Guidance for the Clinical Use of the Breast Cancer Polygenic Risk Scores
Simple Summary
Abstract
1. Introduction
2. Background
2.1. Breast Cancer Prevention and Screening
2.2. Monogenic Breast Cancer Risk
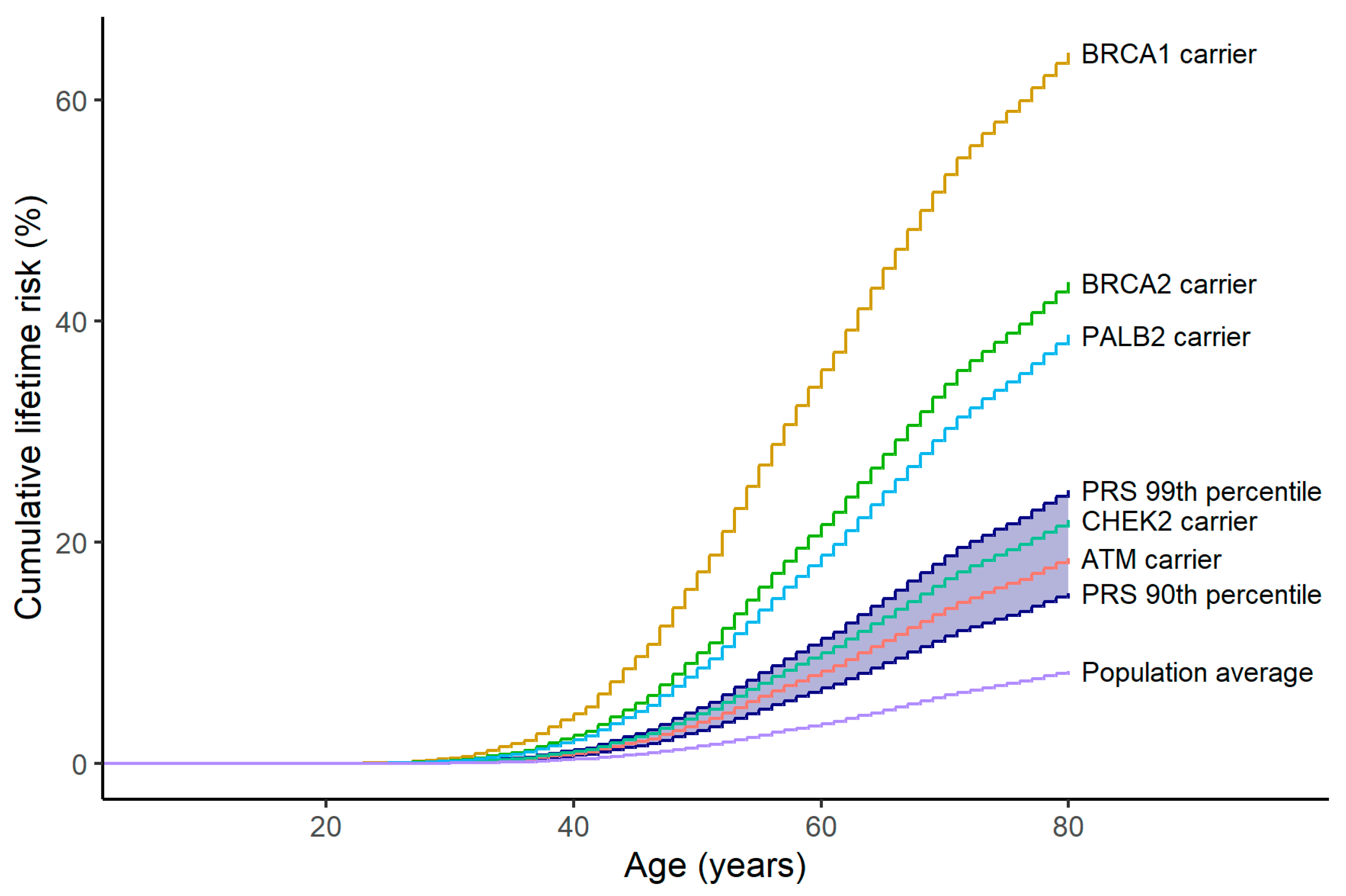
2.3. Polygenic Breast Cancer Risk
2.4. Possibilities to Combine Breast Cancer PRSs with Other Risk Factors
3. Utilising Breast Cancer Polygenic Risk Scores in Clinical Practice
- (1)
- (2)
- (3)
3.1. Personalised Breast Cancer Risk-Based Management of Cancer-Free Women with a Family History of Cancer in Hereditary Cancer Clinics
3.1.1. Women with Negative Breast Cancer MPV Test Findings
3.1.2. Women with Breast Cancer MPV Findings
3.2. Individual Personalised Breast Cancer Prevention and Screening
3.3. Enhancement of Systematic Public Breast Cancer Screening Programs
- Women aged 40–44: no screening;
- Women aged 45–49: screening every 2 or 3 years;
- Women aged 50–69: screening every 2 years;
- Women aged 70–74: screening every 3 years.
