Prognostic Role of Adaptive Immune Microenvironment in Patients with High-Risk Myelodysplastic Syndromes Treated with 5-Azacytidine
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Immunohistochemistry (IHC)
2.3. Digital Imaging and Cell Population Scoring
2.4. Statistical Analysis
3. Results
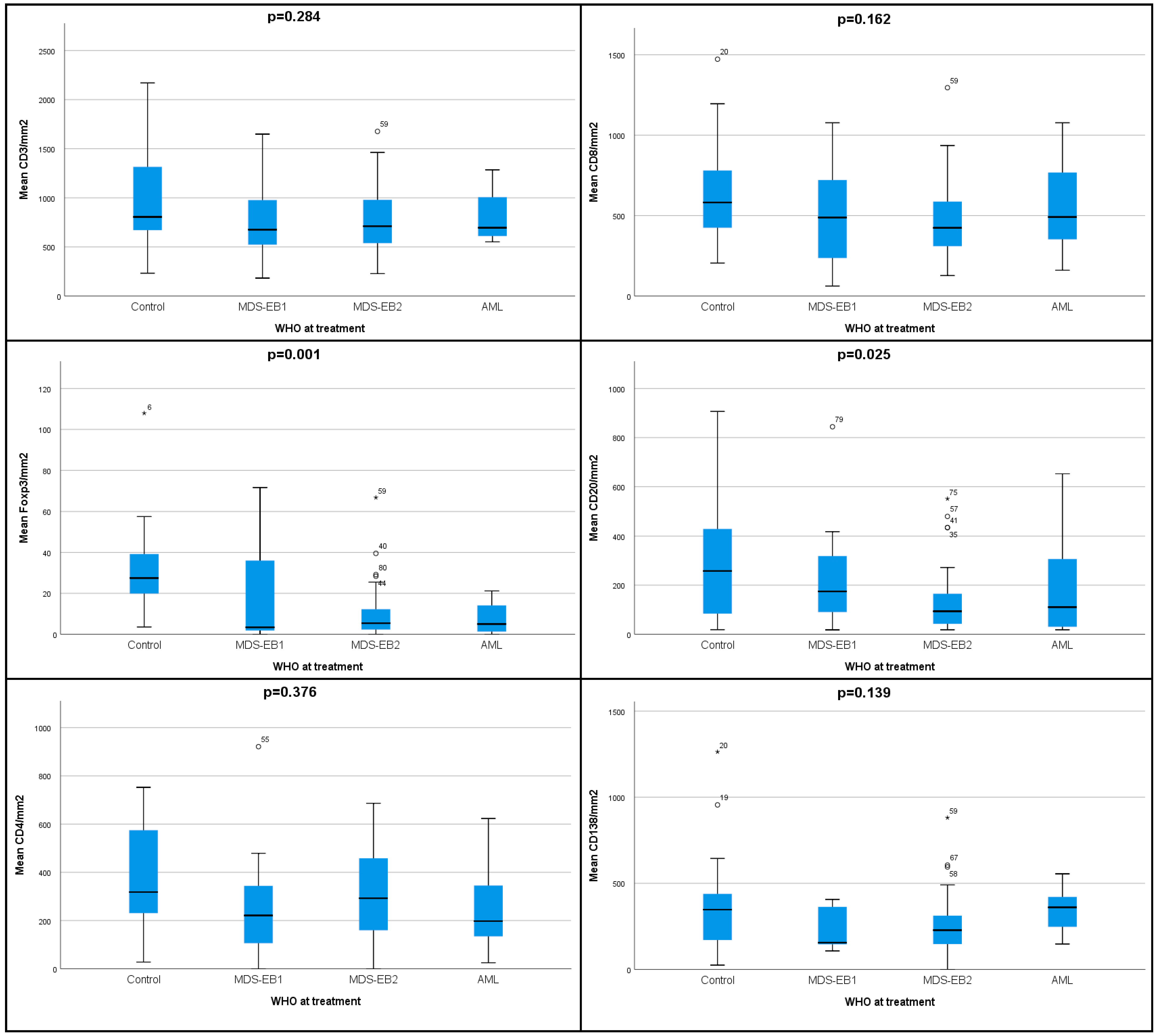
3.1. Lower Densities of Adaptive Immune Cell Subpopulations Characterize the Microenvironment of HR-MDS Compared to Age-Matched Normal Controls
3.2. Higher Densities of CD3+ T Cells and CD4+ Th Cells Correlate with Response to Treatment with 5-AZA
3.3. Immune Cell Densities Can Aid in Response Prediction
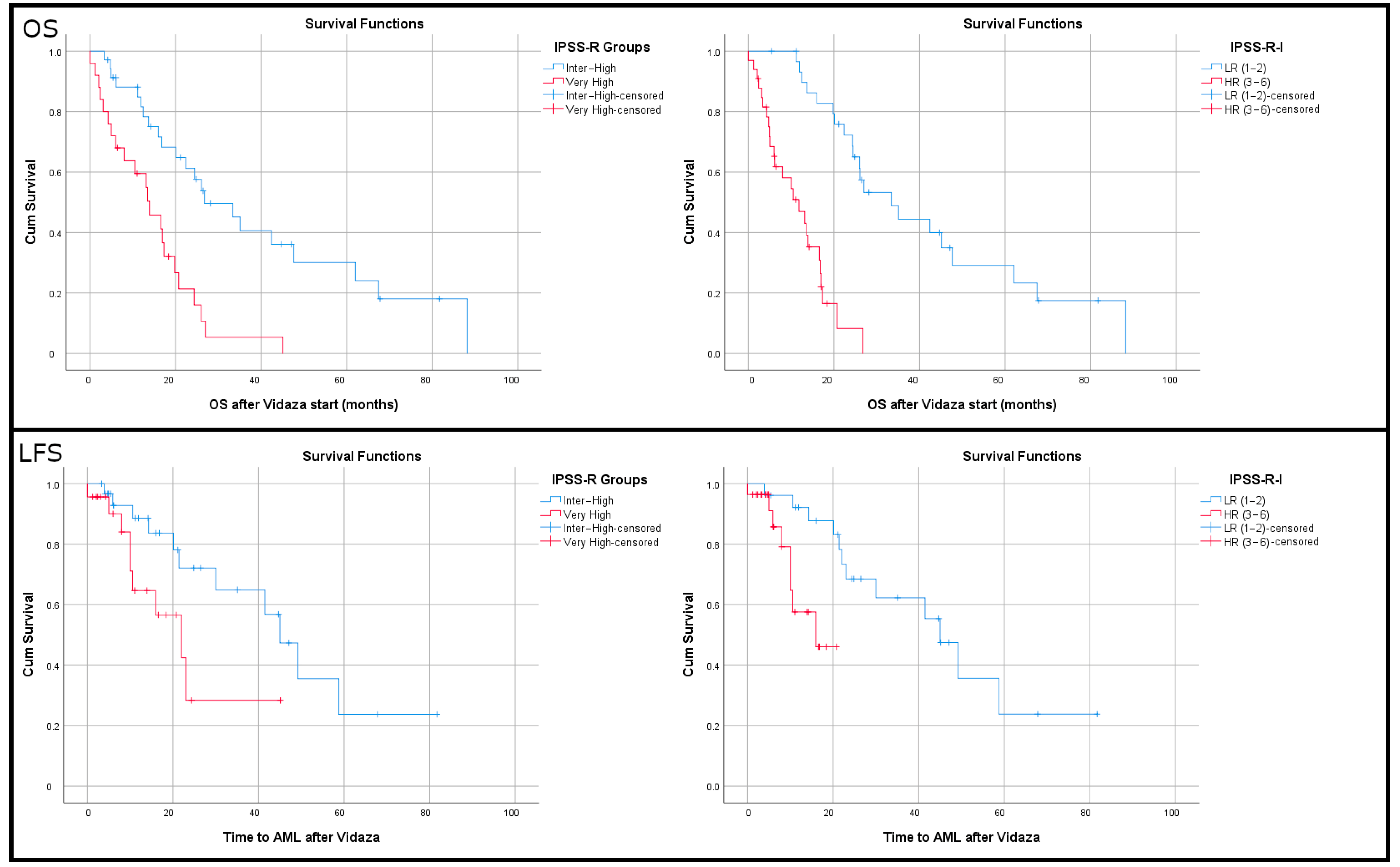
3.4. Immune Cell Densities Correlate with Overall Survival
3.5. Lower Densities of Tregs and Plasma Cells Correlate with Increased Leukemia-Free Survival
3.6. Lower Densities of T Cells and Higher Densities of Tregs Correlate with Increased Hazard Ratios of Death and Transformation into AML
3.7. An Immune-IPSS-R Score for Enhanced OS and LFS Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banerjee, T.; Calvi, L.M.; Becker, M.W.; Liesveld, J.L. Flaming and fanning: The Spectrum of inflammatory influences in myelodysplastic syndromes. Blood Rev. 2019, 36, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Fozza, C.; Crobu, V.; Isoni, M.A.; Dore, F. The immune landscape of myelodysplastic syndromes. Crit. Rev. Oncol. Hematol. 2016, 107, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.; Shoaie, S.; Kordasti, S.; Platzbecker, U. Integrating the “Immunome” in the Stratification of Myelodysplastic Syndromes and Future Clinical Trial Design. J. Clin. Oncol. 2020, 38, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Kouroukli, O.; Symeonidis, A.; Foukas, P.; Maragkou, M.K.; Kourea, E.P. Bone Marrow Immune Microenvironment in Myelodysplastic Syndromes. Cancers 2022, 14, 5656. [Google Scholar] [CrossRef]
- Kordasti, S.Y.; Afzali, B.; Lim, Z.; Ingram, W.; Hayden, J.; Barber, L.; Matthews, K.; Chelliah, R.; Guinn, B.; Lombardi, G.; et al. IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br. J. Haematol. 2009, 145, 64–72. [Google Scholar] [CrossRef]
