The Role of the Systemic Immune-Inflammation Index in Predicting Postoperative Complications in Ovarian Cancer Patients: A Retrospective Cohort Study
Simple Summary
Abstract
1. Introduction
2. Material Method
- -
- Patients with ECOG performance status > 3: 7 patients;
- -
- Patients with known history of cancer: 12 patients (breast, colorectal, and thyroid cancers);
- -
- Patients with a history of venous thromboembolism: 7 patients;
- -
- Patients undergoing dialysis treatment: 2 patients;
- -
- Patients referred for neoadjuvant chemotherapy: 61 patients.
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bryant, A.; Hiu, S.; Kunonga, P.T.; Gajjar, K.; Craig, D.; Vale, L.; Winter-Roach, B.A.; Elattar, A.; Naik, R. Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery. Cochrane Database Syst. Rev. 2022, 26, CD015048. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.Y.; Nam, E.J.; Kim, S.W.; Kim, S.; Kim, Y.T. Rethinking radical surgery in interval debulking surgery for advanced-stage ovarian cancer patients undergoing neoadjuvant chemotherapy. J. Clin. Med. 2020, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Gostout, B.S.; Jones, M.B.; Stanhope, C.R.; Wilson, T.O.; Podratz, K.C.; Cliby, W.A. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet. Gynecol. 2006, 107, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.S.; Eisenhauer, E.L.; Zivanovic, O.; Sonoda, Y.; Abu-Rustum, N.R.; Levine, D.A.; Guile, M.W.; Bristow, R.E.; Aghajanian, C.; Barakat, R.R. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol. Oncol. 2009, 114, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Kengsakul, M.; Nieuwenhuyzen-de Boer, G.M.; Udomkarnjananun, S.; Kerr, S.J.; Niehot, C.D.; van Beekhuizen, H.J. Factors predicting postoperative morbidity after cytoreductive surgery for ovarian cancer: A systematic review and meta-analysis. J. Gynecol. Oncol. 2022, 33, e53. [Google Scholar] [CrossRef]
- Aletti, G.D.; Eisenhauer, E.L.; Santillan, A.; Axtell, A.; Aletti, G.; Holschneider, C.; Chi, D.S.; Bristow, R.E.; Cliby, W.A. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol. Oncol. 2011, 120, 23–28. [Google Scholar] [CrossRef]
- Kumar, A.; Janco, J.M.; Mariani, A.; Bakkum-Gamez, J.N.; Langstraat, C.L.; Weaver, A.L.; McGree, M.E.; Cliby, W.A. Risk-prediction model of severe postoperative complications after primary debulking surgery for advanced ovarian cancer. Gynecol. Oncol. 2016, 140, 15–21. [Google Scholar] [CrossRef]
- Günakan, E.; Tohma, Y.A.; Tunç, M.; Akıllı, H.; Şahin, H.; Ayhan, A. Factors associated with surgical morbidity of primary debulking in epithelial ovarian cancer. Obstet. Gynecol. Sci. 2020, 63, 64–71. [Google Scholar] [CrossRef]
- Zighelboim, I.; Kizer, N.; Taylor, N.P.; Case, A.S.; Gao, F.; Thaker, P.H.; Rader, J.S.; Massad, L.S.; Mutch, D.G.; Powell, M.A. “Surgical Apgar Score” predicts postoperative complications after cytoreduction for advanced ovarian cancer. Gynecol. Oncol. 2010, 116, 370–373. [Google Scholar] [CrossRef]
