; Durand-Panteix, S.; Schlee, M.; Grömminger, S.; Schuhmacher, M.; Hölzel, M.; Laux, G.; Mailhammer, R.; Rosenwald, A.; Staudt, L.M.; et al. c-Myc and Rel/NF-kappaB are the two master transcriptional systems activated in the latency III program of Epstein-Barr virus-immortalized B cells. J. Virol. 2009, 83, 5014–5027. [Google Scholar] [CrossRef]
- Terrin, L.; Dal Col, J.; Rampazzo, E.; Zancai, P.; Pedrotti, M.; Ammirabile, G.; Bergamin, S.; Rizzo, S.; Dolcetti, R.; De Rossi, A. Latent membrane protein 1 of Epstein-Barr virus activates the hTERT promoter and enhances telomerase activity in B lymphocytes. J. Virol. 2008, 82, 10175–10187. [Google Scholar] [CrossRef]
- Norrback, K.F.; Dahlenborg, K.; Carlsson, R.; Roos, G. Telomerase activation in normal B lymphocytes and non-Hodgkin’s lymphomas. Blood 1996, 88, 222–229. [Google Scholar]
- Martens, U.M.; Brass, V.; Sedlacek, L.; Pantic, M.; Exner, C.; Guo, Y.; Engelhardt, M.; Lansdorp, P.M.; Waller, C.F.; Lange, W. Telomere maintenance in human B lymphocytes. Br. J. Haematol. 2002, 119, 810–818. [Google Scholar] [CrossRef]
- Weng, N.P.; Granger, L.; Hodes, R.J. Telomere lengthening and telomerase activation during human B cell differentiation. Proc. Natl. Acad. Sci. USA 1997, 94, 10827–10832. [Google Scholar] [CrossRef] [PubMed]
- Ely, S.A.; Chadburn, A.; Dayton, C.M.; Cesarman, E.; Knowles, D.M. Telomerase activity in B-cell non-Hodgkin lymphoma. Cancer 2000, 89, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Lobetti-Bodoni, C.; Bernocco, E.; Genuardi, E.; Boccadoro, M.; Ladetto, M. Telomeres and telomerase in normal and malignant B-cells. Hematol. Oncol. 2010, 28, 157–167. [Google Scholar] [CrossRef]
- Ladetto, M.; Compagno, M.; Ricca, I.; Pagano, M.; Rocci, A.; Astolfi, M.; Drandi, D.; di Celle, P.F.; Dell’Aquila, M.; Mantoan, B.; et al. Telomere length correlates with histopathogenesis according to the germinal center in mature B-cell lymphoproliferative disorders. Blood 2004, 103, 4644–4649. [Google Scholar] [CrossRef]
- Mochida, A.; Gotoh, E.; Senpuku, H.; Harada, S.; Kitamura, R.; Takahashi, T.; Yanagi, K. Telomere size and telomerase activity in Epstein-Barr virus (EBV)-positive and EBV-negative Burkitt’s lymphoma cell lines. Arch. Virol. 2005, 150, 2139–2150. [Google Scholar] [CrossRef]
- Cuceu, C.; Hempel, W.M.; Sabatier, L.; Bosq, J.; Carde, P.; M’kacher, R. Chromosomal Instability in Hodgkin Lymphoma: An In-Depth Review and Perspectives. Cancers 2018, 10, 91. [Google Scholar] [CrossRef]
- Knecht, H.; Sawan, B.; Lichtensztejn, D.; Lemieux, B.; Wellinger, R.J.; Mai, S. The 3D nuclear organization of telomeres marks the transition from Hodgkin to Reed-Sternberg cells. Leukemia 2009, 23, 565–573. [Google Scholar] [CrossRef]
- Lajoie, V.; Lemieux, B.; Sawan, B.; Lichtensztejn, D.; Lichtensztejn, Z.; Wellinger, R.; Mai, S.; Knecht, H. LMP1 mediates multinuclearity through downregulation of shelterin proteins and formation of telomeric aggregates. Blood 2015, 125, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- M’kacher, R.; Cuceu, C.; Al Jawhari, M.; Morat, L.; Frenzel, M.; Shim, G.; Lenain, A.; Hempel, W.M.; Junker, S.; Girinsky, T.; et al. The Transition between Telomerase and ALT Mechanisms in Hodgkin Lymphoma and Its Predictive Value in Clinical Outcomes. Cancers 2018, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Knecht, H.; Johnson, N.; Bienz, M.N.; Brousset, P.; Memeo, L.; Shifrin, Y.; Alikhah, A.; Louis, S.F.; Mai, S. Analysis by TeloView® Technology Predicts the Response of Hodgkin’s Lymphoma to First-Line ABVD Therapy. Cancers 2024, 16, 2816. [Google Scholar] [CrossRef]
- Kamranvar, S.A.; Chen, X.; Masucci, M.G. Telomere dysfunction and activation of alternative lengthening of telomeres in B-lymphocytes infected by Epstein-Barr virus. Oncogene 2013, 32, 5522–5530. [Google Scholar] [CrossRef] [PubMed]
