Beyond Genetics: Exploring Lifestyle, Microbiome, and Social Determinants in Oral Cancer Development
Simple Summary
Abstract
1. Introduction
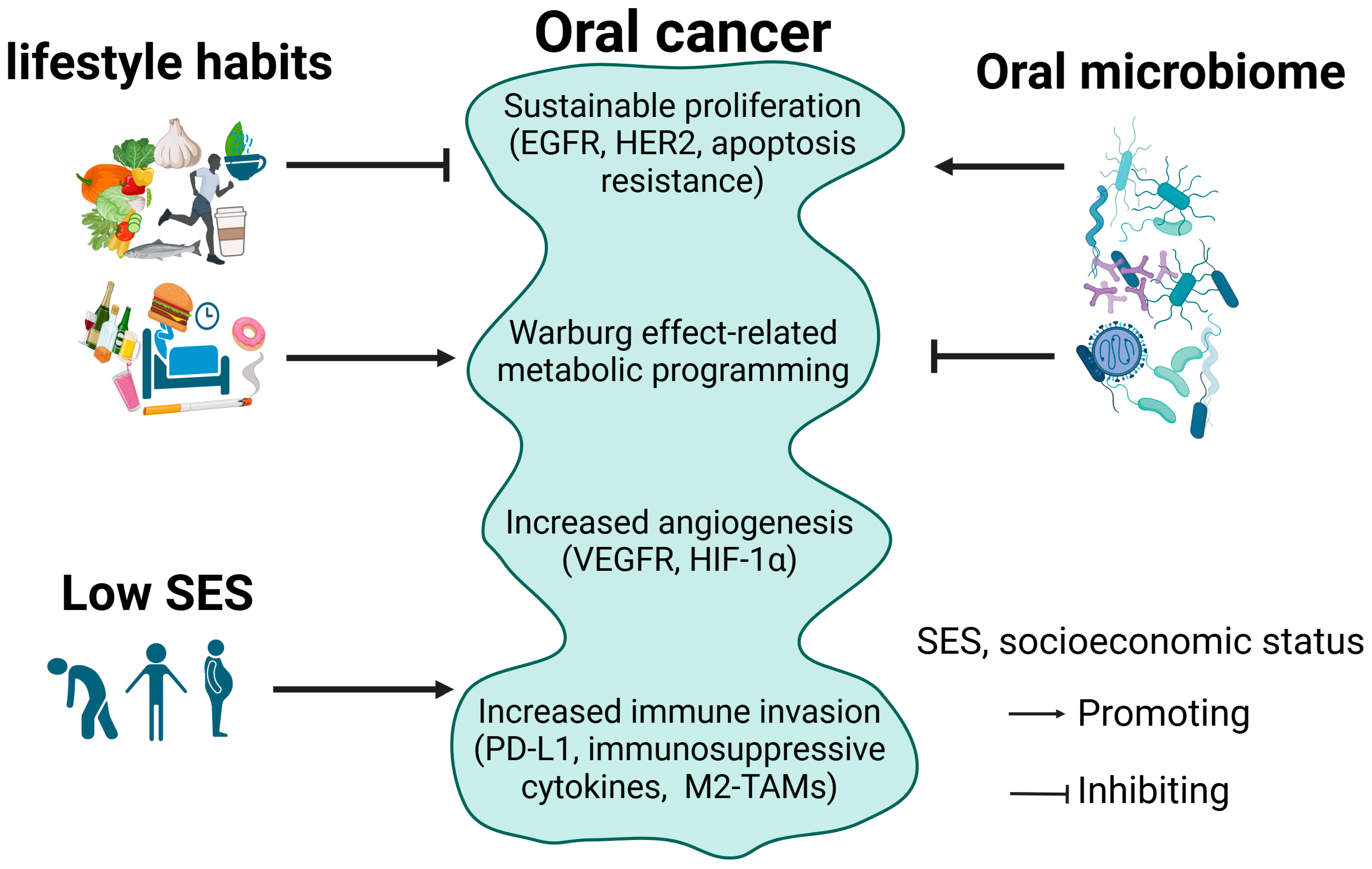
2. Oral Cancer and the Underlying Cellular Mechanisms
2.1. Oral Cancer
2.2. Sustained Proliferation in Oral Cancer
2.3. Metabolic Reprogramming in Oral Cancer
2.4. Angiogenesis in Oral Cancer
2.5. Immune Evasion in Oral Cancer
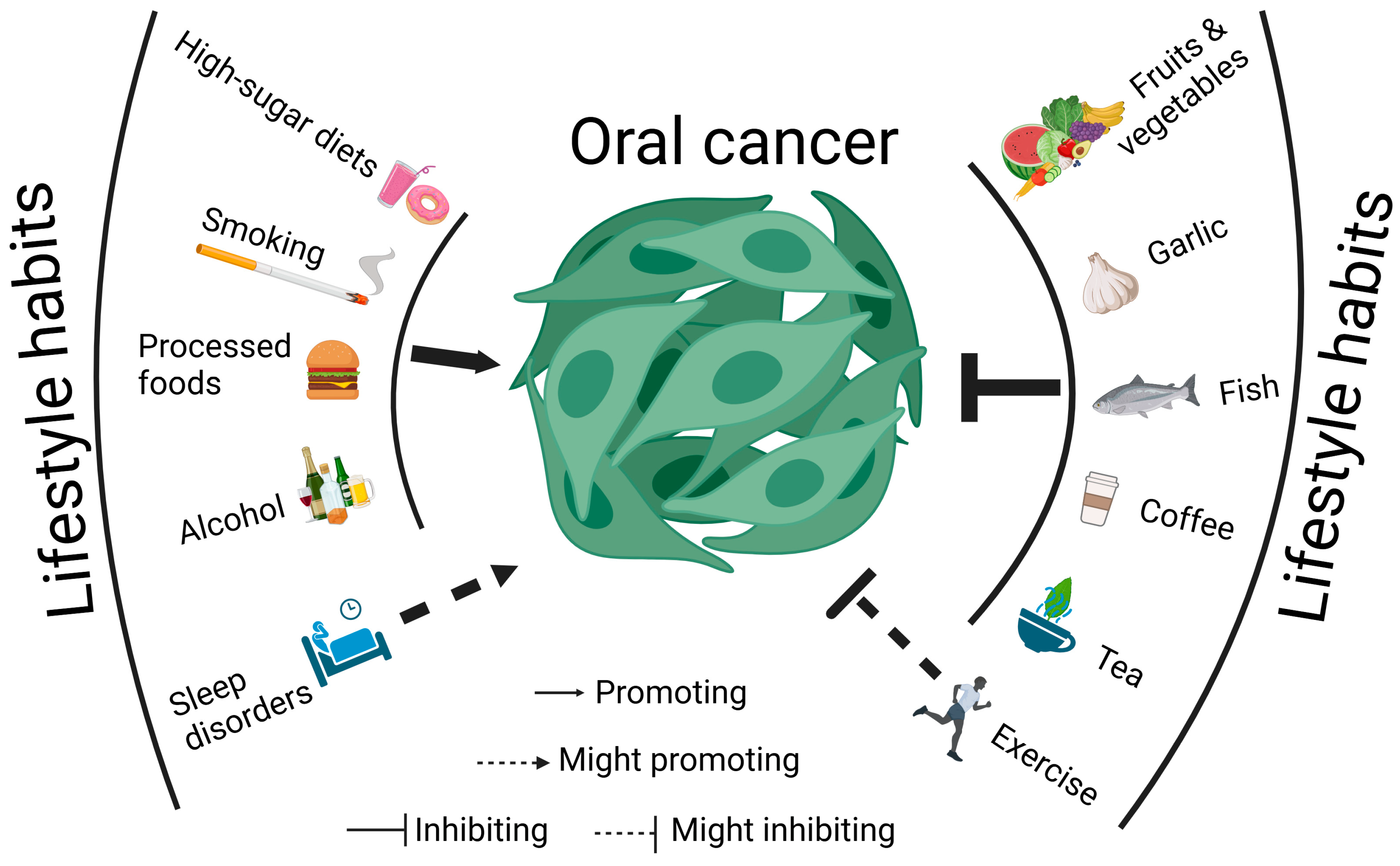
3. Lifestyle Habits and Oral Cancer
3.1. Diet
3.1.1. High-Sugar Diets Promote Oral Cancer Progression
3.1.2. Natural Diets Could Combat Oral Cancer
3.1.3. Tea and Coffee Could Combat Oral Cancer
3.1.4. Excessive Alcohol Consumption Promotes Oral Cancer Progression
3.1.5. Processed Foods Could Promote Oral Cancer Progression
3.1.6. Micronutrients Could Combat Oral Cancer
3.1.7. Diet and TME
3.2. Smoking
3.3. Exercise
3.4. Sleep
4. Oral Microbiome and Oral Cancer
5. Socioeconomic Factors and Oral Cancer
5.1. Inaccessibility to Healthcare Contributes to Delayed Diagnosis and Ineffective Treatment
5.2. Economic Status Affects Oral Cancer Diagnosis and Treatment
5.3. Education Levels Affect Oral Cancer Diagnosis and Treatment
5.4. Reflecting Global Inequalities
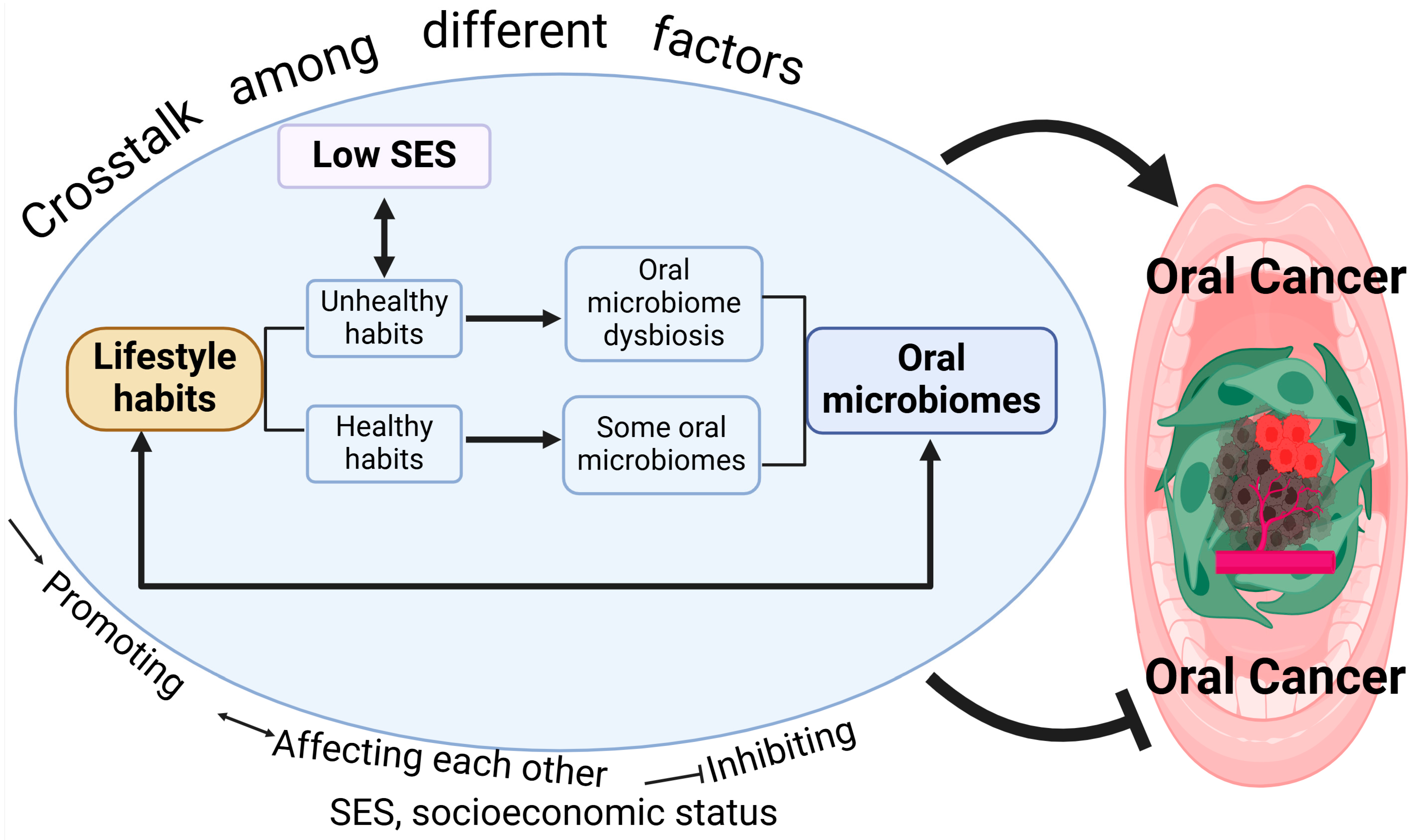
6. Oral Cancer Is Affected by Crosstalk Between Different Factors
6.1. SES and Lifestyle Habits
6.2. Lifestyle Habits and Oral Microbiome
7. Perspectives: Strategies for Oral Cancer Management and Treatment
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | adenomatous polyposis coli |
| ATP | adenosine triphosphate |
| BCL-2 | B-cell lymphoma 2 |
| bFGF | basic fibroblast growth factor |
| BNIP3 | BCL-2/adenovirus E1B 19-kDa-interacting protein 3 |
| BRAF | V-Raf murine sarcoma viral oncogene homolog B |
| CDKN2A | cyclin-dependent kinase inhibitor 1A |
| CTNNB1 | catenin beta 1 |
| DNA | deoxyribonucleic acid |
| EGFR | epidermal growth factor receptor |
| GLUT1 | glucose transporter type 1 |
| GSH | glutathione |
| HER2 | human epidermal growth factor receptor 2 |
| HIF-1α | hypoxia-inducible factor-1α |
| HIF-2α | hypoxia-inducible factor-2α |
| HK2 | hexokinase 2 |
| HNC | head and neck cancer |
| HNSCC | head and neck squamous cell carcinoma |
| HPV | human oral papillomavirus |
| IL-10 | interleukin-10 |
| IL-8 | interleukin-8 |
| LDH-A | lactate dehydrogenase-A |
| M2-TAMs | M2-like tumor-associated macrophages |
| MDSC | myeloid-derived suppressor cell |
| MHC | major histocompatibility complex |
| MMP | matrix metalloproteinase |
| MYB-NFIB | myeloblastosis viral oncogene homolog–nuclear factor I/B |
| NRAS | neuroblastoma RAS viral oncogene homolog |
| OC | oral cancer |
| OPSCC | oropharyngeal squamous cell cancer |
| OSCC | squamous cell carcinoma |
| PD-1 | programmed cell death protein 1 |
| PDGF | platelet-derived growth factor |
| PD-L1 | programmed cell death ligand 1 |
| PKM2 | pyruvate kinase M2 |
| RAS | rat sarcoma virus |
| rDNA | ribosomal DNA |
| RNA | ribonucleic acid |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| rRNA | ribosomal RNA |
| SES | socioeconomic status |
| TAMs | tumor-associated macrophages |
| TGF-β | transforming growth factor-beta |
| TILs | tumor-infiltrating lymphocytes |
| TME | tumor microenvironment |
| TP53 | tumor protein p53 |
| US | United States |
| VEGF | vascular endothelial growth factor |
| WHO | World Health Organization |
References
- The global challenge of cancer. Nat. Cancer 2020, 1, 1–2. [CrossRef]
- Badwelan, M.; Muaddi, H.; Ahmed, A.; Lee, K.T.; Tran, S.D. Oral Squamous Cell Carcinoma and Concomitant Primary Tumors, What Do We Know? A Review of the Literature. Curr. Oncol. 2023, 30, 3721–3734. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, J.; Alade, A.A.; Zhu, W.Y.; Wang, W.; Choi, S.W.; Thomson, P. Efficacy of hypermethylated DNA biomarkers in saliva and oral swabs for oral cancer diagnosis: Systematic review and meta-analysis. Oral Dis. 2022, 28, 541–558. [Google Scholar] [CrossRef]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Tranby, E.P.; Heaton, L.J.; Tomar, S.L.; Kelly, A.L.; Fager, G.L.; Backley, M.; Frantsve-Hawley, J. Oral Cancer Prevalence, Mortality, and Costs in Medicaid and Commercial Insurance Claims Data. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1849–1857. [Google Scholar] [CrossRef]
- Sun, R.; Dou, W.; Liu, W.; Li, J.; Han, X.; Li, S.; Wu, X.; Wang, F.; Xu, X.; Li, J. Global, regional, and national burden of oral cancer and its attributable risk factors from 1990 to 2019. Cancer Med. 2023, 12, 13811–13820. [Google Scholar] [CrossRef]
