Clinical and Pathological Risk Factors for Peritoneal Metastases in a Surgical Series of T4 Colorectal Cancers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
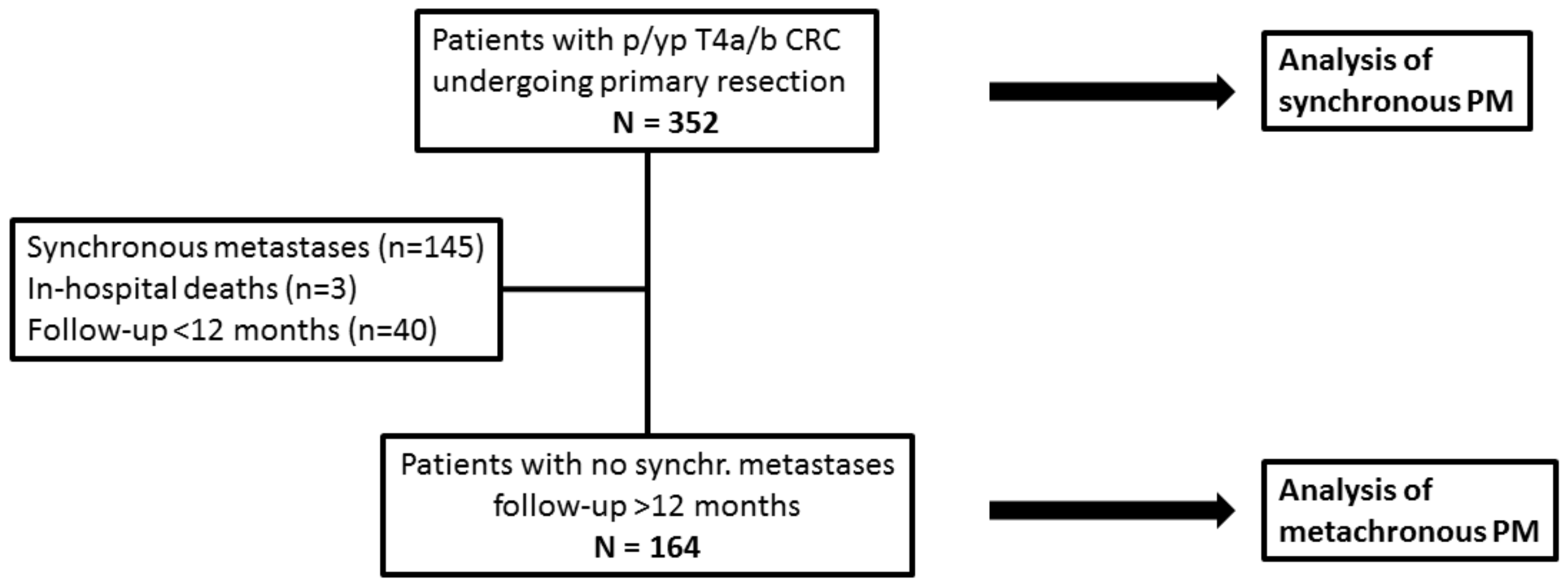
2.1. Patient Population
2.2. Operative Treatment
2.3. Pathological Assessment
2.4. Definitions
- Right-sided tumours: lesions located proximally to the splenic flexure;
- Left-sided tumours: lesions located from the splenic flexure to the peritoneal reflection;
- Extraperitoneal tumours: lesions with the cranial limit below the peritoneal reflection, infiltrating surrounding structures, such as genitourinary organs, anal musculature, sacrum, pelvis sidewall, or floor (T4b).