4. Possibilities for Clinical Recommendations for Personalised Prevention and Screening of Breast Cancer Based on PRS Results
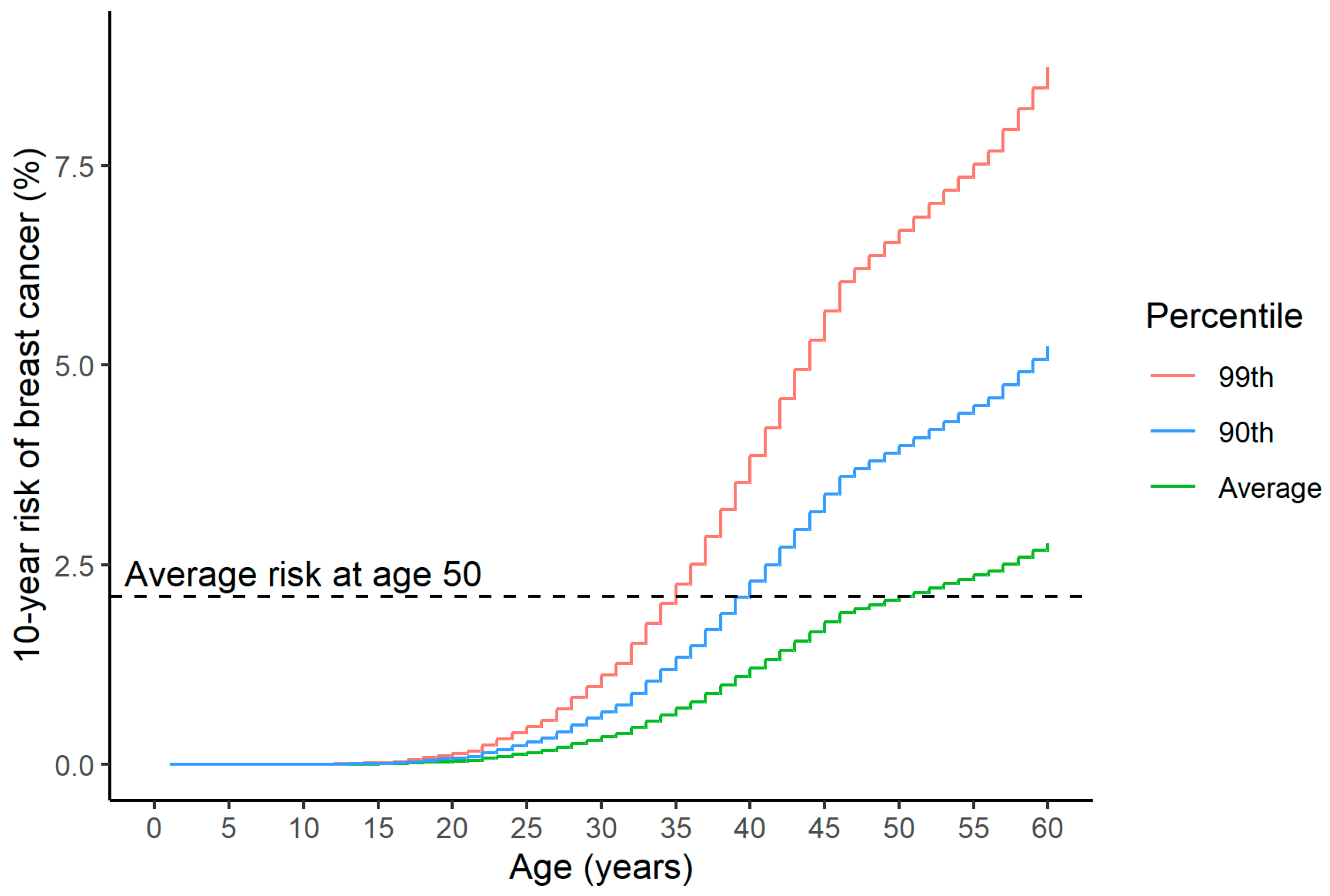
4.1. Comparison with the Average Risk of the Same Population at the Same Age, Combined with a Comparison to the Average Risk upon Initiation of Mammographic Screening
4.2. Comparison with Similar Risk MPVs
4.3. Comparison with Already Existing National Guidelines Based on Other Risk Factors (Not Including PRSs) for Risk-Stratified Breast Cancer Screening According to Different Risk Levels
4.3.1. Guidelines in the United Kingdom
- General population risk: 1.–79. percentiles;
- Moderate risk: 80.–97. percentiles;
- High risk: 98.–99. percentiles.
4.3.2. Guidelines in Germany
4.3.3. Guidelines in Norway
4.3.4. Guidelines in Sweden
4.3.5. Guidelines in Portugal
4.3.6. Guidelines in Estonia
5. The Regulatory and Legal Status of Breast Cancer Risk Estimation Tools in the European Union in the Context of Polygenic Risk Score Testing
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abu-El-Haija, A.; Reddi, H.V.; Wand, H.; Rose, N.C.; Mori, M.; Qian, E.; Murray, M.F.; Practice, A.P.; The ACMG Professional Practice and Guidelines Committee. The clinical application of polygenic risk scores: A points to consider statement of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. Off. J. Am. Coll. Med. Genet. 2023, 25, 100803. [Google Scholar] [CrossRef] [PubMed]
- AnteNOR Project. Available online: https://antegenes.com/antenor/ (accessed on 1 October 2024).
- BRIGHT Project. Available online: https://brightscreening.eu (accessed on 1 October 2024).
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018; Available online: https://gco.iarc.fr/today (accessed on 15 September 2024).