- Glenthøj, A.; Ørskov, A.D.; Hansen, J.W.; Hadrup, S.R.; O’Connell, C.; Grønbæk, K. Immune Mechanisms in Myelodysplastic Syndrome. Int. J. Mol. Sci. 2016, 17, 944. [Google Scholar] [CrossRef]
- Sand, K.; Theorell, J.; Bruserud, O.; Bryceson, Y.T.; Kittang, A.O. Reduced potency of cytotoxic T lymphocytes from patients with high-risk myelodysplastic syndromes. Cancer Immunol. Immunother. 2016, 65, 1135–1147. [Google Scholar]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- van der Leun, A.M.; Thommen, D.S. CD8(+) T cell states in human cancer: Insights from single-cell analysis. Histopathology 2020, 20, 218–232. [Google Scholar] [CrossRef]
- Kordasti, S.Y.; Ingram, W.; Hayden, J.; Darling, D.; Barber, L.; Afzali, B.; Lombardi, G.; Wlodarski, M.W.; Maciejewski, J.P.; Farzaneh, F.; et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS). Blood 2007, 110, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Bouchliou, I.; Miltiades, P.; Nakou, E.; Spanoudakis, E.; Goutzouvelidis, A.; Vakalopoulou, S.; Garypidou, V.; Kotoula, V.; Bourikas, G.; Tsatalas, C.; et al. Th17 and Foxp3(+) T regulatory cell dynamics and distribution in myelodysplastic syndromes. Clin. Immunol. 2011, 139, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Pescia, C.; Boggio, F.; Croci, G.A.; Cassin, R.; Barella, M.; Pettine, L.; Reda, G.; Sabattini, E.; Finelli, C.; Gianelli, U. Lymphocytic Infiltrate and p53 Protein Expression as Predictive Markers of Response and Outcome in Myelodysplastic Syndromes Treated with Azacitidine. J. Clin. Med. 2021, 10, 4809. [Google Scholar] [CrossRef]
- Pulhorn, H.; Herrmann, M.; Harms, H.; Jung, A.; Baumann, I. Apoptotic cells and clonally expanded cytotoxic T cells in bone marrow trephines of patients with myelodysplastic syndrome. Histopathology 2012, 61, 200–211. [Google Scholar] [CrossRef]
- Magalhães, S.M.; Filho, F.D.; Vassallo, J.; Pinheiro, M.P.; Metze, K.; Lorand-Metze, I. Bone marrow lymphoid aggregates in myelodysplastic syndromes: Incidence, immunomorphological characteristics and correlation with clinical features and survival. Leuk. Res. 2002, 26, 525–530, discussion 531. [Google Scholar] [CrossRef]