- Kijowska, J.; Grzegorczyk, J.; Gliwa, K.; Jędras, A.; Sitarz, M. Epidemiology, Diagnostics, and Therapy of Oral Cancer-Update Review. Cancers 2024, 16, 3156. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Nagarathna, P.J.; Patil, S.R.; Veeraraghavan, V.P.; Daniel, S.; Aileni, K.R.; Karobari, M.I. Oral Cancer Stem Cells: A Comprehensive Review of Key Drivers of Treatment Resistance and Tumor Recurrence. Eur. J. Pharmacol. 2025, 989, 177222. [Google Scholar] [CrossRef]
- Alazazi, E.A.; Roslan, A.; Aziz, F.M.A.; Vanoh, D.; Seeni, A.; Ahmed, S.; Munir, S.; Mohammad, J.I.; Murtey, M.D. Chemoprevention of natural product against oral cancer: A comprehensive review. Malays. J. Pathol. 2024, 46, 355–368. [Google Scholar]
- Farina, F.; Cirillo, N. Estimating the Benefits of Oral Cancer Screening: Challenges and Opportunities. Cancers 2024, 16, 4110. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Khoushab, S.; Aghmiuni, M.H.; Anaraki, S.N.; Alimohammadi, M.; Taheriazam, A.; Farahani, N.; Entezari, M. Non-coding RNAs in oral cancer: Emerging biomarkers and therapeutic frontier. Heliyon 2024, 10, e40096. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Lei, J.; Wu, Y. Autophagy in oral cancer: Promises and challenges (Review). Int. J. Mol. Med. 2024, 54, 116. [Google Scholar] [CrossRef] [PubMed]
- Siquara da Rocha, L.O.; de Morais, E.F.; de Oliveira, L.Q.R.; Barbosa, A.V.; Lambert, D.W.; Gurgel Rocha, C.A.; Coletta, R.D. Exploring beyond Common Cell Death Pathways in Oral Cancer: A Systematic Review. Biology 2024, 13, 103. [Google Scholar] [CrossRef]
- Talapko, J.; Erić, S.; Meštrović, T.; Stipetić, M.M.; Juzbašić, M.; Katalinić, D.; Bekić, S.; Muršić, D.; Flam, J.; Belić, D.; et al. The Impact of Oral Microbiome Dysbiosis on the Aetiology, Pathogenesis, and Development of Oral Cancer. Cancers 2024, 16, 2997. [Google Scholar] [CrossRef]
- Bishayee, A.; Penn, A.; Bhandari, N.; Petrovich, R.; DeLiberto, L.K.; Burcher, J.T.; Barbalho, S.M.; Nagini, S. Dietary plants for oral cancer prevention and therapy: A review of preclinical and clinical studies. Phytother. Res. 2024, 38, 5225–5263. [Google Scholar] [CrossRef]
- Ionescu, C.; Kamal, F.Z.; Ciobica, A.; Halitchi, G.; Burlui, V.; Petroaie, A.D. Oxidative Stress in the Pathogenesis of Oral Cancer. Biomedicines 2024, 12, 1150. [Google Scholar] [CrossRef]
- Espressivo, A.; Pan, Z.S.; Usher-Smith, J.A.; Harrison, H. Risk Prediction Models for Oral Cancer: A Systematic Review. Cancers 2024, 16, 617. [Google Scholar] [CrossRef]
- Xu, H.; Gao, Z.; Liu, H.; An, L.; Yang, T.; Zhang, B.; Liu, G.; Sun, D. Associations of lifestyle factors with oral cancer risk: An umbrella review. J. Stomatol. Oral Maxillofac. Surg. 2025, 102234. [Google Scholar] [CrossRef]
- Li, C.C.; Shen, Z.; Bavarian, R.; Yang, F.; Bhattacharya, A. Oral cancer: Genetics and the role of precision medicine. Surg. Oncol. Clin. N. Am. 2020, 29, 127–144. [Google Scholar] [CrossRef]
- Abhinav, R.P.; Williams, J.; Livingston, P.; Anjana, R.M.; Mohan, V. Burden of diabetes and oral cancer in India. J. Diabetes Complicat. 2020, 34, 107670. [Google Scholar] [CrossRef]
- Yete, S.; Saranath, D. MicroRNAs in oral cancer: Biomarkers with clinical potential. Oral Oncol. 2020, 110, 105002. [Google Scholar] [CrossRef] [PubMed]
- Kalele, K.; Nyahatkar, S.; Mirgh, D.; Muthuswamy, R.; Adhikari, M.D.; Anand, K. Exosomes: A cutting-edge theranostics tool for oral cancer. ACS Appl. Bio. Mater. 2024, 7, 1400–1415. [Google Scholar] [CrossRef]
- Usman, S.; Jamal, A.; The, M.T.; Waseem, A. Major Molecular Signaling Pathways in Oral Cancer Associated with Therapeutic Resistance. Front. Oral Health 2020, 1, 603160. [Google Scholar] [CrossRef]
- Aizawa, Y.; Haga, K.; Yoshiba, N.; Yortchan, W.; Takada, S.; Tanaka, R.; Naito, E.; Abé, T.; Maruyama, S.; Yamazaki, M.; et al. Development and Characterization of a Three-Dimensional Organotypic In Vitro Oral Cancer Model with Four Co-Cultured Cell Types, Including Patient-Derived Cancer-Associated Fibroblasts. Biomedicines 2024, 12, 2373. [Google Scholar] [CrossRef]
- Adorno-Farias, D.; Morales-Pisón, S.; Gischkow-Rucatti, G.; Margarit, S.; Fernández-Ramires, R. Genetic and epigenetic landscape of early-onset oral squamous cell carcinoma: Insights of genomic underserved and underrepresented populations. Genet. Mol. Biol. 2024, 47 (Suppl. S1), e20240036. [Google Scholar] [CrossRef]
- Wagner, V.P.; Bingle, C.D.; Bingle, L. MYB-NFIB fusion transcript in adenoid cystic carcinoma: Current state of knowledge and future directions. Crit. Rev. Oncol. Hematol. 2022, 176, 103745. [Google Scholar] [CrossRef]
- Dumaz, N.; Jouenne, F.; Delyon, J.; Mourah, S.; Bensussan, A.; Lebbé, C. Atypical BRAF and NRAS Mutations in Mucosal Melanoma. Cancers 2019, 11, 1133. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Warnakulasuriya, S.; López-Ansio, M.; Ramos-García, P. Hallmarks of Cancer Applied to Oral and Oropharyngeal Carcinogenesis: A Scoping Review of the Evidence Gaps Found in Published Systematic Reviews. Cancers 2022, 14, 3834. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 protein family: Attractive targets for cancer therapy. Apoptosis 2023, 28, 20–38. [Google Scholar] [CrossRef]