- Bacalbasa, N.; Balescu, I.; Dimitriu, M.; Iliescu, L.; Diaconu, C.; Dima, S.; Vilcu, M.; Brezean, I. The Influence of the Preoperative Status on the Risk of Postoperative Complications After Cytoreductive Surgery for Advanced-stage Ovarian Cancer. In Vivo 2020, 34, 839–844. [Google Scholar] [CrossRef]
- Balescu, I.; Eftimie, M.; Petrea, S.; Diaconu, C.; Gaspar, B.; Pop, L.; Varlas, V.; Hasegan, A.; Martac, C.; Bolca, C.; et al. Prognostic significance of preoperative inflammation markers on the long-term outcomes in peritoneal carcinomatosis from ovarian cancer. Cancers 2024, 16, 254. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Wu, F.; Zhang, L. Prognostic significance of pretreatment neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, or monocyte-to-lymphocyte ratio in endometrial neoplasms: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 734948. [Google Scholar] [CrossRef]
- Guo, J.; Lv, W.; Wang, Z.; Shang, Y.; Yang, F.; Zhang, X.; Xiao, K.; Zhang, S.; Pan, X.; Han, Y.; et al. Prognostic value of inflammatory and nutritional markers for patients with early-stage poorly-to moderately-differentiated cervical squamous cell carcinoma. Cancer Control. 2023, 30, 10732748221148913. [Google Scholar] [CrossRef]
- Yuce, E.; Karakullukcu, S.; Bulbul, H.; Alandag, C.; Saygin, I.; Kavgaci, H. The effect of the change in hemoglobin-albumin-lymphocyte-platelet scores occurring with neoadjuvant chemotherapy on clinical and pathological responses in breast cancer. Bratisl. Lek. Listy. 2023, 124, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Liu, Z.; Song, J.; Li, J. The inflammation score predicts the prognosis of gastric cancer patients undergoing Da Vinci robot surgery. J. Robot. Surg. 2024, 18, 131. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, X.L.; Ma, X.L.; Yang, X.F.; Liu, Y.Y.; Yu, Y.J. Preoperative neutrophil-to-lymphocyte ratio predicts symptomatic anastomotic leakage in elderly colon cancer patients: Multicenter propensity score-matched analysis. World J. Gastrointest. Surg. 2024, 16, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Liu, H.Y.; Liu, W.C.; Hou, M.C.; Tai, Y.H. A predictive model incorporating inflammation markers for high-grade surgical complications following liver resection for hepatocellular carcinoma. J. Chin. Med. Assoc. 2022, 85, 845–852. [Google Scholar] [CrossRef]
- Shevchenko, I.; Grigorescu, C.C.; Serban, D.; Cristea, B.M.; Simion, L.; Gherghiceanu, F.; Costea, A.C.; Dumitrescu, D.; Alius, C.; Tudor, C.; et al. The value of systemic inflammatory indices for predicting early postoperative complications in colorectal cancer. Medicina 2024, 60, 1481. [Google Scholar] [CrossRef]
