Exploring the Genetic Orchestra of Cancer: The Interplay Between Oncogenes and Tumor-Suppressor Genes
Simple Summary
Abstract
1. Introduction
2. Historical Perspective
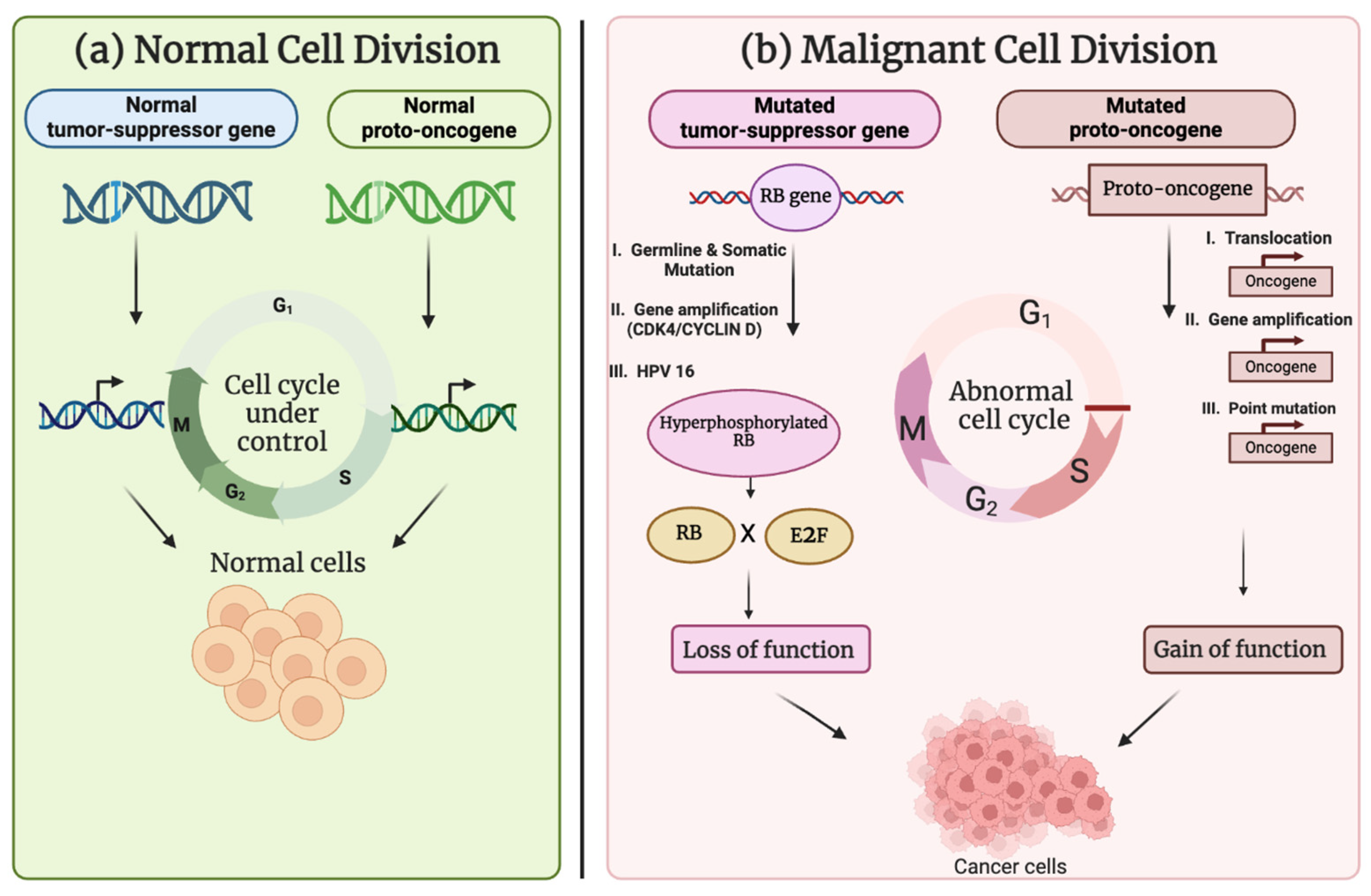
3. Molecular Factors Driving Oncogenic Activation and Tumor-Suppressor Gene Inactivation
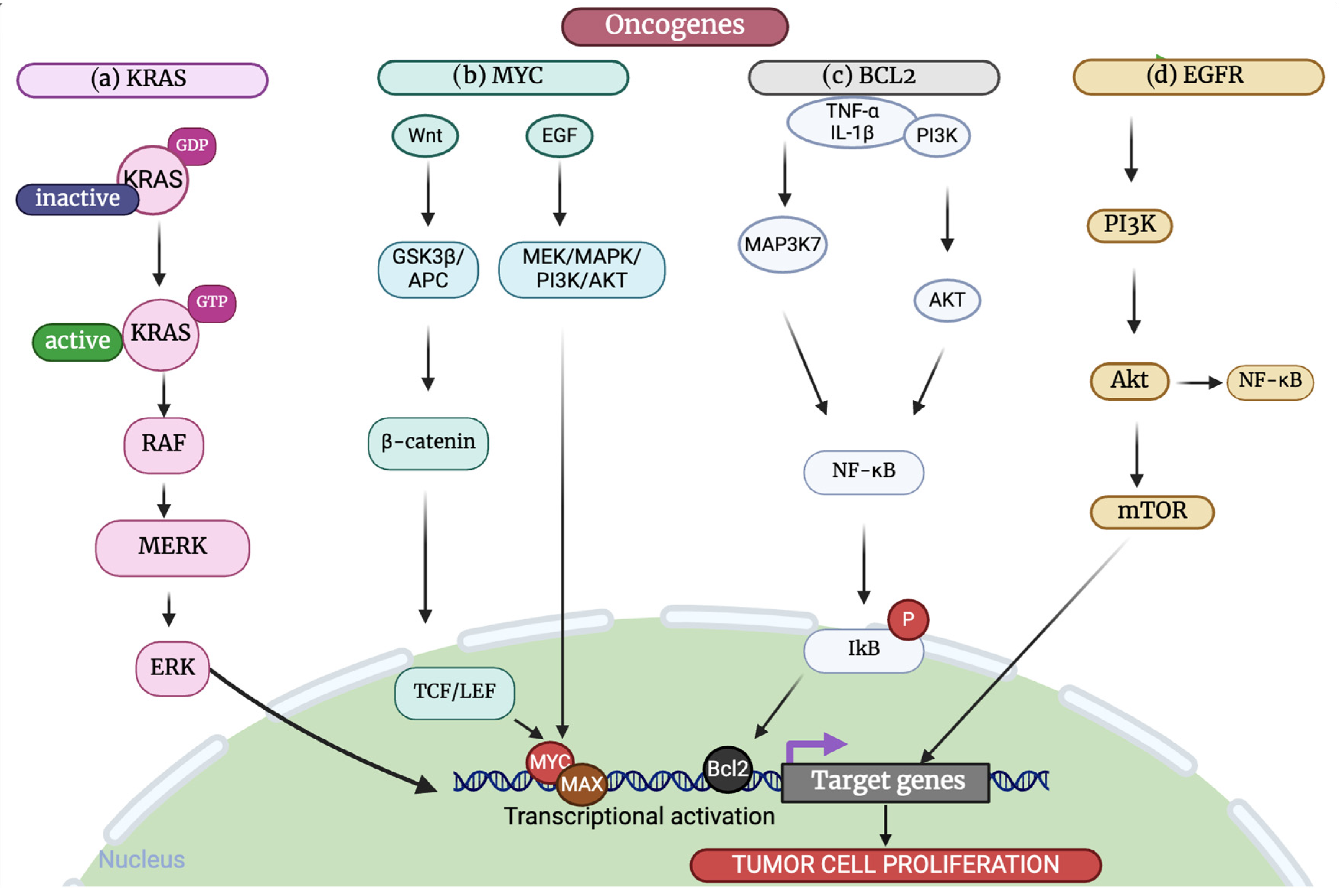
3.1. Oncogenes
3.1.1. MYC
3.1.2. HER2
3.1.3. KRAS
3.1.4. BRAF
3.1.5. Bcl-2
3.2. Tumor-Suppressor Genes
3.2.1. TP53
3.2.2. RB1
3.2.3. NF1
3.2.4. APC
4. Oncogenes and Tumor-Suppressor Gene Regulation
4.1. Cell Cycle Regulation
4.1.1. Cyclin D
4.1.2. Cyclin A
4.1.3. Cyclin B
4.1.4. Cyclin E
5. The Interplay Between Oncogenes and Tumor-Suppressor Genes
5.1. Uncontrolled Cell Proliferation
5.1.1. Inhibition of Apoptosis
5.1.2. Genomic Instability
5.1.3. Cancer Metabolism
5.1.4. Epigenetic Modifications
5.1.5. Long-Non-Coding RNAs
5.1.6. MicroRNAs
5.1.7. Proto-Oncogene Activation
6. Signaling Pathways
6.1. MAPK/ERK Pathway
6.2. Wnt/β Signaling Pathway
6.3. PI3K/AKT/mTOR Pathway
6.4. p53 Pathway
6.5. Notch Pathway
7. Feedback Loops
7.1. Negative Feedback Loops
7.2. Positive Feedback Loops
8. Artificial Intelligence in Cancer Research
9. Therapeutic Advances and Challenges
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| ALL | Acute lymphoblastic leukemia |
| APC | Adenomatous polyposis coli |
| BRAF | B-raf proto-OG, serine/threonine kinase |
| CDK | Cyclin-dependent kinases |
| CML | Chronic myeloid leukemia |
| CRC | Colorectal cancer |
| DBD | DNA-binding domain |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-to-mesenchymal transition |
| FGFR | Fibroblast growth factor receptor |
| GAP | GTPase-activating protein |
| GPCR | G-protein coupled receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| HGFR | Hepatocyte growth factor receptor |
| IGFR | Insulin-like growth factor receptor |
| ING1 | Inhibitor of growth family member 1 |
| JNK | c-Jun NH2-terminal kinase |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| lncRNA | Long non-coding RNA |
| MDM2 | Murine double minute 2 |
| MIIP | Migration and invasion inhibitory protein |
| miRNA | MicroRNA |
| NGS | Next-generation sequencing |
| NKRF | NF-kB repressing factor |
| NSCLC | Non-small cell lung carcinoma |
| OG | Oncogene |
| PDAC | Pancreatic ductal adenocarcinoma |
| PDGFR | Platelet-derived growth factor receptor |
| PDK1 | Phosphoinositide-dependent kinase 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP3 | Phosphatidylinositol 3,4,5-trisphosphate |
| PRLR | Prolactin receptor |
| Proto-OG | Proto-oncogene |
| Rb | Retinoblastoma (protein) |
| RB1 | Retinoblastoma (gene) |
| RSV | Rous sarcoma virus |
| RTK | Receptor tyrosine kinase |
| SCFR | Stem cell factor receptor |
| SMT | Somatic mutation theory of carcinogenesis |
| sncRNA | Small non-coding RNA |
| TCF/LEF | T-cell factor/lymphoid enhancer factor |
| TME | Tumor microenvironment |
| TK | Tyrosine kinase |
| TSG | Tumor-suppressor gene |
| VEGF | Vascular endothelial growth factor receptor |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.H.; Knudson, A.G.; Pandolfi, P.P. A continuum model for tumour suppression. Nature 2011, 476, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lipsick, J. A History of Cancer Research: Tumor Suppressor Genes. Cold Spring Harb. Perspect. Biol. 2020, 12, a035907. [Google Scholar] [CrossRef] [PubMed]
- Vogt, P.K. Retroviral oncogenes: A historical primer. Nat. Rev. Cancer 2012, 12, 639–648. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef]
- Knudson, A.G., Jr. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef]
- Weinberg, R.A. Tumor suppressor genes. Science 1991, 254, 1138–1146. [Google Scholar] [CrossRef]
- Burkhart, D.L.; Sage, J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat. Rev. Cancer 2008, 8, 671–682. [Google Scholar] [CrossRef]
- Chernoff, J. The two-hit theory hits 50. Mol. Biol. Cell 2021, 32, rt1. [Google Scholar] [CrossRef]
- Lietman, C.D.; Johnson, M.L.; McCormick, F.; Lindsay, C.R. More to the RAS Story: KRAS(G12C) Inhibition, Resistance Mechanisms, and Moving Beyond KRAS(G12C). Am. Soc. Clin. Oncol. Educ. Book. 2022, 42, 205–217. [Google Scholar] [CrossRef]
- Tsuchida, N.; Murugan, A.K.; Grieco, M. Kirsten Ras* oncogene: Significance of its discovery in human cancer research. Oncotarget 2016, 7, 46717–46733. [Google Scholar] [CrossRef] [PubMed]
- Garnett, M.J.; Marais, R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell 2004, 6, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef]
- Sabapathy, K.; Lane, D.P. Therapeutic targeting of p53: All mutants are equal, but some mutants are more equal than others. Nat. Rev. Clin. Oncol. 2018, 15, 13–30. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Kalkat, M.; De Melo, J.; Hickman, K.A.; Lourenco, C.; Redel, C.; Resetca, D.; Tamachi, A.; Tu, W.B.; Penn, L.Z. MYC Deregulation in Primary Human Cancers. Genes 2017, 8, 151. [Google Scholar] [CrossRef]
- Conacci-Sorrell, M.; McFerrin, L.; Eisenman, R.N. An Overview of MYC and Its Interactome. Cold Spring Harb. Perspect. Med. 2014, 4, a014357. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Deutzmann, A.; Mahauad-Fernandez, W.D.; Hansen, A.S.; Gouw, A.M.; Felsher, D.W. The MYC oncogene—The grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol. 2022, 19, 23–36. [Google Scholar] [CrossRef]
- Schaub, F.X.; Dhankani, V.; Berger, A.C.; Trivedi, M.; Richardson, A.B.; Shaw, R.; Zhao, W.; Zhang, X.; Ventura, A.; Liu, Y.; et al. Pan-cancer Alterations of the MYC Oncogene and Its Proximal Network across the Cancer Genome Atlas. Cell Syst. 2018, 6, 282–300.e2. [Google Scholar] [CrossRef]