- NCCN Clinical Practice Guidelines in Oncology. Breast Cancer Risk Reduction. Version 2. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf (accessed on 30 May 2024).
- Myers, E.R.; Moorman, P.; Gierisch, J.M.; Havrilesky, L.J.; Grimm, L.J.; Ghate, S.; Davidson, B.; Mongtomery, R.C.; Crowley, M.J.; McCrory, D.C.; et al. Benefits and Harms of Breast Cancer Screening. JAMA 2015, 314, 1615–1634. [Google Scholar] [CrossRef] [PubMed]
- Tabár, L.; Dean, P.B.; Chen, T.H.-H.; Yen, A.M.-F.; Chen, S.L.-S.; Fann, J.C.-Y.; Chiu, S.Y.-H.; Ku, M.M.-S.; Wu, W.Y.-Y.; Hsu, C.-Y.; et al. The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening. Cancer 2018, 125, 515–523. [Google Scholar] [CrossRef]
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: An independent review. Lancet 2012, 380, 1778–1786. [Google Scholar] [CrossRef]
- WHO Position Paper on Mammography Screening; WHO Guidelines Approved by the Guidelines Review Committee; WHO: Geneva, Switzerland, 2014.
- European Guidelines on Breast Cancer Screening and Diagnosis. Available online: https://healthcare-quality.jrc.ec.europa.eu/ecibc/european-breast-cancer-guidelines (accessed on 30 August 2024).
- Schousboe, J.T.; Kerlikowske, K.; Loh, A.; Cummings, S.R. Personalizing mammography by breast density and other risk factors for breast cancer: Analysis of health benefits and cost-effectiveness. Ann. Intern. Med. 2011, 155, 10–20. [Google Scholar] [CrossRef]
- Shieh, Y.; Eklund, M.; Sawaya, G.F.; Black, W.C.; Kramer, B.S.; Esserman, L.J. Population-based screening for cancer: Hope and hype. Nat. Rev. Clin. Oncol. 2016, 13, 550–565. [Google Scholar] [CrossRef]
- Mucci, L.A.; Hjelmborg, J.B.; Harris, J.R.; Czene, K.; Havelick, D.J.; Scheike, T.; Graff, R.E.; Holst, K.; Moller, S.; Unger, R.H.; et al. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA 2016, 315, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, P.; Fostira, F. Hereditary breast cancer: The era of new susceptibility genes. BioMed Res. Int. 2013, 2013, 747318. [Google Scholar] [CrossRef]
- Rowlands, C.F.; Allen, S.; Balmaña, J.; Domchek, S.M.; Evans, D.G.; Hanson, H.; Hoogerbrugge, N.; James, P.A.; Nathanson, K.L.; Robson, M.; et al. Population-based germline breast cancer gene association studies and meta-analysis to inform wider mainstream testing. Ann. Oncol. 2024, 35, 892–901. [Google Scholar] [CrossRef]
- Ghoussaini, M.; Pharoah, P.D. Polygenic susceptibility to breast cancer: Current state-of-the-art. Future Oncol. 2009, 5, 689–701. [Google Scholar] [CrossRef]
- Mavaddat, N.; Pharoah, P.D.; Michailidou, K.; Tyrer, J.; Brook, M.N.; Bolla, M.K.; Wang, Q.; Dennis, J.; Dunning, A.M.; Shah, M.; et al. Prediction of breast cancer risk based on profiling with common genetic variants. J. Natl. Cancer Inst. 2015, 107, djv036. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Association Consortium. Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439. [Google Scholar] [CrossRef]
- Hu, C.; Hart, S.N.; Gnanaolivu, R.; Huang, H.; Lee, K.Y.; Na, J.; Gao, C.; Lilyquist, J.; Yadav, S.; Boddicker, N.J.; et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N. Engl. J. Med. 2021, 384, 440–451. [Google Scholar] [CrossRef]
- Foulkes, W.D. The ten genes for breast (and ovarian) cancer susceptibility. Nat. Rev. Clin. Oncol. 2021, 18, 259–260. [Google Scholar] [CrossRef]
- McDevitt, T.; Durkie, M.; Arnold, N.; Burghel, G.J.; Butler, S.; Claes, K.B.M.; Logan, P.; Robinson, R.; Sheils, K.; Wolstenholme, N.; et al. EMQN best practice guidelines for genetic testing in hereditary breast and ovarian cancer. Eur. J. Hum. Genet. 2024, 32, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Familial Breast Cancer: Classification, Care and Managing Breast Cancer and Related Risks in People with a Family History of Breast Cancer. Available online: https://www.nice.org.uk/guidance/cg164/chapter/recommendations#breast-cancer-risk-category (accessed on 11 June 2017).