- da Silva Rosa, S.C.; Alizadeh, J.; Vitorino, R.; Surendran, A.; Ravandi, A.; Kidane, B.; Ghavami, S. A Lipidomics Approach to Determine the Role of Lipids and Its Crosstalk with Autophagy in Lung Cancer Metastasis. Methods Mol. Biol. 2025, 2879, 239–260. [Google Scholar] [CrossRef]
- Alizadeh, J.; Kavoosi, M.; Singh, N.; Lorzadeh, S.; Ravandi, A.; Kidane, B.; Ahmed, N.; Mraiche, F.; Mowat, M.R.; Ghavami, S. Regulation of Autophagy via Carbohydrate and Lipid Metabolism in Cancer. Cancers 2023, 15, 2195. [Google Scholar] [CrossRef]
- Jaworska, M.; Szczudło, J.; Pietrzyk, A.; Shah, J.; Trojan, S.E.; Ostrowska, B.; Kocemba-Pilarczyk, K.A. The Warburg effect: A score for many instruments in the concert of cancer and cancer niche cells. Pharmacol. Rep. 2023, 75, 876–890. [Google Scholar] [CrossRef]
- Gonçalves, F.A.; da Silva Bittencourt, L.; Barbosa, S.; Diel, L.F.; Bernardi, L.; Matte, C.; Lamers, M.L. Energy Metabolic Profile in Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma: A Preliminary Landscape of Warburg Effect in Oral Cancer. Mol. Carcinog. 2025, 64, 126–137. [Google Scholar] [CrossRef]
- Mathew, M.; Nguyen, N.T.; Bhutia, Y.D.; Sivaprakasam, S.; Ganapathy, V. Metabolic Signature of Warburg Effect in Cancer: An Effective and Obligatory Interplay between Nutrient Transporters and Catabolic/Anabolic Pathways to Promote Tumor Growth. Cancers 2024, 16, 504. [Google Scholar] [CrossRef]
- Kurihara-Shimomura, M.; Sasahira, T.; Nakashima, C.; Kuniyasu, H.; Shimomura, H.; Kirita, T. The Multifarious Functions of Pyruvate Kinase M2 in Oral Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2907. [Google Scholar] [CrossRef]
- Alizadeh, J.; da Silva Rosa, S.C.; Weng, X.; Jacobs, J.; Lorzadeh, S.; Ravandi, A.; Vitorino, R.; Pecic, S.; Zivkovic, A.; Stark, H.; et al. Ceramides and ceramide synthases in cancer: Focus on apoptosis and autophagy. Eur. J. Cell Biol. 2023, 102, 151337. [Google Scholar] [CrossRef]
- Mat Lazim, N.; Yousaf, A.; Abusalah, M.A.H.; Sulong, S.; Mohd Ismail, Z.I.; Mohamud, R.; Abu-Harirah, H.A.; AlRamadneh, T.N.; Hassan, R.; Abdullah, B. The Epigenesis of Salivary Glands Carcinoma: From Field Cancerization to Carcinogenesis. Cancers 2023, 15, 2111. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, D.; Huang, M.; Ji, J.; Xu, X.; Wang, F.; Zhou, L.; Bao, B.; Jiang, F.; Xu, W.; et al. Glycolysis in the tumor microenvironment: A driver of cancer progression and a promising therapeutic target. Front. Cell Dev. Biol. 2024, 12, 1416472. [Google Scholar] [CrossRef]
- Avagliano, A.; Fiume, G.; Pelagalli, A.; Sanità, G.; Ruocco, M.R.; Montagnani, S.; Arcucci, A. Metabolic Plasticity of Melanoma Cells and Their Crosstalk with Tumor Microenvironment. Front. Oncol. 2020, 10, 722. [Google Scholar] [CrossRef]
- Kuphal, S.; Winklmeier, A.; Warnecke, C.; Bosserhoff, A.K. Constitutive HIF-1 activity in malignant melanoma. Eur. J. Cancer 2010, 46, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Dong, Q.; Li, J.; Niu, W.; Liu, T. Extracellular vesicle-bound VEGF in oral squamous cell carcinoma and its role in resistance to Bevacizumab Therapy. Cancer Cell Int. 2024, 24, 296. [Google Scholar] [CrossRef]
- Nasry, W.H.S.; Rodriguez-Lecompte, J.C.; Martin, C.K. Role of COX-2/PGE2 Mediated Inflammation in Oral Squamous Cell Carcinoma. Cancers 2018, 10, 348. [Google Scholar] [CrossRef]
- Liu, J.; Shao, C.; Tan, M.L.; Mu, D.; Ferris, R.L.; Ha, P.K. Molecular biology of adenoid cystic carcinoma. Head Neck 2012, 34, 1665–1677. [Google Scholar] [CrossRef]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef]
- Pollock, N.I.; Grandis, J.R. HER2 as a therapeutic target in head and neck squamous cell carcinoma. Clin. Cancer Res. 2015, 21, 526–533. [Google Scholar] [CrossRef]
- Hu, A.; Sun, L.; Lin, H.; Liao, Y.; Yang, H.; Mao, Y. Harnessing innate immune pathways for therapeutic advancement in cancer. Signal. Transduct. Target. Ther. 2024, 9, 68. [Google Scholar] [CrossRef]
- Anderson, T.S.; Wooster, A.L.; Piersall, S.L.; Okpalanwaka, I.F.; Lowe, D.B. Disrupting cancer angiogenesis and immune checkpoint networks for improved tumor immunity. Semin. Cancer Biol. 2022, 86, 981–996. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Hashemi, M.; Karami, S.; Sarabandi, S.; Moazeni-Roodi, A.; Małecki, A.; Ghavami, S.; Wiechec, E. Association between PD-1 and PD-L1 Polymorphisms and the Risk of Cancer: A Meta-Analysis of Case-Control Studies. Cancers 2019, 11, 1150. [Google Scholar] [CrossRef]