2.5. Statistics and Study Design
3. Results
3.1. Synchronous Peritoneal Metastases
3.2. Risk Factors for Synchronous Peritoneal Metastases
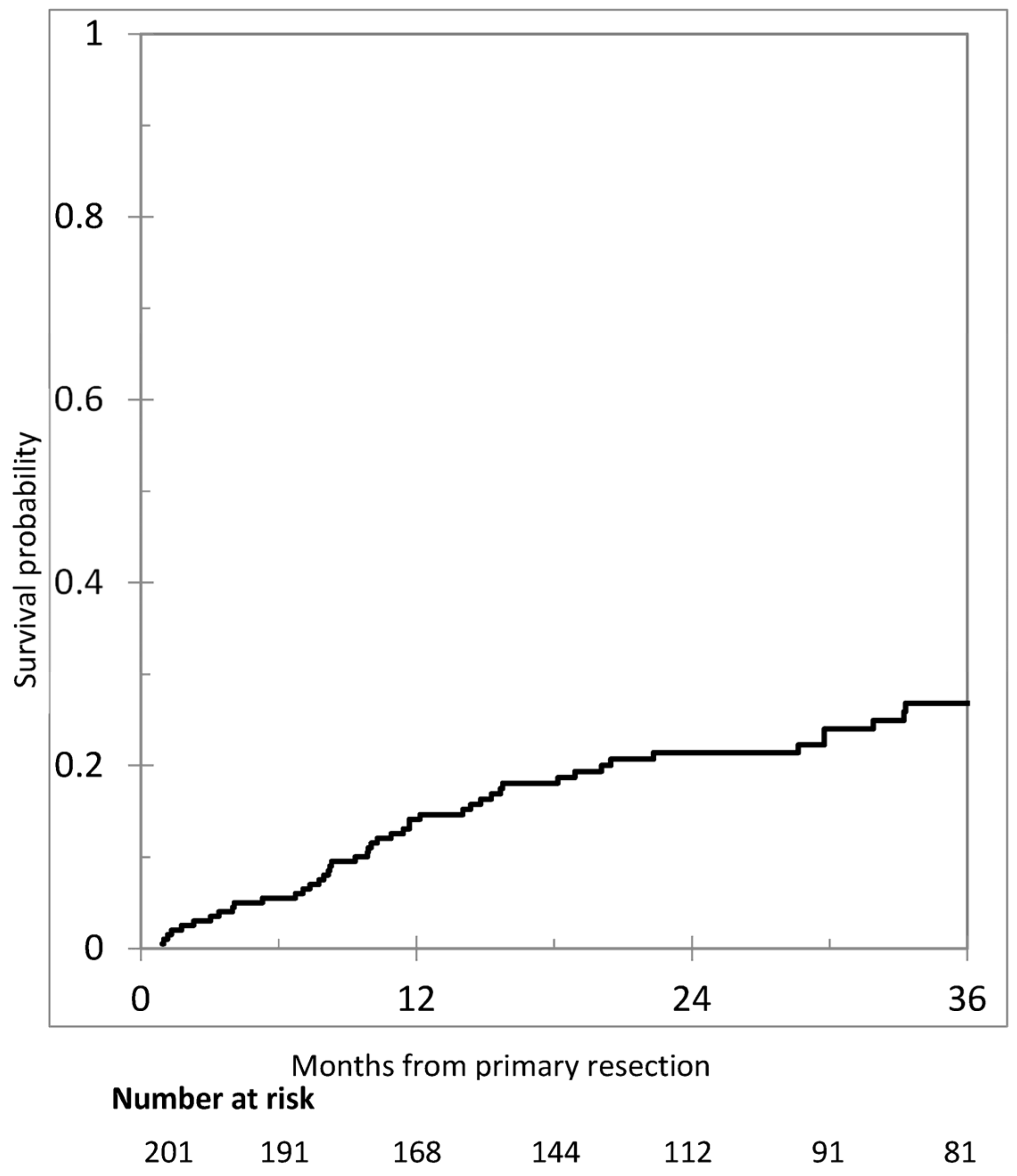
3.3. Metachronous Peritoneal Metastases
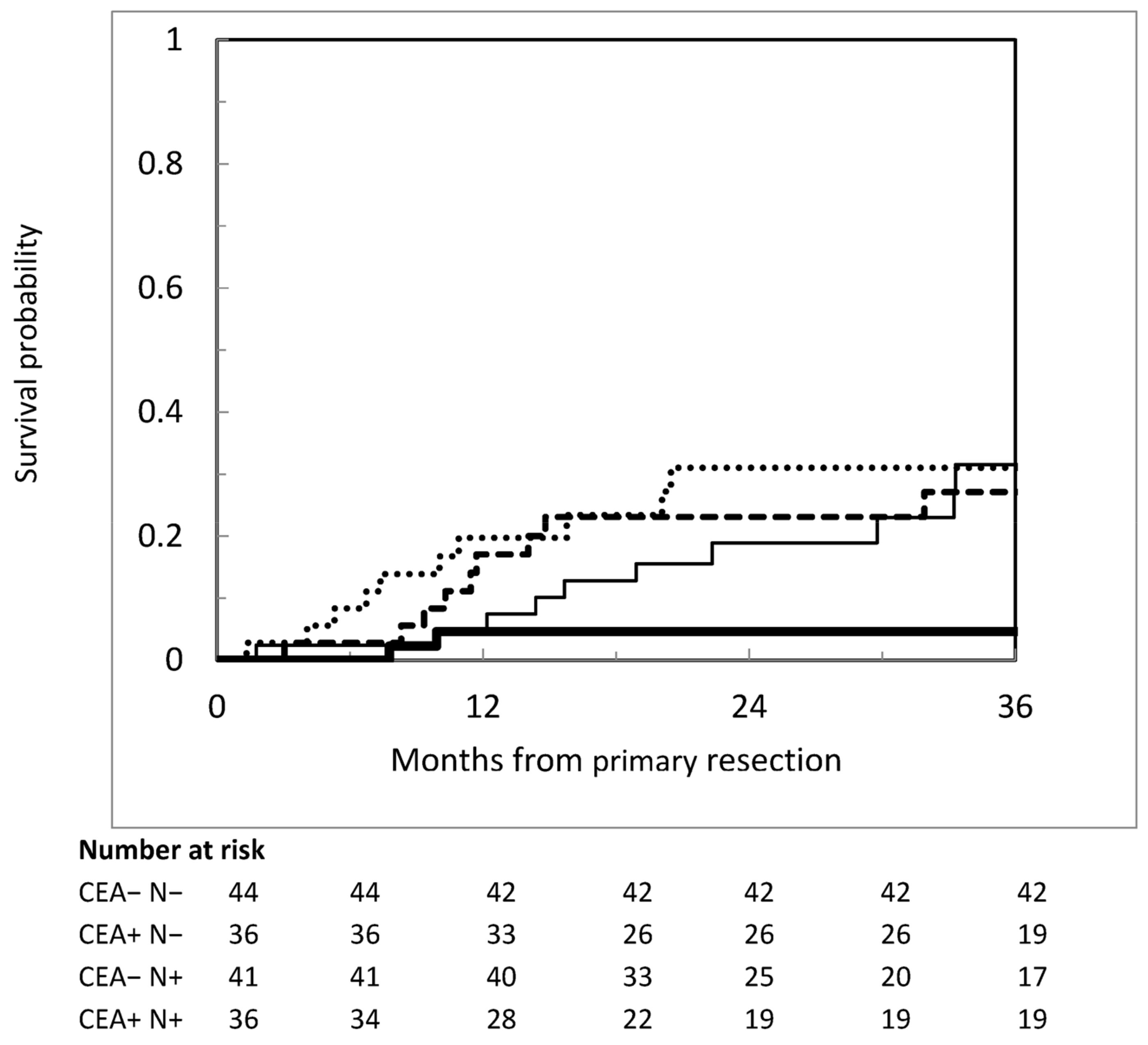
3.4. Risk Factors for Metachronous Peritoneal Metastases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| CRS | cytoreductive surgery |
| CT | computed tomography |
| HIPEC | hyperthermic intraperitoneal chemotherapy |
| MMR | mismatch repair |
| MSI | microsatellite instability |
| PIPAC | Pressurized Intraperitoneal Aerosolized Chemotherapy |
| PM | peritoneal metastases |
| RT | radiotherapy |
| S-CT | systemic chemotherapy |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| TNM | Tumour Node Metastasis |
| UICC | The International Union Against Cancer |
| WHO | World Health Organization |
| VLS | videolaparoscopic surgery |
References
- GBD 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, V.E.; Klaver, Y.L.; Verwaal, V.J.; Rutten, H.J.; Coebergh, J.W.; de Hingh, I.H.J.T. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: A population-based study. Int. J. Cancer 2011, 128, 2717–2725. [Google Scholar]
- Segelman, J.; Granath, F.; Holm, T.; Machado, M.; Mahteme, H.; Martling, A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2012, 99, 699–705. [Google Scholar] [PubMed]
- Kerscher, A.G.; Chua, T.C.; Gasser, M.; Maeder, U.; Kunzmann, V.; Isbert, C.; Germer, C.T.; Pelz, J.O.W. Impact of peritoneal carcinomatosis in the disease history of colorectal cancer management: A longitudinal experience of 2406 patients over two decades. Br. J. Cancer 2013, 108, 1432–1439. [Google Scholar] [PubMed]