- Sessa, C.; Balmana, J.; Bober, S.L.; Cardoso, M.J.; Colombo, N.; Curigliano, G.; Domchek, S.M.; Evans, D.G.; Fischerova, D.; Harbeck, N.; et al. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Wappenschmidt, B.; Hauke, J.; Faust, U.; Niederacher, D.; Wiesmuller, L.; Schmidt, G.; Gross, E.; Gehrig, A.; Sutter, C.; Ramser, J.; et al. Criteria of the German Consortium for Hereditary Breast and Ovarian Cancer for the Classification of Germline Sequence Variants in Risk Genes for Hereditary Breast and Ovarian Cancer. Geburtshilfe Frauenheilkd 2020, 80, 410–429. [Google Scholar] [CrossRef]
- Rhiem, K.; Auber, B.; Briest, S.; Dikow, N.; Ditsch, N.; Dragicevic, N.; Grill, S.; Hahnen, E.; Horvath, J.; Jaeger, B.; et al. Consensus Recommendations of the German Consortium for Hereditary Breast and Ovarian Cancer. Breast Care 2022, 17, 199–207. [Google Scholar] [CrossRef]
- Bröstcancer. Nationellt Vårdprogram. 2024-02-07. Version: 4.4. Available online: https://kunskapsbanken.cancercentrum.se/globalassets/cancerdiagnoser/brost/vardprogram/nationellt-vardprogram-brostcancer.pdf (accessed on 7 February 2024).
- NHS National Genomic Test Directory. Testing Criteria for Rare and Inherited Disease. Version 6. 2024. Available online: https://www.england.nhs.uk/wp-content/uploads/2024/07/national-genomic-test-directory-rare-and-inherited-disease-eligibility-criteria-v7.pdf (accessed on 8 June 2024).
- Reddi, H.V.; Wand, H.; Funke, B.; Zimmermann, M.T.; Lebo, M.S.; Qian, E.; Shirts, B.H.; Zou, Y.S.; Zhang, B.M.; Rose, N.C.; et al. Laboratory perspectives in the development of polygenic risk scores for disease: A points to consider statement of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. Off. J. Am. Coll. Med. Genet. 2023, 25, 100804. [Google Scholar] [CrossRef]
- Mavaddat, N.; Michailidou, K.; Dennis, J.; Lush, M.; Fachal, L.; Lee, A.; Tyrer, J.P.; Chen, T.H.; Wang, Q.; Bolla, M.K.; et al. Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. Am. J. Hum. Genet. 2019, 104, 21–34. [Google Scholar] [CrossRef]
- Michailidou, K.; Lindstrom, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemacon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Sieh, W.; Rothstein, J.H.; McGuire, V.; Whittemore, A.S. The role of genome sequencing in personalized breast cancer prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2322–2327. [Google Scholar] [CrossRef]
- Hughes, E.; Judkins, T.; Wagner, S.; Wenstrup, R.J.; Lanchbury, J.S.; Gutin, A. Development and validation of a residual risk score to predict breast cancer risk in unaffected women negative for mutations on a multi-gene hereditary cancer panel. J. Clin. Oncol. 2017, 35, 1579. [Google Scholar] [CrossRef]
- Khera, A.V.; Chaffin, M.; Aragam, K.G.; Haas, M.E.; Roselli, C.; Choi, S.H.; Natarajan, P.; Lander, E.S.; Lubitz, S.A.; Ellinor, P.T.; et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 2018, 50, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, M.; Gribble, S.; Pashayan, N.; Easton, D.F.; Antoniou, A.C.; Lee, A.; van Katwyk, S.; Simard, J. Potential of polygenic risk scores for improving population estimates of women’s breast cancer genetic risks. Genet. Med. Off. J. Am. Coll. Med. Genet. 2021, 23, 2114–2121. [Google Scholar] [CrossRef]
- Lee, A.; Mavaddat, N.; Wilcox, A.N.; Cunningham, A.P.; Carver, T.; Hartley, S.; Babb de Villiers, C.; Izquierdo, A.; Simard, J.; Schmidt, M.K.; et al. BOADICEA: A comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet. Med. Off. J. Am. Coll. Med. Genet. 2019, 21, 1708–1718. [Google Scholar] [CrossRef]
- Lewis, C.M.; Vassos, E. Prospects for using risk scores in polygenic medicine. Genome Med. 2017, 9, 96. [Google Scholar] [CrossRef]
- Pashayan, N.; Morris, S.; Gilbert, F.J.; Pharoah, P.D. Cost-effectiveness and benefit-to-harm ratio of risk-stratified screening for breast cancer: A life-table model. JAMA Oncol. 2018, 4, 1504–1510. [Google Scholar]
- Maas, P.; Barrdahl, M.; Joshi, A.D.; Auer, P.L.; Gaudet, M.M.; Milne, R.L.; Schumacher, F.R.; Anderson, W.F.; Check, D.; Chattopadhyay, S.; et al. Breast Cancer Risk From Modifiable and Nonmodifiable Risk Factors Among White Women in the United States. JAMA Oncol. 2016, 2, 1295–1302. [Google Scholar] [CrossRef]
- Wright, S.J.; Eden, M.; Ruane, H.; Byers, H.; Evans, D.G.; Harvie, M.; Howell, S.J.; Howell, A.; French, D.; Payne, K. Estimating the Cost of 3 Risk Prediction Strategies for Potential Use in the United Kingdom National Breast Screening Program. MDM Policy Pract. 2023, 8, 23814683231171363. [Google Scholar] [CrossRef]
- Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Doubeni, C.A.; Epling, J.W.; Kubik, M.; Landefeld, C.S.; et al. Medication Use to Reduce Risk of Breast Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2019, 322, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.A.; Gil, L.; Jupp, S.; Ritchie, S.C.; Xu, Y.; Buniello, A.; McMahon, A.; Abraham, G.; Chapman, M.; Parkinson, H.; et al. The Polygenic Score Catalog as an open database for reproducibility and systematic evaluation. Nat. Genet. 2021, 53, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Howell, S.; Evans, D.G. Polygenic risk scores and breast cancer risk prediction. Breast 2023, 67, 71–77. [Google Scholar] [CrossRef]
- Chatterjee, N.; Shi, J.; Garcia-Closas, M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat. Rev. Genet. 2016, 17, 392–406. [Google Scholar] [CrossRef]
- Padrik, P.; Puustusmaa, M.; Tõnisson, N.; Kolk, B.; Saar, R.; Padrik, A.; Tasa, T. Implementation of Risk-Stratified Breast Cancer Prevention With a Polygenic Risk Score Test in Clinical Practice. Breast Cancer Basic Clin. Res. 2023, 17, 11782234231205700. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz, B.C.; Mattingsdal, M.; Dominguez-Valentin, M.; Frei, O.; Shadrin, A.; Puustusmaa, M.; Saar, R.; Sõber, S.; Møller, P.; Andreassen, O.A.; et al. A Breast Cancer Polygenic Risk Score Is Feasible for Risk Stratification in the Norwegian Population. Cancers 2023, 15, 4124. [Google Scholar] [CrossRef]
- Costantino, J.P.; Gail, M.H.; Pee, D.; Anderson, S.; Redmond, C.K.; Benichou, J.; Wieand, H.S. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J. Natl. Cancer Inst. 1999, 91, 1541–1548. [Google Scholar] [CrossRef]
- Gail, M.H.; Brinton, L.A.; Byar, D.P.; Corle, D.K.; Green, S.B.; Schairer, C.; Mulvihill, J.J. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J. Natl. Cancer Inst. 1989, 81, 1879–1886. [Google Scholar] [CrossRef]
- Tyrer, J.; Duffy, S.W.; Cuzick, J. A breast cancer prediction model incorporating familial and personal risk factors. Stat. Med. 2004, 23, 1111–1130. [Google Scholar] [CrossRef]
- Lee, A.; Mavaddat, N.; Cunningham, A.; Carver, T.; Ficorella, L.; Archer, S.; Walter, F.M.; Tischkowitz, M.; Roberts, J.; Usher-Smith, J.; et al. Enhancing the BOADICEA cancer risk prediction model to incorporate new data on RAD51C, RAD51D, BARD1 updates to tumour pathology and cancer incidence. J. Med. Genet. 2022, 59, 1206–1218. [Google Scholar] [CrossRef]
- Carver, T.; Hartley, S.; Lee, A.; Cunningham, A.P.; Archer, S.; Babb de Villiers, C.; Roberts, J.; Ruston, R.; Walter, F.M.; Tischkowitz, M.; et al. CanRisk Tool-A Web Interface for the Prediction of Breast and Ovarian Cancer Risk and the Likelihood of Carrying Genetic Pathogenic Variants. Cancer Epidemiol. Biomark. Prev. 2021, 30, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.R.; van Veen, E.M.; Harkness, E.F.; Brentnall, A.R.; Astley, S.M.; Byers, H.; Woodward, E.R.; Sampson, S.; Southworth, J.; Howell, S.J.; et al. Breast cancer risk stratification in women of screening age: Incremental effects of adding mammographic density, polygenic risk, and a gene panel. Genet. Med. Off. J. Am. Coll. Med. Genet. 2022, 24, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, A.R.; van Veen, E.M.; Harkness, E.F.; Rafiq, S.; Byers, H.; Astley, S.M.; Sampson, S.; Howell, A.; Newman, W.G.; Cuzick, J.; et al. A case-control evaluation of 143 single nucleotide polymorphisms for breast cancer risk stratification with classical factors and mammographic density. Int. J. Cancer 2020, 146, 2122–2129. [Google Scholar] [CrossRef]
- Martin, A.R.; Kanai, M.; Kamatani, Y.; Okada, Y.; Neale, B.M.; Daly, M.J. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 2019, 51, 584–591. [Google Scholar] [CrossRef]
- van den Broek, J.J.; Schechter, C.B.; van Ravesteyn, N.T.; Janssens, A.; Wolfson, M.C.; Trentham-Dietz, A.; Simard, J.; Easton, D.F.; Mandelblatt, J.S.; Kraft, P.; et al. Personalizing Breast Cancer Screening Based on Polygenic Risk and Family History. J. Natl. Cancer Inst. 2021, 113, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.R.; Harkness, E.F.; Brentnall, A.R.; van Veen, E.M.; Astley, S.M.; Byers, H.; Sampson, S.; Southworth, J.; Stavrinos, P.; Howell, S.J.; et al. Breast cancer pathology and stage are better predicted by risk stratification models that include mammographic density and common genetic variants. Breast Cancer Res. Treat. 2019, 176, 141–148. [Google Scholar] [CrossRef]
- Evans, D.G.; Brentnall, A.; Byers, H.; Harkness, E.; Stavrinos, P.; Howell, A.; Newman, W.G.; Cuzick, J. The impact of a panel of 18 SNPs on breast cancer risk in women attending a UK familial screening clinic: A case-control study. J. Med. Genet. 2017, 54, 111–113. [Google Scholar] [CrossRef]
- Lakeman, I.M.M.; Hilbers, F.S.; Rodriguez-Girondo, M.; Lee, A.; Vreeswijk, M.P.G.; Hollestelle, A.; Seynaeve, C.; Meijers-Heijboer, H.; Oosterwijk, J.C.; Hoogerbrugge, N.; et al. Addition of a 161-SNP polygenic risk score to family history-based risk prediction: Impact on clinical management in non-BRCA1/2 breast cancer families. J. Med. Genet. 2019, 56, 581–589. [Google Scholar] [CrossRef]