- Mungan, İ.; Dicle, Ç.B.; Bektaş, Ş.; Sarı, S.; Yamanyar, S.; Çavuş, M.; Turan, S.; Bostancı, E.B. Does the preoperative platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict morbidity after gastrectomy for gastric cancer? Mil. Med. Res. 2020, 7, 9. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Elyashiv, O.; Graham, R.; Counsell, N.; Jayanth, N.; Berg, L.; Howard, K.; Gleeson, J.T.; Doufekas, K.; Macdonald, N.D.; Ledermann, J.A.; et al. Survival outcomes following cytoreductive surgery in advanced ovarian cancer patients: Prognosis is better predicted by the completeness of resection than by disease stage. Int. J. Gynecol. Cancer. 2024, 34, A33–A34. [Google Scholar]
- Wang, R.H.; Wen, W.X.; Jiang, Z.P.; Du, Z.P.; Ma, Z.H.; Lu, A.L.; Li, H.P.; Yuan, F.; Wu, S.B.; Guo, J.W.; et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front Immunol. 2023, 13, 1115031. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Ferreira, J. The Benjamini-Hochberg Method in the Case of Discrete Test Statistics. Int. J. Biostat. 2007, 3, 11. [Google Scholar] [CrossRef]
- Barakat, P.; Nieroda, C.; Diaz-Montes, T.; Gushchin, V. Peritoneal metastases from rare ovarian cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC). Pleura Peritoneum. 2023, 27, 15–22. [Google Scholar] [CrossRef]
- Narasimhulu, D.M.; Thannickal, A.; Kumar, A.; Weaver, A.L.; McGree, M.E.; Langstraat, C.L.; Cliby, W.A. Appropriate triage allows aggressive primary debulking surgery with rates of morbidity and mortality comparable to interval surgery after chemotherapy. Gynecol. Oncol. 2021, 160, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Inci, M.G.; Rasch, J.; Woopen, H.; Mueller, K.; Richter, R.; Sehouli, J. ECOG and BMI as preoperative risk factors for severe postoperative complications in ovarian cancer patients: Results of a prospective study (RISC-GYN-trial). Arch. Gynecol. Obstet. 2021, 304, 1323–1333. [Google Scholar] [CrossRef]
- Savant, S.S.; Sriramkumar, S.; O’Hagan, H.M. The Role of Inflammation and Inflammatory Mediators in the Development, Progression, Metastasis, and Chemoresistance of Epithelial Ovarian Cancer. Cancers 2018, 30, 251. [Google Scholar] [CrossRef]
- Stoiber, D.; Assinger, A. Platelet-Leukocyte Interplay in Cancer Development and Progression. Cells 2020, 9, 855. [Google Scholar] [CrossRef]
- Browning, L.; Patel, M.R.; Horvath, E.B.; Tawara, K.; Jorcyk, C.L. IL-6 and ovarian cancer: Inflammatory cytokines in promotion of metastasis. Cancer Manag. Res. 2018, 5, 6685–6693. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten, D.P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Ménétrier-Caux, C.; Ray-Coquard, I.; Blay, J.Y.; Caux, C. Lymphopenia in Cancer Patients and its Effects on Response to Immunotherapy: An opportunity for combination with Cytokines? J. Immunother. Cancer 2019, 28, 85. [Google Scholar] [CrossRef]