- van Gestel, Y.R.; Thomassen, I.; Lemmens, V.E.; Pruijt, J.F.M.; van Herk-Sukel, M.P.P.; Rutten, H.J.T.; Creemers, G.J.; de Hingh, I.H.J.T. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur. J. Surg. Oncol. 2014, 40, 963–969. [Google Scholar]
- Kobayashi, H.; Kotake, K.; Sugihara, K. Outcomes of surgery without HIPEC for synchronous peritoneal metastasis from colorectal cancer: Data from a multi-center registry. Int. J. Clin. Oncol. 2014, 19, 98–105. [Google Scholar]
- Lurvink, R.; Bakkers, C.; Rijken, A.; van Erning, F.N.; Nienhuijs, S.W.; Burger, J.W.; Creemers, G.J.; Verhoef, C.; Lemmens VE de Hingh, I.H.J.T. Increase in the incidence of synchronous and metachronous peritoneal metastases in patients with colorectal cancer: A nationwide study. Eur. J. Surg. Oncol. 2021, 47, 1026–1033. [Google Scholar]
- Baratti, D.; Kusamura, S.; Pietrantonio, F.; Guaglio, M.; Niger, M.; Deraco, M. Progress in treatments for colorectal cancer peritoneal metastases during the years 2010–2015: A systematic review. Crit. Rev. Oncol. Hematol. 2016, 100, 209–222. [Google Scholar]
- Baratti, D.; Kusamura, S.; Iusco DGimondi, S.; Pietrantonio, F.; Milione, M.; Guaglio, M.; Bonomi, S.; Grassi, A.; Virzì, S.; Leo, E.; et al. Hyperthermic Intraperitoneal chemotherapy (HIPEC) at the time of primary curative surgery in patients with colorectal cancer at high risk for metachronous Peritoneal Metastases. Ann. Surg. Oncol. 2017, 24, 167–175. [Google Scholar]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar]