- Lacoste, S.; Wiechec, E.; Dos Santos Silva, A.G.; Guffei, A.; Williams, G.; Lowbeer, M.; Benedek, K.; Henriksson, M.; Klein, G.; Mai, S. Chromosomal rearrangements after ex vivo Epstein-Barr virus (EBV) infection of human B cells. Oncogene 2010, 29, 503–515. [Google Scholar] [CrossRef]
- Sugimoto, M.; Tahara, H.; Ide, T.; Furuichi, Y. Steps involved in immortalization and tumorigenesis in human B-lymphoblastoid cell lines transformed by Epstein-Barr virus. Cancer Res. 2004, 64, 3361–3364. [Google Scholar] [CrossRef]
- Jeon, J.P.; Nam, H.Y.; Shim, S.M.; Han, B.G. Sustained viral activity of epstein-Barr virus contributes to cellular immortalization of lymphoblastoid cell lines. Mol. Cells 2009, 27, 143–148. [Google Scholar] [CrossRef]
- Terrin, L.; Dolcetti, R.; Corradini, I.; Indraccolo, S.; Dal Col, J.; Bertorelle, R.; Bonaldi, L.; Esposito, G.; De Rossi, A. hTERT inhibits the Epstein-Barr virus lytic cycle and promotes the proliferation of primary B lymphocytes: Implications for EBV-driven lymphomagenesis. Int. J. Cancer 2007, 121, 576–587. [Google Scholar] [CrossRef]
- da Mota, T.H.A.; Camargo, R.; Biojone, E.R.; Guimarães, A.F.R.; Pittella-Silva, F.; de Oliveira, D.M. The Relevance of Telomerase and Telomere-Associated Proteins in B-Acute Lymphoblastic Leukemia. Genes 2023, 14, 691. [Google Scholar] [CrossRef]
- Nogueira, B.M.D.; da Costa Pantoja, L.; da Silva, E.L.; Mello Júnior, F.A.R.; Teixeira, E.B.; Wanderley, A.V.; da Silva Maués, J.H.; de Moraes Filho, M.O.; de Moraes, M.E.A.; Montenegro, R.C.; et al. Telomerase (hTERT) Overexpression Reveals a Promising Prognostic Biomarker and Therapeutical Target in Different Clinical Subtypes of Pediatric Acute Lymphoblastic Leukaemia. Genes 2021, 12, 1632. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, M.; Sun, X.; Sun, J. Telomerase activity and telomere length in acute leukemia: Correlations with disease progression, subtypes and overall survival. Int. J. Lab. Hematol. 2010, 32, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Ohyashiki, J.H.; Ohyashiki, K.; Iwama, H.; Hayashi, S.; Toyama, K.; Shay, J.W. Clinical implications of telomerase activity levels in acute leukemia. Clin. Cancer Res. 1997, 3, 619–625. [Google Scholar] [PubMed]
- Allegra, A.; Innao, V.; Penna, G.; Gerace, D.; Allegra, A.G.; Musolino, C. Telomerase and telomere biology in hematological diseases: A new therapeutic target. Leuk. Res. 2017, 56, 60–74. [Google Scholar] [CrossRef]
- Li, Y.; Tergaonkar, V. Noncanonical functions of telomerase: Implications in telomerase-targeted cancer therapies. Cancer Res. 2014, 74, 1639–1644. [Google Scholar] [CrossRef]
- Park, J.I.; Venteicher, A.S.; Hong, J.Y.; Choi, J.; Jun, S.; Shkreli, M.; Chang, W.; Meng, Z.; Cheung, P.; Ji, H.; et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 2009, 460, 66–72. [Google Scholar] [CrossRef]
- Ghosh, A.; Saginc, G.; Leow, S.C.; Khattar, E.; Shin, E.M.; Yan, T.D.; Wong, M.; Zhang, Z.; Li, G.; Sung, W.K.; et al. Telomerase directly regulates NF-κB-dependent transcription. Nat. Cell Biol. 2012, 14, 1270–1281. [Google Scholar] [CrossRef]
- Koh, C.M.; Khattar, E.; Leow, S.C.; Liu, C.Y.; Muller, J.; Ang, W.X.; Li, Y.; Franzoso, G.; Li, S.; Guccione, E.; et al. Telomerase regulates MYC-driven oncogenesis independent of its reverse transcriptase activity. J. Clin. Investig. 2015, 125, 2109–2122. [Google Scholar] [CrossRef]
- Amin, A.; Morello, M.; Petrara, M.R.; Rizzo, B.; Argenton, F.; De Rossi, A.; Giunco, S. Short-Term TERT Inhibition Impairs Cellular Proliferation via a Telomere Length-Independent Mechanism and Can Be Exploited as a Potential Anticancer Approach. Cancers 2023, 15, 2673. [Google Scholar] [CrossRef]