- Williams, P.; Basu, S.; Garcia-Manero, G.; Hourigan, C.S.; Oetjen, K.A.; Cortes, J.E.; Ravandi, F.; Jabbour, E.J.; Al-Hamal, Z.; Konopleva, M.; et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer 2019, 125, 1470–1481. [Google Scholar] [CrossRef]
- Lopes, M.R.; Traina, F.; Campos Pde, M.; Pereira, J.K.; Machado-Neto, J.A.; Machado Hda, C.; Gilli, S.C.; Saad, S.T.; Favaro, P. IL10 inversely correlates with the percentage of CD8+ cells in MDS patients. Leukemia Res. 2013, 37, 541–546. [Google Scholar] [CrossRef]
- Motz, G.T.; Coukos, G. The parallel lives of angiogenesis and immunosuppression: Cancer and other tales. Nat. Rev. Immunol. 2011, 11, 702–711. [Google Scholar] [CrossRef]
- Fang, J.; Lu, Y.; Zheng, J.; Jiang, X.; Shen, H.; Shang, X.; Lu, Y.; Fu, P. Exploring the crosstalk between endothelial cells, immune cells, and immune checkpoints in the tumor microenvironment: New insights and therapeutic implications. Cell. Death Dis. 2023, 14, 586. [Google Scholar] [CrossRef]
- Giovazzino, A.; Leone, S.; Rubino, V.; Palatucci, A.T.; Cerciello, G.; Alfinito, F.; Pane, F.; Ruggiero, G.; Terrazzano, G. Reduced regulatory T cells (Treg) in bone marrow preferentially associate with the expansion of cytotoxic T lymphocytes in low risk MDS patients. Br. J. Haematol. 2019, 185, 357–360. [Google Scholar] [CrossRef]
- Zheng, Z.; Qianqiao, Z.; Qi, H.; Feng, X.; Chunkang, C.; Xiao, L. In vitro deprivation of CD8(+)CD57(+)T cells promotes the malignant growth of bone marrow colony cells in patients with lower-risk myelodysplastic syndrome. Exp. Hematol. 2010, 38, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.A.; Srivastava, P.; Matsuzaki, J.; Brumberger, Z.; Wang, E.S.; Kocent, J.; Miller, A.; Roloff, G.W.; Wong, H.Y.; Paluch, B.E.; et al. NY-ESO-1 Vaccination in Combination with Decitabine Induces Antigen-Specific T-lymphocyte Responses in Patients with Myelodysplastic Syndrome. Clin. Cancer Res. 2018, 24, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Keilholz, U.; Letsch, A.; Busse, A.; Asemissen, A.M.; Bauer, S.; Blau, I.W.; Hofmann, W.K.; Uharek, L.; Thiel, E.; Scheibenbogen, C. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood 2009, 113, 6541–6548. [Google Scholar] [CrossRef] [PubMed]
