Post-Transplant Cyclophosphamide-Based Prophylaxis and Its Impact on Infectious Complications and Immune Reconstitution According to Donor Type
Simple Summary
Abstract
1. Introduction
2. Materials and Method
2.1. Patient Selection
2.2. Transplant Information and GVHD Prophylaxis
2.3. Infectious Prophylaxis, Monitoring, Treatment, and Main Definitions
2.4. Definitions and Supportive Care
2.5. Statistical Methods
3. Results
3.1. Patients and Allo-HCT Baseline Information
3.2. Main Post-Transplant Information and Outcomes
3.3. Incidence of Infectious Complications According to Donor Type
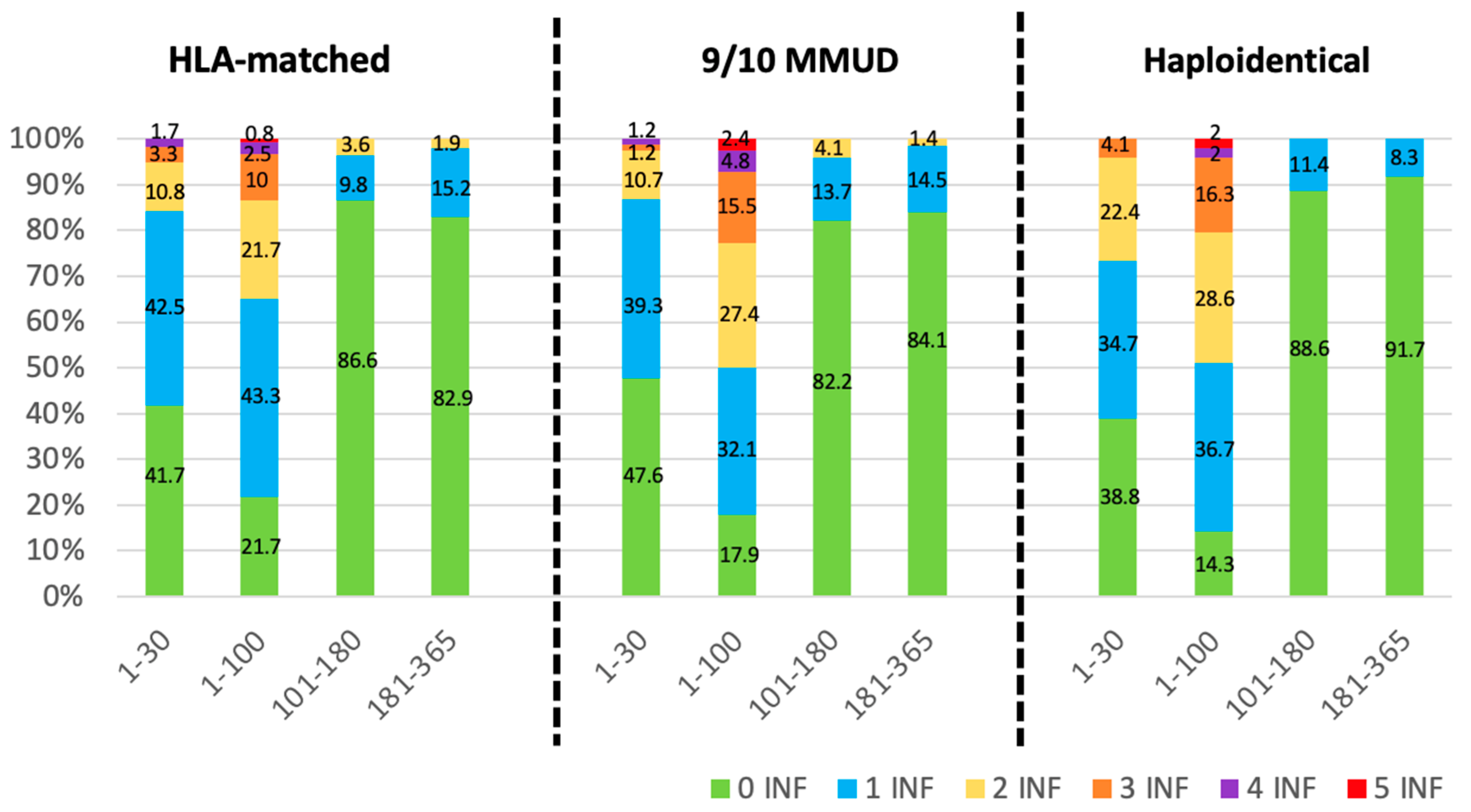
3.4. Infectious Density
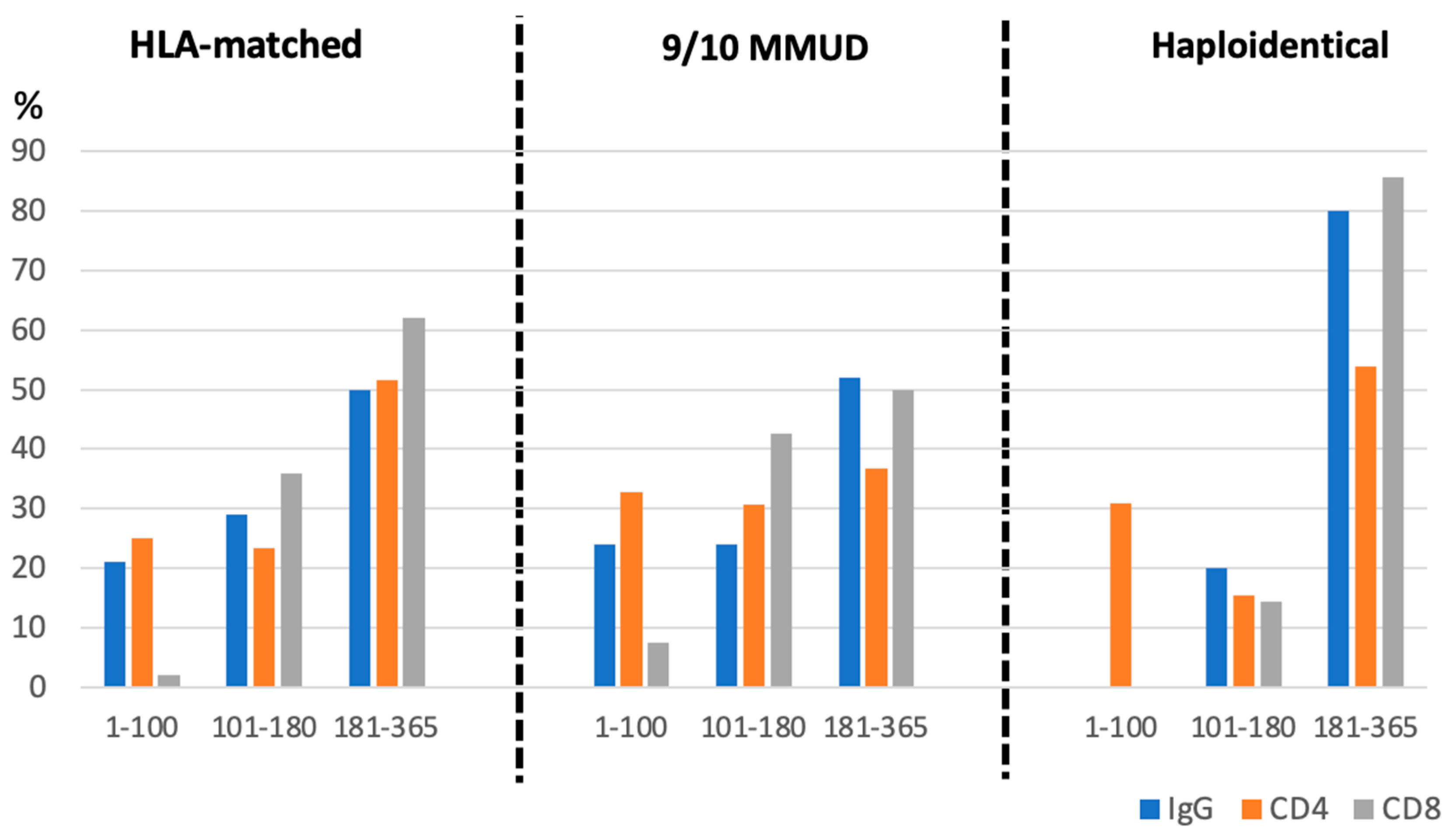
3.5. Immune Reconstitution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luznik, L.; O’Donnell, P.V.; Symons, H.J.; Chen, A.R.; Leffell, M.S.; Zahurak, M.; Gooley, T.A.; Piantadosi, S.; Kaup, M.; Ambinder, R.F.; et al. HLA-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biol. Blood Marrow Transplant. 2008, 14, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Nakamae, H. Systematic overview of HLA-matched allogeneic hematopoietic cell transplantation with post-transplantation cyclophosphamide. Int. J. Hematol. 2022, 116, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Luznik, L.; Fuchs, E.J. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol. Res. 2010, 47, 65–77. [Google Scholar] [CrossRef]
- Moiseev, I.S.; Pirogova, O.V.; Alyanski, A.L.; Babenko, E.V.; Gindina, T.L.; Darskaya, E.I.; Slesarchuk, O.A.; Bondarenko, S.N.; Afanasyev, B.V. Graft-versus-Host Disease Prophylaxis in Unrelated Peripheral Blood Stem Cell Transplantation with Post-Transplantation Cyclophosphamide, Tacrolimus, and Mycophenolate Mofetil. Biol. Blood Marrow Transplant. 2016, 22, 1037–1042. [Google Scholar] [CrossRef]
- Bhamidipati, P.K.; DiPersio, J.F.; Stokerl-Goldstein, K.; Rashidi, A.; Gao, F.; Uy, G.L.; Westervelt, P.; Vij, R.; A Schroeder, M.; Abboud, C.N.; et al. Haploidentical transplantation using G-CSF-mobilized T-cell replete PBSCs and post-transplantation CY after non-myeloablative conditioning is safe and is associated with favorable outcomes. Bone Marrow Transplant. 2014, 49, 1124–1126. [Google Scholar] [CrossRef]
- Esquirol, A.; Pascual, M.J.; Kwon, M.; Pérez, A.; Parody, R.; Ferra, C.; Cadenas, I.G.; Herruzo, B.; Dorado, N.; Hernani, R.; et al. Severe infections and infection-related mortality in a large series of haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide. Bone Marrow Transplant. 2021, 56, 2432–2444. [Google Scholar] [CrossRef] [PubMed]
- Oltolini, C.; Greco, R.; Galli, L.; Clerici, D.; Lorentino, F.; Xue, E.; Stanghellini, M.T.L.; Giglio, F.; Uhr, L.; Ripa, M.; et al. Infections after Allogenic Transplant with Post-Transplant Cyclophosphamide: Impact of Donor HLA Matching. Biol. Blood Marrow Transplant. 2020, 26, 1179–1188. [Google Scholar] [CrossRef]