- Haendeler, J.; Hoffmann, J.; Brandes, R.P.; Zeiher, A.M.; Dimmeler, S. Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707. Mol. Cell. Biol. 2003, 23, 4598–4610. [Google Scholar] [CrossRef]
- Haendeler, J.; Hoffmann, J.; Diehl, J.F.; Vasa, M.; Spyridopoulos, I.; Zeiher, A.M.; Dimmeler, S. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ. Res. 2004, 94, 768–775. [Google Scholar] [CrossRef]
- Santos, J.H.; Meyer, J.N.; Skorvaga, M.; Annab, L.; Van Houten, B. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell 2004, 3, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Meyer, J.N.; Van Houten, B. Mitochondrial localization of telomerase as a determinant for hydrogen peroxide-induced mitochondrial DNA damage and apoptosis. Hum. Mol. Genet. 2006, 15, 1757–1768. [Google Scholar] [CrossRef]
- Ahmed, S.; Passos, J.F.; Birket, M.J.; Beckmann, T.; Brings, S.; Peters, H.; Birch-Machin, M.A.; von Zglinicki, T.; Saretzki, G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J. Cell Sci. 2008, 121, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Reyes, A.; Green, P.; Caron, M.J.; Bonini, M.G.; Gordon, D.M.; Holt, I.J.; Santos, J.H. Human telomerase acts as a hTR-independent reverse transcriptase in mitochondria. Nucleic Acids Res. 2012, 40, 712–725. [Google Scholar] [CrossRef]
- Kovalenko, O.A.; Caron, M.J.; Ulema, P.; Medrano, C.; Thomas, A.P.; Kimura, M.; Bonini, M.G.; Herbig, U.; Santos, J.H. A mutant telomerase defective in nuclear-cytoplasmic shuttling fails to immortalize cells and is associated with mitochondrial dysfunction. Aging Cell 2010, 9, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Martens, A.; Schmid, B.; Akintola, O.; Saretzki, G. Telomerase Does Not Improve DNA Repair in Mitochondria upon Stress but Increases MnSOD Protein under Serum-Free Conditions. Int. J. Mol. Sci. 2019, 21, 27. [Google Scholar] [CrossRef]
- Indran, I.; Hande, M.P.; Pervaiz, S. hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Res. 2011, 71, 266–276. [Google Scholar] [CrossRef]
- Mukherjee, S.; Firpo, E.J.; Wang, Y.; Roberts, J.M. Separation of telomerase functions by reverse genetics. Proc. Natl. Acad. Sci. USA 2011, 108, E1363–E1371. [Google Scholar] [CrossRef]
- Giunco, S.; Dolcetti, R.; Keppel, S.; Celeghin, A.; Indraccolo, S.; Dal Col, J.; Mastorci, K.; De Rossi, A. hTERT inhibition triggers Epstein-Barr virus lytic cycle and apoptosis in immortalized and transformed B cells: A basis for new therapies. Clin. Cancer Res. 2013, 19, 2036–2047. [Google Scholar] [CrossRef]
- Giunco, S.; Celeghin, A.; Gianesin, K.; Dolcetti, R.; Indraccolo, S.; De Rossi, A. Cross talk between EBV and telomerase: The role of TERT and NOTCH2 in the switch of latent/lytic cycle of the virus. Cell Death Dis. 2015, 6, e1774. [Google Scholar] [CrossRef]
- Celeghin, A.; Giunco, S.; Freguja, R.; Zangrossi, M.; Nalio, S.; Dolcetti, R.; De Rossi, A. Short-term inhibition of TERT induces telomere length-independent cell cycle arrest and apoptotic response in EBV-immortalized and transformed B cells. Cell Death. Dis. 2016, 7, e2562. [Google Scholar] [CrossRef] [PubMed]
- Giunco, S.; Zangrossi, M.; Dal Pozzolo, F.; Celeghin, A.; Ballin, G.; Petrara, M.R.; Amin, A.; Argenton, F.; Godinho Ferreira, M.; De Rossi, A. Anti-Proliferative and Pro-Apoptotic Effects of Short-Term Inhibition of Telomerase In Vivo and in Human Malignant B Cells Xenografted in Zebrafish. Cancers 2020, 12, 2052. [Google Scholar] [CrossRef]
- Long, C.; Xu, Q.B.; Ding, L.; Yang, L.; Ji, W.; Gao, F.; Ji, Y. Triptolide inhibits human telomerase reverse transcriptase by downregulating translation factors SP1 and c-Myc in Epstein-Barr virus-positive B lymphocytes. Oncol. Lett. 2021, 21, 280. [Google Scholar] [CrossRef] [PubMed]