- Xie, H.; Yuan, G.; Huang, S.; Kuang, J.; Yan, L.; Ruan, G.; Tang, S.; Gan, J. The prognostic value of combined tumor markers and systemic immune-inflammation index in colorectal cancer patients. Langenbecks Arch. Surg. 2020, 405, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Xu, R.; Lin, L.; Liao, X. Effect of the systemic immune-inflammation index on postoperative complications and the long-term prognosis of patients with colorectal cancer: A retrospective cohort study. J. Gastrointest. Oncol. 2022, 13, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Wenpei, G.; Yuan, L.; Liangbo, L.; Jingjun, M.; Bo, W.; Zhiqiang, N.; Yijie, N.; Lixin, L. Predictive value of preoperative inflammatory indexes for postoperative early recurrence of hepatitis B-related hepatocellular carcinoma. Front. Oncol. 2023, 13, 1142168. [Google Scholar] [CrossRef]
- Hung, H.C.; Hsu, P.J.; Chang, T.C.; Chou, H.H.; Huang, K.G.; Lai, C.H.; Lee, C.W.; Yu, M.C.; You, J.F.; Hsu, J.T.; et al. Neutrophil-to-lymphocyte-ratio-based perioperative prognosis prediction model on early mortality after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Asian J. Surg. 2022, 45, 2676–2685. [Google Scholar] [CrossRef]
- Medina Fernández, F.J.; Muñoz-Casares, F.C.; Arjona-Sánchez, A.; Casado-Adam, A.; Gómez-Luque, I.; Garcilazo Arismendi, D.J.; Thoelecke, H.; Rufián, P.S.; Briceño, D.J. Postoperative time course and utility of inflammatory markers in patients with ovarian peritoneal carcinomatosis treated with neoadjuvant chemotherapy, cytoreductive surgery, and HIPEC. Ann. Surg. Oncol. 2015, 22, 1332–1340. [Google Scholar] [CrossRef]
- Williams, K.A.; Labidi-Galy, S.I.; Terry, K.L.; Vitonis, A.F.; Welch, W.R.; Goodman, A.; Cramer, D.W. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol. 2014, 132, 542–550. [Google Scholar] [CrossRef]
- Baert, T.; Van Camp, J.; Vanbrabant, L.; Busschaert, P.; Laenen, A.; Han, S.; Van Nieuwenhuysen, E.; Vergote, I.; Coosemans, A. Influence of CA125, platelet count and neutrophil to lymphocyte ratio on the immune system of ovarian cancer patients. Gynecol. Oncol. 2018, 150, 31–37. [Google Scholar] [CrossRef]
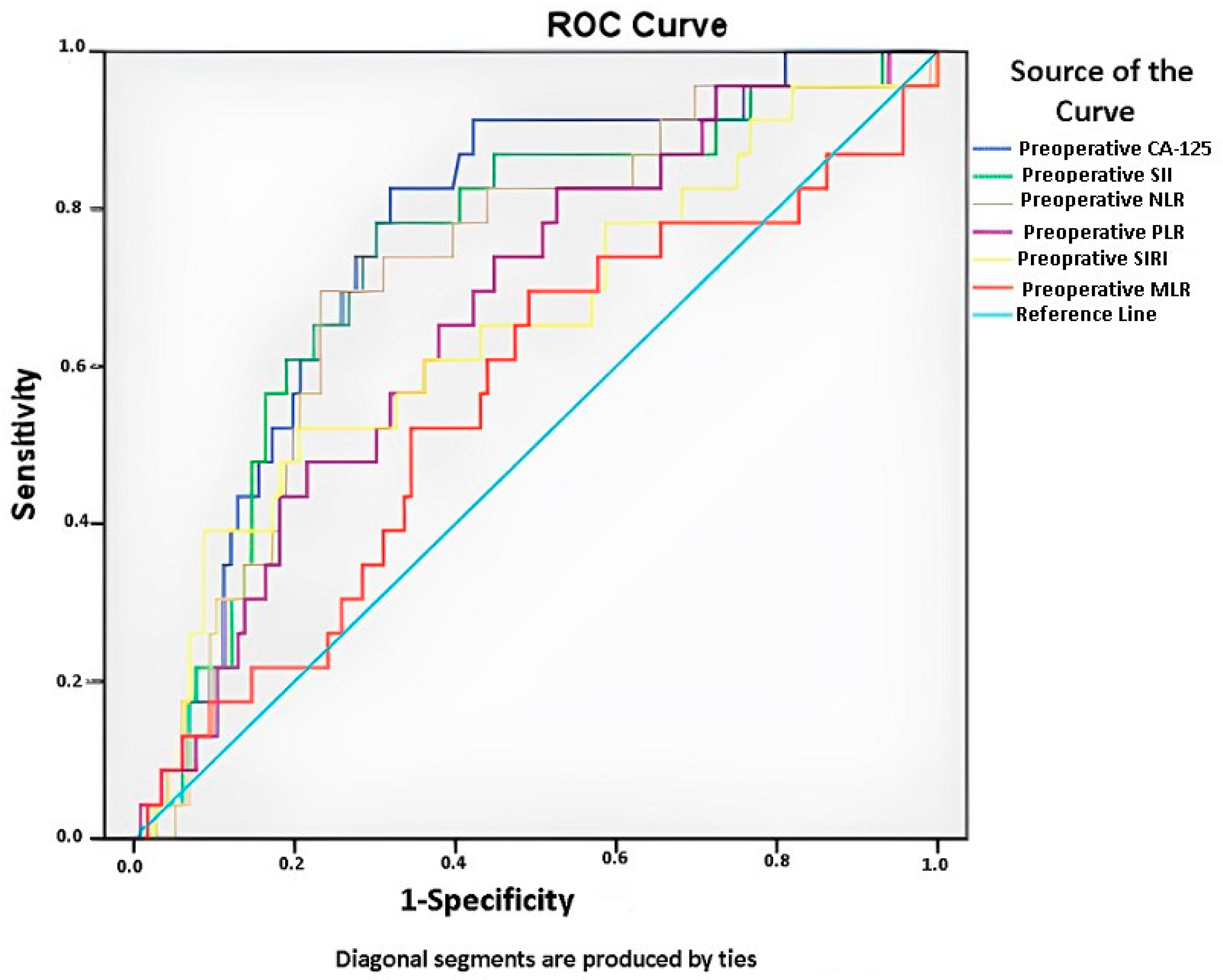
| Items | Preoperative Ca125 Value | Preoperative SII | Preoperative NLR | Preoperative PLR | Preoperative SIRI | Preoperative MLR |
|---|---|---|---|---|---|---|
| AUC | 0.766 | 0.739 | 0.718 | 0.652 | 0.652 | 0.560 |
| SE | 0.05 | 0.056 | 0.057 | 0.058 | 0.067 | 0.068 |
| P | <0.001 * | <0.001 * | 0.001 * | 0.011 * | 0.022 * | 0.374 |
| %95 CI | 0.661–0.857 | 0.622–0.837 | 0.598–0.824 | 0.522–0.772 | 0.521–0.772 | 0.427–0.689 |
| Associated criterion | 838.5 | 1151.2 | 4.16 | 167.4 | 1.5 | 0.2 |
| Sensitivity (%) | 82.6 | 78.3 | 69.6 | 82.6 | 60.9 | 69.6 |
| Specificity (%) | 68.1 | 69.8 | 76.7 | 47.4 | 63.8 | 50.9 |
| 1 − β | 0.98 | 0.96 | 0.94 | 0.76 | 0.70 | 0.04 |
| Variables | Total | Ca125 | p | SII | p | NLR | p | |||
|---|---|---|---|---|---|---|---|---|---|---|
| <838.50 | ≥838.50 | <1151.21 | ≥1151.21 | <4.15 | ≥4.15 | |||||
| Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | ||||
| Hemoglobin (g/dL) | 11.80 [2.1] | 11.80 [2.00] | 11.65 [2.35] | 0.427 | 11.95 [1.92] | 11.00 [2.51] | 0.041 | 11.80 [1.80] | 11.50 [3.18] | 0.278 |