- Suwabe, T.; Shibasaki, Y.; Sato, H.; Tamura, S.; Katagiri, T.; Nemoto, H.; Kasami, T.; Kozakai, T.; Nanba, A.; Kitajima, T.; et al. WT1-specific CD8 + cytotoxic T cells with the capacity for antigen-specific expansion accumulate in the bone marrow in MDS. Int. J. Hematol. 2021, 113, 723–734. [Google Scholar] [CrossRef]
- Tanaka, T.N.; Ferrari, V.; Tarke, A.; Fields, H.; Ferrari, L.; Ferrari, F.; McCarthy, C.L.; Sanchez, A.P.; Vitiello, A.; Lane, T.A.; et al. Adoptive transfer of neoantigen-specific T-cell therapy is feasible in older patients with higher-risk myelodysplastic syndrome. Cytotherapy 2021, 23, 236–241. [Google Scholar] [CrossRef]
- Kotsianidis, I.; Bouchliou, I.; Nakou, E.; Spanoudakis, E.; Margaritis, D.; Christophoridou, A.V.; Anastasiades, A.; Tsigalou, C.; Bourikas, G.; Karadimitris, A.; et al. Kinetics, function and bone marrow trafficking of CD4+CD25+FOXP3+ regulatory T cells in myelodysplastic syndromes (MDS). Leukemia 2009, 23, 510–518. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Guo, J.; Xu, F.; He, Q.; Zhao, Y.; Yang, Y.; Gu, S.; Zhang, Y.; Wu, L.; et al. Interleukin-17 enhances the production of interferon-γ and tumour necrosis factor-α by bone marrow T lymphocytes from patients with lower risk myelodysplastic syndromes. Eur. J. Haematol. 2013, 90, 375–384. [Google Scholar] [CrossRef]
- Ben Khelil, M.; Godet, Y.; Abdeljaoued, S.; Borg, C.; Adotevi, O.; Loyon, R. Harnessing Antitumor CD4(+) T Cells for Cancer Immunotherapy. Cancers 2022, 14, 260. [Google Scholar] [CrossRef]
- Li, T.; Wu, B.; Yang, T.; Zhang, L.; Jin, K. The outstanding antitumor capacity of CD4(+) T helper lymphocytes. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188439. [Google Scholar] [CrossRef]
- Sternberg, A.; Killick, S.; Littlewood, T.; Hatton, C.; Peniket, A.; Seidl, T.; Soneji, S.; Leach, J.; Bowen, D.; Chapman, C.; et al. Evidence for reduced B-cell progenitors in early (low-risk) myelodysplastic syndrome. Blood 2005, 106, 2982–2991. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.M.; Jilani, I.; Estey, E.H.; Keating, M.J.; Dey, A.L.; Manshouri, T.; Kantarjian, H.M.; Estrov, Z.; Cortes, J.E.; Thomas, D.A.; et al. Increased apoptosis in bone marrow B lymphocytes but not T lymphocytes in myelodysplastic syndrome. Blood 2003, 102, 1866–1868. [Google Scholar] [CrossRef]