- Wu, T.; Tang, C.; Tao, R.; Yong, X.; Jiang, Q.; Feng, C. PD-L1-Mediated Immunosuppression in Oral Squamous Cell Carcinoma: Relationship With Macrophage Infiltration and Epithelial to Mesenchymal Transition Markers. Front. Immunol. 2021, 12, 693881. [Google Scholar] [CrossRef]
- Porcheri, C.; Meisel, C.T.; Mitsiadis, T.A. Molecular and Cellular Modelling of Salivary Gland Tumors Open New Landscapes in Diagnosis and Treatment. Cancers 2020, 12, 3107. [Google Scholar] [CrossRef]
- Tie, Y.; Tang, F.; Wei, Y.Q.; Wei, X.W. Immunosuppressive cells in cancer: Mechanisms and potential therapeutic targets. J. Hematol. Oncol. 2022, 15, 61. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and hot tumors: From molecular mechanisms to targeted therapy. Signal. Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef]
- Saba, N.F.; Chen, Z.G.; Haigentz, M.; Bossi, P.; Rinaldo, A.; Rodrigo, J.P.; Mäkitie, A.A.; Takes, R.P.; Strojan, P.; Vermorken, J.B.; et al. Targeting the EGFR and Immune Pathways in Squamous Cell Carcinoma of the Head and Neck (SCCHN): Forging a New Alliance. Mol. Cancer Ther. 2019, 18, 1909–1915. [Google Scholar] [CrossRef]
- Mittelman, S.D. The Role of Diet in Cancer Prevention and Chemotherapy Efficacy. Annu. Rev. Nutr. 2020, 40, 273–297. [Google Scholar] [CrossRef]
- Rodríguez-Molinero, J.; Migueláñez-Medrán, B.D.C.; Puente-Gutiérrez, C.; Delgado-Somolinos, E.; Martín Carreras-Presas, C.; Fernández-Farhall, J.; López-Sánchez, A.F. Association between Oral Cancer and Diet: An Update. Nutrients 2021, 13, 1299. [Google Scholar] [CrossRef]
- La Vecchia, C.; Tavani, A.; Franceschi, S.; Levi, F.; Corrao, G.; Negri, E. Epidemiology and prevention of oral cancer. Oral Oncol. 1997, 33, 302–312. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K. Stress, food, and inflammation: Psychoneuroimmunology and nutrition at the cutting edge. Psychosom. Med. 2010, 72, 365–369. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair 2019, 83, 102673. [Google Scholar]
- Hua, R.; Liang, G.; Yang, F. Meta-analysis of the association between dietary inflammatory index (DII) and upper aerodigestive tract cancer risk. Medicine 2020, 99, e19879. [Google Scholar] [CrossRef] [PubMed]
- Anil, A.; Raheja, R.; Gibu, D.; Raj, A.S.; Spurthi, S. Uncovering the Links Between Dietary Sugar and Cancer: A Narrative Review Exploring the Impact of Dietary Sugar and Fasting on Cancer Risk and Prevention. Cureus 2024, 16, e67434. [Google Scholar] [CrossRef]
- Chazelas, E.; Srour, B.; Desmetz, E.; Kesse-Guyot, E.; Julia, C.; Deschamps, V.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; Latino-Martel, P.; et al. Sugary drink consumption and risk of cancer: Results from NutriNet-Sante prospective cohort. BMJ 2019, 366, l2408. [Google Scholar] [CrossRef]
- Cetinkaya, H.; Romaniuk, P. Relationship between consumption of soft and alcoholic drinks and oral health problems. Cent. Eur. J. Public Health 2020, 28, 94–102. [Google Scholar] [CrossRef]
- Epner, M.; Yang, P.; Wagner, R.W.; Cohen, L. Understanding the Link between Sugar and Cancer: An Examination of the Preclinical and Clinical Evidence. Cancers 2022, 14, 6042. [Google Scholar] [CrossRef]
- Arthur, A.E.; Goss, A.M.; Demark-Wahnefried, W.; Mondul, A.M.; Fontaine, K.R.; Chen, Y.T.; Carroll, W.R.; Spencer, S.A.; Rogers, L.Q.; Rozek, L.S.; et al. Higher carbohydrate intake is associated with increased risk of all-cause and disease-specific mortality in head and neck cancer patients: Results from a prospective cohort study. Int. J. Cancer 2018, 143, 1105–1113. [Google Scholar] [CrossRef]
- Reis, M.G.; Lopes, L.C.; Sanches, A.B.A.M.A.; Guimarães, N.S.; Martins-Chaves, R.R. Diet and Oral Squamous Cell Carcinoma: A Scoping Review. Int. J. Environ. Res. Public Health 2024, 21, 1199. [Google Scholar] [CrossRef]
- Malathi, N.; Rajan, S.T.; Warnakulasuriya, S. Natural products and diet for the prevention of oral cancer: Research from south and southeast Asia. Oral Dis. 2024. [Google Scholar] [CrossRef]
- Hu, S.; Yu, J.; Wang, Y.; Li, Y.; Chen, H.; Shi, Y.; Ma, X. Fish consumption could reduce the risk of oral cancer in Europeans: A meta-analysis. Arch. Oral Biol. 2019, 107, 104494. [Google Scholar] [CrossRef]
- Pai, M.H.; Kuo, Y.H.; Chiang, E.P.; Tang, F.Y. S-Allylcysteine inhibits tumour progression and the epithelial-mesenchymal transition in a mouse xenograft model of oral cancer. Br. J. Nutr. 2012, 108, 28–38. [Google Scholar] [CrossRef]
- Tang, F.Y.; Chiang, E.P.; Chung, J.G.; Lee, H.Z.; Hsu, C.Y. S-allylcysteine modulates the expression of E-cadherin and inhibits the malignant progression of human oral cancer. J. Nutr. Biochem. 2009, 20, 1013–1020. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Cui, D. Association between coffee consumption and the risk of oral cancer: A meta-analysis of observational studies. Int. J. Clin. Exp. Med. 2015, 8, 11657–11665. [Google Scholar]