- Bashash, D.; Ghaffari, S.H.; Mirzaee, R.; Alimoghaddam, K.; Ghavamzadeh, A. Telomerase inhibition by non-nucleosidic compound BIBR1532 causes rapid cell death in pre-B acute lymphoblastic leukemia cells. Leuk. Lymphoma 2013, 54, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Bashash, D.; Zareii, M.; Safaroghli-Azar, A.; Omrani, M.D.; Ghaffari, S.H. Inhibition of telomerase using BIBR1532 enhances doxorubicin-induced apoptosis in pre-B acute lymphoblastic leukemia cells. Hematology 2017, 22, 330–340. [Google Scholar] [CrossRef]
- Ghasemimehr, N.; Farsinejad, A.; Mirzaee Khalilabadi, R.; Yazdani, Z.; Fatemi, A. The telomerase inhibitor MST-312 synergistically enhances the apoptotic effect of doxorubicin in pre-B acute lymphoblastic leukemia cells. Biomed. Pharmacother. 2018, 106, 1742–1750. [Google Scholar] [CrossRef]
- Katoueezadeh, M.; Maleki, P.; Torabizadeh, S.A.; Farsinejad, A.; Khalilabadi, R.M.; Valandani, H.M.; Nurain, I.O.; Ashoub, M.H.; Fatemi, A. Combinatorial targeting of telomerase and DNA-PK induces synergistic apoptotic effects against Pre-B acute lymphoblastic leukemia cells. Mol. Biol. Rep. 2024, 51, 163. [Google Scholar] [CrossRef]
- Ashoub, M.H.; Afgar, A.; Farsinejad, A.; Razavi, R.; Anvari, S.; Fatemi, A. siRNA-mediated inhibition of hTERT enhances the effects of curcumin in promoting cell death in precursor-B acute lymphoblastic leukemia cells: An in silico and in vitro study. Sci. Rep. 2025, 15, 3083. [Google Scholar] [CrossRef]
- Ameri, Z.; Ghiasi, S.; Farsinejad, A.; Hassanshahi, G.; Ehsan, M.; Fatemi, A. Telomerase inhibitor MST-312 induces apoptosis of multiple myeloma cells and down-regulation of anti-apoptotic, proliferative and inflammatory genes. Life Sci. 2019, 228, 66–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Zhou, H.; Yao, G.; Zhou, L.; Qian, C. BIBR1532 inhibits proliferation and enhances apoptosis in multiple myeloma cells by reducing telomerase activity. PeerJ 2023, 11, e16404. [Google Scholar] [CrossRef]
- Brennan, S.K.; Wang, Q.; Tressler, R.; Harley, C.; Go, N.; Bassett, E.; Huff, C.A.; Jones, R.J.; Matsui, W. Telomerase inhibition targets clonogenic multiple myeloma cells through telomere length-dependent and independent mechanisms. PLoS ONE 2010, 5, e12487. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, D.; Onozawa, M.; Miyashita, N.; Yokoyama, S.; Nakagawa, M.; Hashimoto, D.; Teshima, T. Short-term treatment with imetelstat sensitizes hematopoietic malignant cells to a genotoxic agent via suppression of the telomerase-mediated DNA repair process. Leuk. Lymphoma 2020, 61, 2722–2732. [Google Scholar] [CrossRef]
- Rohan, P.; Binato, R.; Abdelhay, E. NF-ΚB Activation as a Key Driver in Chronic Lymphocytic Leukemia Evolution to Richter’s Syndrome: Unraveling the Influence of Immune Microenvironment Dynamics. Genes 2024, 15, 1434. [Google Scholar] [CrossRef]
- Balaji, S.; Ahmed, M.; Lorence, E.; Yan, F.; Nomie, K.; Wang, M. NF-κB signaling and its relevance to the treatment of mantle cell lymphoma. J. Hematol. Oncol. 2018, 11, 83. [Google Scholar] [CrossRef]
- de Barrios, O.; Meler, A.; Parra, M. MYC’s Fine Line Between B Cell Development and Malignancy. Cells 2020, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.M.; Kanda, K.; Zhang, L.; Boxer, L.M. Activation of the c-myc p1 promoter in Burkitt’s lymphoma by the hs3 immunoglobulin heavy-chain gene enhancer. Leukemia 2007, 21, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Kanda, K.; Hu, H.M.; Zhang, L.; Grandchamps, J.; Boxer, L.M. NF-kappa B activity is required for the deregulation of c-myc expression by the immunoglobulin heavy chain enhancer. J. Biol. Chem. 2000, 275, 32338–32346. [Google Scholar] [CrossRef]