| Hematocrit (%) | 36.10 [6.00] | 36.70 [5.70] | 35.45 [6.27] | 0.203 | 36.65 [4.45] | 33.90 [7.50] | 0.046 | 36.20 [5.30] | 35.70 [8.20] | 0.220 |
| Monocyte (×103/µL) | 0.41 [0.24] | 0.38 [0.25] | 0.42 [0.25] | 0.411 | 0.38 [0.24] | 0.45 [0.24] | 0.077 | 0.38 [0.24] | 0.45 [0.24] | 0.113 |
| Leukocyte (×103/µL) | 7.11 [3.44] | 6.82 [3.74] | 7.37 [2.85] | 0.125 | 6.33 [2.60] | 8.41 [3.32] | <0.001 | 6.82 [2.90] | 8.24 [4.92] | 0.013 |
| Platelet (×103/µL) | 284.0 [148.0] | 266.0 [147.0] | 328.5 [185.0] | <0.001 | 266.0 [114.2] | 381.0 [199.0] | <0.001 | 281.0 [113.0] | 351.0 [239.0] | 0.005 |
| Lymphocyte (×103/µL) | 1.59 [0.81] | 1.64 [0.77] | 1.50 [0.79] | 0.230 | 1.75 [0.80] | 1.34 [0.82] | <0.001 | 1.75 [0.76] | 0.97 [0.78] | <0.001 |
| Neutrophil (×103/µL) | 4.91 [2.99] | 4.63 [3.16] | 5.14 [2.99] | 0.060 | 4.05 [1.99] | 7.10 [3.07] | <0.001 | 4.31 [2.21] | 7.74 [3.06] | <0.001 |
| Variables | Total | PLR | p | SIRI | p | |||||
| <167.38 | ≥167.38 | <1.53 | ≥1.53 | |||||||
| Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | ||||||
| Hemoglobin (g/dL) | 11.80 [2.10] | 12.00 [2.00] | 11.35 [2.20] | 0.028 | 11.85 [1.82] | 11.60 [2.76] | 0.374 | |||
| Hematocrit (%) | 36.10 [6.00] | 37.20 [4.50] | 35.45 [6.05] | 0.021 | 36.40 [5.30] | 35.70 [7.80] | 0.349 | |||
| Monocyte (×103/µL) | 0.41 [0.24] | 0.41 [0.25] | 0.39 [0.25] | 0.907 | 0.35 [0.17] | 0.53 [0.26] | <0.001 | |||
| Leukocyte (×103/µL) | 7.11 [3.44] | 6.82 [3.16] | 7.34 [3.52] | 0.681 | 6.43 [2.71] | 8.31 [3.45] | <0.001 | |||
| Platelet (×103/µL) | 284.00 [148.00] | 250.00 [94.00] | 336.00 [188.75] | <0.001 | 274.50 [129.75] | 332.00 [208.00] | 0.001 | |||
| Lymphocyte (×103/µL) | 1.59 [0.81] | 1.99 [0.86] | 1.35 [0.75] | <0.001 | 1.76 [0.78] | 1.36 [0.81] | <0.001 | |||
| Neutrophil (×103/µL) | 4.91 [2.99] | 4.22 [2.86] | 5.51 [3.13] | 0.001 | 4.21 [2.24] | 7.08 [3.74] | <0.001 | |||
| Variables | Total | Ca125 | p | NLR | p | PLR | p | |||
|---|---|---|---|---|---|---|---|---|---|---|
| <838.50 | ≥838.50 | <4.15 | ≥4.15 | <167.38 | ≥167.38 | |||||
| Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | ||||
| Age | 60.00 [16.00] | 58.00 [16.00] | 63.00 [15.00] | 0.008 | 59.00 [16.00] | 61.00 [12.00] | 0.270 | 58.00 [16.00] | 61.50 [15.75] | 0.195 |
| Hypoalbuminemia | 0.002 | 0.012 | <0.001 | |||||||
| None | 42 (30.2) | 17 (20.5) | 25 (44.6) | 22 (23.2) | 19 (44.2) | 7 (11.9) | 35 (43.8) | |||
| Yes | 97 (69.8) | 66 (79.5) | 31 (55.4) | 73 (76.8) | 24 (55.8) | 52 (88.1) | 45 (56.3) | |||
| MLR | 0.25 [0.20] | 0.23 [0.22] | 0.29 [0.17] | 0.293 | 0.22 [0.12] | 0.41 [0.18] | <0.001 | 0.20 [0.11] | 0.34 [0.22] | <0.001 |
| Residual disease | 0.005 | 0.009 | 0.092 | |||||||
| 0 | 115(83.3) | 75 (91.5) | 40 (71.4) | 84 (89.4) | 30 (69.8) | 53 (91.4) | 62 (77.5) | |||
| 1 | 16 (11.6) | 6 (7.3) | 10 (17.9) | 8 (8.5) | 8 (18.6) | 4 (6.9) | 12 (15.0) | |||
| 2 | 7 (5.1) | 1 (1.2) | 6 (10.7) | 2 (2.1) | 5 (11.6) | 1 (1.7) | 6 (7.5) | |||