- Li, Y.M.; Peng, J.; Li, L.Z. Coffee consumption associated with reduced risk of oral cancer: A meta-analysis. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2016, 121, 381–389.e1. [Google Scholar] [CrossRef]
- Hildebrand, J.S.; Patel, A.V.; McCullough, M.L.; Gaudet, M.M.; Chen, A.Y.; Hayes, R.B.; Gapstur, S.M. Coffee, tea, and fatal oral/pharyngeal cancer in a large prospective US cohort. Am. J. Epidemiol. 2013, 177, 50–58. [Google Scholar] [CrossRef]
- Goldstein, B.Y.; Chang, S.C.; Hashibe, M.; La Vecchia, C.; Zhang, Z.F. Alcohol consumption and cancers of the oral cavity and pharynx from 1988 to 2009: An update. Eur. J. Cancer Prev. 2010, 19, 431–465. [Google Scholar] [CrossRef]
- O’Grady, I.; O’Sullivan, J. Alcohol consumption modulates Candida albicans-induced oral carcinogenesis and progression. J. Oral Biosci. 2023, 65, 293–304. [Google Scholar] [CrossRef]
- Kliemann, N.; Al Nahas, A.; Vamos, E.P.; Touvier, M.; Kesse-Guyot, E.; Gunter, M.J.; Millett, C.; Huybrechts, I. Ultra-processed foods and cancer risk: From global food systems to individual exposures and mechanisms. Br. J. Cancer 2022, 127, 14–20. [Google Scholar] [CrossRef]
- Bulanda, S.; Lau, K.; Nowak, A.; Łyko-Morawska, D.; Kotylak, A.; Janoszka, B. The Risk of Oral Cancer and the High Consumption of Thermally Processed Meat Containing Mutagenic and Carcinogenic Compounds. Nutrients 2024, 16, 1084. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Richie, J.P., Jr.; Kleinman, W.; Marina, P.; Abraham, P.; Wynder, E.L.; Muscat, J.E. Blood iron, glutathione, and micronutrient levels and the risk of oral cancer. Nutr. Cancer 2008, 60, 474–482. [Google Scholar] [CrossRef]
- Hung, M.; Almpani, K.; Thao, B.; Sudweeks, K.; Lipsky, M.S. Vitamin D in the Prevention and Treatment of Oral Cancer: A Scoping Review. Nutrients 2023, 15, 2346. [Google Scholar] [CrossRef]
- Verma, A.; Vincent-Chong, V.K.; DeJong, H.; Hershberger, P.A.; Seshadri, M. Impact of dietary vitamin D on initiation and progression of oral cancer. J. Steroid. Biochem. Mol. Biol. 2020, 199, 105603. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y.; Qian, J.; Wang, S.; Li, Y.; Xu, M.; Chen, F.; Wang, J.; Qiu, Y.; Lin, L.; et al. Relationship Between Dietary Fiber and Vitamin C Intake and Oral Cancer. Front. Public Health 2022, 10, 880506. [Google Scholar] [CrossRef]
- Iqubal, M.A.; Khan, M.; Kumar, P.; Kumar, A.; Ajai, K. Role of vitamin e in prevention of oral cancer: A review. J. Clin. Diagn. Res. 2014, 8, ZE05–ZE07. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Peng, J.; Hu, Q.; Chen, X.; Wang, C.; Zhang, J.; Ren, X.; Wang, Y.; Tao, X.; Li, H.; Song, M.; et al. Diet-induced obesity accelerates oral carcinogenesis by recruitment and functional enhancement of myeloid-derived suppressor cells. Cell Death Dis. 2021, 12, 946. [Google Scholar] [CrossRef]
- Fraga, M.; Yáñez, M.; Sherman, M.; Llerena, F.; Hernandez, M.; Nourdin, G.; Álvarez, F.; Urrizola, J.; Rivera, C.; Lamperti, L.; et al. Immunomodulation of T Helper Cells by Tumor Microenvironment in Oral Cancer Is Associated with CCR8 Expression and Rapid Membrane Vitamin D Signaling Pathway. Front. Immunol. 2021, 12, 643298. [Google Scholar] [CrossRef]
- Freitas, R.D.S.; Campos, M.M. Protective Effects of Omega-3 Fatty Acids in Cancer-Related Complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Vakili, S.; Behrooz, A.B.; Whichelo, R.; Fernandes, A.; Emwas, A.H.; Jaremko, M.; Markowski, J.; Los, M.J.; Ghavami, S.; Vitorino, R. Progress in Precision Medicine for Head and Neck Cancer. Cancers 2024, 16, 3716. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.I.; Petticrew, M.; Marlborough, H.; Berthiller, J.; Hashibe, M.; Macpherson, L.M. Socioeconomic inequalities and oral cancer risk: A systematic review and meta-analysis of case-control studies. Int. J. Cancer 2008, 122, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, C.; Leatherdale, S.; Cooke, M. Tobacco, alcohol and marijuana use among Indigenous youth attending off-reserve schools in Canada: Cross-sectional results from the Canadian Student Tobacco, Alcohol and Drugs Survey. Health Promot. Chronic. Dis. Prev. Can. 2019, 39, 207–215. [Google Scholar] [CrossRef]
- Booth, C.M.; Li, G.; Zhang-Salomons, J.; Mackillop, W.J. The impact of socioeconomic status on stage of cancer at diagnosis and survival: A population-based study in Ontario, Canada. Cancer 2010, 116, 4160–4167. [Google Scholar] [CrossRef]
- Yu, V.X.; Long, S.; Tassler, A. Smoking and Head and Neck Cancer. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 470. [Google Scholar] [CrossRef]