- Gudiol, C.; Garcia-Vidal, C.; Arnan, M.; Sánchez-Ortega, I.; Patiño, B.; Duarte, R.; Carratalà, J. Etiology, clinical features and outcomes of pre-engraftment and post-engraftment bloodstream infection in hematopoietic SCT recipients. Bone Marrow Transplant. 2014, 49, 824–830. [Google Scholar] [CrossRef]
- Jorge, A.S.; Suárez-Lledó, M.; Pereira, A.; Gutierrez, G.; Fernández-Avilés, F.; Rosiñol, L.; Llobet, N.; Solano, T.; Urbano-Ispízua, Á.; Rovira, M.; et al. Single Antigen–Mismatched Unrelated Hematopoietic Stem Cell Transplantation Using High-Dose Post-Transplantation Cyclophosphamide Is a Suitable Alternative for Patients Lacking HLA-Matched Donors. Biol. Blood Marrow Transplant. 2018, 24, 1196–1202. [Google Scholar] [CrossRef]
- Pedraza, A.; Jorge, S.; Suárez-Lledó, M.; Pereira, A.; Gutiérrez-García, G.; Fernández-Avilés, F.; Rosiñol, L.; Llobet, N.; Solano, T.; Urbano-Ispízua, Á.; et al. High-Dose Cyclophosphamide and Tacrolimus as Graft-versus-Host Disease Prophylaxis for Matched and Mismatched Unrelated Donor Transplantation. Transplant. Cell. Ther. 2021, 27, 619.e1–619.e8. [Google Scholar] [CrossRef]
- Tomblyn, M.; Chiller, T.; Einsele, H.; Gress, R.; Sepkowitz, K.; Storek, J.; Wingard, J.R.; Young, J.-A.H.; Boeckh, M.A. Guidelines for Preventing Infectious Complications among Hematopoietic Cell Transplantation Recipients: A Global Perspective. Biol. Blood Marrow Transplant. 2009, 15, 1143–1238. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, G.; Bullinger, L.; Garcia-Vidal, C.; Herbrecht, R.; Maertens, J.; Menna, P.; Pagano, L.; Thiebaut-Bertrand, A.; Calandra, T. Infectious complications of targeted drugs and biotherapies in acute leukemia. Clinical practice guidelines by the European Conference on Infections in Leukemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the International Immunocompromised Host Society (ICHS) and the European Leukemia Net (ELN). Leukemia 2022, 36, 1215–1226. [Google Scholar] [CrossRef]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Flörl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017, 102, 433–444. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Schmidt-Hieber, M.; Bertz, H.; Heinz, W.J.; Kiehl, M.; Krüger, W.; Mousset, S.; Neuburger, S.; Neumann, S.; Penack, O.; et al. Infectious diseases in allogeneic haematopoietic stem cell transplantation: Prevention and prophylaxis strategy guidelines 2016. Ann. Hematol. 2016, 95, 1435–1455. [Google Scholar] [CrossRef]
- Ljungman, P.; de la Camara, R.; Cordonnier, C.; Einsele, H.; Engelhard, D.; Reusser, P.; Styczynski, J.; Ward, K.N. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant. 2008, 42, 227–240. [Google Scholar] [CrossRef]