- Tergaonkar, V. NFκB drives TERT promoter reactivation in cancer. Cell Cycle 2016, 15, 156–157. [Google Scholar] [CrossRef]
- Bu, D.X.; Johansson, M.E.; Ren, J.; Xu, D.W.; Johnson, F.B.; Edfeldt, K.; Yan, Z.Q. Nuclear factor {kappa}B-mediated transactivation of telomerase prevents intimal smooth muscle cell from replicative senescence during vascular repair. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2604–2610. [Google Scholar] [CrossRef]
- Sheng, W.Y.; Chen, Y.R.; Wang, T.C. A major role of PKC theta and NFkappaB in the regulation of hTERT in human T lymphocytes. FEBS Lett. 2006, 580, 6819–6824. [Google Scholar] [CrossRef]
- Bryan, C.; Rice, C.; Hoffman, H.; Harkisheimer, M.; Sweeney, M.; Skordalakes, E. Structural Basis of Telomerase Inhibition by the Highly Specific BIBR1532. Structure 2015, 23, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Shirgahi Talari, F.; Bagherzadeh, K.; Golestanian, S.; Jarstfer, M.; Amanlou, M. Potent Human Telomerase Inhibitors: Molecular Dynamic Simulations, Multiple Pharmacophore-Based Virtual Screening, and Biochemical Assays. J. Chem. Inf. Model. 2015, 55, 2596–2610. [Google Scholar] [CrossRef] [PubMed]
- Pascolo, E.; Wenz, C.; Lingner, J.; Hauel, N.; Priepke, H.; Kauffmann, I.; Garin-Chesa, P.; Rettig, W.J.; Damm, K.; Schnapp, A. Mechanism of human telomerase inhibition by BIBR1532, a synthetic, non-nucleosidic drug candidate. J. Biol. Chem. 2002, 277, 15566–15572. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Koundrioukoff, S.; Carignon, S.; Técher, H.; Letessier, A.; Brison, O.; Debatisse, M. Stepwise activation of the ATR signaling pathway upon increasing replication stress impacts fragile site integrity. PLoS Genet. 2013, 9, e1003643. [Google Scholar] [CrossRef]
- Limon, J.J.; Fruman, D.A. Akt and mTOR in B Cell Activation and Differentiation. Front. Immunol. 2012, 3, 228. [Google Scholar] [CrossRef]
- Giunco, S.; Petrara, M.R.; Zangrossi, M.; Celeghin, A.; De Rossi, A. Extra-telomeric functions of telomerase in the pathogenesis of Epstein-Barr virus-driven B-cell malignancies and potential therapeutic implications. Infect. Agent. Cancer 2018, 13, 14. [Google Scholar] [CrossRef]
- Rasouli, S.; Dakic, A.; Wang, Q.E.; Mitchell, D.; Blakaj, D.M.; Putluri, N.; Li, J.; Liu, X. Noncanonical functions of telomerase and telomeres in viruses-associated cancer. J. Med. Virol. 2024, 96, e29665. [Google Scholar] [CrossRef]
- Schrank, Z.; Khan, N.; Osude, C.; Singh, S.; Miller, R.; Merrick, C.; Mabel, A.; Kuckovic, A.; Puri, N. Oligonucleotides Targeting Telomeres and Telomerase in Cancer. Molecules 2018, 23, 2267. [Google Scholar] [CrossRef]
- Sugarman, E.T.; Zhang, G.; Shay, J.W. In perspective: An update on telomere targeting in cancer. Mol. Carcinog. 2019, 58, 1581–1588. [Google Scholar] [CrossRef]
- Dratwa, M.; Wysoczańska, B.; Łacina, P.; Kubik, T.; Bogunia-Kubik, K. TERT-Regulation and Roles in Cancer Formation. Front. Immunol. 2020, 11, 589929. [Google Scholar] [CrossRef]
- Guterres, A.N.; Villanueva, J. Targeting telomerase for cancer therapy. Oncogene 2020, 39, 5811–5824. [Google Scholar] [CrossRef] [PubMed]
- Salloum, R.; Hummel, T.R.; Kumar, S.S.; Dorris, K.; Li, S.; Lin, T.; Daryani, V.M.; Stewart, C.F.; Miles, L.; Poussaint, T.Y.; et al. A molecular biology and phase II study of imetelstat (GRN163L) in children with recurrent or refractory central nervous system malignancies: A pediatric brain tumor consortium study. J. Neurooncol. 2016, 129, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Chiappori, A.A.; Kolevska, T.; Spigel, D.R.; Hager, S.; Rarick, M.; Gadgeel, S.; Blais, N.; Von Pawel, J.; Hart, L.; Reck, M.; et al. A randomized phase II study of the telomerase inhibitor imetelstat as maintenance therapy for advanced non-small-cell lung cancer. Ann. Oncol. 2015, 26, 354–362. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Komrokji, R.; Palandri, F.; Martino, B.; Niederwieser, D.; Reiter, A.; Scott, B.L.; Baer, M.R.; Hoffman, R.; Odenike, O.; et al. Randomized, Single-Blind, Multicenter Phase II Study of Two Doses of Imetelstat in Relapsed or Refractory Myelofibrosis. J. Clin. Oncol. 2021, 39, 2881–2892. [Google Scholar] [CrossRef]