- Jung, M.; Russell, A.J.; Liu, B.; George, J.; Liu, P.Y.; Liu, T.; DeFazio, A.; Bowtell, D.D.L.; Oberthuer, A.; London, W.B.; et al. A Myc Activity Signature Predicts Poor Clinical Outcomes in Myc-Associated Cancers. Cancer Res. 2017, 77, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, R.; van der Minne, C.; Plomp, A.; Sijts, A.; van Leeuwen, A.; Schrier, P. N-myc expression switched off and class I human leukocyte antigen expression switched on after somatic cell fusion of neuroblastoma cells. Mol. Cell. Biol. 1990, 10, 5416–5423. [Google Scholar] [CrossRef] [PubMed]
- Hatzi, E.; Murphy, C.; Zoephel, A.; Ahorn, H.; Tontsch, U.; Bamberger, A.M.; Yamauchi-Takihara, K.; Schweigerer, L.; Fotsis, T. N-myc oncogene overexpression down-regulates leukemia inhibitory factor in neuroblastoma. Eur. J. Biochem. 2002, 269, 3732–3741. [Google Scholar] [CrossRef]
- Posternak, V.; Cole, M.D. Strategically targeting MYC in cancer. F1000Research 2016, 5, F1000. [Google Scholar] [CrossRef]
- Castell, A.; Larsson, L.-G. Targeting MYC Translation in Colorectal Cancer. Cancer Discov. 2015, 5, 701–703. [Google Scholar] [CrossRef]
- Toh, P.P.C.; Luo, S.; Menzies, F.M.; Raskó, T.; Wanker, E.E.; Rubinsztein, D.C. Myc inhibition impairs autophagosome formation. Hum. Mol. Genet. 2013, 22, 5237–5248. [Google Scholar] [CrossRef]
- Ismail, M.S.; Downward, J. Abstract LB-A17: Identification and Characterization of Cyclic Peptide Inhibitors for Ras/PI3K and Ras/Raf Protein Complexes. Mol. Cancer Ther. 2018, 17, LB-A17. [Google Scholar] [CrossRef]
- Valencia, A.; Kjeldgaard, M.; Pai, E.F.; Sander, C. GTPase domains of ras p21 oncogene protein and elongation factor Tu: Analysis of three-dimensional structures, sequence families, and functional sites. Proc. Natl. Acad. Sci. USA 1991, 88, 5443–5447. [Google Scholar] [CrossRef]
- Zenonos, K. RAS signaling pathways, mutations and their role in colorectal cancer. World J. Gastrointest. Oncol. 2013, 5, 97. [Google Scholar] [CrossRef]
- Mainiero, F.; Gismondi, A.; Soriani, A.; Cippitelli, M.; Palmieri, G.; Jacobelli, J.; Piccoli, M.; Frati, L.; Santoni, A. Integrin-mediated Ras–Extracellular Regulated Kinase (ERK) Signaling Regulates Interferon γ Production in Human Natural Killer Cells. J. Exp. Med. 1998, 188, 1267–1275. [Google Scholar] [CrossRef]
- Dong, X.; Akuetteh, P.D.P.; Song, J.; Ni, C.; Jin, C.; Li, H.; Jiang, W.; Si, Y.; Zhang, X.; Zhang, Q.; et al. Major Vault Protein (MVP) Associated With BRAFV600E Mutation Is an Immune Microenvironment-Related Biomarker Promoting the Progression of Papillary Thyroid Cancer via MAPK/ERK and PI3K/AKT Pathways. Front. Cell Dev. Biol. 2022, 9, 688370. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Huang, P. Regulation of CD137 expression through K-Ras signaling in pancreatic cancer cells. Cancer Commun. 2019, 39, 41. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; He, J.; Yuan, Z.; Wu, Z.; Liu, B.; Lin, X.; Guan, J. PLK1 contributes to autophagy by regulating MYC stabilization in osteosarcoma cells. OncoTargets Ther. 2019, 12, 7527–7536. [Google Scholar] [CrossRef]
- Chaillou, T.; Kirby, T.J.; McCarthy, J.J. Ribosome Biogenesis: Emerging Evidence for a Central Role in the Regulation of Skeletal Muscle Mass. J. Cell. Physiol. 2014, 229, 1584–1594. [Google Scholar] [CrossRef]
- Guo, Y.E.; Young, R. Abstract A166: Biogenesis and regulatory functions of super-enhancer RNAs in cancer cells of the immune system. Cancer Immunol. Res. 2016, 4, A166. [Google Scholar] [CrossRef]
- Demyanenko, S.; Sharifulina, S. The Role of Post-Translational Acetylation and Deacetylation of Signaling Proteins and Transcription Factors after Cerebral Ischemia: Facts and Hypotheses. Int. J. Mol. Sci. 2021, 22, 7947. [Google Scholar] [CrossRef]
- Zhang, W.-D.; Chen, H.-X.; Wang, Y.-X.; Chen, Z.-P.; Shan, Z.-J.; Xu, G. Bioinformatic analysis of c-Myc target from laryngeal cancer cell gene of laryngeal cancer. J. Cancer Res. Ther. 2016, 12, 58–61. [Google Scholar] [CrossRef]
- Pupa, S.M.; Ligorio, F.; Cancila, V.; Franceschini, A.; Tripodo, C.; Vernieri, C.; Castagnoli, L. HER2 Signaling and Breast Cancer Stem Cells: The Bridge behind HER2-Positive Breast Cancer Aggressiveness and Therapy Refractoriness. Cancers 2021, 13, 4778. [Google Scholar] [CrossRef]
- Kavarthapu, R.; Anbazhagan, R.; Dufau, M.L. Crosstalk between PRLR and EGFR/HER2 Signaling Pathways in Breast Cancer. Cancers 2021, 13, 4685. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, Y.; Sheng, S.; Yuan, H.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; et al. HER2 somatic mutations are associated with poor survival in HER2-negative breast cancers. Cancer Sci. 2017, 108, 671–677. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Zheng, Z.-Q.; Jia, S.C.; Liu, S.-N.; Xiao, X.-F.; Chen, G.-Y.; Liang, W.-Q.; Lu, X.-F. Trastuzumab resistance in HER2-positive breast cancer: Mechanisms, emerging biomarkers and targeting agents. Front. Oncol. 2022, 12, 1006429. [Google Scholar] [CrossRef] [PubMed]
- Villunger, A.; Xiao, Q.; Hu, Y.; Liu, Y.; Wang, Z.; Geng, H.; Hu, L.; Xu, D.; Wang, K.; Zheng, L.; et al. BEX1 Promotes Imatinib-Induced Apoptosis by Binding to and Antagonizing BCL-2. PLoS ONE 2014, 9, e91782. [Google Scholar] [CrossRef]
- Sudhakar, N.; George Priya Doss, C.; Thirumal Kumar, D.; Chakraborty, C.; Anand, K.; Suresh, M. Deciphering the impact of somatic mutations in exon 20 and exon 9 ofPIK3CAgene in breast tumors among Indian women through molecular dynamics approach. J. Biomol. Struct. Dyn. 2015, 34, 29–41. [Google Scholar] [CrossRef]
- Shi, X.; Qu, M.; Jin, X.; Liu, L.; Meng, F.; Shen, H. Relationship between TSHR, BRAF and PIK3CA gene copy number variations and thyroid nodules. Endocrine 2021, 73, 116–124. [Google Scholar] [CrossRef]