- Saade, A.; Styczynski, J.; Cesaro, S. BK virus infection in allogeneic hematopoietic cell transplantation: An update on pathogenesis, immune responses, diagnosis and treatments: BK polyoma virus infection. J. Infect. 2020, 81, 372–382. [Google Scholar] [CrossRef] [PubMed]
- BMT CTN Technical Document Infectious Diseases. Available online: www.astct.org/learn/practice-guidelines (accessed on 1 December 2024).
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin. Infect. Dis. 2017, 64, 87–91. [Google Scholar] [PubMed]
- Hill, J.A. Human herpesvirus 6 in transplant recipients: An update on diagnostic and treatment strategies. Curr. Opin. Infect. Dis. 2019, 32, 584–590. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Battipaglia, G.; Galimard, J.-E.; Labopin, M.; Raiola, A.M.; Blaise, D.; Ruggeri, A.; Koc, Y.; Gülbas, Z.; Vitek, A.; Sica, S.; et al. Post-transplant cyclophosphamide in one-antigen mismatched unrelated donor transplantation versus haploidentical transplantation in acute myeloid leukemia: A study from the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2022, 57, 562–571. [Google Scholar] [CrossRef]
- Lee, C.J.; Savani, B.N.; Mohty, M.; Labopin, M.; Ruggeri, A.; Schmid, C.; Baron, F.; Esteve, J.; Gorin, N.C.; Giebel, S.; et al. Haploidentical hematopoietic cell transplantation for adult acute myeloid leukemia: A position statement from the acute leukemia working party of the European society for blood and marrow transplantation. Haematologica 2017, 102, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- Penack, O.; Abouqateb, M.; Peczynski, C.; Boreland, W.; Gülbas, Z.; Gedde-Dahl, T.; Castilla-Llorente, C.; Kröger, N.; Eder, M.; Rambaldi, A.; et al. PTCy versus ATG as graft-versus-host disease prophylaxis in mismatched unrelated stem cell transplantation. Blood Cancer J. 2024, 14, 45. [Google Scholar] [CrossRef]
- Lorentino, F.; Labopin, M.; Ciceri, F.; Vago, L.; Fleischhauer, K.; Afanasyev, B.; Kröger, N.; Cornelissen, J.J.; Lovira, M.; Meijer, E.; et al. Post-transplantation cyclophosphamide GvHD prophylaxis after hematopoietic stem cell transplantation from 9/10 or 10/10 HLA-matched unrelated donors for acute leukemia. Leukemia 2021, 35, 585–594. [Google Scholar] [CrossRef]
- Gaballa, S.; Ge, I.; El Fakih, R.; Brammer, J.E.; Kongtim, P.; Tomuleasa, C.; Wang, S.A.; Lee, D.; Petropoulos, D.; Cao, K.; et al. Results of a 2-arm, phase 2 clinical trial using post-transplantation cyclophosphamide for the prevention of graft-versus-host disease in haploidentical donor and mismatched unrelated donor hematopoietic stem cell transplantation. Cancer 2016, 122, 3316–3326. [Google Scholar] [CrossRef]