- Proia, N.K.; Paszkiewicz, G.M.; Nasca, M.A.; Franke, G.E.; Pauly, J.L. Smoking and smokeless tobacco-associated human buccal cell mutations and their association with oral cancer—A review. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 1061–1077. [Google Scholar] [CrossRef]
- Petros, W.P.; Younis, I.R.; Ford, J.N.; Weed, S.A. Effects of tobacco smoking and nicotine on cancer treatment. Pharmacotherapy 2012, 32, 920–931. [Google Scholar] [CrossRef]
- Smith, J.; Nastasi, D.; Tso, R.; Vangaveti, V.; Renison, B.; Chilkuri, M. The effects of continued smoking in head and neck cancer patients treated with radiotherapy: A systematic review and meta-analysis. Radiother. Oncol. 2019, 135, 51–57. [Google Scholar] [CrossRef]
- von Kroge, P.R.; Bokemeyer, F.; Ghandili, S.; Bokemeyer, C.; Seidel, C. The Impact of Smoking Cessation and Continuation on Recurrence and Survival in Patients with Head and Neck Cancer: A Systematic Review of the Literature. Oncol. Res. Treat. 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Raj, A.T.; Sujatha, G.; Muruganandhan, J.; Kumar, S.S.; Bharkavi, S.I.; Varadarajan, S.; Patil, S.; Awan, K.H. Reviewing the oral carcinogenic potential of E-cigarettes using the Bradford Hill criteria of causation. Transl. Cancer Res. 2020, 9, 3142–3152. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Lee, W.T.; Ou, C.Y.; Hsiao, J.R.; Huang, C.C.; Huang, J.S.; Wong, T.Y.; Chen, K.C.; Tsai, S.T.; Fang, S.Y.; et al. Regular recreational physical activity and risk of head and neck cancer. BMC Cancer 2017, 17, 286. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Wang, C.P.; Chen, Y.J.; Lou, P.J.; Ko, J.Y.; Lin, J.J.; Chen, M.R.; Lai, Y.H. Physical activity and fitness in survivors of head and neck cancer. Support Care Cancer 2021, 29, 6807–6817. [Google Scholar] [CrossRef]
- Karczewska-Lindinger, M.; Tuomi, L.; Fridolfsson, J.; Arvidsson, D.; Börjesson, M.; Finizia, C. Low physical activity in patients diagnosed with head and neck cancer. Laryngoscope Investig. Otolaryngol. 2021, 6, 747–755. [Google Scholar] [CrossRef]
- Capozzi, L.C.; Nishimura, K.C.; McNeely, M.L.; Lau, H.; Culos-Reed, S.N. The impact of physical activity on health-related fitness and quality of life for patients with head and neck cancer: A systematic review. Br. J. Sports Med. 2016, 50, 325–338. [Google Scholar] [CrossRef]
- Avancini, A.; Borsati, A.; Belluomini, L.; Giannarelli, D.; Nocini, R.; Insolda, J.; Sposito, M.; Schena, F.; Milella, M.; Pilotto, S. Effect of exercise across the head and neck cancer continuum: A systematic review of randomized controlled trials. Support Care Cancer 2023, 31, 670. [Google Scholar] [CrossRef]
- Fang, H.F.; Miao, N.F.; Chen, C.D.; Sithole, T.; Chung, M.H. Risk of Cancer in Patients with Insomnia, Parasomnia, and Obstructive Sleep Apnea: A Nationwide Nested Case-Control Study. J. Cancer 2015, 15, 1140–1147. [Google Scholar] [CrossRef]
- Mogavero, M.P.; DelRosso, L.M.; Fanfulla, F.; Bruni, O.; Ferri, R. Sleep disorders and cancer: State of the art and future perspectives. Sleep Med. Rev. 2021, 56, 101409. [Google Scholar] [CrossRef]
- Santoso, A.M.M.; Jansen, F.; de Vries, R.; Leemans, C.R.; van Straten, A.; Verdonck-de Leeuw, I.M. Prevalence of sleep disturbances among head and neck cancer patients: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 47, 62–73. [Google Scholar] [CrossRef]
- Iovoli, A.J.; Smith, K.; Yu, H.; Kluczynski, M.A.; Jungquist, C.R.; Ray, A.D.; Farrugia, M.K.; Gu, F.; Singh, A.K. Association of Insomnia and Obstructive Sleep Apnea with Worse Oral Mucositis and Quality of Life in Head and Neck Cancer Patients Undergoing Radiation Therapy. Cancers 2024, 16, 1335. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Zhang, L. Role of the microbiome in oral cancer occurrence, progression and therapy. Microb. Pathog. 2022, 169, 105638. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tan, X.; Cheng, J.; Liu, Z.; Zhou, H.; Liao, J.; Wang, X.; Liu, H. Oral microbiome and its relationship with oral cancer. J. Cancer Res. Ther. 2024, 20, 1141–1149. [Google Scholar] [CrossRef]
- Wang, J.; Gao, B. Mechanisms and Potential Clinical Implications of Oral Microbiome in Oral Squamous Cell Carcinoma. Curr. Oncol. 2023, 31, 168–182. [Google Scholar] [CrossRef]
- Smędra, A.; Berent, J. The Influence of the Oral Microbiome on Oral Cancer: A Literature Review and a New Approach. Biomolecules 2023, 13, 815. [Google Scholar] [CrossRef]
- Li, R.; Xiao, L.; Gong, T.; Liu, J.; Li, Y.; Zhou, X.; Li, Y.; Zheng, X. Role of oral microbiome in oral oncogenesis, tumor progression, and metastasis. Mol. Oral Microbiol. 2023, 38, 9–22. [Google Scholar] [CrossRef]