- Yi, Z.; Rong, G.; Guan, Y.; Li, J.; Chang, L.; Li, H.; Liu, B.; Wang, W.; Guan, X.; Ouyang, Q.; et al. Molecular landscape and efficacy of HER2-targeted therapy in patients with HER2-mutated metastatic breast cancer. NPJ Breast Cancer 2020, 6, 59. [Google Scholar] [CrossRef]
- Oh, D.Y.; Bang, Y.J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Engelman, J.A. ERBB receptors: From oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014, 25, 282–303. [Google Scholar] [CrossRef]
- Li, X.; Gu, X.; Xu, J.; Chen, L.; Li, H.; Meng, D.; Bai, H.; Yang, J.; Qian, J. Sustained Clinical Benefit of Pyrotinib Combined with Capecitabine Rescue Therapy After Trastuzumab Resistance in HER2-Positive Advanced Gastric Cancer: A Case Report. OncoTargets Ther. 2021, 14, 3983–3989. [Google Scholar] [CrossRef]
- Qin, S.; Ji, J.; Xu, R.-H.; Wang, W.; Tang, Y.; Bi, F.; Li, J.; Wang, K.; Xu, J.-m.; Fan, Q.; et al. Treatment Patterns and Outcomes in Chinese Patients with Gastric Cancer by HER2 Status: A Noninterventional Registry Study (EVIDENCE). Oncologist 2021, 26, e1567–e1580. [Google Scholar] [CrossRef]
- Sun, G.-Y.; Jing, H.; Wang, S.-L.; Song, Y.-W.; Jin, J.; Fang, H.; Liu, Y.-P.; Ren, H.; Tang, Y.; Zhao, X.-R.; et al. Trastuzumab Provides a Comparable Prognosis in Patients with HER2-Positive Breast Cancer to Those with HER2-Negative Breast Cancer: Post Hoc Analyses of a Randomized Controlled Trial of Post-Mastectomy Hypofractionated Radiotherapy. Front. Oncol. 2021, 10, 605750. [Google Scholar] [CrossRef]
- Li, B.T.; Ross, D.S.; Aisner, D.L.; Chaft, J.E.; Hsu, M.; Kako, S.L.; Kris, M.G.; Varella-Garcia, M.; Arcila, M.E. HER2 Amplification and HER2 Mutation Are Distinct Molecular Targets in Lung Cancers. J. Thorac. Oncol. 2016, 11, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, Z.; Wang, F.; Fu, L. KRAS mutation: From undruggable to druggable in cancer. Signal Transduct. Target. Ther. 2021, 6, 386. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Herrmann, R.; Christofori, G. Kras in metastatic colorectal cancer. Swiss. Med. Wkly. 2010, 140, w13112. [Google Scholar] [CrossRef]
- Tie, J.; Lipton, L.; Desai, J.; Gibbs, P.; Jorissen, R.N.; Christie, M.; Drummond, K.J.; Thomson, B.N.J.; Usatoff, V.; Evans, P.M.; et al. KRAS Mutation Is Associated with Lung Metastasis in Patients with Curatively Resected Colorectal Cancer. Clin. Cancer Res. 2011, 17, 1122–1130. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, C.; Zhang, R.; Yao, J.; Bo, M. Wild kras inhibit the migration and invasion of pancreatic cancer through Wnt/β-catenin pathway. 2022; preprint. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Zhang, J.; Liu, P.; Jiao, B.; Wang, Z.; Ren, R. Focal Adhesion Kinase (FAK) Inhibition Synergizes with KRAS G12C Inhibitors in Treating Cancer through the Regulation of the FAK–YAP Signaling. Adv. Sci. 2021, 8, 2100250. [Google Scholar] [CrossRef]
- Chida, K.; Kotani, D.; Masuishi, T.; Kawakami, T.; Kawamoto, Y.; Kato, K.; Fushiki, K.; Sawada, K.; Kumanishi, R.; Shirasu, H.; et al. The Prognostic Impact of KRAS G12C Mutation in Patients with Metastatic Colorectal Cancer: A Multicenter Retrospective Observational Study. Oncologist 2021, 26, 845–853. [Google Scholar] [CrossRef]
- Degirmenci, U.; Wang, M.; Hu, J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198. [Google Scholar] [CrossRef]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2016, 109, 314–341. [Google Scholar] [CrossRef]
- Guo, W.; Huang, J.; Shi, T.; Duan, H.; Chen, X.; Huang, Z. Genotypes of Papillary Thyroid Carcinoma With High Lateral Neck Metastasis in Chinese Population. Front. Oncol. 2022, 12, 816897. [Google Scholar] [CrossRef]
- Roden, A.C.; Hu, X.; Kip, S.; Parrilla Castellar, E.R.; Rumilla, K.M.; Vrana, J.A.; Vassallo, R.; Ryu, J.H.; Yi, E.S. BRAF V600E Expression in Langerhans Cell Histiocytosis. Am. J. Surg. Pathol. 2014, 38, 548–551. [Google Scholar] [CrossRef]
- Kim, C.H.; Choi, Y.J.; Choi, S.H.; Rho, M.-H.; Kook, S.-H.; Chung, E.C.; Chae, S.W.; Kim, D.-H.; Sohn, J.-H.; Yun, J.-S. Prevalence of the B Type Raf Kinase V600E Mutation in Cytologically Indeterminate Thyroid Nodules: Correlation with Ultrasonographic and Pathologic Features. J. Korean Soc. Radiol. 2012, 66, 17–26. [Google Scholar] [CrossRef][Green Version]
- Ernstoff, M.S. Been There, Not Done That—Melanoma in the Age of Molecular Therapy. N. Engl. J. Med. 2011, 364, 2547–2548. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yuan, X.; Du, R.; Cheung, S.-H.; Zhang, G.; Wei, J.; Zhao, Y.; Feng, Y.; Peng, H.; Zhang, Y.; et al. BGB-283, a Novel RAF Kinase and EGFR Inhibitor, Displays Potent Antitumor Activity in BRAF-Mutated Colorectal Cancers. Mol. Cancer Ther. 2015, 14, 2187–2197. [Google Scholar] [CrossRef]
- Kim, A.; Cohen, M.S. The discovery of vemurafenib for the treatment of BRAF-mutated metastatic melanoma. Expert Opin. Drug Discov. 2016, 11, 907–916. [Google Scholar] [CrossRef]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Finger, L.R.; Yunis, J.; Nowell, P.C.; Croce, C.M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 1984, 226, 1097–1099. [Google Scholar] [CrossRef]
- Delbridge, A.R.; Grabow, S.; Strasser, A.; Vaux, D.L. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer 2016, 16, 99–109. [Google Scholar] [CrossRef]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef]
- Montero, J.; Letai, A. Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ. 2018, 25, 56–64. [Google Scholar] [CrossRef]
- Davids, M.S. Targeting BCL-2 in B-cell lymphomas. Blood 2017, 130, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Macleod, K. Tumor suppressor genes. Curr. Opin. Genet. Dev. 2000, 10, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Malarkey, D.E.; Maronpot, R.R. Carcinogenesis. In Encyclopedia of Toxicology; Academic Press: Cambridge, MA, USA, 2005; pp. 445–466. [Google Scholar]