- Goéré, D.; Glehen, O.; Quenet, F.; Guilloit, J.M.; Bereder, J.M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.J.; et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Sánchez, A.; Espinosa-Redondo, E.; Gutiérrez-Calvo, A.; Segura-Sampedro, J.J.; Pérez-Viejo, E.; Concepción-Martín, V.; Sánchez-García, S.; García-Fadrique, A.; Prieto-Nieto, I.; Barrios-Sanchez, P.; et al. Efficacy and Safety of Intraoperative Hyperthermic Intraperitoneal Chemotherapy for Locally Advanced Colon Cancer: A Phase 3 Randomized Clinical Trial. JAMA Surg. 2023, 158, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Honore, C.; Goere, D.; Souadka, A.; Dumont, F.; Elias, D. Definition of patients presenting a high risk of developing peritoneal carcinomatosisafter curative surgery for colorectal cancer: A systematic review. Ann. Surg. Oncol. 2013, 20, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, X.; Chen, W.; Liu, D.; Luo, J.; Wang, H.; Wang, H. Risk factors for developing peritoneal metastases after curative surgery for colorectal cancer: A systematic review and meta-analysis. Color. Dis. 2021, 23, 2846–2858. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Baratti, D.; Battaglia, L.; Belli, F.; Bonfanti, G.; Cesa Bianchi, A.; Deraco, M.; Guaglio, M.; Kusamura, S.; Sorrentino, L.; Vitellaro, M.; et al. Preliminary results of a program for the implementation of laparoscopic colorectal surgery in an Italian comprehensive cancer center during the COVID-19 pandemic. Updates Surg. 2022, 74, 1271–1279. [Google Scholar] [CrossRef]
- Deraco, M.; Baratti, D.; Kusamura, S.; Laterza, B.; Balestra, M.R. Surgical technique of parietal and visceral peritonectomy for peritoneal surface malignancies. J. Surg. Oncol. 2009, 100, 321–328. [Google Scholar] [CrossRef]
- Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 12 December 2024).