| Acid (L) | 1.25 [1.60] | 0.95 [0.85] | 2.15 [2.34] | <0.001 | 1.20 [1.10] | 1.70 [4.00] | 0.002 | 1.10 [10.50] | 1.40 [2.51] | 0.083 |
| PCI | <0.001 | 0.005 | 0.116 | |||||||
| <10 | 55 (39.6) | 42 (50.6) | 13 (23.2) | 46 (48.4) | 9 (20.9) | 26 (44.1) | 29 (36.3) | |||
| 10–15 | 48 (34.5) | 30 (36.1) | 18 (32.1) | 30 (31.6) | 17 (39.5) | 23 (39.0) | 25 (31.3) | |||
| >15 | 36 (25.9) | 11 (13.3) | 25 (44.6) | 19 (20.0) | 17 (39.5) | 10 (16.9) | 26 (32.5) | |||
| Amount of bleeding (L) | 0.35 [0.25] | 0.30 [0.20] | 0.42 [0.30] | 0.008 | 0.30 [0.17] | 0.45 [0.35] | 0.133 | 0.30 [0.20] | 0.40 [0.35] | 0.077 |
| ASA | 0.001 | 0.051 | 0.166 | |||||||
| 1 | 12 (8.6) | 7(8.4) | 5 (8.9) | 11 (11.6) | 1 (2.3) | 6 (10.2) | 6 (7.5) | |||
| 2 | 87 (62.6) | 62 (74.7) | 25 (44.6) | 62 (65.3) | 25 (58.1) | 41 (69.5) | 46 (57.5) | |||
| 3 | 40 (28.8) | 14 (16.9) | 26 (46.4) | 22 (23.2) | 17 (39.5) | 12 (20.3) | 28 (35.0) | |||
| Operation time (min) | 240.0 [80.0] | 230.0 [50.0] | 260.0 [80.0] | <0.001 | 230.0 [60.0] | 260.0 [70.0] | 0.008 | 230.0 [60.0] | 240.0 [70.0] | 0.140 |
| Variables | Total | SII | p | SIRI | p | |||||
| <1151.21 | ≥1151.21 | <1.53 | ≥1.53 | |||||||
| Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | ||||||
| Age | 60.00 [16.00] | 59.50 [16.50] | 61.00 [2.00] | 0.374 | 59.00 [16.50] | 61.00 [13.00] | 0.447 | |||
| Hypoalbuminemia | <0.001 | 0.030 | ||||||||
| None | 42 (30.2) | 16 (18.6) | 26 (49.1) | 19 (23.2) | 23 (40.4) | |||||
| Yes | 97 (69.8) | 70 (81.4) | 27 (50.9) | 63 (76.8) | 34 (59.6) | |||||
| MLR | 0.25 [0.20] | 0.22 [0.12] | 0.37 [0.21] | <0.001 | 0.20 [0.08] | 0.42 [0.16] | <0.001 | |||
| Residual disease | 0.041 | 0.248 | ||||||||
| 0 | 115(83.3) | 76 (89.4) | 39 (73.6) | 70 (85.4) | 45 (80.4) | |||||
| 1 | 16 (11.6) | 7 (8.2) | 9 (17.0) | 10 (12.2) | 6 (10.7) | |||||
| 2 | 7 (5.1) | 2 (2.4) | 5 (9.4) | 2 (2.4) | 5 (8.9) | |||||
| Acid (L) | 1.25 [1.60] | 1.20 [1.10] | 1.40 [3.00] | 0.058 | 1.20 [1.25] | 1.40 [2.60] | 0.077 | |||
| PCI | 0.026 | 0.433 | ||||||||
| <10 | 55 (39.6) | 41 (47.7) | 14 (26.4) | 36 (43.9) | 19 (33.3) | |||||
| 10–15 | 48 (34.5) | 28 (32.6) | 20 (37.7) | 27 (32.9) | 21 (36.9) | |||||
| >15 | 36 (25.9) | 17 (19.8) | 19 (35.8) | 19 (23.2) | 17 (29.8) | |||||
| Amount of bleeding (L) | 0.35 [0.25] | 0.30 [0.15] | 0.40 [0.35] | 0.135 | 0.30 [0.23] | 0.35 [0.32] | 0.704 | |||
| ASA | 0.018 | 0.092 | ||||||||
| 1 | 12 (8.6) | 10 (11.6) | 2 (3.8) | 7 (8.5) | 5 (8.8) | |||||
| 2 | 87 (62.6) | 58 (67.4) | 29 (54.7) | 57 (69.5) | 30 (52.6) | |||||
| 3 | 40 (28.8) | 18 (20.9) | 22 (41.5) | 18 (22.0) | 22 (38.6) | |||||
| Operation time | 240.0 [80.0] | 230.0 [60.0] | 250.0 [75.0] | 0.019 | 235.0 [70.0] | 250.0 [75.0] | 0.278 | |||
| Variables | Total | Ca125 | p | NLR | p | PLR | p | |||
|---|---|---|---|---|---|---|---|---|---|---|
| <838.50 | ≥838.50 | <4.15 | ≥4.15 | <167.38 | ≥167.38 | |||||
| Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | ||||
| Duration of hospital stay | 60.0 [3.0] | 6.0 [3.0] | 8.0 [4.0] | 0.001 | 6.0 [3.0] | 8.0 [4.0] | 0.002 | 6.0 [3.0] | 7.0 [4.0] | 0.034 |
| ICU | 0.028 | 0.815 | 0.662 | |||||||
| Yes | 21 (15.1) | 8 (9.6) | 13 (23.2) | 14 (14.7) | 7 (16.3) | 8 (13.6) | 13 (16.3) | |||
| No | 118 (84.9) | 75 (90.4) | 43 (76.8) | 81 (85.3) | 36 (83.7) | 51 (86.4) | 67 (83.3) | |||
| Intensive care unit length of stay | 0 [5.0] | 0 [5.0] | 0 [5.0] | 0.026 | 0 [4.0] | 0 [5.0] | 0.713 | 0 [4.0] | 0 [5.0] | 0.622 |
| Complications | <0.001 * | <0.001 * | 0.018* | |||||||
| None | 117 (84.2) | 79 (95.2) | 38 (67.9) | 88 (92.6) | 28 (65.1) | 55(93.2) | 62 (77.5) | |||
| Cardiovascular | 4 (2.9) | - | 4 (7.19 | - | 4 (9.3) | - | 4 (5.0) | |||
| Surgery site | 4 (2.9) | 1 (1.2) | 3 (5.4) | - | 4 (9.3) | - | 4 (5.0) | |||
| Respiratory | 4 (2.9) | 1(1.2) | 3 (5.4) | 2 (2.1) | 2 (4.7) | 1 (1.7) | 3 (3.8) | |||
| Infections | 4 (2.9) | - | 4 (7.1) | 3 (3.2) | 1 (2.3) | 3 (5.1) | 1 (1.3) | |||
| Gastrointestinal | 3 (2.2) | 1 (1.2) | 2 (3.6) | 2 (2.1) | 1 (2.3) | - | 3 (3.8) | |||
| Anastomotic Leaks | 3 (2.2) | 1 (1.2) | 2 (3.6) | - | 3 (7.0) | - | 3 (3.8) | |||
| Complication | <0.001 | <0.001 | 0.008 | |||||||
| 0–2 | 116 (83.5) | 79 (95.2) | 37 (66.1) | 88 (92.6) | 27 (62.8) | 55 (93.2) | 61 (76.3) | |||
| ≥3a | 23 (16.50) | 4 (4.8) | 19 (33.9) | 7 (7.4) | 16 (37.2) | 4 (6.8) | 19 (23.8) | |||
| Operation-related death | 0.222 | 0.389 | ||||||||
| Yes | 1(0.7) | - | 1 (1.8) | - | 1 (2.3) | 0.136 | - | 1 (1.3) | ||
| No | 138 (99.3) | 83 (100.0) | 55 (98.2) | 95 (100.0) | 42 (97.7) | 59 (100.0) | 79 (98.8) | |||
| Variables | Total | SII | p | SIRI | p | |||||
| <1151.21 | ≥1151.21 | <1.53 | ≥1.53 | |||||||
| Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | ||||||
| Duration of hospital stay | 60.0 [3.0] | 6.0 [3.0] | 8.0 [4.50] | 0.003 | 6.0 [3.0] | 7.0 [4.0] | 0.396 | |||
| ICU | 0.331 | 0.082 | ||||||||
| Yes | 21 (15.1) | 11 (12.8) | 10 (18.9) | 16 (19.5) | 5 (8.8) | |||||
| No | 118 (84.9) | 75 (87.2) | 43 (81.1) | 66 (80.5) | 52 (91.2) | |||||
| Intensive care unit length of stay | 0 [5.0] | 0 [4.0] | 0 [5.0] | 0.319 | 0 [4.0] | 0 [5.0] | 1.000 | |||
| Complications | <0.001 * | 0.020 * | ||||||||
| None | 117(84.2) | 81 (94.2) | 36 (67.9) | 73 (89.0) | 44 (77.2) | |||||
| Cardiovascular | 4 (2.9) | - | 4 (7.5) | 1 (1.2) | 3 (5.3) | |||||
| Surgery site | 4 (2.9) | - | 4 (7.5) | - | 4 (7.0) | |||||
| Respiratory | 4 (2.9) | 2 (2.3) | 2 (3.8) | 3 (3.7) | 1 (1.8) | |||||
| Infections | 4 (2.9) | 2 (2.3) | 2 (3.8) | 3 (3.7) | 1(1.8) | |||||
| Gastrointestinal | 3 (2.2) | 1 (81.2) | 2 (3.8) | 2 (2.4) | 1 (1.89) | |||||
| Anastomotic Leaks | 3 (2.2) | - | 3 (5.7) | - | 3 (5.3) | |||||
| Complication | <0.001 | 0.034 | ||||||||
| 0–2 | 116 (83.5) | 81 (94.2) | 35 (66.0) | 73 (89.0) | 43 (75.4) | |||||
| ≥3a | 23 (16.50) | 5 (5.8) | 18 (34.0) | 9 (11.0) | 14 (24.6) | |||||