- Ruggeri, A.; Labopin, M.; Bacigalupo, A.; Afanasyev, B.; Cornelissen, J.J.; Elmaagacli, A.; Itälä-Remes, M.; Blaise, D.; Meijer, E.; Koc, Y.; et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J. Hematol. Oncol. 2018, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Bejanyan, N.; Brunstein, C.G.; Cao, Q.; Lazaryan, A.; Luo, X.; Curtsinger, J.; Mehta, R.S.; Warlick, E.; Cooley, S.A.; Blazar, B.R.; et al. Delayed immune reconstitution after allogeneic transplantation increases the risks of mortality and chronic GVHD. Blood Adv. 2018, 2, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Irene, G.-C.; Albert, E.; Anna, B.-V.; Rahinatu, A.; Silvana, N.; Silvana, S.; Ana, G.; Jordi, L.; Carolina, C.A.; Miquel, G.; et al. Patterns of infection and infectious-related mortality in patients receiving post-transplant high dose cyclophosphamide as graft-versus-host-disease prophylaxis: Impact of HLA donor matching. Bone Marrow Transplant. 2021, 56, 818–827. [Google Scholar] [CrossRef]
- Carreira, A.S.; Salas, M.Q.; Remberger, M.; Basso, I.N.; Law, A.D.; Lam, W.; Pasic, I.; Kim, D.; Michelis, F.V.; Viswabandya, A.; et al. Bloodstream Infections and Outcomes Following Allogeneic Hematopoietic Cell Transplantation: A Single-Center Study. Transplant. Cell Ther. 2022, 28, 50.e1–50.e8. [Google Scholar] [CrossRef]
- Atilla, E.; Atilla, P.A.; Bozdağ, S.C.; Demirer, T. A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection 2017, 45, 403–411. [Google Scholar] [CrossRef]
- Yan, C.-H.; Wang, Y.; Mo, X.-D.; Sun, Y.-Q.; Wang, F.-R.; Fu, H.-X.; Chen, Y.; Han, T.-T.; Kong, J.; Cheng, Y.-F.; et al. Incidence, Risk Factors, Microbiology and Outcomes of Pre-engraftment Bloodstream Infection after Haploidentical Hematopoietic Stem Cell Transplantation and Comparison With HLA-identical Sibling Transplantation. Clin. Infect. Dis. 2018, 67, S162–S173. [Google Scholar] [CrossRef]
- Fayard, A.; Daguenet, E.; Blaise, D.; Chevallier, P.; Labussière, H.; Berceanu, A.; Yakoub-Agha, I.; Socié, G.; Charbonnier, A.; Suarez, F.; et al. Evaluation of infectious complications after haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide following reduced-intensity and myeloablative conditioning: A study on behalf of the Francophone Society of Stem Cell Transplantation and Cellular Therapy (SFGM-TC). Bone Marrow Transplant. 2019, 54, 1586–1594. [Google Scholar] [CrossRef]
- Escribano-Serrat, S.; Pedraza, A.; Suárez-Lledó, M.; Charry, P.; De Moner, B.; Martinez-Sanchez, J.; Ramos, A.; Ventosa-Capell, H.; Moreno, C.; Guardia, L.; et al. Safety and efficacy of G-CSF after allogeneic hematopoietic cell transplantation using post-transplant cyclophosphamide: Clinical and in vitro examination of endothelial activation. Bone Marrow Transplant. 