- Wouters, M.C.A.; Nelson, B.H. Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin. Cancer Res. 2018, 24, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Book, R.; Ben-Ezra, J.; Glait Santar, C.; Kay, S.; Stemer, G.; Oster, H.S.; Mittelman, M. Lymphoid aggregates in the bone marrow biopsies of patients with myelodysplastic syndromes—A potential prognostic marker? Front. Oncol. 2022, 12, 988998. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Ren, Y.; Ba, Y.; Liu, S.; Zuo, A.; Xu, H.; Weng, S.; Han, X.; Liu, Z. Tertiary lymphoid structural heterogeneity determines tumour immunity and prospects for clinical application. Mol. Cancer 2024, 23, 75. [Google Scholar] [CrossRef]
- Bauer, M.; Vaxevanis, C.; Al-Ali, H.K.; Jaekel, N.; Naumann, C.L.H.; Schaffrath, J.; Rau, A.; Seliger, B.; Wickenhauser, C. Altered Spatial Composition of the Immune Cell Repertoire in Association to CD34(+) Blasts in Myelodysplastic Syndromes and Secondary Acute Myeloid Leukemia. Cancers 2021, 13, 186. [Google Scholar] [CrossRef]
- Papageorgiou, S.G.; Kotsianidis, I.; Bouchla, A.; Symeonidis, A.; Galanopoulos, A.; Viniou, N.A.; Hatzimichael, E.; Vassilakopoulos, T.P.; Gogos, D.; Megalakaki, A.; et al. Serum ferritin and ECOG performance status predict the response and improve the prognostic value of IPSS or IPSS-R in patients with high-risk myelodysplastic syndromes and oligoblastic acute myeloid leukemia treated with 5-azacytidine: A retrospective analysis of the Hellenic national registry of myelodysplastic and hypoplastic syndromes. Ther. Adv. Hematol. 2020, 11, 2040620720966121. [Google Scholar] [CrossRef]
- van de Loosdrecht, A.A.; Kern, W.; Porwit, A.; Valent, P.; Kordasti, S.; Cremers, E.; Alhan, C.; Duetz, C.; Dunlop, A.; Hobo, W.; et al. Clinical application of flow cytometry in patients with unexplained cytopenia and suspected myelodysplastic syndrome: A report of the European LeukemiaNet International MDS-Flow Cytometry Working Group. Cytometry B Clin. Cytom. 2023, 104, 77–86. [Google Scholar] [CrossRef]
- Uy, G.; Godwin, J.; Rettig, M.; Vey, N.; Foster, M.; Arellano, M.; Rizzieri, D.; Topp, M.; Huls, G.; Lowenberg, B.; et al. Preliminary Results of a Phase 1 Study of Flotetuzumab, a CD123 x CD3 Bispecific Dart® Protein, in Patients with Relapsed/Refractory Acute Myeloid Leukemia and Myelodysplastic Syndrome. Blood 2017, 130, 637. [Google Scholar] [CrossRef]