| Operation-related death | ||||||||||
| Yes | 1 (0.7) | - | 1 (1.8) | 0.201 | - | 56 (98.2) | 0.229 | |||
| No | 138 (99.3) | 86 (100.0) | 52 (98.1) | 82 (100.0) | 1 (1.8) | |||||
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| RR (%95 CI) | p | RR (%95 GA) | p | |
| Age | 1.045 (1.000–1.091) | 0.048 | 0.993 (0.911–1.082) | 0.870 |
| Hypoalbuminemia | ||||
| (Yes) | 6.181 (2.366–16.143) | <0.001 | - | - |
| Residual disease (RD) | ||||
| 0 | 1 | <0.001 | 1 | 0.134 |
| 1 | 5.673 (1.730–18.603) | 0.004 | 1.428 (0.225–9.078) | 0.706 |
| 2 | 56.727 (6.246–515.212) | <0.001 | 16.430 (1.065–253.369) | 0.045 |
| Acid (Yes) | 1.001 (1.001–1.001) | <0.001 | - | - |
| PCI | <0.001 | - | - | |
| ≤15 | 1 | |||
| >15 | 19.600 (6.453–59.533) | |||
| Amount of bleeding | 1.007 (1.005–1.010) | <0.001 | - | - |
| ASA | <0.001 | - | - | |
| 1–2 | 1 | |||
| 3 | 21.488 (6.621–69.738) | |||
| Operation time (dk) | 1.056 (1.033–1.080) | <0.001 | 1.052 (1.024–1.080) | <0.001 |
| Duration of hospital stay (day) | 5.865 (2.493–13.797) | <0.001 | - | - |
| ICU (Yes) | 17.550 (5.881–52.373) | <0.001 | - | - |
| ICU length of stay (day) | 3.717 (2.192–6.303) | <0.001 | - | - |
| Ca125 | ||||
| ≥838.50 | 10.142 (3.222–31.926) | <0.001 | 1.901 (0.386–9.361) | 0.429 |
| NLR | ||||
| ≥4.15 | 7.450 (2.776–19.995) | <0.001 | - | - |
| PLR | ||||
| ≥167.38 | 4.283 (1.372–13.366) | 0.012 | - | - |
| SII | ||||
| ≥1151.21 | 8.331 (2.866–24.221) | <0.001 | 8.498 (1.498–48.200) | 0.016 |
| SIRI | ||||
| ≥1.53 | 2.641 (1.054–6.615) | 0.038 | - | - |
| Variables | Preoperative Ca125 | |
|---|---|---|
| r | p | |
| Preoperative SII | 0.263 | 0.002 |
| Preoperative NLR | 0.217 | 0.010 |
| Preoperative PLR | 0.282 | 0.001 |
| Preoperative SIRI | 0.119 | 0.164 |
| Preoperative MLR | 0.086 | 0.313 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köse, O.; Köse, E.; Gök, K.; Bostancı, M.S. The Role of the Systemic Immune-Inflammation Index in Predicting Postoperative Complications in Ovarian Cancer Patients: A Retrospective Cohort Study. Cancers 2025, 17, 1124. https://doi.org/10.3390/cancers17071124
Köse O, Köse E, Gök K, Bostancı MS. The Role of the Systemic Immune-Inflammation Index in Predicting Postoperative Complications in Ovarian Cancer Patients: A Retrospective Cohort Study. Cancers. 2025; 17(7):1124. https://doi.org/10.3390/cancers17071124
Chicago/Turabian StyleKöse, Osman, Elif Köse, Koray Gök, and Mehmet Sühha Bostancı. 2025. "The Role of the Systemic Immune-Inflammation Index in Predicting Postoperative Complications in Ovarian Cancer Patients: A Retrospective Cohort Study" Cancers 17, no. 7: 1124. https://doi.org/10.3390/cancers17071124
APA StyleKöse, O., Köse, E., Gök, K., & Bostancı, M. S. (2025). The Role of the Systemic Immune-Inflammation Index in Predicting Postoperative Complications in Ovarian Cancer Patients: A Retrospective Cohort Study. Cancers, 17(7), 1124. https://doi.org/10.3390/cancers17071124






