Clinical Outcomes and Genomic Alterations in Gleason Score 10 Prostate Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Comparison of Clinical Factors Stratified by Gleason Score
2.3. Covariate-Adjusted Hazard Ratios and Estimates of Time to Biochemical Recurrence
2.4. Genomic Alterations and Gene Expression Analysis
2.5. Construction of Protein–Protein Interaction Network and Identification of Hub Genes
2.6. The Prognostic Value of the Altered and Hub Genes
2.7. Functional Enrichment Analysis
3. Results
3.1. Comparison of the Distribution of Clinical Factors Stratified by Gleason Score
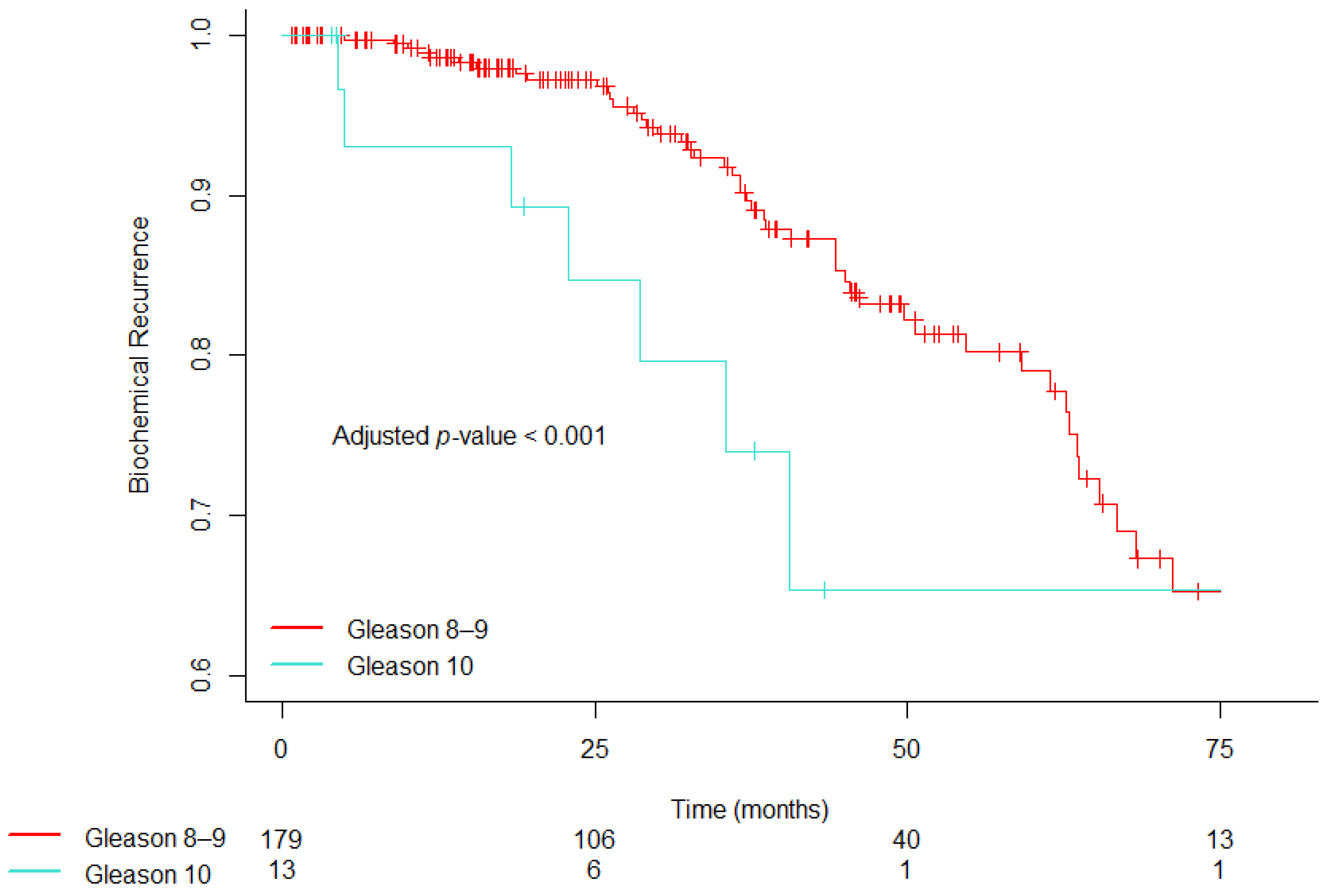
3.2. Covariate-Adjusted Hazard Ratios and Estimates of Time to Biochemical Recurrence