| Patient Characteristics | Groups | No. | Percent | Median (Min–Max) |
|---|---|---|---|---|
| Sex | Male | 51 | 60.7% | |
| Female | 33 | 39.3% | ||
| Age (N = 84) | 73 (43–90) | |||
| Age groups | ≤70 | 30 | 35.7% | |
| >71 | 54 | 64.3% | ||
| WHO-defined entities | EB1 | 12 | 18.75% | |
| EB2 | 44 | 68.75% | ||
| AML | 8 | 12.50% | ||
| Performance status (ECOG) | 0–1 | 38 | 84.4% | |
| 2+ | 7 | 15.6% | ||
| Karyotype risk groups | Good | 40 | 61.5% | |
| Intermediate | 8 | 12.3% | ||
| Poor | 17 | 26.2% | ||
| Neutrophils (109/Lt) (N = 64) | 0.95 (0.1–17.76) | |||
| Monocytes (109/Lt) (N = 63) | 0.17 (0–11.23) | |||
| Lymphocytes (109/Lt) (N = 63) | 1.29 (0.11–5.13) | |||
| Lymphocyte (109/Lt) groups | <1.0 | 18 | 28.6% | |
| >1.0 | 45 | 71.4% | ||
| Ratio lymphocytes/monocytes (N = 61) | 7.64 (0.32–157) | |||
| Ratio lymphocytes /neutrophils (N = 62) | 1.15 (0.13–7.6) | |||
| Ratio lymphocytes/neutrophils (X-tile) | <0.4 | 9 | 14.5% | |
| >0.4 | 53 | 85.5% | ||
| Platelets (N = 61) | 70 (8–534) | |||
| Feritin (mg/mL) baseline (N = 53) | 440 (34–4255) | |||
| Hb (g/dL) (N = 64) | 8.85 (5.9–11.8) | |||
| Hb (g/dL) groups | >10 | 13 | 20.3% | |
| 8–10 | 37 | 57.8% | ||
| <8 | 14 | 21.9% | ||
| No. of cytopenias (N = 62) | 2 (0–3) | |||
| BM blasts (%) (N = 63) | 11 (1–50) | |||
| IPSS-R score (N = 60) | 6.0 (4–9.5) | |||
| IPSS-R groups | Intermediate | 3 | 5.0% | |
| High | 32 | 53.3% | ||
| Very High | 25 | 41.7% | ||
| LDH (N = 65) | 280 (129–1267) | |||
| LDH groups | Normal | 21 | 32.3% | |
| Abnormal | 44 | 67.7% | ||
| Overall response groups (N = 65) | CR/PR/HI | 20 | 30.8% | |
| SD | 29 | 44.6% | ||
| PD | 16 | 24.6% | ||
| Time of best response (months) (N = 18) | 6 (1–12) | |||
| Total cycles of Aza given (N = 57) | 8 (1–49) | |||
| Transformation into AML (N = 55) | No | 34 | 61.8% | |
| Yes | 21 | 38.2% | ||
| Time to AML after Vidaza (N = 55) | 14.2 (0–81.7) | |||
| Status (N = 65) | Alive | 18 | 27.7% | |
| Dead | 47 | 72.3% | ||
| Follow-up OS after Vidaza start (months) (N = 64) | 16.59 (0–88.16) | |||