- Abreu Velez, A.M.; Howard, M.S. Tumor-suppressor Genes, Cell Cycle Regulatory Checkpoints, and the Skin. N. Am. J. Med. Sci. 2015, 7, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.L.; Podlevsky, J.D. Telomeres and Telomerase. In Encyclopedia of Cell Biology; Academic Press: Cambridge, MA, USA, 2016; pp. 418–425. [Google Scholar]
- Pyndiah, S.; Sakamuro, D. Restoration of tumor suppressor functions by small-molecule inhibitors. Mol. Cell Oncol. 2015, 2, e991225. [Google Scholar] [CrossRef][Green Version]
- Gao, L.; Wu, Z.-X.; Assaraf, Y.G.; Chen, Z.-S.; Wang, L. Overcoming anti-cancer drug resistance via restoration of tumor suppressor gene function. Drug Resist. Updates 2021, 57, 100770. [Google Scholar] [CrossRef] [PubMed]
- Zawacka-Pankau, J.E. The Undervalued Avenue to Reinstate Tumor Suppressor Functionality of the p53 Protein Family for Improved Cancer Therapy-Drug Repurposing. Cancers 2020, 12, 2717. [Google Scholar] [CrossRef]
- Thomas, A.F.; Kelly, G.L.; Strasser, A. Of the many cellular responses activated by TP53, which ones are critical for tumour suppression? Cell Death Differ. 2022, 29, 961–971. [Google Scholar] [CrossRef]
- Hernandez Borrero, L.J.; El-Deiry, W.S. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef]
- Demidenko, Z.N.; Korotchkina, L.G.; Gudkov, A.V.; Blagosklonny, M.V. Paradoxical suppression of cellular senescence by p53. Proc. Natl. Acad. Sci. USA 2010, 107, 9660–9664. [Google Scholar] [CrossRef]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef]
- Shi, D.; Jiang, P. A Different Facet of p53 Function: Regulation of Immunity and Inflammation During Tumor Development. Front. Cell Dev. Biol. 2021, 9, 762651. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, A.V.; Gurova, K.V.; Komarova, E.A. Inflammation and p53: A Tale of Two Stresses. Genes Cancer 2011, 2, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Pradhan, M.R.; Siau, J.W.; Kannan, S.; Nguyen, M.N.; Ouaray, Z.; Kwoh, C.K.; Lane, D.P.; Ghadessy, F.; Verma, C.S. Simulations of mutant p53 DNA binding domains reveal a novel druggable pocket. Nucleic Acids Res. 2019, 47, 1637–1652. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef]
- Zhang, S.; Carlsen, L.; Hernandez Borrero, L.; Seyhan, A.A.; Tian, X.; El-Deiry, W.S. Advanced Strategies for Therapeutic Targeting of Wild-Type and Mutant p53 in Cancer. Biomolecules 2022, 12, 548. [Google Scholar] [CrossRef]
- Wiman, K.G. Restoration of Wild-Type p53 Function in Human Tumors: Strategies for Efficient Cancer Therapy. Adv. Cancer Res. 2007, 97, 321–338. [Google Scholar]
- Chasov, V.; Zaripov, M.; Mirgayazova, R.; Khadiullina, R.; Zmievskaya, E.; Ganeeva, I.; Valiullina, A.; Rizvanov, A.; Bulatov, E. Promising New Tools for Targeting p53 Mutant Cancers: Humoral and Cell-Based Immunotherapies. Front. Immunol. 2021, 12, 707734. [Google Scholar] [CrossRef]
- Hassin, O.; Oren, M. Drugging p53 in cancer: One protein, many targets. Nat. Rev. Drug Discov. 2022, 22, 127–144. [Google Scholar] [CrossRef]
- Chasov, V.; Mirgayazova, R.; Zmievskaya, E.; Khadiullina, R.; Valiullina, A.; Stephenson Clarke, J.; Rizvanov, A.; Baud, M.G.J.; Bulatov, E. Key Players in the Mutant p53 Team: Small Molecules, Gene Editing, Immunotherapy. Front. Oncol. 2020, 10, 1460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Allis, C.D.; Wang, G.G. The Language of Chromatin Modification in Human Cancers. Nat. Rev. Cancer 2021, 21, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Freed-Pastor, W.A.; Prives, C. Mutant p53: One Name, Many Proteins. Genes Dev. 2012, 26, 1268–1286. [Google Scholar] [CrossRef]
- Wang, Z.; Strasser, A.; Kelly, G.L. Should Mutant TP53 Be Targeted for Cancer Therapy? Cell Death Differ. 2022, 29, 911–920. [Google Scholar] [CrossRef]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef]
- Jabar, A.; Jan, N.; Jan, I.; Mir, M.A. Genetic Alterations Affecting p53 and Emerging Perspectives on Epigenetic Control of p53 in Breast Cancer. In p53 in Breast Cancer: Molecular Mechanisms, Clinical Implications, and Therapeutic Targets; Mir, M.A., Ed.; CRC Press: Boca Raton, FL, USA, 2024; p. 27. [Google Scholar] [CrossRef]
- Wade, M.; Li, Y.C.; Wahl, G.M. MDM2, MDMX and p53 in Oncogenesis and Cancer Therapy. Nat. Rev. Cancer 2013, 13, 83–96. [Google Scholar] [CrossRef]
- Oliner, J.D.; Kinzler, K.W.; Meltzer, P.S.; George, D.L.; Vogelstein, B. Amplification of a Gene Encoding a p53-Associated Protein in Human Sarcomas. Nature 1992, 358, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Albadari, N.; Du, Y.; Fowler, J.F.; Sang, H.T.; Xian, W.; McKeon, F.; Li, W.; Zhou, J.; Zhang, R. MDM2 Inhibitors for Cancer Therapy: The Past, Present, and Future. Pharmacol. Rev. 2024, 76, 414–453. [Google Scholar] [CrossRef]
- Portman, N.; Milioli, H.H.; Alexandrou, S.; Coulson, R.; Yong, A.; Fernandez, K.J.; Chia, K.M.; Halilovic, E.; Segara, D.; Parker, A.; et al. MDM2 Inhibition in Combination with Endocrine Therapy and CDK4/6 Inhibition for the Treatment of ER-Positive Breast Cancer. Breast Cancer Res. 2020, 22, 87. [Google Scholar] [CrossRef]
- Dimaras, H.; Corson, T.W.; Cobrinik, D.; White, A.; Zhao, J.; Munier, F.L.; Abramson, D.H.; Shields, C.L.; Chantada, G.L.; Njuguna, F.; et al. Retinoblastoma. Nat. Rev. Dis. Primers 2015, 1, 15021. [Google Scholar] [CrossRef]
- Weinberg, R.A. The retinoblastoma protein and cell cycle control. Cell 1995, 81, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, L.; Hei, R.; Li, X.; Cai, H.; Wu, X.; Zheng, Q.; Cai, C. CDK inhibitors in cancer therapy, an overview of recent development. Am. J. Cancer Res. 2021, 11, 1913–1935. [Google Scholar] [PubMed]