- Dite, G.S.; MacInnis, R.J.; Bickerstaffe, A.; Dowty, J.G.; Allman, R.; Apicella, C.; Milne, R.L.; Tsimiklis, H.; Phillips, K.A.; Giles, G.G.; et al. Breast Cancer Risk Prediction Using Clinical Models and 77 Independent Risk-Associated SNPs for Women Aged Under 50 Years: Australian Breast Cancer Family Registry. Cancer Epidemiol. Biomark. Prev. 2016, 25, 359–365. [Google Scholar] [CrossRef]
- Li, H.; Feng, B.; Miron, A.; Chen, X.; Beesley, J.; Bimeh, E.; Barrowdale, D.; John, E.M.; Daly, M.B.; Andrulis, I.L.; et al. Breast cancer risk prediction using a polygenic risk score in the familial setting: A prospective study from the Breast Cancer Family Registry and kConFab. Genet. Med. Off. J. Am. Coll. Med. Genet. 2017, 19, 30–35. [Google Scholar] [CrossRef]
- Lakeman, I.M.M.; Rodriguez-Girondo, M.D.M.; Lee, A.; Celosse, N.; Braspenning, M.E.; van Engelen, K.; van de Beek, I.; van der Hout, A.H.; Gomez Garcia, E.B.; Mensenkamp, A.R.; et al. Clinical applicability of the Polygenic Risk Score for breast cancer risk prediction in familial cases. J. Med. Genet. 2022, 60, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Mars, N.; Widen, E.; Kerminen, S.; Meretoja, T.; Pirinen, M.; Della Briotta Parolo, P.; Palta, P.; FinnGen; Palotie, A.; Kaprio, J.; et al. The role of polygenic risk and susceptibility genes in breast cancer over the course of life. Nat. Commun. 2020, 11, 6383. [Google Scholar] [CrossRef] [PubMed]
- Stiller, S.; Drukewitz, S.; Lehmann, K.; Hentschel, J.; Strehlow, V. Clinical Impact of Polygenic Risk Score for Breast Cancer Risk Prediction in 382 Individuals with Hereditary Breast and Ovarian Cancer Syndrome. Cancers 2023, 15, 3938. [Google Scholar] [CrossRef] [PubMed]
- Tuchler, A.; De Pauw, A.; Ernst, C.; Anota, A.; Lakeman, I.M.M.; Dick, J.; van der Stoep, N.; van Asperen, C.J.; Maringa, M.; Herold, N.; et al. Clinical implications of incorporating genetic and non-genetic risk factors in CanRisk-based breast cancer risk prediction. Breast 2024, 73, 103615. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; McGuffog, L.; Barrowdale, D.; Lee, A.; Soucy, P.; Dennis, J.; Domchek, S.M.; Robson, M.; Spurdle, A.B.; Ramus, S.J.; et al. Evaluation of Polygenic Risk Scores for Breast and Ovarian Cancer Risk Prediction in BRCA1 and BRCA2 Mutation Carriers. J. Natl. Cancer Inst. 2017, 109, djw302. [Google Scholar] [CrossRef]
- Gallagher, S.; Hughes, E.; Wagner, S.; Tshiaba, P.; Rosenthal, E.; Roa, B.B.; Kurian, A.W.; Domchek, S.M.; Garber, J.; Lancaster, J.; et al. Association of a Polygenic Risk Score With Breast Cancer Among Women Carriers of High- and Moderate-Risk Breast Cancer Genes. JAMA Netw. Open 2020, 3, e208501. [Google Scholar] [CrossRef]
- Gao, C.; Polley, E.C.; Hart, S.N.; Huang, H.; Hu, C.; Gnanaolivu, R.; Lilyquist, J.; Boddicker, N.J.; Na, J.; Ambrosone, C.B.; et al. Risk of Breast Cancer Among Carriers of Pathogenic Variants in Breast Cancer Predisposition Genes Varies by Polygenic Risk Score. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 2564–2573. [Google Scholar] [CrossRef]
- Schreurs, M.A.C.; Ramón y Cajal, T.; Adank, M.A.; Collée, J.M.; Hollestelle, A.; van Rooij, J.; Schmidt, M.K.; Hooning, M.J. The benefit of adding polygenic risk scores, lifestyle factors, and breast density to family history and genetic status for breast cancer risk and surveillance classification of unaffected women from germline CHEK2 c.1100delC families. Breast 2024, 73, 103611. [Google Scholar] [CrossRef]
- Mars, N.; Kerminen, S.; Tamlander, M.; Pirinen, M.; Jakkula, E.; Aaltonen, K.; Meretoja, T.; Heinävaara, S.; Widén, E.; Ripatti, S.; et al. Comprehensive Inherited Risk Estimation for Risk-Based Breast Cancer Screening in Women. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2024, 42, 1477–1487. [Google Scholar] [CrossRef]
- Huntley, C.; Torr, B.; Sud, A.; Rowlands, C.F.; Way, R.; Snape, K.; Hanson, H.; Swanton, C.; Broggio, J.; Lucassen, A.; et al. Utility of polygenic risk scores in UK cancer screening: A modelling analysis. Lancet Oncol. 2023, 24, 658–668. [Google Scholar] [CrossRef]
- Huntley, C.; Torr, B.; Sud, A.; Houlston, R.S.; Hingorani, A.D.; Jones, M.E.; Turnbull, C. The impact of risk stratification by polygenic risk and age on breast cancer screening in women aged 40-49 years: A modelling study. Lancet 2023, 402 (Suppl. S1), S54. [Google Scholar] [CrossRef] [PubMed]
- Tamm, M.; Padrik, P.; Paas, A.; Lepland, A.; Kruuv-Käo, K.; Sõber, S.; Roht, L.; Ojamaa, K.; Pajusalu, S.; Padrik, A.; et al. Implementation of Genetics-Based Precision Prevention in Breast Cancer: Results from the Estonian Arm of the BRIGHT Study. Poster P18.048.C. In Proceedings of the European Society of Human Genetics Conference, Berlin, Germany, 1–4 June 2024. [Google Scholar]