2024, 59, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Cieri, N.; Greco, R.; Crucitti, L.; Morelli, M.; Giglio, F.; Levati, G.; Assanelli, A.; Carrabba, M.G.; Bellio, L.; Milani, R.; et al. Post-transplantation Cyclophosphamide and Sirolimus after Haploidentical Hematopoietic Stem Cell Transplantation Using a Treosulfan-based Myeloablative Conditioning and Peripheral Blood Stem Cells. Biol. Blood Marrow Transplant. 2015, 21, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.R.; Fuchs, E.J.; Bashey, A.; Ciurea, S.O.; Singh, A.K.; Ganguly, S.; Taplitz, R.; Mulroney, C.; Maziarz, R.T.; Kim, S.; et al. Incidence and Impact of Cytomegalovirus Infection in Haploidentical and Matched-Related Donors Receiving Post-Transplant Cyclophosphamide (PTCy): A CIBMTR Analysis. Biol. Blood Marrow Transplant. 2020, 26, S69–S70. [Google Scholar] [CrossRef]
- Teira, P.; Battiwalla, M.; Ramanathan, M.; Barrett, A.J.; Ahn, K.W.; Chen, M.; Green, J.S.; Saad, A.; Antin, J.H.; Savani, B.N.; et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: A CIBMTR analysis. Blood 2016, 127, 2427–2438. [Google Scholar] [CrossRef]
- Brusosa, M.; Ruiz, S.; Monge, I.; Solano, M.T.; Rosiñol, L.; Esteve, J.; Carreras, E.; Marcos, M.Á.; Riu, G.; Carcelero, E.; et al. Impact of letermovir prophylaxis in CMV reactivation and disease after allogenic hematopoietic cell transplantation: A real-world, observational study. Ann. Hematol. 2024, 103, 609–621. [Google Scholar] [CrossRef]
- Marty, F.M.; Ljungman, P.; Chemaly, R.F.; Maertens, J.; Dadwal, S.S.; Duarte, R.F.; Haider, S.; Ullmann, A.J.; Katayama, Y.; Brown, J.; et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2017, 377, 2433–2444. [Google Scholar] [CrossRef]
- Kampouri, E.; Zamora, D.; Kiem, E.S.; Liu, W.; Ibrahimi, S.; Blazevic, R.L.; Lovas, E.A.; Kimball, L.E.; Huang, M.-L.; Jerome, K.R.; et al. Human herpesvirus-6 reactivation and disease after allogeneic haematopoietic cell transplantation in the era of letermovir for cytomegalovirus prophylaxis. Clin. Microbiol. Infect. 2023, 29, 1450.e1–1450.e7. [Google Scholar] [CrossRef]
- Kampouri, E.; Handley, G.; Hill, J.A. Human Herpes Virus-6 (HHV-6) Reactivation after Hematopoietic Cell Transplant and Chimeric Antigen Receptor (CAR)- T Cell Therapy: A Shifting Landscape. Viruses 2024, 16, 498. [Google Scholar] [CrossRef]
- Singh, A.; Dandoy, C.E.; Chen, M.; Kim, S.; Mulroney, C.M.; Kharfan-Dabaja, M.A.; Ganguly, S.; Maziarz, R.T.; Kanakry, C.G.; Kanakry, J.A.; et al. Post-Transplantation Cyclophosphamide Is Associated with an Increase in Non-Cytomegalovirus Herpesvirus Infections in Patients with Acute Leukemia and Myelodysplastic Syndrome. Transplant. Cell. Ther. 2022, 28, 48.e1–48.e10. [Google Scholar] [CrossRef]