- Steensma, D.P.; Fenaux, P.; Van Eygen, K.; Raza, A.; Santini, V.; Germing, U.; Font, P.; Diez-Campelo, M.; Thepot, S.; Vellenga, E.; et al. Imetelstat Achieves Meaningful and Durable Transfusion Independence in High Transfusion-Burden Patients With Lower-Risk Myelodysplastic Syndromes in a Phase II Study. J. Clin. Oncol. 2021, 39, 48–56. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Harrison, C.N.; Kiladjian, J.J.; Komrokji, R.S.; Koschmieder, S.; Vannucchi, A.M.; Berry, T.; Redding, D.; Sherman, L.; Dougherty, S.; et al. Imetelstat in intermediate-2 or high-risk myelofibrosis refractory to JAK inhibitor: IMpactMF phase III study design. Future Oncol. 2022, 18, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Santini, V.; Fenaux, P.; Sekeres, M.A.; Savona, M.R.; Madanat, Y.F.; Díez-Campelo, M.; Valcárcel, D.; Illmer, T.; Jonášová, A.; et al. Imetelstat in patients with lower-risk myelodysplastic syndromes who have relapsed or are refractory to erythropoiesis-stimulating agents (IMerge): A multinational, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2024, 403, 249–260. [Google Scholar] [CrossRef]
- Damm, K.; Hemmann, U.; Garin-Chesa, P.; Hauel, N.; Kauffmann, I.; Priepke, H.; Niestroj, C.; Daiber, C.; Enenkel, B.; Guilliard, B.; et al. A highly selective telomerase inhibitor limiting human cancer cell proliferation. EMBO J. 2001, 20, 6958–6968. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Yan, L.; You, Y.; Zhao, F.; Cheng, J.; Yang, L.; Sun, Y.; Chang, Q.; Liu, R.; et al. Zeolitic Imidazolate Framework-8 (ZIF-8) as a Drug Delivery Vehicle for the Transport and Release of Telomerase Inhibitor BIBR 1532. Nanomaterials 2023, 13, 1779. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, S. Evaluation of the efficacy of MST-312, as a telomerase inhibitor, in the treatment of patients with multiple myeloma after stem cell transplantation. Cell. Mol. Biol. 2022, 67, 115–120. [Google Scholar] [CrossRef]
- Kim, M.Y.; Vankayalapati, H.; Shin-Ya, K.; Wierzba, K.; Hurley, L.H. Telomestatin, a potent telomerase inhibitor that interacts quite specifically with the human telomeric intramolecular g-quadruplex. J. Am. Chem. Soc. 2002, 124, 2098–2099. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, X.; Li, Y.; Xu, S.; Ma, C.; Wu, X.; Cheng, Y.; Yu, Z.; Zhao, G.; Chen, Y. Telomere targeting with a novel G-quadruplex-interactive ligand BRACO-19 induces T-loop disassembly and telomerase displacement in human glioblastoma cells. Oncotarget 2016, 7, 14925–14939. [Google Scholar] [CrossRef]
- Xu, H.; Di Antonio, M.; McKinney, S.; Mathew, V.; Ho, B.; O’Neil, N.J.; Santos, N.D.; Silvester, J.; Wei, V.; Garcia, J.; et al. CX-5461 is a DNA G-quadruplex stabilizer with selective lethality in BRCA1/2 deficient tumours. Nat. Commun. 2017, 8, 14432. [Google Scholar] [CrossRef] [PubMed]
- Mender, I.; Gryaznov, S.; Dikmen, Z.G.; Wright, W.E.; Shay, J.W. Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2′-deoxyguanosine. Cancer Discov. 2015, 5, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, Y.; Tazawa, H.; Tanabe, S.; Kanaya, N.; Noma, K.; Koujima, T.; Kashima, H.; Kato, T.; Kuroda, S.; Kikuchi, S.; et al. Phase I dose-escalation study of endoscopic intratumoral injection of OBP-301 (Telomelysin) with radiotherapy in oesophageal cancer patients unfit for standard treatments. Eur. J. Cancer 2021, 153, 98–108. [Google Scholar] [CrossRef]