| Follow-up LFS after Vidaza start (months) (N = 54) | 14.07 (0–81.70) |
| Parameters | Patients | Controls | p (Mann–Whitney U) |
|---|---|---|---|
| CD3 (mean/mm2) | 764 | 977 | 0.048 |
| CD4 (mean/mm2) | 286 | 376 | 0.143 |
| CD8 (mean/mm2) | 477 | 640 | 0.036 |
| Foxp3 (mean/mm2) | 11.5 | 32.4 | 0.000 |
| CD20 (mean/mm2) | 161 | 308 | 0.009 |
| FoxP3/CD3 (%) | 1.64% | 3.42% | 0.000 |
| FoxP3/CD8 | 0.025 | 0.052 | 0.001 |
| Parameter | CR/PR/HI | SD | PD | p (Kruskal–Wallis) |
|---|---|---|---|---|
| CD3 (mean/mm2) | 852 | 776 | 647 | 0.018 |
| CD4 (mean/mm2) | 344 | 292 | 212 | 0.020 |
| Lymphocytes/monocytes | 24 | 10.6 | 5.8 | 0.005 |
| Hb (g/dL) | 9.3 | 8.9 | 8.4 | 0.010 |
| Time to AML (months) | 34.8 | 19.2 | 8.3 | 0.012 |
| OS (months) | 36.6 | 16.1 | 11.6 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsakiraki, Z.; Spathis, A.; Bouchla, A.; Pouliakis, A.; Vryttia, P.; Panayiotides, I.G.; Pappa, V.; Papageorgiou, S.G.; Foukas, P.G. Prognostic Role of Adaptive Immune Microenvironment in Patients with High-Risk Myelodysplastic Syndromes Treated with 5-Azacytidine. Cancers 2025, 17, 1104. https://doi.org/10.3390/cancers17071104
Tsakiraki Z, Spathis A, Bouchla A, Pouliakis A, Vryttia P, Panayiotides IG, Pappa V, Papageorgiou SG, Foukas PG. Prognostic Role of Adaptive Immune Microenvironment in Patients with High-Risk Myelodysplastic Syndromes Treated with 5-Azacytidine. Cancers. 2025; 17(7):1104. https://doi.org/10.3390/cancers17071104
Chicago/Turabian StyleTsakiraki, Zoi, Aris Spathis, Anthi Bouchla, Abraham Pouliakis, Pinelopi Vryttia, Ioannis G. Panayiotides, Vasiliki Pappa, Sotiris G. Papageorgiou, and Periklis G. Foukas. 2025. "Prognostic Role of Adaptive Immune Microenvironment in Patients with High-Risk Myelodysplastic Syndromes Treated with 5-Azacytidine" Cancers 17, no. 7: 1104. https://doi.org/10.3390/cancers17071104
APA StyleTsakiraki, Z., Spathis, A., Bouchla, A., Pouliakis, A., Vryttia, P., Panayiotides, I. G., Pappa, V., Papageorgiou, S. G., & Foukas, P. G. (2025). Prognostic Role of Adaptive Immune Microenvironment in Patients with High-Risk Myelodysplastic Syndromes Treated with 5-Azacytidine. Cancers, 17(7), 1104. https://doi.org/10.3390/cancers17071104