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gres, D.M.; Byrd, D.R.; Winchester, D.P. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Baratti, D.; Kusamura, S.; Niger, M.; Perrone, F.; Milione, M.; Cattaneo, L.; Guaglio, M.; Bartolini, V.; Pietrantonio, F.; Deraco, M. Prognostic Impact of Primary Side and RAS/RAF Mutations in a Surgical Series of Colorectal Cancer with Peritoneal Metastases. Ann. Surg. Oncol. 2021, 28, 3332–3342. [Google Scholar] [CrossRef]
- Johnson, G.R.J.; Park, J.; Helewa, R.M.; Goldenberg, B.A.; Nashed, M.; Hyun, E. Total neoadjuvant therapy for rectal cancer: A guide for surgeons. Can. J. Surg. 2023, 66, E196–E201. [Google Scholar] [CrossRef]
- Morton, D.; Seymour, M.; Magill, L.; Handley, K.; Glasbey, J.; Glimelius, B.; Palmer, A.; Seligmann, J.; Laurberg, S.; Murakami, K.; et al. Preoperative chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J. Clin. Oncol. 2023, 41, 1541–1552. [Google Scholar] [PubMed]
- Hompes, D.; Tiek, J.; Wolthuis, A. HIPEC in T4a colon cancer: A defendable treatment to improve the oncologic outcome? Ann. Oncol. 2012, 23, 3123–3129. [Google Scholar] [PubMed]
- van Santvoort, H.C.; Braam, H.J.; Spekreijse, K.R.; Koning, N.R.; de Bruin, P.C.; de Vries Reilingh, T.S.; Boerma, D.; Smits, A.B.; Wiezer, M.J.; van Ramshorst, B. Peritoneal carcinomatosis in t4 colorectal cancer: Occurrence and risk factors. Ann. Surg. Oncol. 2014, 21, 1686–1691. [Google Scholar] [PubMed]
- Klaver, C.E.L.; van Huijgevoort, N.C.M.; de Buck van Overstraeten, A.; Wolthuis, A.M.; Tanis, P.J.; van der Bilt, J.D.W.; Sagaert, X.; D’Hoore, A.D. Locally Advanced Colorectal Cancer: True Peritoneal Tumor Penetration is Associated with Peritoneal Metastases. Ann. Surg. Oncol. 2018, 25, 212–220. [Google Scholar]
- Arrizabalaga, N.B.; Navascués, J.M.E.; Echaniz, G.E.; Saralegui Ansorena, Y.; Placer Galán, C.; Arteaga Martín, X.; Velaz Pardo, L. Prophylactic HIPEC in pT4 Colon Tumors: Proactive Approach or Overtreatment? Ann. Surg. Oncol. 2020, 27, 1094–1100. [Google Scholar]
- Tsai, T.Y.; You, J.F.; Hsu, Y.J.; Jhuang, J.R.; Chern, Y.J.; Hung, H.Y.; Yeh, C.Y.; Hsieh, P.S.; Chiang, S.F.; Lai, C.C.; et al. A Prediction Model for Metachronous Peritoneal Carcinomatosis in Patients with Stage T4 Colon Cancer after Curative Resection. Cancers 2021, 13, 2808. [Google Scholar] [CrossRef]
- Bastiaenen, V.P.; Aalbers, A.G.J.; Arjona-Sánchez, A.; Bellato, V.; van der Bilt, J.D.W.; D’Hoore, A.D.; Espinosa-Redondo, E.; Klaver, C.E.L.; Nagtegaal, I.D.; van Ramshorst, B.; et al. Risk of metachronous peritoneal metastases in patients with pT4a versus pT4b colon cancer: An international multicentre cohort study. Eur. J. Surg. Oncol. 2021, 47, 2405–2413. [Google Scholar] [CrossRef]
- Li, T.; Yu, J.; Chen, J.; Liu, R.; Liu, R.; Li, Y.; Wang, Y.X.; Wang, J.J.; Zhu, Z. Preventive intraperitoneal hyperthermic perfusion chemotherapy for patients with T4 stage colon adenocarcinoma. Tech. Coloproctol. 2021, 25, 683–691. [Google Scholar] [CrossRef]
- Uppal, A.; Helmink, B.; Grotz, T.E.; Konishi, T.; Fournier, K.F.; Nguyen, S.; Taggart, M.W.; Shen, J.P.; Bednarski, B.K.; You, Y.Q.N.; et al. What is the risk for peritoneal metastases and survival afterwards in T4 colon cancers? Ann. Surg. Oncol. 2022, 29, 4224–4233. [Google Scholar]
- Cerdán-Santacruz, C.; Cano-Valderrama, Ó.; Peña Ros, E.; Serrano Del Moral, A.; Pereira Pérez, F.; Flor Lorente, B.; Biondo, S. Epidemiology, oncologic results and risk stratification model for metachronous peritoneal metastases after surgery for pT4 colon cancers: Results from an observational retrospective multicentre long-term follow-up study. Tech. Coloproctol. 2023, 27, 1025–1036. [Google Scholar]
- Klaver, C.E.L.; Gietelink, L.; Bemelman, W.A.; Wouters, M.W.J.M.; Wiggers, T.; Tollenaar, R.A.E.M.; Tanis, P.J.; Dutch Surgical Colorectal Audit Group. Locally advanced colon cancer: Evaluation of current clinical practice and treatment outcomes at the population level. J. Natl. Compr. Cancer Netw. 2017, 15, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Bastiaenen, V.P.; Klaver, C.E.L.; Kok, N.F.M.; de Wilt, J.H.W.; de Hingh, I.H.J.T.; Aalbers, A.G.J.; Boerma, D.; Bremers, A.J.A.; Burger, J.W.A.; van Duyn, E.B.; et al. Second and third look laparoscopy in pT4 colon cancer patients for early detection of peritoneal metastases; the COLOPEC 2 randomized multicentre trial. BMC Cancer 2019, 19, 254. [Google Scholar]
- Adjuvant Pressurized Intraperitoneal Aerosol Chemotherapy to Prevent Colorectal Peritoneal Metastases (ProphyPIPAC). Available online: https://clinicaltrials.gov/study/NCT06091683 (accessed on 12 December 2024).