- Heo, J.; Liang, J.D.; Kim, C.W.; Woo, H.Y.; Shih, I.L.; Su, T.H.; Lin, Z.Z.; Yoo, S.Y.; Chang, S.; Urata, Y.; et al. Safety and dose escalation of the targeted oncolytic adenovirus OBP-301 for refractory advanced liver cancer: Phase I clinical trial. Mol Ther. 2023, 31, 2077–2088. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Chen, G.; Shao, M. Clinical research progress of telomerase targeted cancer immunotherapy: A literature review. Transl. Cancer Res. 2024, 13, 3904–3921. [Google Scholar] [CrossRef]
- Ellingsen, E.B.; Mangsbo, S.M.; Hovig, E.; Gaudernack, G. Telomerase as a Target for Therapeutic Cancer Vaccines and Considerations for Optimizing Their Clinical Potential. Front Immunol. 2021, 12, 682492. [Google Scholar] [CrossRef]
- Sandri, S.; De Sanctis, F.; Lamolinara, A.; Boschi, F.; Poffe, O.; Trovato, R.; Fiore, A.; Sartori, S.; Sbarbati, A.; Bondanza, A.; et al. Effective control of acute myeloid leukaemia and acute lymphoblastic leukaemia progression by telomerase specific adoptive T-cell therapy. Oncotarget 2017, 8, 86987–87001. [Google Scholar] [CrossRef]
- Condoluci, A.; Rossi, D. Genomic Instability and Clonal Evolution in Chronic Lymphocytic Leukemia: Clinical Relevance. J. Natl. Compr. Cancer Netw. 2020, 19, 227–233. [Google Scholar] [CrossRef]
- Lupatov, A.Y.; Yarygin, K.N. Telomeres and Telomerase in the Control of Stem Cells. Biomedicines 2022, 10, 2335. [Google Scholar] [CrossRef] [PubMed]
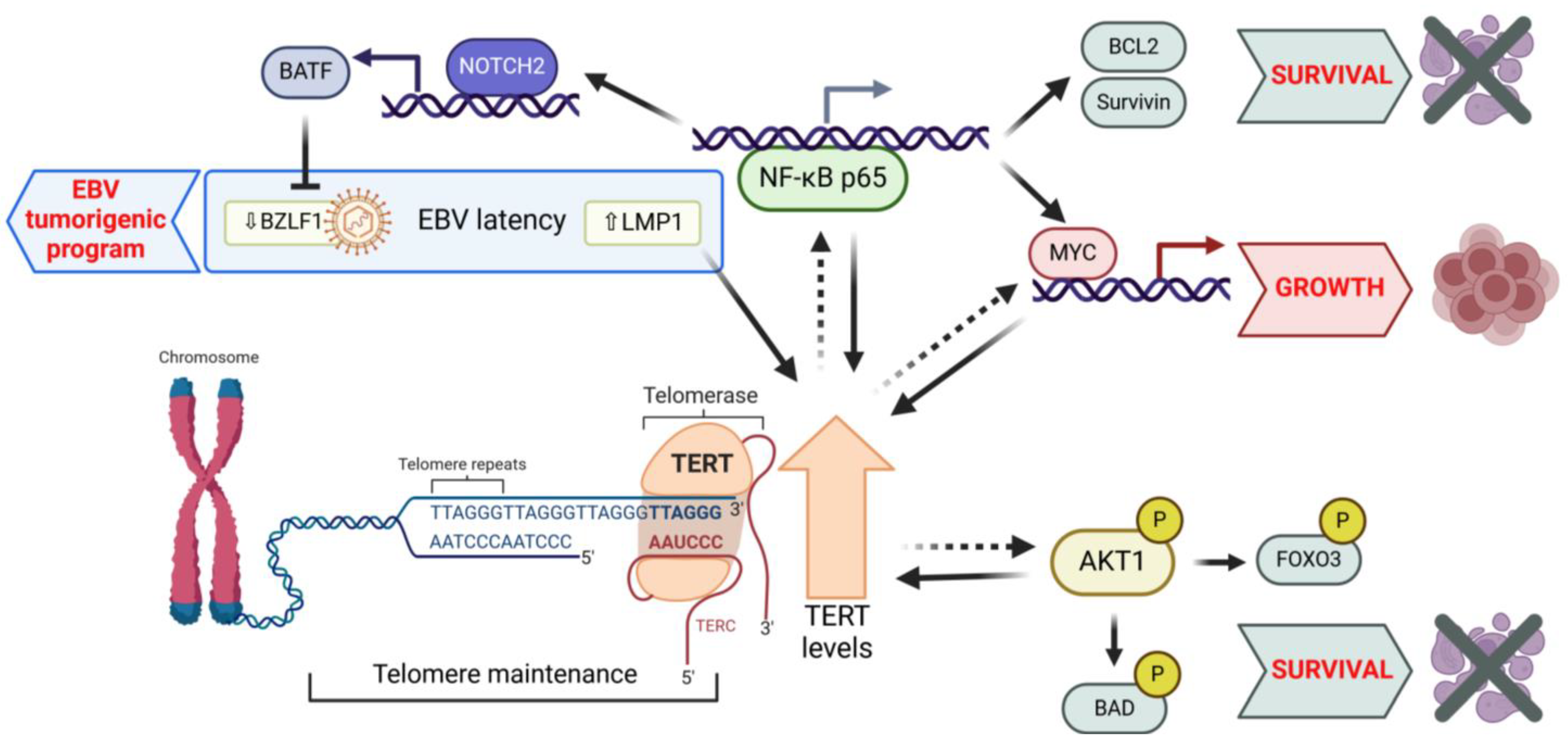
| B-Malignancy | TERT’s Extratelomeric Function(s) | Reference |
|---|---|---|
| EBV-immortalized lymphoblastoma cell lines | Increased TERT levels promote EBV latency program, increase resistance to lytic cycle induction, and enhance in vitro growth properties. | [68] |
| EBV-immortalized lymphoblastoma cell lines; EBV-negative and -positive BL cell lines | TERT inhibition via shTERT RNA, decreasing BATF and increasing BZLF1 expression, induces the EBV lytic cycle. In both EBV-positive and -negative cells, TERT inhibition reduces proliferation and triggers AKT1/FOXO3/NOXA-dependent apoptosis. | [89] |
| EBV-immortalized lymphoblastoma cell lines | High TERT levels activate NOTCH2 through the NF-κB signaling pathway. In turn, NOTCH2 induces BATF, which suppresses BZLF1 viral expression, thereby promoting EBV latency. | [90] |
| EBV-immortalized lymphoblastoma cell lines; EBV-positive and -negative BL cell lines | Short-term inhibition of TERT by BIBR1532 causes cell cycle arrest and apoptosis, associated with activation of DDR independently of telomere shortening. TERT inhibition sensitizes cells to the pro-apoptotic effects of chemotherapeutic agents. | [91] |
| EBV-immortalized lymphoblastoma cell lines; EBV-negative BL cell lines | Short-term TERT inhibition by BIBR1532 reduces proliferation and impairs the viability of LCL and BL cells xenografted in zebrafish through cell cycle arrest and apoptosis driven by DDR activation, independently of telomere shortening. | [92] |
| EBV-immortalized lymphoblastoma cell lines; EBV-negative BL cell lines | Short-term inhibition of TERT by BIBR1532 impairs cell growth by downregulating MYC via NF-κB signaling. Combined treatment with TERT inhibitor and chemotherapeutic agents induces a cumulative inhibitory effect on the proliferation of LCL and BL cells xenografted in zebrafish. | [78] |
| EBV-positive B-cell lymphoma cell line | Triptolide inhibits TERT expression and activity by downregulating SP1 and MYC. Triptolide promotes the lytic cycle of EBV. | [93] |
| Lymphoblastoma cell line and primary leukemic cells from B-ALL | Short-term inhibition of TERT by shTERT RNA decreases MYC protein stability, leading to the reduced transcription of its target genes and impairing cell viability without affecting the telomere length. | [77] |