- Peter, T.K.; Withanage, M.H.H.; Comnick, C.L.; Pendleton, C.; Dabdoub, S.; Ganesan, S.; Drake, D.; Banas, J.; Xie, X.J.; Zeng, E. Systematic review and meta-analysis of oral squamous cell carcinoma associated oral microbiome. Front. Microbiol. 2022, 13, 968304. [Google Scholar] [CrossRef]
- Kessis, T.D.; Connolly, D.C.; Hedrick, L.; Cho, K.R. Expression of HPV16 E6 or E7 increases integration of foreign DNA. Oncogene 1996, 13, 427–431. [Google Scholar]
- Hawley-Nelson, P.; Vousden, K.H.; Hubbert, N.L.; Lowy, D.R.; Schiller, J.T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989, 8, 3905–3910. [Google Scholar] [CrossRef]
- Mohd Fuad, A.S.; Amran, N.A.; Nasruddin, N.S.; Burhanudin, N.A.; Dashper, S.; Arzmi, M.H. The Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics in Oral Cancer Management. Probiotics Antimicrob. Proteins 2023, 15, 1298–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Pan, W.; Ming, X.; Wu, J.; Zhang, X.; Miao, J.; Cui, W. The effect of probiotics on severe oral mucositis in cancer patients undergoing chemotherapy and/or radiotherapy: A meta-analysis. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101983. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.J.; Young, C.D.; Zhou, H.M.; Wang, X.J. Mouse Models for Studying Oral Cancer: Impact in the Era of Cancer Immunotherapy. J. Dent. Res. 2018, 97, 683–690. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, L.; Puram, S.V.; Mazul, A.L. Neighborhood Socioeconomic Status and Racial and Ethnic Survival Disparities in Oral Cavity and Laryngeal Cancer. Cancer Epidemiol. Biomarkers Prev. 2023, 32, 642–652. [Google Scholar] [CrossRef]
- Ramadan, S.; Lee, J.J.; Wang, R.; Jackson, R.S.; Pipkorn, P.; Rich, J.; Harbison, R.A.; Zolkind, P.; Kang, S.Y.; Puram, S.V.; et al. Neighborhood socioeconomic status and race are associated with incidence disparities in oral cavity cancers. Oral Oncol. 2023, 147, 106607. [Google Scholar] [CrossRef]
- Akashanand; Zahiruddin, Q.S.; Jena, D.; Ballal, S.; Kumar, S.; Bhat, M.; Sharma, S.; Kumar, M.R.; Rustagi, S.; Gaidhane, A.M.; et al. Burden of oral cancer and associated risk factors at national and state levels: A systematic analysis from the global burden of disease in India, 1990–2021. Oral Oncol. 2024, 159, 107063. [Google Scholar] [CrossRef]
- Asmin, P.K.; Nusrath, F.; Divakar, D.D. Occurrence and Distribution of Cancers with Emphasis Upon Oral Cancers in Registered Oncology Institutes of South India—A Retrospective Study. Indian J. Community Med. 2024, 49, 120–130. [Google Scholar] [CrossRef]
- Coelho, K.R. Challenges of the oral cancer burden in India. J. Cancer Epidemiol. 2012, 2012, 701932. [Google Scholar] [CrossRef]
- Yangzom, K.; Masoud, H.; Hahmann, T. Primary Health Care Access Among First Nations People Living Off Reserve, Métis and Inuit, 2017 to 2020. Statistics Canada, 2023. Available online: https://www150.statcan.gc.ca/n1/pub/41-20-0002/412000022023005-eng.htm (accessed on 7 March 2025).
- Vela, M.B.; Erondu, A.I.; Smith, N.A.; Peek, M.E.; Woodruff, J.N.; Chin, M.H. Eliminating Explicit and Implicit Biases in Health Care: Evidence and Research Needs. Annu. Rev. Public Health. 2022, 43, 477–501. [Google Scholar] [CrossRef]
- Bourgeois, A.; Horrill, T.; Mollison, A.; Stringer, E.; Lambert, L.K.; Stajduhar, K. Barriers to cancer treatment for people experiencing socioeconomic disadvantage in high-income countries: A scoping review. BMC Health Serv. Res. 2024, 24, 670. [Google Scholar] [CrossRef]
- Horrill, T.C.; Dahl, L.; Sanderson, E.; Munro, G.; Garson, C.; Taylor, C.; Fransoo, R.; Thompson, G.; Cook, C.; Linton, J.; et al. Comparing cancer incidence, stage at diagnosis and outcomes of First Nations and all other Manitobans: A retrospective analysis. BMC Cancer 2019, 19, 1055. [Google Scholar] [CrossRef]
- The Atlases of Health Variation Publications Head and Neck Cancer—2024. Available online: https://fingertips.phe.org.uk/profile/atlas-of-variation/supporting-information/health-atlases (accessed on 10 March 2025).
- Nassani, M.Z.; Alsalhani, A.; Alali, F.M.; Rastam, S.; Alqhtani, N.R.; Alqahtahni, A.S.; Robaian, A.; Alhedyan, F.S.; Bin Nabhan, A.; Alenazi, A.; et al. Public awareness and knowledge of oral cancer in 13 Middle Eastern and North African Countries. JAMA Netw. Open. 2025, 8, e250522. [Google Scholar] [CrossRef] [PubMed]
- Muallah, D.; Matschke, J.; Muallah, S.; Klimova, A.; Kroschwald, L.M.; Schröder, T.A.; Lauer, G.; Haim, D. Socioeconomic disparities between oral cavity cancer patients in Germany. Front. Public Health 2022, 10, 831479. [Google Scholar] [CrossRef]
- Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030. Summary of the WHO European Region; WHO Regional Office for Europe: Copenhagen, Denmark, 2023; License: CC BY-NC-SA 3.0 IGO.