- Santos Carreira, A.; Salas, M.Q.; Remberger, M.; Novitzky-Basso, I.; Law, A.D.; Lam, W.; Pasic, I.; Mazzull, T.; Cserti-Gazdewich, C.; Kim, D.; et al. Interaction Between High-Dose Intravenous Busulfan and Post-Transplantation Cyclophosphamide on Hemorrhagic Cystitis After Allogeneic Hematopoietic Cell Transplantation. Transpl. Cell. Ther. 2023, 29, 581.e1–581.e8. [Google Scholar] [CrossRef] [PubMed]
- Mac, S.; Ngo, D.; Yang, D.; Chen, J.; Ali, H.; Arslan, S.; Dadwal, S.; Salhotra, A.; Cao, T.; Karras, N.; et al. Use of high-dose mesna and hyperhydration leads to lower incidence of hemorrhagic cystitis after posttransplant cyclophosphamide-based allogeneic transplantation. Bone Marrow Transplant. 2021, 56, 2464–2470. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.; Samuels, D.; Chen, J.; Koller, P.B.; Al Malki, M.M. A Clinical Review of the Different Strategies to Minimize Hemorrhagic Cystitis Associated with the Use of Post-Transplantation Cyclophosphamide in an Allogeneic Transplant. Transplant. Cell. Ther. 2022, 28, 349–354. [Google Scholar] [PubMed]
- Crocchiolo, R.; Bramanti, S.; Vai, A.; Sarina, B.; Mineri, R.; Casari, E.; Tordato, F.; Mauro, E.; Timofeeva, I.; Lugli, E.; et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl. Infect. Dis. 2015, 17, 242–249. [Google Scholar]
- Khimani, F.; Ranspach, P.; Elmariah, H.; Kim, J.; Whiting, J.; Nishihori, T.; Locke, F.L.; Perez, A.P.; Dean, E.; Mishra, A.; et al. Increased Infections and Delayed CD4+ T Cell but Faster B Cell Immune Reconstitution after Post-Transplantation Cyclophosphamide Compared to Conventional GVHD Prophylaxis in Allogeneic Transplantation. Transplant. Cell. Ther. 2021, 27, 940–948. [Google Scholar] [CrossRef]
| HLA-Matched (n = 120) | 9/10 MMUD (n = 84) | Haploidentical (n = 49) | p Value | |
|---|---|---|---|---|
| Age Median (range) ≥65 years | 53 (23–70) 22 (18.3) | 52 (18–68) 12 (14.3) | 53 (20–70) 10 (20.4) | 0.464 0.325 |
| Sex Female | 49 (40.8) | 33 (39.3) | 23 (46.9) | 0.674 |
| Baseline Diagnosis AML MDS MPN ALL Lymphoproliferative disorders CML PCD Others | 38 (31.7) 23 (19.2) 9 (7.5) 21 (17.5) 13 (10.8) 1 (0.8) 13 (10.8) 2 (1.7) | 28 (33.3) 20 (23.8) 4 (4.8) 17 (20.2) 9 (10.7) 4 (4.8) 2 (2.4) 0 | 24 (49.0) 3 (6.1) 0 8 (16.3) 13 (26.5) 0 1 (2.0) 0 | N/A |
| HCT-CI > 3 | 38 (31.7) | 15 (17.9) | 8 (16.3) | 0.028 |
| Karnofsky Performance Status 70–80% ≥90% | 28 (23.4) 92 (76.6) | 23 (27.4) 61 (72.6) | 10 (20.4) 39 (79.6) | 0.675 |
| CMV Risk Status Low risk Intermediate High | 17 (14.16) 91 (75.83) 12 (10.01) | 8 (9.52) 73 (86.91) 3 (3.57) | 4 (8.16) 40 (81.63) 5 (10.2) | 0.48 |
| Donor/Recipient characteristics Donor female -> male patient | 3 (2.5) | 1 (1.2) | 0 | 0.467 |
| Intensity Myeloablative Reduced intensity | 46 (38.3) 74 (61.7) | 37 (44) 47 (56) | 20 (40.8) 29 (59.2) | |