| Primary leukemic cells from ALL | Telomerase inhibition with MST-312 for 48 hours significantly reduces levels of IL6 in primary leukemic cells by inhibiting NF-κB signaling. | [76] |
| Pre-B ALL cell line | Treatment with BIBR1532 for 48 h impairs cell proliferation and causes cell death, likely by reducing Survivin-mediated MYC and TERT levels. | [94] |
| Pre-B ALL cell line | Treatment with BIBR1532 for 24 h enhances ROS production and increases the anti-proliferative and pro-apoptotic effects of doxorubicin by upregulating TP73 and p21 and downregulating MYC and TERT. | [95] |
| Pre-B ALL cell lines | The telomerase inhibitor MST-312 shows dose-dependent cytotoxic and apoptotic effects on pre-B ALL cells. A 48 h combination with doxorubicin enhances cytotoxicity and apoptosis, linked to reductions in BCL2, MYC, and TERT and an increase in BAX. | [96] |
| Pre-B ALL cell line | A 48 h combined treatment with MST-312 and NU7441, a DNA-PK inhibitor, synergistically induces anti-proliferative and pro-apoptotic effects, downregulating MYC, TERT, and BCL2 and upregulating BAX. | [97] |
| Pre-B ALL cell lines | Short-term TERT inhibition by siRNA reduces proliferation and viability, associated with the upregulation of BAX and downregulation of BCL2. The cytotoxicity of TERT inhibition is characterized by the upregulation of ferroptosis promoters (lipid-ROS, ACSL4) and suppression of inhibitors (SLC7A11). | [98] |
| Human multiple myeloma cell line | Treatment with MST-312 for 48 h induces anti-proliferative and pro-apoptotic effects, by downregulating MYC, TERT, BCL2, IL6, and TNFα and upregulating BAX. | [99] |
| Multiple myeloma cell lines | Treatment with BIBR1532 for 48 h inhibits cell proliferation and promotes apoptosis associated with the downregulation of TERT, MYC, BCL-XL, and Survivin; increased BAD levels; the dephosphorylation of PI3K, AKT1, and mTOR; and the increased phosphorylation of ERK1/2 and MAPK. BIBR1532, combined with doxorubicin or bortezomib, exhibits a synergistic pro-apoptotic effect. | [100] |
| Multiple myeloma cancer stem cells from cell lines and primary clinical specimens | Short-term telomerase inhibition by Imetelstat reduces clonogenic growth and promotes differentiation by downregulating stemness-related genes, without affecting the telomere length. | [101] |
| Philadelphia chromosome-positive B-lymphoblastic leukemia cell line | Short-term treatment with the telomerase inhibitor Imetelstat demonstrates dose-dependent suppression of cell proliferation, unrelated to telomere length: at higher concentrations, it induces increased levels of γH2AX. | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giunco, S.; Petrara, M.R.; Indraccolo, S.; Ciminale, V.; De Rossi, A. Beyond Telomeres: Unveiling the Extratelomeric Functions of TERT in B-Cell Malignancies. Cancers 2025, 17, 1165. https://doi.org/10.3390/cancers17071165
Giunco S, Petrara MR, Indraccolo S, Ciminale V, De Rossi A. Beyond Telomeres: Unveiling the Extratelomeric Functions of TERT in B-Cell Malignancies. Cancers. 2025; 17(7):1165. https://doi.org/10.3390/cancers17071165
Chicago/Turabian StyleGiunco, Silvia, Maria Raffaella Petrara, Stefano Indraccolo, Vincenzo Ciminale, and Anita De Rossi. 2025. "Beyond Telomeres: Unveiling the Extratelomeric Functions of TERT in B-Cell Malignancies" Cancers 17, no. 7: 1165. https://doi.org/10.3390/cancers17071165
APA StyleGiunco, S., Petrara, M. R., Indraccolo, S., Ciminale, V., & De Rossi, A. (2025). Beyond Telomeres: Unveiling the Extratelomeric Functions of TERT in B-Cell Malignancies. Cancers, 17(7), 1165. https://doi.org/10.3390/cancers17071165