- Hingorani, A.D.; Gratton, J.; Finan, C.; Schmidt, A.F.; Patel, R.; Sofat, R.; Kuan, V.; Langenberg, C.; Hemingway, H.; Morris, J.K.; et al. Performance of polygenic risk scores in screening, prediction, and risk stratification: Secondary analysis of data in the Polygenic Score Catalog. BMJ Med. 2023, 2, e000554. [Google Scholar] [CrossRef]
- Kachuri, L.; Chatterjee, N.; Hirbo, J.; Schaid, D.J.; Martin, I.; Kullo, I.J.; Kenny, E.E.; Pasaniuc, B.; Polygenic Risk Methods in Diverse Populations (PRIMED) Consortium Methods Working Group; Witte, J.S.; et al. Principles and methods for transferring polygenic risk scores across global populations. Nat. Rev. Genet. 2024, 25, 8–25. [Google Scholar] [CrossRef]
- Sawyer, S.; Mitchell, G.; McKinley, J.; Chenevix-Trench, G.; Beesley, J.; Chen, X.Q.; Bowtell, D.; Trainer, A.H.; Harris, M.; Lindeman, G.J.; et al. A role for common genomic variants in the assessment of familial breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 4330–4336. [Google Scholar] [CrossRef]
- Bahcall, O. Common variation and heritability estimates for breast, ovarian and prostate cancers. Nat. Genet. 2013, 10, 304. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Niksic, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Esteve, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Precision-HBOC. Stratifying Risk for Early Detection in Hereditary Breast and Ovarian Cancer. Available online: https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/precision-hboc/ (accessed on 8 June 2024).
- Crouch, D.J.M.; Bodmer, W.F. Polygenic inheritance, GWAS, polygenic risk scores, and the search for functional variants. Proc. Natl. Acad. Sci. USA 2020, 117, 18924–18933. [Google Scholar] [CrossRef]
- Peintinger, F. National Breast Screening Programs across Europe. Breast Care 2019, 14, 354–358. [Google Scholar] [CrossRef]
- Hurson, A.N.; Pal Choudhury, P.; Gao, C.; Husing, A.; Eriksson, M.; Shi, M.; Jones, M.E.; Evans, D.G.R.; Milne, R.L.; Gaudet, M.M.; et al. Prospective evaluation of a breast-cancer risk model integrating classical risk factors and polygenic risk in 15 cohorts from six countries. Int. J. Epidemiol. 2022, 50, 1897–1911. [Google Scholar] [CrossRef]
- Lakeman, I.M.M.; Rodriguez-Girondo, M.; Lee, A.; Ruiter, R.; Stricker, B.H.; Wijnant, S.R.A.; Kavousi, M.; Antoniou, A.C.; Schmidt, M.K.; Uitterlinden, A.G.; et al. Validation of the BOADICEA model and a 313-variant polygenic risk score for breast cancer risk prediction in a Dutch prospective cohort. Genet. Med. Off. J. Am. Coll. Med. Genet. 2020, 22, 1803–1811. [Google Scholar] [CrossRef]
- Vachon, C.M.; Scott, C.G.; Tamimi, R.M.; Thompson, D.J.; Fasching, P.A.; Stone, J.; Southey, M.C.; Winham, S.; Lindström, S.; Lilyquist, J.; et al. Joint association of mammographic density adjusted for age and body mass index and polygenic risk score with breast cancer risk. Breast Cancer Res. 2019, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Bolze, A.; Cirulli, E.T.; Hajek, C.; Schnell Blitstein, J.M.; Grzymski, J.J. The Potential of Genetics in Identifying Women at Lower Risk of Breast Cancer. JAMA Oncol. 2024, 10, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Tamm, M.; Padrik, P.; Ojamaa, K.; Paas, A.; Lepland, A.; Kruuv-Käo, K.; Leitsalu, L.; Sõber, S.; Roht, L.; Pajusalu, S.; et al. An implementation study of the service model for genetic risk-based stratified breast cancer screening—Estonian results of the BRIGHT project. medRxiv 2024. [Google Scholar] [CrossRef]
- Sampaio, F.; Padrik, P.; Kruuv-Käo, K.; Lutsar, K.; Tõnisson, N.; Feldman, I. Cost-Effectiveness of a Polygenic Risk Score Based Breast Cancer Screening Program in Estonia. Poster P18.018.A. In Proceedings of the European Society of Human Genetics Conference, Berlin, Germany, 1–4 June 2024. [Google Scholar]
- Shieh, Y.; Eklund, M.; Madlensky, L.; Sawyer, S.D.; Thompson, C.K.; Stover Fiscalini, A.; Ziv, E.; Van’t Veer, L.J.; Esserman, L.J.; Tice, J.A. Breast Cancer Screening in the Precision Medicine Era: Risk-Based Screening in a Population-Based Trial. J. Natl. Cancer Inst. 2017, 109, djw290. [Google Scholar] [CrossRef]
- Lowry, K.P.; Geuzinge, H.A.; Stout, N.K.; Alagoz, O.; Hampton, J.; Kerlikowske, K.; de Koning, H.J.; Miglioretti, D.L.; van Ravesteyn, N.T.; Schechter, C.; et al. Breast Cancer Screening Strategies for Women With ATM, CHEK2, and PALB2 Pathogenic Variants: A Comparative Modeling Analysis. JAMA Oncol. 2022, 8, 587–596. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2024. Available online: https://www.nccn.org/guidelines/category_2 (accessed on 30 May 2024).