- Diaz-Moralli, S.; Tarrado-Castellarnau, M.; Miranda, A.; Cascante, M. Targeting cell cycle regulation in cancer therapy. Pharmacol. Ther. 2013, 138, 255–271. [Google Scholar] [CrossRef]
- Dick, F.A.; Rubin, S.M. Molecular mechanisms underlying RB protein function. Nat. Rev. Mol. Cell Biol. 2013, 14, 297–306. [Google Scholar] [CrossRef]
- Chen, H.Z.; Tsai, S.Y.; Leone, G. Emerging roles of E2Fs in cancer: An exit from cell cycle control. Nat. Rev. Cancer 2009, 9, 785–797. [Google Scholar] [CrossRef]
- Park, J.A.; Ahn, J.W.; Kim, Y.K.; Kim, S.J.; Kim, J.K.; Kim, W.T.; Pai, H.S. Retinoblastoma protein regulates cell proliferation, differentiation, and endoreduplication in plants. Plant J. 2005, 42, 153–163. [Google Scholar] [CrossRef]
- Staedtke, V.; Topilko, P.; Le, L.Q.; Grimes, K.; Largaespada, D.A.; Cagan, R.L.; Steensma, M.R.; Stemmer-Rachamimov, A.; Blakeley, J.O.; Rhodes, S.D.; et al. Existing and Developing Preclinical Models for Neurofibromatosis Type 1−Related Cutaneous Neurofibromas. J. Investig. Dermatol. 2023, 143, 1378–1387. [Google Scholar] [CrossRef]
- Ko, J.M.; Sohn, Y.B.; Jeong, S.Y.; Kim, H.-J.; Messiaen, L.M. Mutation Spectrum of NF1 and Clinical Characteristics in 78 Korean Patients With Neurofibromatosis Type 1. Pediatr. Neurol. 2013, 48, 447–453. [Google Scholar] [CrossRef]
- Kiuru, M.; Busam, K.J. The NF1 gene in tumor syndromes and melanoma. Lab. Investig. 2017, 97, 146–157. [Google Scholar] [CrossRef]
- Zhang, L.; Theodoropoulos, P.C.; Eskiocak, U.; Wang, W.; Moon, Y.-A.; Posner, B.; Williams, N.S.; Wright, W.E.; Kim, S.B.; Nijhawan, D.; et al. Selective targeting of mutant adenomatous polyposis coli (APC) in colorectal cancer. Sci. Transl. Med. 2016, 8, 361ra140. [Google Scholar] [CrossRef]
- Zhang, L.; Shay, J.W. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2017, 109, djw332. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.; Näthke, I.S. Interactions and functions of the adenomatous polyposis coli (APC) protein at a glance. J. Cell Sci. 2013, 126, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Svitkina, T.M. Adenomatous Polyposis Coli (APC) in cell migration. Eur. J. Cell Biol. 2022, 101, 151228. [Google Scholar] [CrossRef]
- Advani, D.; Kumar, P. Uncovering Cell Cycle Dysregulations and Associated Mechanisms in Cancer and Neurodegenerative Disorders: A Glimpse of Hope for Repurposed Drugs. Mol. Neurobiol. 2024, 61, 8600–8630. [Google Scholar] [CrossRef]
- Wang, J.; Su, W.; Zhang, T.; Zhang, S.; Lei, H.; Ma, F.; Shi, M.; Shi, W.; Xie, X.; Di, C. Aberrant Cyclin D1 splicing in cancer: From molecular mechanism to therapeutic modulation. Cell Death Dis. 2023, 14, 244. [Google Scholar] [CrossRef]
- Sachdeva, P.; Ghosh, S.; Ghosh, S.; Han, S.; Banerjee, J.; Bhaskar, R.; Sinha, J.K. Childhood Obesity: A Potential Key Factor in the Development of Glioblastoma Multiforme. Life 2022, 12, 1673. [Google Scholar] [CrossRef]
- Sharaky, M.; El Kiki, S.M.; Effat, H.; Mansour, H.H. Effect of Palliative Radiotherapy and Cyclin-Dependent Kinase 4/6 Inhibitor on Breast Cancer Cell Lines. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025; advance online publication. [Google Scholar] [CrossRef]
- Hume, S.; Dianov, G.L.; Ramadan, K. A unified model for the G1/S cell cycle transition. Nucleic Acids Res. 2020, 48, 12483–12501. [Google Scholar] [CrossRef]
- Bertoli, C.; Skotheim, J.M.; de Bruin, R.A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef]
- Amani, J.; Gorjizadeh, N.; Younesi, S.; Najafi, M.; Ashrafi, A.M.; Irian, S.; Gorjizadeh, N.; Azizian, K. Cyclin-dependent kinase inhibitors (CDKIs) and the DNA damage response: The link between signaling pathways and cancer. DNA Repair 2021, 102, 103103. [Google Scholar] [CrossRef]
- Wesierska-Gadek, J.; Mauritz, M. Why (multi)targeting of cyclin-dependent kinases is a promising therapeutic option for hormone-positive breast cancer and beyond. Future Med. Chem. 2016, 8, 55–72. [Google Scholar] [CrossRef]
- Schraml, P.; Bucher, C.; Bissig, H.; Nocito, A.; Haas, P.; Wilber, K.; Seelig, S.; Kononen, J.; Mihatsch, M.J.; Dirnhofer, S.; et al. Cyclin E overexpression and amplification in human tumours. J. Pathol. 2003, 200, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Jorge, J.; Lapa, B.S.; Ribeiro, I.P.; Teles, P.; Góis, I.N.; Santos, L.; Gomes, C.; Ribeiro, A.B.S.; Gonçalves, A.C. Altered cell cycle regulation in the development of resistance. In Resistance in Hematologic Malignancies and Cancer; Elsevier: Amsterdam, The Netherlands, 2025; pp. 193–213. [Google Scholar]
- Nenclares, P.; Harrington, K.J. The biology of cancer. Medicine 2020, 48, 67–72. [Google Scholar] [CrossRef]
- Reynolds, M.R.; Lane, A.N.; Robertson, B.; Kemp, S.; Liu, Y.; Hill, B.G.; Dean, D.C.; Clem, B.F. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene 2013, 33, 556–566. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Kanwal, R.; Gupta, S. Epigenetic modifications in cancer. Clin. Genet. 2011, 81, 303–311. [Google Scholar] [CrossRef]
- Anderson, M.W.; Reynolds, S.H.; You, M.; Maronpot, R.M. Role of proto-oncogene activation in carcinogenesis. Environ. Health Perspect. 1992, 98, 13–24. [Google Scholar] [CrossRef]
- Yip, H.Y.K.; Papa, A. Signaling Pathways in Cancer: Therapeutic Targets, Combinatorial Treatments, and New Developments. Cells 2021, 10, 659. [Google Scholar] [CrossRef]
- Delire, B.; Starkel, P. The Ras/MAPK pathway and hepatocarcinoma: Pathogenesis and therapeutic implications. Eur. J. Clin. Investig. 2015, 45, 609–623. [Google Scholar] [CrossRef]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef]