- Saberzadeh-Ardestani, B.; Foster, N.R.; Lee, H.E.; Shi, Q.; Albert, S.R.; Smyrk, T.C.; Sinicro, F.A. Association of tumor-infiltrating lymphocytes with survival depends on primary tumor sidedness in stage III colon cancers (NCCTG N0147) [Alliance]. Ann. Oncol. 2022, 33, 1159–1167. [Google Scholar] [PubMed]
| Variables | Categories | N. | % | Synchronous Peritoneal Metastases | ||||
|---|---|---|---|---|---|---|---|---|
| yes N. | (n = 73) % | no N. | (n = 279) % | p Value | ||||
| Sex | Male Female | 178 174 | 50.6 49.4 | 36 37 | 20.2 21.3 | 142 137 | 79.8 78.7 | 0.896 |
| Age | Median, IQ range | 65.5 55.1–74.2 | 58.4 | 48.6–71.7 | 65.5 | 57.3–75.6 | <0.001 | |
| Race | White Asian | 349 3 | 99.1 0.9 | 73 0 | 20.9 - | 276 3 | 79.1 100.0 | 1.000 |
| Site of primary tumour | Right-sided Left-sided Below perit. refl. | 111 183 58 | 31.5 52.0 16.5 | 31 41 1 | 27.9 22.4 1.7 | 80 142 57 | 72.1 77.6 98.3 | <0.001 |
| T category | p/yp 4a p/yp 4b | 230 122 | 65.3 34.7 | 47 26 | 64.4 35.6 | 183 96 | 65.6 34.4 | 0.890 |
| N category | 0 1a/b/c 2a/b | 123 123 102 | 35.3 35.3 29.4 | 13 27 31 | 10.6 22.0 30.4 | 110 96 71 | 89.4 78.0 69.6 | <0.001 |
| M category | 0 1a 1b 1c | 207 65 7 73 | 58.8 18.5 2.0 20.7 | 0 0 0 73 | - - - 100.0 | 207 65 7 0 | 74.2 23.3 2.5 - | NA |
| Extraperitoneal disease | Present Absent | 100 252 | 28.4 71.6 | 29 44 | 29.0 17.5 | 71 208 | 71.0 82.5 | 0.020 |
| CEA | >5.0 ng/mL ≤5.0 ng/mL | 189 159 | 54.3 45.7 | 51 21 | 35.6 13.2 | 138 138 | 64.4 86.8 | 0.002 |
| CA19.9 | >37.0 UI/mL ≤37.0 UI/mL | 104 244 | 41.9 58.1 | 37 35 | 51.4 48.6 | 67 209 | 24.3 75.7 | <0.001 |
| Preoperative CT | Conducted Not conducted | 86 266 | 24.4 75.6 | 23 50 | 26.7 18.8 | 63 216 | 73.3 81.2 | 0.127 |
| Preoperative RT | Conducted Not conducted | 61 291 | 17.3 82.7 | 5 68 | 8.2 23.4 | 56 223 | 91.8 76.6 | 0.008 |
| Surgery | Right/transverse Left/sigmoid Total/subtotal RAR Hartmann APR Other | 110 87 5 99 27 21 3 | 31.2 24.7 1.4 28.1 7.7 6.0 0.8 | 29 26 0 10 7 0 1 | 29.1 21.9 1.8 31.9 7.1 7.5 0.7 | 81 61 5 89 20 21 2 | 39.7 35.6 0 13.7 9.6 0 1.4 | NA |
| Postoperative complications | Yes No | 69 283 | 19.6 80.4 | 10 63 | 14.5 22.3 | 59 220 | 85.5 77.7 | 0.186 |
| Postoperative CT | Conducted Not conducted | 244 59 | 80.5 19.5 | 51 12 | 20.9 20.3 | 193 47 | 79.1 79.7 | NA |
| Postoperative RT | Conducted Not conducted | 29 323 | 8.2 91.8 | - 22.6 | 29 250 | 100.0 77.4 | NA | |
| Variables | Categories | OR | 95%CI | p Value |
|---|---|---|---|---|
| Age | ≤65.5 vs. >65.5 | 1.85 | 1.04–3.33 | 0.037 |
| Site of primary tumour | Below perit. reflection vs. left-sided vs. right-sided | 2.08 | 1.30–3.33 | 0.002 |
| CEA | >5.0 ng/mL vs. ≤5.0 ng/mL | 1.63 | 0.86–3.12 | 0.136 |