| Conditioning Regimen (Extended) Flu/Bu (4) (± TBI) Flu/TBI (12 Gy) TBF Cy/Flu/TBI (2 Gy) Flu/TBI (8 Gy) Flu/Bu (3) Flu/Mel Sequential RIC allo-HCT Other | 23 (19.1) 18 (15) 4 (3.3) 1 (0.8) 8 (6.7) 45 (37.5) 13 (10.8) 6 (5.0) 3 (2.4) | 21 (25.0) 14 (16.7) 1 (1.2) 1 (1.2) 3 (3.6) 30 (35.7) 2 (2.4) 6 (7.1) 6 (7.1) | 11 (22.4) 6 (12.2) 1 (2.0) 11 (22.4) 1 (2.0) 17 (34.7) 0 1 (2.0) 1 (2.0) | N/A |
| Median Follow-Up: Months (IQR) | 25 (10.16–51.63) | 33.06 (7.66–60.96) | 30.26 (5.52–68) |
| HLA-Matched (n = 120) | 9/10 MMUD (n = 84) | Haploidentical (n = 49) | p Value | |
|---|---|---|---|---|
| Median Days of Transplant Hospitalization (IQR) Post-Transplant Information: SOS TMA | 30 (17–79) 1 (0.8) 4 (3.3) | 30 (9–155) 2 (2.4) 6 (7.1) | 30 (17–169) 1 (2) 4 (8.2) | 0.62 0.467 0.674 |
| Engraftment Information Median days neutrophil engraftment (IQR) Median days platelet engraftment (IQR) Primary graft failure (%) | 19 (11–62) 18 (6–128) 5 (4.1) | 19 (12–89) 18 (8–141) 6 (7.1) | 19 (14–103) 18 (9–63) 1 (0.02) | 0.692 0.651 0.467 |
| Cumulative Incidence Infection Complications: Bacterial Bloodstream Infections: - Day + 30 - Day + 100 - Day + 180 - Day + 365 CMV Reactivation: - Day + 30 - Day + 100 - Day + 180 - Day + 365 CMV Disease: - Day + 30 - Day + 100 - Day + 180 - Day + 365 Grade 2–4 BK Hemorrhagic Cystitis: - Day + 30 - Day + 100 - Day + 180 - Day + 365 VHH6 Infection: - Day + 30 - Day + 100 - Day + 180 - Day + 365 Respiratory Viral Infection: - Day + 30 - Day + 100 - Day + 180 - Day + 365 Fungal Infection: - Day + 30 - Day + 100 - Day + 180 - Day + 365 | 49.2 (39.9–57.8) 53.3 (44.0–61.8) 54.2 (44.8–62.6) 56.8 (47.4–65.2) 9.2 (4.8–15.2) 39.2 (30.4–47.8) 40.9 (32.0–49.5) 40.9 (32.0–49.5) 1.7 (0.3–5.4) 5.8 (2.6–11.0) 6.7 (3.1–12.1) 6.7 (3.1–12.1) 5.0 (2.0–10.0) 7.6 (3.7–13.2) 10.3 (5.6–16.6) 10.3 (5.6–16.6) 2.5 (0.7–6.6) 7.5 (3.7–13.1) 9.2 (4.9–15.2) 11.9 (6.8–18.4) 7.5 (3.7–13.1) 10.8 (6.1–17.2) 19.2 (12.7–26.7) 26.9 (19.2–35.1) 5.9 (2.6–11.1) 6.7 (3.1–12.2) 6.7 (3.1–12.2) 11.1 (6.2–17.6) | 38.1 (27.7–48.4) 46.4 (35.4–56.7) 50.0 (38.8–60.2) 51.2 (40.0–61.3) 8.3 (3.6–15.5) 59.5 (48.1–69.2) 61.9 (50.5–71.4) 61.9 (50.5–71.4) 2.4 (0.5–7.5) 6.0 (2.2–12.4) 8.3 (3.6–15.5) 11.9 (6.1–19.9) 2.4 (0.5–7.6) 22.3 (13.9–32.0) 23.7(15.0–33.6) 23.7(15.0–33.6) 8.3 (3.6–15.5) 9.5 (4.4–17.0) 10.7 (5.2–18.5) 11.9 (6.1–19.9) 4.8 (1.5–10.9) 9.5 (4.4–17.0) 17.9 (10.5–26.8) 25.0 (16.3–34.7) 5.2 (1.7–11.8) 13.0 (6.6–21.6) 13.0 (6.6–21.6) 14.3 (7.6–23.1) | 34.7 (21.7–48.0) 38.8 (25.1–52.2) 38.8 (25.1–52.2) 38.8 (25.1–52.2) 20.4 (10.4–32.7) 55.1 (40.0–67.9) 55.1 (40.0–67.9) 55.1 (40.0–67.9) 4.1 (0.7–12.5) 6.1 (1.6–15.3) 8.2 (2.6–18.0) 8.2 (2.6–18.0) 4.1 (0.7–12.6) 14.8 (6.4–26.5) 14.8 (6.4–26.5) 14.8 (6.4–26.5) 14.3 (6.2–25.6) 22.4 (11.9–35.0) 24.5 (13.5–37.2) 24.5 (13.5–37.2) 8.2 (2.6–18.0) 16.3 (7.6–28.0) 20.4 (10.4–32.7) 24.6 (13.5–37.5) 6.4 (1.6–15.9) 8.5 (2.7–18.7) 10.6 (3.8–21.4) 12.8 (5.1–24.2) | 0.073 0.033 0.449 0.056 0.068 0.744 0.640 |