- Reis, M.; Liebner, S. Wnt signaling in the vasculature. Exp. Cell Res. 2013, 319, 1317–1323. [Google Scholar] [CrossRef]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Barton, M.C. p53: Emerging roles in stem cells, development and beyond. Development 2018, 145, dev158360. [Google Scholar] [CrossRef]
- Bouaoun, L.; Sonkin, D.; Ardin, M.; Hollstein, M.; Byrnes, G.; Zavadil, J.; Olivier, M. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum. Mutat. 2016, 37, 865–876. [Google Scholar] [CrossRef]
- Drosten, M.; Sum, E.Y.M.; Lechuga, C.G.; Simón-Carrasco, L.; Jacob, H.K.C.; García-Medina, R.; Huang, S.; Beijersbergen, R.L.; Bernards, R.; Barbacid, M. Loss of p53 induces cell proliferation via Ras-independent activation of the Raf/Mek/Erk signaling pathway. Proc. Natl. Acad. Sci. USA 2014, 111, 15155–15160. [Google Scholar] [CrossRef]
- Cooks, T.; Pateras, I.S.; Tarcic, O.; Solomon, H.; Schetter, A.J.; Wilder, S.; Lozano, G.; Pikarsky, E.; Forshew, T.; Rosenfeld, N.; et al. Mutant p53 prolongs NF-kappaB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 2013, 23, 634–646. [Google Scholar] [CrossRef]
- Manchester, K.L. Theodor Boveri and the origin of malignant tumours. Trends Cell Biol. 1995, 5, 384–387. [Google Scholar] [CrossRef]
- Niu, Y.; Jin, Y.; Deng, S.C.; Deng, S.J.; Zhu, S.; Liu, Y.; Li, X.; He, C.; Liu, M.L.; Zeng, Z.; et al. MiRNA-646-mediated reciprocal repression between HIF-1alpha and MIIP contributes to tumorigenesis of pancreatic cancer. Oncogene 2018, 37, 1743–1758. [Google Scholar] [CrossRef]
- Szybowska, P.; Kostas, M.; Wesche, J.; Wiedlocha, A.; Haugsten, E.M. Cancer Mutations in FGFR2 Prevent a Negative Feedback Loop Mediated by the ERK1/2 Pathway. Cells 2019, 8, 518. [Google Scholar] [CrossRef]
- di Magliano, M.P.; Logsdon, C.D. Roles for KRAS in Pancreatic Tumor Development and Progression. Gastroenterology 2013, 144, 1220–1229. [Google Scholar] [CrossRef]
- Park, J.S.; Lim, M.A.; Cho, M.L.; Ryu, J.G.; Moon, Y.M.; Jhun, J.Y.; Byun, J.K.; Kim, E.K.; Hwang, S.Y.; Ju, J.H.; et al. p53 Controls Autoimmune Arthritis via STAT-Mediated Regulation of the Th17 Cell/Treg Cell Balance in Mice. Arthritis Rheum. 2013, 65, 949–959. [Google Scholar] [CrossRef]
- Schulz-Heddergott, R.; Stark, N.; Edmunds, S.J.; Li, J.; Conradi, L.-C.; Bohnenberger, H.; Ceteci, F.; Greten, F.R.; Dobbelstein, M.; Moll, U.M. Therapeutic Ablation of Gain-of-Function Mutant p53 in Colorectal Cancer Inhibits Stat3-Mediated Tumor Growth and Invasion. Cancer Cell 2018, 34, 298–314.e297. [Google Scholar] [CrossRef] [PubMed]
- Elemento, O.; Leslie, C.; Lundin, J.; Tourassi, G. Artificial Intelligence in Cancer Research, Diagnosis and Therapy. Nat. Rev. Cancer 2021, 21, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Feretzakis, G.; Juliebø-Jones, P.; Tsaturyan, A.; Sener, T.E.; Verykios, V.S.; Karapiperis, D.; Bellos, T.; Katsimperis, S.; Angelopoulos, P.; Varkarakis, I.; et al. Emerging Trends in AI and Radiomics for Bladder, Kidney, and Prostate Cancer: A Critical Review. Cancers 2024, 16, 810. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Lemus, E.; Ochoa, S. Methods for Multi-Omic Data Integration in Cancer Research. Front. Genet. 2024, 15, 1425456. [Google Scholar] [CrossRef]
- Kather, J.N.; Pearson, A.T.; Halama, N.; Jäger, D.; Krause, J.; Loosen, S.H.; Marx, A.; Boor, P.; Tacke, F.; Neumann, U.P.; et al. Deep Learning Can Predict Microsatellite Instability Directly from Histology in Gastrointestinal Cancer. Nat. Med. 2019, 25, 1054–1056. [Google Scholar] [CrossRef]
- Lu, M.Y.; Williamson, D.F.K.; Chen, T.Y.; Chen, R.J.; Barbieri, M.; Mahmood, F. Data-Efficient and Weakly Supervised Computational Pathology on Whole-Slide Images. Nat. Biomed. Eng. 2021, 5, 555–570. [Google Scholar] [CrossRef]
- Zhavoronkov, A.; Ivanenkov, Y.A.; Aliper, A.; Veselov, M.S.; Aladinskiy, V.A.; Aladinskaya, A.V.; Terentiev, V.A.; Polykovskiy, D.A.; Kuznetsov, M.D.; Asadulaev, A.; et al. Deep Learning Enables Rapid Identification of Potent DDR1 Kinase Inhibitors. Nat. Biotechnol. 2019, 37, 1038–1040. [Google Scholar] [CrossRef]
- Stokes, J.M.; Yang, K.; Swanson, K.; Jin, W.; Cubillos-Ruiz, A.; Donghia, N.M.; MacNair, C.R.; French, S.; Carfrae, L.A.; Bloom-Ackermann, Z.; et al. A Deep Learning Approach to Antibiotic Discovery. Cell 2020, 180, 688–702.e13. [Google Scholar] [CrossRef]
- Pagano, D.; Barresi, V.; Tropea, A.; Galvano, A.; Bazan, V.; Caldarella, A.; Sani, C.; Pompeo, G.; Russo, V.; Liotta, R.; et al. Clinical Validation of a Machine Learning-Based Biomarker Signature to Predict Response to Cytotoxic Chemotherapy Alone or Combined with Targeted Therapy in Metastatic Colorectal Cancer Patients: A Study Protocol and Review. Life 2025, 15, 320. [Google Scholar] [CrossRef]
- Sheller, M.J.; Edwards, B.; Reina, G.A.; Martin, J.; Pati, S.; Kotrotsou, A.; Milchenko, M.; Xu, W.; Marcus, D.; Colen, R.R.; et al. Federated Learning in Medicine: Facilitating Multi-Institutional Collaborations Without Sharing Patient Data. Sci. Rep. 2020, 10, 12598. [Google Scholar] [CrossRef]
- Youssef, E.; Fletcher, B.; Palmer, D. Enhancing precision in cancer treatment: The role of gene therapy and immune modulation in oncology. Front. Med. 2025, 11, 1527600. [Google Scholar] [CrossRef] [PubMed]
- Labrie, M.; Brugge, J.S.; Mills, G.B.; Zervantonakis, I.K. Therapy resistance: Opportunities created by adaptive responses to targeted therapies in cancer. Nat. Rev. Cancer 2022, 22, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.A.; AboulMagd, A.M.; Abbas, S.H.; Abdel-Rahman, H.M.; Abdel-Aziz, M. Insights into fourth generation selective inhibitors of (C797S) EGFR mutation combating non-small cell lung cancer resistance: A critical review. RSC Adv. 2023, 13, 18825–18853. [Google Scholar] [CrossRef] [PubMed]