| CA19.9 | >37.0 UI/mL vs. ≤37.0 UI/mL | 2.93 | 1.62–5.30 | <0.001 |
| Histological type | Intestinal vs. mucinous/SRC | 3.08 | 1.58–5.98 | <0.001 |
| N category | 2 vs. 1 vs. 0 | 1.70 | 1.18–2.46 | 0.005 |
| Extraperitoneal disease | Absent vs. present | 1.29 | 0.67–2.50 | 0.443 |
| Preoperative RT | Conducted vs. not conducted | 0.75 | 0.24–2.36 | 0.626 |
| Variables | Categories | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| Sex | Male vs. female | 0.94 | 0.70–1.27 | 0.696 | |||
| Age | ≤65.5 vs. >65.5 | 1.39 | 1.03–1.88 | 0.033 | 1.37 | 0.98–1.89 | 0.052 |
| Primary site | Below perit. reflection Right-sided Left-sided | Ref. 0.75 0.80 | 0.51–1.11 0.64–0.99 | 0.153 0.045 | 0.94 | 0.75–1.17 | 0.564 |
| CEA | >5 ng/mL vs. ≤5 ng/mL | 1.43 | 1.06–1.95 | 0.021 | |||
| CA19.9 | >37 UI/mL vs. ≤37 UI/mL | 1.33 | 0.91–1.94 | 0.139 | |||
| Histological type | Intest. vs. mucinous/other | 1.22 | 0.78–1.90 | 0.382 | |||
| Grading | 3 vs. 1/2 | 1.26 | 0.91–1.74 | 0.165 | |||
| T category | 4a vs. 4b | 0.73 | 0.52–1.02 | 0.066 | |||
| N category | 2 vs. 1 vs. 0 | 1.31 | 1.08–1.59 | 0.006 | 1.27 | 1.02–1.59 | 0.033 * |
| Extraperit. disease | Yes vs. no | 2.1 | 1.51–3.08 | <0.001 | 2.07 | 1.40–3.06 | <0.001 |
| Ulceration | Yes vs. no | 0.89 | 0.44–1.75 | 0.727 | |||
| Intratumoral vascular invasion | Yes vs. no | 0.98 | 0.61–1.57 | 0.927 | |||
| Peritumoral vascular invasion | Yes vs. no | 0.84 | 0.53–1.32 | 0.442 | |||
| Neural invasion | Yes vs. no | 1.22 | 0.76–1.95 | 0.406 | |||
| Pattern of invasiveness | Infiltrative vs. expansive | 0.82 | 0.54–1.22 | 0.324 | |||
| Peritumoral infiltrat. lymph. | Absent/mild vs. moderate/severe | 0.85 | 0.61–1.20 | 0.366 | |||
| Intratumoral infiltrat. lymph. | Absent/mild vs. moderate/severe | 1.12 | 0.79–1.60 | 0.528 | |||
| Crohn’s like lymphoid reaction | Yes vs. no | 1.18 | 0.73–1.92 | 0.490 | |||
| Margins | R1/2 vs. R0 | 1.91 | 1.19–3.09 | 0.008 | 2.01 | 1.20–3.39 | 0.008 |
| Preoperative CT | Conducted vs. not conducted | 0.94 | 0.67–1.33 | 0.731 | |||
| Postoperative CT | Conducted vs. not conducted | 0.67 | 0.46–0.97 | 0.035 | 0.51 | 0.33–0.77 | 0.001 |
| Preoperative RT | Conducted vs. not conducted | 0.88 | 0.62–1.26 | 0.494 | |||
| MSS | MSI-H/dMMR vs. MSS/pMMR | 0.95 | 0.51–1.75 | 0.857 | |||
| KRAS | Mut. vs. wild-type | 0.81 | 0.52–1.27 | 0.366 | |||
| NRAS | Mut. vs. wild-type | 0.85 | 0.26–2.71 | 0.779 | |||
| BRAF | Mut. vs. wild-type | 1.27 | 0.60–2.66 | 0.531 | |||
| Complications | Yes vs. no | 1.13 | 0.77–1.66 | 0.518 | |||
| Author (Year) | Study Design | Site of Primary Tumour | N. of pts | PM | % | Timing |
|---|---|---|---|---|---|---|