| Cumulative Incidence GVHD [% (95% CI)] Grade 2–4 acute GVHD at day +100 Grade 3–4 acute GVHD at day +100 Moderate/severe chronic GVHD at 2 years | 19.2 (12.7–26.7) 6.7 (3.1–12.1) 3.6 (1.2–8.4) | 31.0 (21.4–41.0) 8.3 (3.6–15.5) 13.6 (6.9–22.6) | 22.4 (11.9–35.0) 4.1 (0.7–12.5) 9.6 (3.0–21.0) | 0.055 0.848 0.062 |
| Median Days to IS Discontinuation (IQR) | 185 (39–675) | 197.5 (34–1883) | 186 (62–824) | 0.842 |
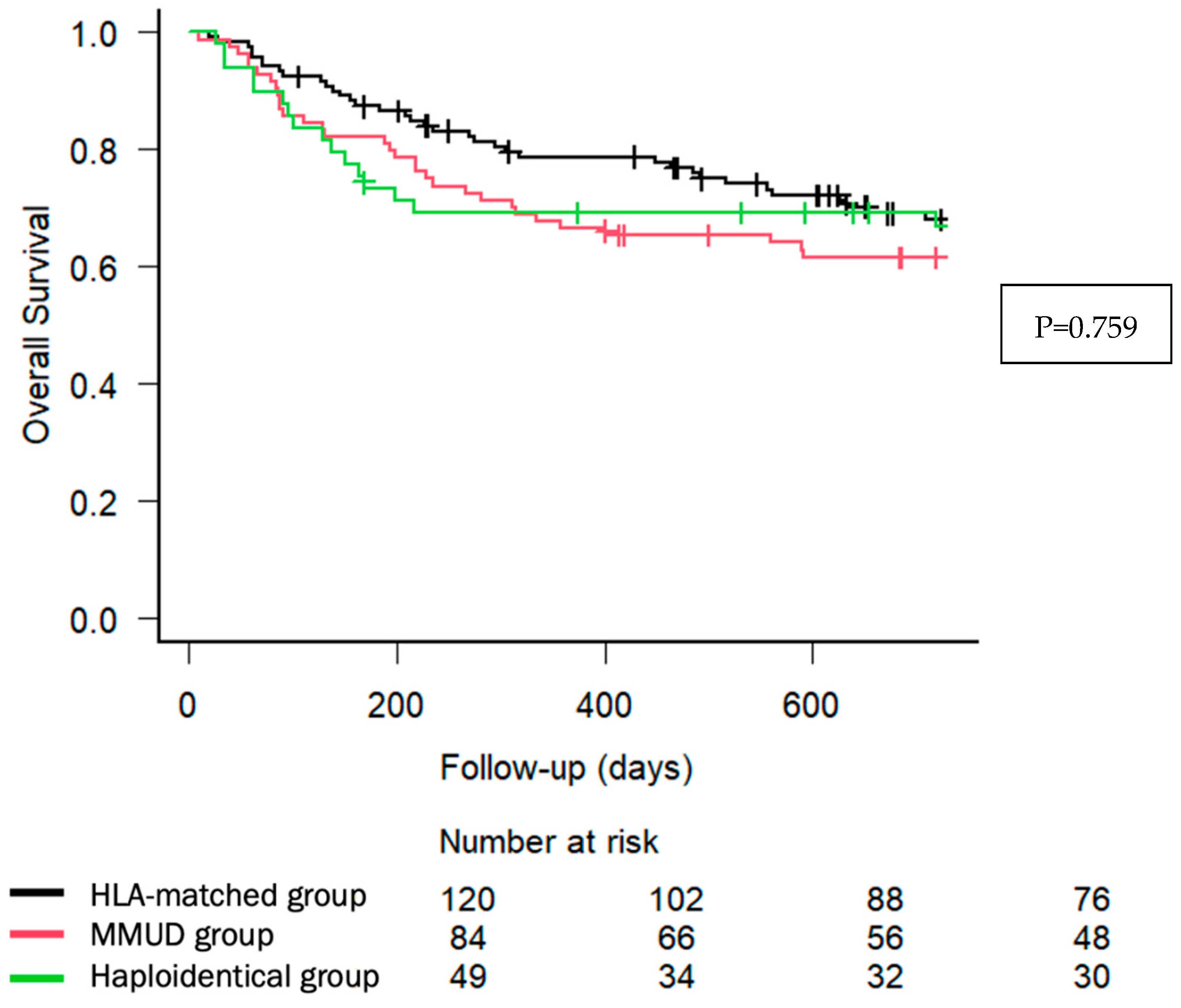
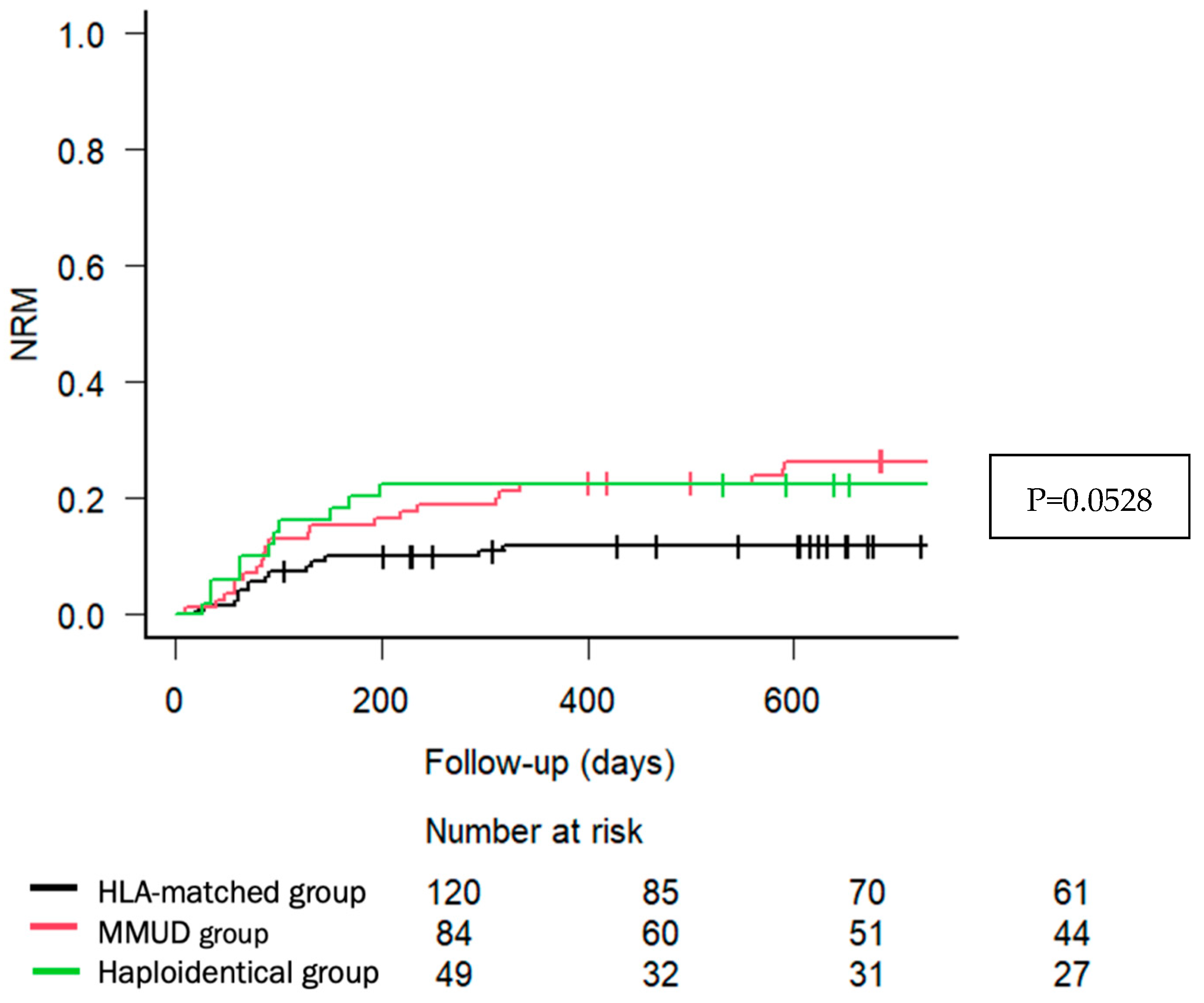
| Main Post-Transplant Outcomes (% (95% CI)) Overall survival 2 years Relapse-free survival 2 years Non-relapse mortality 2 years Cumulative incidence of relapse 2 years GRFS 2 years | 68.1 (58.5–75.9) 55.2 (45.6–63.8) 11.8 (6.8–18.4) 33.0 (24.5–41.7) 51.0 (41.5–59.7) | 61.6 (50.3–71.1) 53.1 (41.9–63.2) 26.4 (17.4–36.3) 20.4 (12.5–29.7) 42.3 (31.5–52.6) | 66.8 (51.6–78.2) 54.4 (39.3–67.2) 22.4 (11.9–35.0) 23.2 (12.3–36.1) 46.0 (31.5–59.4) | 0.759 0.995 0.0528 0.0972 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merchán-Muñoz, B.; Suárez-Lledó, M.; Rodríguez-Lobato, L.G.; Aiello, T.F.; Gallardo-Pizarro, A.; Charry, P.; Cid, J.; Lozano, M.; Pedraza, A.; Martínez-Roca, A.; et al. Post-Transplant Cyclophosphamide-Based Prophylaxis and Its Impact on Infectious Complications and Immune Reconstitution According to Donor Type. Cancers 2025, 17, 1109. https://doi.org/10.3390/cancers17071109
Merchán-Muñoz B, Suárez-Lledó M, Rodríguez-Lobato LG, Aiello TF, Gallardo-Pizarro A, Charry P, Cid J, Lozano M, Pedraza A, Martínez-Roca A, et al. Post-Transplant Cyclophosphamide-Based Prophylaxis and Its Impact on Infectious Complications and Immune Reconstitution According to Donor Type. Cancers. 2025; 17(7):1109. https://doi.org/10.3390/cancers17071109
Chicago/Turabian StyleMerchán-Muñoz, Beatriz, María Suárez-Lledó, Luis Gerardo Rodríguez-Lobato, Tommaso Francesco Aiello, Antonio Gallardo-Pizarro, Paola Charry, Joan Cid, Miquel Lozano, Alexandra Pedraza, Alexandra Martínez-Roca, and et al. 2025. "Post-Transplant Cyclophosphamide-Based Prophylaxis and Its Impact on Infectious Complications and Immune Reconstitution According to Donor Type" Cancers 17, no. 7: 1109. https://doi.org/10.3390/cancers17071109
APA StyleMerchán-Muñoz, B., Suárez-Lledó, M., Rodríguez-Lobato, L. G., Aiello, T. F., Gallardo-Pizarro, A., Charry, P., Cid, J., Lozano, M., Pedraza, A., Martínez-Roca, A., Guardia, A., Guardia, L., Moreno, C., Carreras, E., Rosiñol, L., García-Vidal, C., Fernández-Avilés, F., Martínez, C., Rovira, M., & Salas, M. Q. (2025). Post-Transplant Cyclophosphamide-Based Prophylaxis and Its Impact on Infectious Complications and Immune Reconstitution According to Donor Type. Cancers, 17(7), 1109. https://doi.org/10.3390/cancers17071109