- Cancer Research UK. Breast Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer#heading-Zero (accessed on 1 December 2024).
- Interdisziplinäre S3-Leitlinie für die Früherkennung, Diagnostik, Therapie und Nachsorge des Mammakarzinoms. Available online: https://register.awmf.org/assets/guidelines/032-045OLl_S3_Mammakarzinom_2021-07.pdf (accessed on 24 May 2024).
- AGO Breast Cancer Risk, Genetics and Prevention. Available online: https://www.ago-online.de/fileadmin/ago-online/downloads/_leitlinien/kommission_mamma/2024/englisch/Einzeldateien_Literatur/AGO_2024E_02_Genetics_REF.pdf (accessed on 30 May 2024).
- Deutsches Konsortium Familiärer Brust- und Eierstockkrebs. Available online: https://www.konsortium-familiaerer-brustkrebs.de/ (accessed on 30 June 2024).
- Nasjonalt Handlingsprogram Med Retningslinjer for Diagnostikk, Behandling og Oppfølging av Pasienter Med Brystkreft. Available online: https://www.helsebiblioteket.no/innhold/nasjonal-faglig-retningslinje/brystkreft-handlingsprogram (accessed on 30 May 2024).
- Sociedade Portuguesa de Oncologia. Available online: https://www.sponcologia.pt/web/home.php (accessed on 15 October 2024).
- Personaalmeditsiini Juhtprojekti Eeluuring. Available online: https://www.sm.ee/personaalmeditsiini-juhtprojekti-eeluuring (accessed on 30 August 2024).
- Estonian Health Insurance News. Eestis on Kanda kinnitamas uued Sõeluuringud. Available online: https://www.tervisekassa.ee/uudised/eestis-kanda-kinnitamas-uued-soeluuringud (accessed on 5 June 2024).
- Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on In Vitro Diagnostic Medical Devices. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0746 (accessed on 30 June 2024).
| Breast Cancer Risk Category | |||
|---|---|---|---|
| Near Population Risk | Moderate Risk | High Risk | |
| Lifetime risk from age 20 | Less than 17% | 17% or greater but less than 30% | 30% or greater |
| Risk between ages 40 and 50 | Less than 3% | 3–8% | Greater than 8% |
| Corresponding relative risk | Less than 1.5 | 1.5–2.7 | Greater than 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padrik, P.; Tõnisson, N.; Hovda, T.; Sahlberg, K.K.; Hovig, E.; Costa, L.; Nogueira da Costa, G.; Feldman, I.; Sampaio, F.; Pajusalu, S.; et al. Guidance for the Clinical Use of the Breast Cancer Polygenic Risk Scores. Cancers 2025, 17, 1056. https://doi.org/10.3390/cancers17071056
Padrik P, Tõnisson N, Hovda T, Sahlberg KK, Hovig E, Costa L, Nogueira da Costa G, Feldman I, Sampaio F, Pajusalu S, et al. Guidance for the Clinical Use of the Breast Cancer Polygenic Risk Scores. Cancers. 2025; 17(7):1056. https://doi.org/10.3390/cancers17071056
Chicago/Turabian StylePadrik, Peeter, Neeme Tõnisson, Tone Hovda, Kristine Kleivi Sahlberg, Eivind Hovig, Luís Costa, Gonçalo Nogueira da Costa, Inna Feldman, Filipa Sampaio, Sander Pajusalu, and et al. 2025. "Guidance for the Clinical Use of the Breast Cancer Polygenic Risk Scores" Cancers 17, no. 7: 1056. https://doi.org/10.3390/cancers17071056
APA StylePadrik, P., Tõnisson, N., Hovda, T., Sahlberg, K. K., Hovig, E., Costa, L., Nogueira da Costa, G., Feldman, I., Sampaio, F., Pajusalu, S., Ojamaa, K., Kallak, K., Tihamäe, A.-T., Roht, L., Kahre, T., Lepland, A., Sõber, S., Kruuv-Käo, K., Tamm, M., ... Evans, D. G., on behalf of the AnteNOR and BRIGHT Research Consortia. (2025). Guidance for the Clinical Use of the Breast Cancer Polygenic Risk Scores. Cancers, 17(7), 1056. https://doi.org/10.3390/cancers17071056