- Katayama, R. Drug resistance in anaplastic lymphoma kinase-rearranged lung cancer. Cancer Sci. 2018, 109, 572–580. [Google Scholar] [CrossRef]
- Al-Lazikani, B.; Banerji, U.; Workman, P. Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 2012, 30, 679–692. [Google Scholar] [CrossRef]
- Coleman, N.; Hong, L.; Zhang, J.; Heymach, J.; Hong, D.; Le, X. Beyond epidermal growth factor receptor: MET amplification as a general resistance driver to targeted therapy in oncogene-driven non-small-cell lung cancer. ESMO Open 2021, 6, 100319. [Google Scholar] [CrossRef]
- Chae, Y.K.; Chang, S.; Ko, T.; Anker, J.; Agte, S.; Iams, W.; Choi, W.M.; Lee, K.; Cruz, M. Epithelial-mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci. Rep. 2018, 8, 2918. [Google Scholar] [CrossRef]
- Li, Y.R.; Halladay, T.; Yang, L. Immune evasion in cell-based immunotherapy: Unraveling challenges and novel strategies. J. Biomed. Sci. 2024, 31, 5. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Rek, M.M.; Karpowicz, K.; Górska, M.; Polityńska, B.; Wojtukiewicz, A.M.; Moniuszko, M.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Inhibitors of immune checkpoints-PD-1, PD-L1, CTLA-4-new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021, 40, 949–982. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Vidotto, T.; Melo, C.M.; Castelli, E.; Koti, M.; Dos Reis, R.B.; Squire, J.A. Emerging role of PTEN loss in evasion of the immune response to tumors. Br. J. Cancer 2020, 122, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, J.H.; Chao, S.X.; Pelka, K.; Giannakis, M.; Hess, J.; Burke, K.; Jorgji, V.; Sindurakar, P.; Braverman, J.; et al. Combined PD-1, BRAF and MEK inhibition in BRAFV600E colorectal cancer: A phase 2 trial. Nat. Med. 2023, 29, 458–466. [Google Scholar] [CrossRef] [PubMed]
| Gene | Main Functions | Mechanism of Action | Signaling Pathways Involved | Role in Normal Development | Regulation | Interactions with Other Genes/Proteins | Protein(s) Structural Features | Frequent Mutations | Associated Cancer Types | Clinical Significance |
|---|---|---|---|---|---|---|---|---|---|---|
| RAS | Regulates cell growth, proliferation, differentiation, and survival [27]; acts as a molecular switch in various cellular functions [28] | Cycles between active GTP-bound and inactive GDP-bound states [28]; activates downstream effector pathways when in GTP-bound form [27] | MAPK [27], PI3K [29] | Controls signaling pathways regulating cellular functions [27]; involved in growth control, protein biosynthesis, and membrane traffic [28] | Regulated by GTP/GDP exchange factors [28]; controlled by GTPase-activating proteins (GAPs) [28] | Forms complexes with Shc, Grb2, and proline-rich tyrosine kinase [30]; interacts with PI3K and Raf kinases [27] | Contains a GTPase domain functioning as a molecular switch [28]; includes conserved structural core common to GTPase domains [28] | Oncogenic mutations present in almost 25% of human cancers [27]; BRAF V600E mutation associated with RAS signaling in some cancers [31] | Colorectal cancer [29]; papillary thyroid cancer (associated with BRAF V600E mutation) [31]; pancreatic cancer [32] | Crucial target for cancer therapy due to its involvement in multiple signaling pathways [27]; RAS mutations can affect treatment response and prognosis in colorectal cancer [29] |
| MYC | Modulates transcription of thousands of genes; coordinates cellular processes essential for growth, proliferation, differentiation, self-renewal, and apoptosis [33] | Acts as a transcription factor; forms complexes with other protein to control gene expression | Wnt/β-catenin [34], mTORC1 [34] | Regulates ribosome biogenesis [34]; controls cell identity and disease [35] | Regulated by post-translational modifications, including acetylation and deacetylation [36]; protein stability controlled by PLK1 [33] | Interacts with PLK1, which contributes to MYC protein stabilization [33]; forms complexes with transcription factors and co-activators at super-enhancers [35] | Contains DNA-binding domains and protein–protein interaction regions; subject to post-translational modifications affecting its function and stability | Overexpression of MYC is a hallmark of many human cancers [33]; mutations in MYC regulatory regions can lead to its dysregulation | Multiple myeloma [35]; osteosarcoma [33]; laryngeal cancer [37] | High MYC expression often correlates with poor prognosis in cancer [33]; serves as a potential biomarker and therapeutic target in various cancers |
| EGFR | Regulates cell growth, proliferation, differentiation, and survival; mediates signal transduction in response to growth factors | Activates upon binding of ligands like EGF; undergoes dimerization and autophosphorylation, triggering downstream signaling cascades | MAPK/ERK, PI3K/AKT, STAT | Crucial for embryonic development and tissue homeostasis; involved in the development of various organs, including the brain | Controlled by ligand availability and receptor internalization; regulated by post-translational modifications and protein–protein interactions | Forms complexes with proteins like Grb2 and SOS; interacts with SRC tyrosine kinase upon activation | Consists of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain; contains multiple phosphorylation sites in the intracellular domain | Overexpression or activating mutations common in various cancers; EGFR gene amplification observed in some tumors | Non-small cell lung cancer; glioblastoma; head and neck squamous cell carcinoma | EGFR status used as a prognostic and predictive biomarker in cancer; overexpression often correlates with poor prognosis and treatment resistance |