- Mihor, A.; Tomsic, S.; Zagar, T.; Lokar, K.; Zadnik, V. Socioeconomic inequalities in cancer incidence in Europe: A comprehensive review of population-based epidemiological studies. Radio. Onco. 2020, 54, 1–13. [Google Scholar] [CrossRef]
- Tetzlaff, F.; Nowossadeck, E.; Jansen, L.; Michalski, N.; Barnes, B.; Kraywinkel, K.; Hoebel, J. Widening area-based socioeconomic inequalities in cancer mortality in Germany between 2003 and 2019. Sci. Rep. 2023, 13, 17833. [Google Scholar] [CrossRef]
- Collins, S.E. Associations Between Socioeconomic Factors and Alcohol Outcomes. Alcohol Res. 2016, 38, 83–94. [Google Scholar]
- Ranganathan, K.; Kavitha, L. Clinical aspects of oral cancer and potentially malignant disorders in South and Southeast Asia. Oral Dis. 2024. [Google Scholar] [CrossRef]
- Rumgay, H.; Nethan, S.T.; Shah, R.; Vignat, J.; Ayo-Yusuf, O.; Chaturvedi, P.; Guerra, E.N.S.; Gupta, P.C.; Gupta, R.; Liu, S.; et al. Global burden of oral cancer in 2022 attributable to smokeless tobacco and areca nut consumption: A population attributable fraction analysis. Lancet Oncol. 2024, 25, 1413–1423. [Google Scholar] [CrossRef]
- Office of the Chief Dental Officer of Canada. Human papillomavirus and oral health. Can. Commun. Dis. Rep. 2020, 46, 380–383. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Miller, K.D.; Tossas, K.Y.; Winn, R.A.; Jemal, A.; Siegel, R.L. Cancer statistics for African American/Black People 2022. CA Cancer J. Clin. 2022, 72, 202–229. [Google Scholar] [CrossRef]
- Hoare, A.; Soto, C.; Rojas-Celis, V.; Bravo, D. Chronic Inflammation as a Link between Periodontitis and Carcinogenesis. Mediat. Inflamm. 2019, 2019, 1029857. [Google Scholar] [CrossRef]
- Custodio, M.C.; Ynion, J.; Samaddar, A.; Cuevas, R.P.; Mohanty, S.K.; Ray Chakravarti, A.; Demont, M. Unraveling heterogeneity of consumers’ food choice: Implications for nutrition interventions in eastern India. Glob. Food Sec. 2021, 28, 100497. [Google Scholar] [CrossRef] [PubMed]
- Batal, M.; Chan, H.M.; Fediuk, K.; Ing, A.; Berti, P.R.; Mercille, G.; Sadik, T.; Johnson-Down, L. First Nations households living on-reserve experience food insecurity: Prevalence and predictors among ninety-two First Nations communities across Canada. Can. J. Public Health 2021, 112 (Suppl. S1), 52–63. [Google Scholar]
- Chan, A.K.Y.; Tsang, Y.C.; Jiang, C.M.; Leung, K.C.M.; Lo, E.C.M.; Chu, C.H. Diet, Nutrition, and Oral Health in Older Adults: A Review of the Literature. Dent. J. 2023, 11, 222. [Google Scholar] [CrossRef]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef]
- Pignatelli, P.; Romei, F.M.; Bondi, D.; Giuliani, M.; Piattelli, A.; Curia, M.C. Microbiota and Oral Cancer as A Complex and Dynamic Microenvironment: A Narrative Review from Etiology to Prognosis. Int. J. Mol. Sci. 2022, 23, 8323. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Malayil, L.; Chopyk, J.; Smyth, E.; Kulkarni, P.; Raspanti, G.; Thomas, S.B.; Sapkota, A.; Mongodin, E.F.; Sapkota, A.R. Oral microbiome dysbiosis among cigarette smokers and smokeless tobacco users compared to non-users. Sci. Rep. 2024, 14, 10394. [Google Scholar] [CrossRef]
- Pan, C.; Liu, C.; Jia, W.; Zhao, D.; Chen, X.; Zhu, X.; Yang, M.; Wang, L. Alcohol drinking alters oral microbiota to modulate the progression of alcohol-related liver disease. iScience 2023, 26, 107977. [Google Scholar] [CrossRef]
- Ramshankar, V.; Krishnamurthy, A. Chemoprevention of oral cancer: Green tea experience. J. Nat. Sci. Biol. Med. 2014, 5, 3–7. [Google Scholar] [CrossRef]
- Yuan, X.; Long, Y.; Ji, Z.; Gao, J.; Fu, T.; Yan, M.; Zhang, L.; Su, H.; Zhang, W.; Wen, X.; et al. Green Tea Liquid Consumption Alters the Human Intestinal and Oral Microbiome. Mol. Nutr. Food Res. 2018, 62, e1800178. [Google Scholar] [CrossRef]
- Urban, S.; Chmura, O.; Wątor, J.; Panek, P.; Zapała, B. The intensive physical activity causes changes in the composition of gut and oral microbiota. Sci. Rep. 2024, 14, 20858. [Google Scholar] [CrossRef] [PubMed]
- Sotozono, M.; Kuriki, N.; Asahi, Y.; Noiri, Y.; Hayashi, M.; Motooka, D.; Nakamura, S.; Yamaguchi, M.; Iida, T.; Ebisu, S. Impact of sleep on the microbiome of oral biofilms. PLoS ONE 2021, 16, e0259850. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; de’Angelis, N.; Gavriilidis, P.; Sobhani, I.; de’Angelis, G.L.; Carra, M.C. Oral microbiota in obstructive sleep apnea patients: A systematic review. Sleep Breath 2023, 27, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Yang, C.; Zhi, L.; Steven Xu, X.; Yuan, M. Oral microbiome diversity shapes the association between sleep duration and depression. Front. Neurol. 2024, 15, 1442557. [Google Scholar] [CrossRef]
- Hosseini, B.; Berthon, B.S.; Saedisomeolia, A.; Starkey, M.R.; Collison, A.; Wark, P.A.B.; Wood, L.G. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: A systematic literature review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 136–155. [Google Scholar] [CrossRef]
- Prado, R.P.; dos Santos, B.F.; Pinto, C.L.; de Assis, K.R.; Salvadori, D.M.; Ladeira, M.S. Influence of diet on oxidative DNA damage, uracil misincorporation and DNA repair capability. Mutagenesis 2010, 25, 483–487. [Google Scholar] [CrossRef]
- Budi, H.S.; Farhood, B. Tumor microenvironment remodeling in oral cancer: Application of plant derived-natural products and nanomaterials. Environ. Res. 2023, 233, 116432. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menon, A.; Mutalik, V.S.; Chen, Y.; Ponamgi, S.; Peela, S.; Schroth, R.J.; Ghavami, S.; Chelikani, P. Beyond Genetics: Exploring Lifestyle, Microbiome, and Social Determinants in Oral Cancer Development. Cancers 2025, 17, 1094. https://doi.org/10.3390/cancers17071094
Menon A, Mutalik VS, Chen Y, Ponamgi S, Peela S, Schroth RJ, Ghavami S, Chelikani P. Beyond Genetics: Exploring Lifestyle, Microbiome, and Social Determinants in Oral Cancer Development. Cancers. 2025; 17(7):1094. https://doi.org/10.3390/cancers17071094
Chicago/Turabian StyleMenon, Anil, Vimi S. Mutalik, Yongqiang Chen, SPD. Ponamgi, Sujatha Peela, Robert J. Schroth, Saeid Ghavami, and Prashen Chelikani. 2025. "Beyond Genetics: Exploring Lifestyle, Microbiome, and Social Determinants in Oral Cancer Development" Cancers 17, no. 7: 1094. https://doi.org/10.3390/cancers17071094
APA StyleMenon, A., Mutalik, V. S., Chen, Y., Ponamgi, S., Peela, S., Schroth, R. J., Ghavami, S., & Chelikani, P. (2025). Beyond Genetics: Exploring Lifestyle, Microbiome, and Social Determinants in Oral Cancer Development. Cancers, 17(7), 1094. https://doi.org/10.3390/cancers17071094