| Lemmens 2011 [2] | Pop.-based | C + R | 1986 | 324 | 16.2 | Synchr. |
| Segelman 2012 [3] | Pop.-based | C + R | 1138 | 281 | 24.7 | Synchr. + metachr. |
| Hompes 2012 [24] | Surg. series | C | 19 | 8 | 42.1 | Metachr. |
| Kerscher 2013 [4] | Surg. series | C + R | 378 306 | 72 48 | 19.0 15.7 | Synchr. Metachr. |
| Van Gestel 2014 [5] | Pop.-based | C + R | 487 | 9 | 10.1 | Metachr. |
| Van Santvoort 2014 [25] | Surg. series | C + R | 200 154 200 | 46 33 79 | 23.0 21.2 39.5 | Synchr. Metachr. Synchr. + metachr. |
| Klaver 2018 [26] | Surg. series | C | 159 130 | 29 30 | 18.2 23.1 | Synchr. Metachr. |
| Klaver 2019 [10] | Rand. trial | C | 100 102 | 19 23 | 19.0 22.5 | Metachr. Metachr. |
| Arrizabalaga 2020 [27] | Surg. series | C | 125 98 | 15 21 | 12.0 21.4 | Synchr. Metachr. |
| Bastianen 2021 [29] | Surg. series | C | 852 | 156 | 18.3 | Metachr. |
| Tsai 2021 [28] | Surg. series | C | 2003 | 246 | 12.3 | Metachr. |
| Lurvink 2021 [7] | Pop.-based | C | 192 119 | 19 19 | 9.9 16.0 | Synchr. Metachr. |
| Uppal 2021 [31] | Surg. series | C | 151 | 27 | 17.9 | Metachr. |
| Li 2021 [30] | Surg. series | C | 195 | 73 | 37.4 | Metachr. |
| Arjona-Sánchez 2022 [12] | Rand. trial | C | 62 | 10 | 16.1 | Metachr. |
| Cerdán-Santacruz 2022 [32] | Surg. series | C | 1356 | 185 | 13.6 | Metachr. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratti, D.; Riva, C.G.; Guaglio, M.; Cavalleri, T.; Colletti, G.; Kusamura, S.; Sabella, G.; Milione, M.; Kuhn, E.; Nava, F.L.; et al. Clinical and Pathological Risk Factors for Peritoneal Metastases in a Surgical Series of T4 Colorectal Cancers. Cancers 2025, 17, 1103. https://doi.org/10.3390/cancers17071103
Baratti D, Riva CG, Guaglio M, Cavalleri T, Colletti G, Kusamura S, Sabella G, Milione M, Kuhn E, Nava FL, et al. Clinical and Pathological Risk Factors for Peritoneal Metastases in a Surgical Series of T4 Colorectal Cancers. Cancers. 2025; 17(7):1103. https://doi.org/10.3390/cancers17071103
Chicago/Turabian StyleBaratti, Dario, Carlo Galdino Riva, Marcello Guaglio, Tommaso Cavalleri, Gaia Colletti, Shigeki Kusamura, Giovanna Sabella, Massimo Milione, Elisabetta Kuhn, Francesca Laura Nava, and et al. 2025. "Clinical and Pathological Risk Factors for Peritoneal Metastases in a Surgical Series of T4 Colorectal Cancers" Cancers 17, no. 7: 1103. https://doi.org/10.3390/cancers17071103
APA StyleBaratti, D., Riva, C. G., Guaglio, M., Cavalleri, T., Colletti, G., Kusamura, S., Sabella, G., Milione, M., Kuhn, E., Nava, F. L., & Deraco, M. (2025). Clinical and Pathological Risk Factors for Peritoneal Metastases in a Surgical Series of T4 Colorectal Cancers. Cancers, 17(7), 1103. https://doi.org/10.3390/cancers17071103