| HER2 | Regulates cell growth, proliferation, differentiation, and survival [38]; plays a role in mammary gland development and breast carcinogenesis [39] | Activates upon dimerization with other HER family members; triggers downstream signaling cascades through autophosphorylation [38] | MAPK/ERK, PI3K/AKT, STAT | Crucial for mammary gland development [39]; involved in embryonic development and tissue homeostasis | Controlled by ligand availability and receptor internalization; regulated by post-translational modifications and protein–protein interactions | Forms complexes with other HER family members; interacts with prolactin receptor (PRLR) signaling pathways [39] | Consists of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain; exists in full-length, splice variant (d16HER2), and truncated (p95HER2) forms [38] | HER2 somatic mutations occur in about 2% of breast cancers [40]; ERBB2 mutations can lead to trastuzumab resistance [41] | HER2-positive breast cancer (15–20% of breast cancers) [38]; some gastric and gastroesophageal cancers | HER2 overexpression/amplification is associated with poor survival in breast cancer patients [40]; used as a prognostic and predictive biomarker in cancer |
| BCL-ABL | Promotes cell proliferation and survival; inhibits apoptosis in leukemic cells | Constitutively active tyrosine kinase; activates multiple signaling pathways, promoting cell growth and survival | MAPK/ERK, PI3K/AKT STAT | Not present in normal development; result of chromosomal translocation | Regulated by post-translational modifications; controlled by protein–protein interactions | Interacts with BCL-2, potentially influencing apoptosis regulation [42]; forms complexes with various signaling proteins | Contains an intrinsically disordered region essential for protein function and stability; includes the tyrosine kinase domain from ABL and regulatory domains from BCR | The fusion itself is the primary mutation; secondary mutations can occur, leading to drug resistance | Chronic myeloid leukemia (CML); some cases of acute lymphoblastic leukemia (ALL) | Presence of BCR-ABL is diagnostic for CML; used as a target for monitoring treatment response and disease progression |
| BRAF | Regulates cell growth, proliferation, and survival; mediates cellular responses to growth signals | Activates the MEK-ERK signaling cascade; phosphorylates downstream targets to promote cell proliferation | MAPK/ERK, PI3K/AKT (indirectly) | Essential for embryonic development; involved in cell differentiation and organ development | Activated by RAS proteins; regulated by phosphorylation and protein–protein interactions | Interacts with MEK1/2, its primary downstream targets; Forms complexes with scaffold proteins like KSR | Contains a kinase domain and regulatory regions; includes an activation segment that regulates kinase activity | V600E mutation accounts for about 90% of BRAF mutations in cancer; other mutations include V600K, V600R, and K601E | Melanoma (40–60% of cases); colorectal cancer (5–10% of cases); papillary thyroid cancer (40–45% of cases) | BRAF mutation status is a prognostic and predictive biomarker in several cancers; used to guide treatment decisions, particularly in melanoma |
| PIK3CA | Regulates cell growth, proliferation, survival, and migration; generates 3′-phosphoinositides that activate various cellular targets | Catalyzes the production of phosphatidylinositol 3,4,5-trisphosphate (PIP3); activates downstream signaling cascades through PIP3 generation | PI3K/AKT/mTOR, MAPK (indirectly) | Essential for embryonic development; involved in cell differentiation and organ development | Controlled by growth factor receptor tyrosine kinases; regulated by PTEN, a tumor suppressor that counteracts PI3K activity | Interacts with regulatory subunits of PI3K; forms complexes with RAS proteins | Contains a kinase domain and regulatory regions; includes hotspot mutation sites in the helical and kinase domains | Hotspot mutations include E542K, E545K (helical domain), and H1047R (kinase domain) [43]; mutations can lead to constitutive activation of the PI3K pathway | Breast cancer; CRC; thyroid cancer [44] | PIK3CA mutations serve as prognostic and predictive biomarkers in various cancers; mutation status guides treatment decisions, particularly in breast cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.R.; Bhaskar, R.; Ghosh, S.; Yarlagadda, B.; Singh, K.K.; Verma, P.; Sengupta, S.; Mladenov, M.; Hadzi-Petrushev, N.; Stojchevski, R.; et al. Exploring the Genetic Orchestra of Cancer: The Interplay Between Oncogenes and Tumor-Suppressor Genes. Cancers 2025, 17, 1082. https://doi.org/10.3390/cancers17071082
Singh SR, Bhaskar R, Ghosh S, Yarlagadda B, Singh KK, Verma P, Sengupta S, Mladenov M, Hadzi-Petrushev N, Stojchevski R, et al. Exploring the Genetic Orchestra of Cancer: The Interplay Between Oncogenes and Tumor-Suppressor Genes. Cancers. 2025; 17(7):1082. https://doi.org/10.3390/cancers17071082
Chicago/Turabian StyleSingh, Sajal Raj, Rakesh Bhaskar, Shampa Ghosh, Bhuvaneshwar Yarlagadda, Krishna Kumar Singh, Prashant Verma, Sonali Sengupta, Mitko Mladenov, Nikola Hadzi-Petrushev, Radoslav Stojchevski, and et al. 2025. "Exploring the Genetic Orchestra of Cancer: The Interplay Between Oncogenes and Tumor-Suppressor Genes" Cancers 17, no. 7: 1082. https://doi.org/10.3390/cancers17071082
APA StyleSingh, S. R., Bhaskar, R., Ghosh, S., Yarlagadda, B., Singh, K. K., Verma, P., Sengupta, S., Mladenov, M., Hadzi-Petrushev, N., Stojchevski, R., Sinha, J. K., & Avtanski, D. (2025). Exploring the Genetic Orchestra of Cancer: The Interplay Between Oncogenes and Tumor-Suppressor Genes. Cancers, 17(7), 1082. https://doi.org/10.3390/cancers17071082