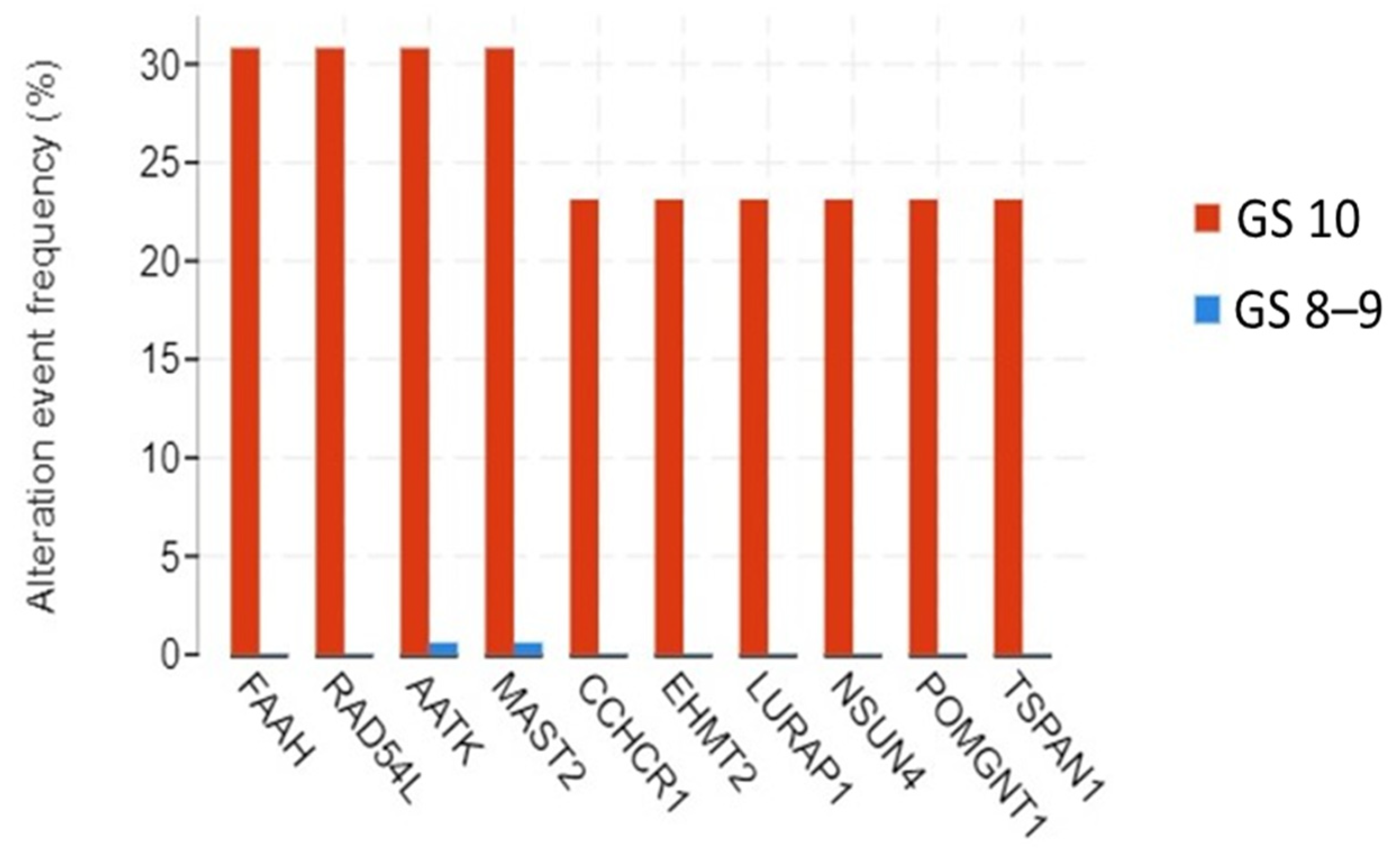
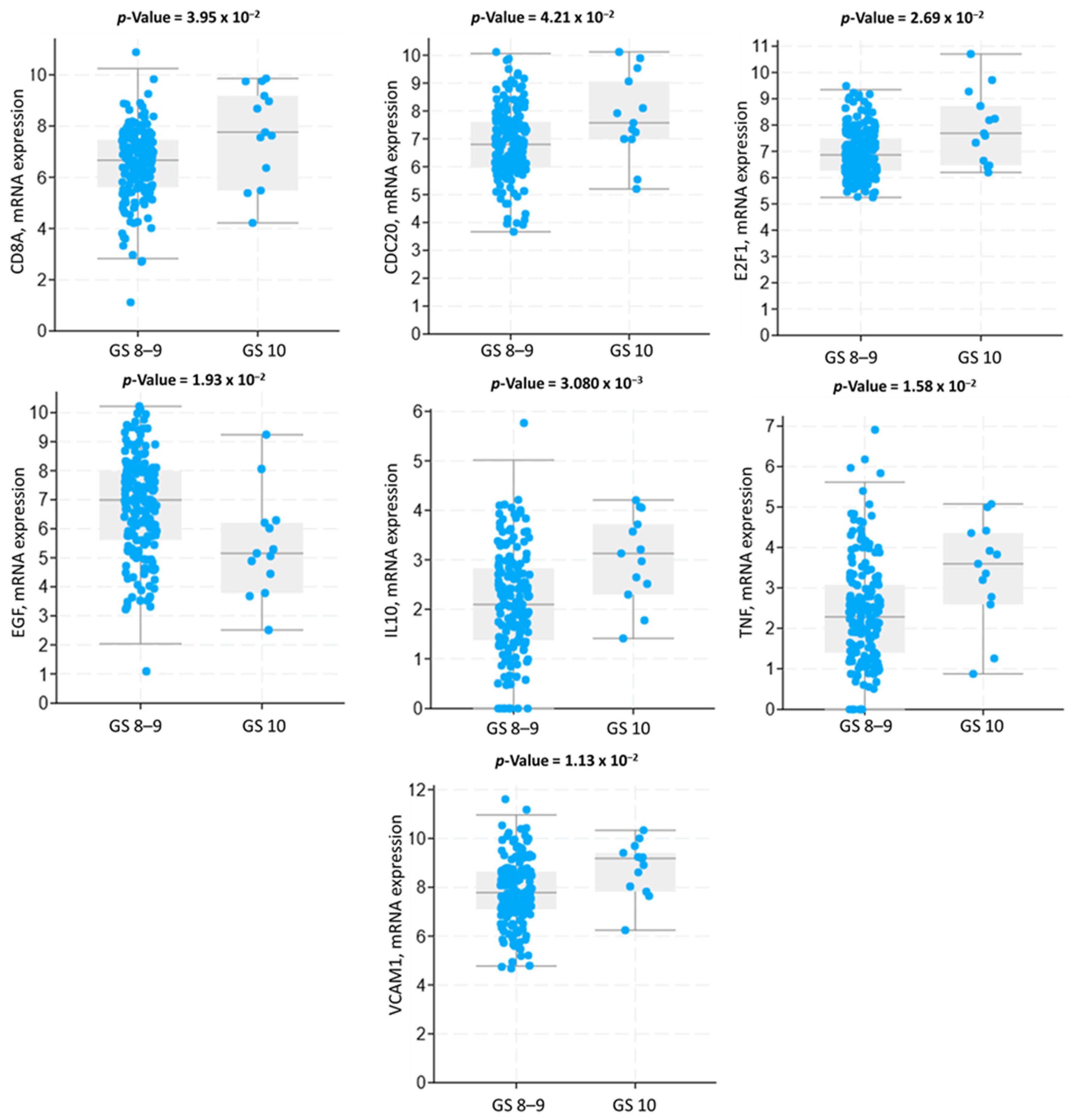
3.3. Genomic Alterations and Gene Expression Analysis
3.4. Protein–Protein Interaction Network and Hub Genes
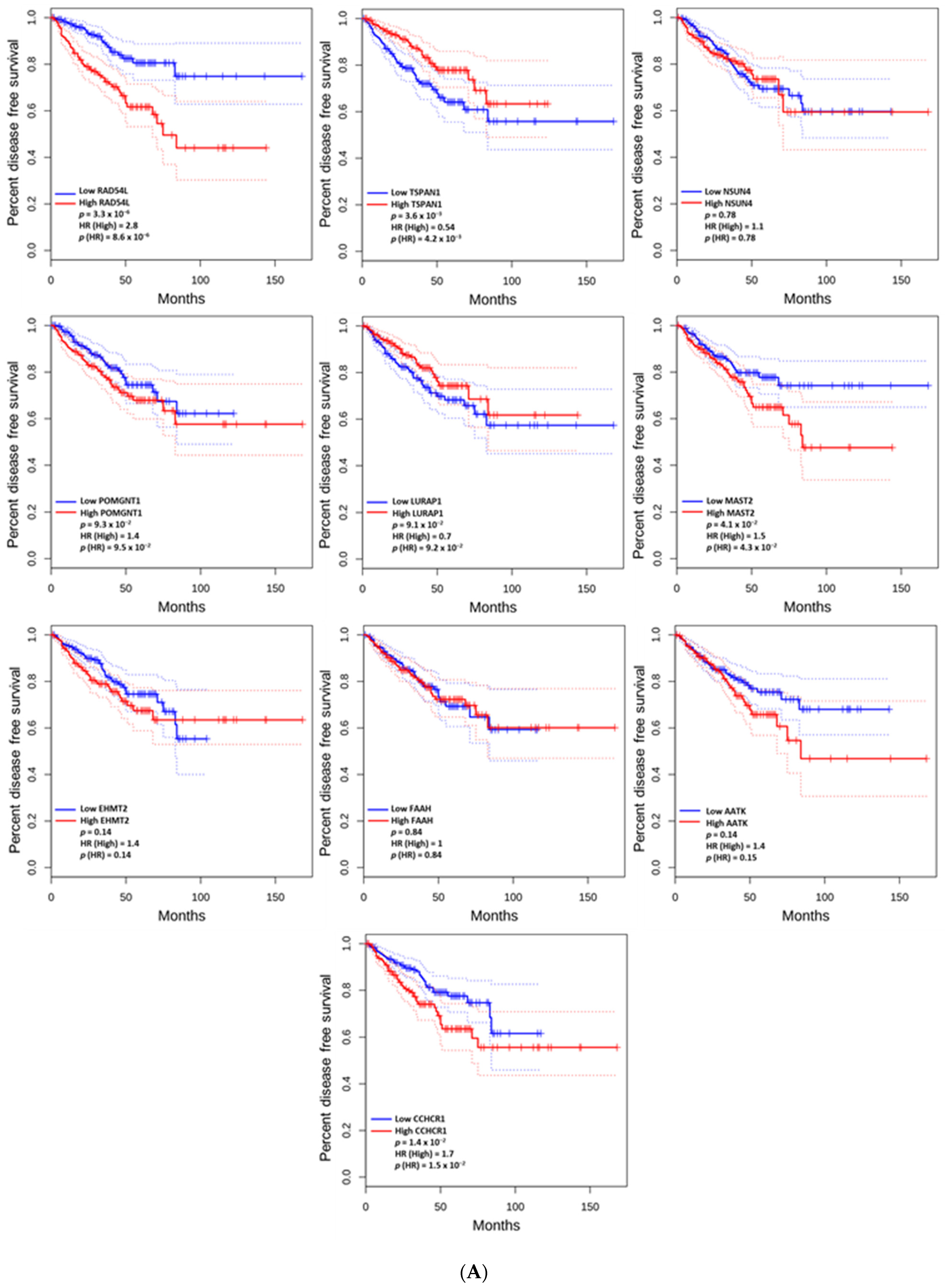
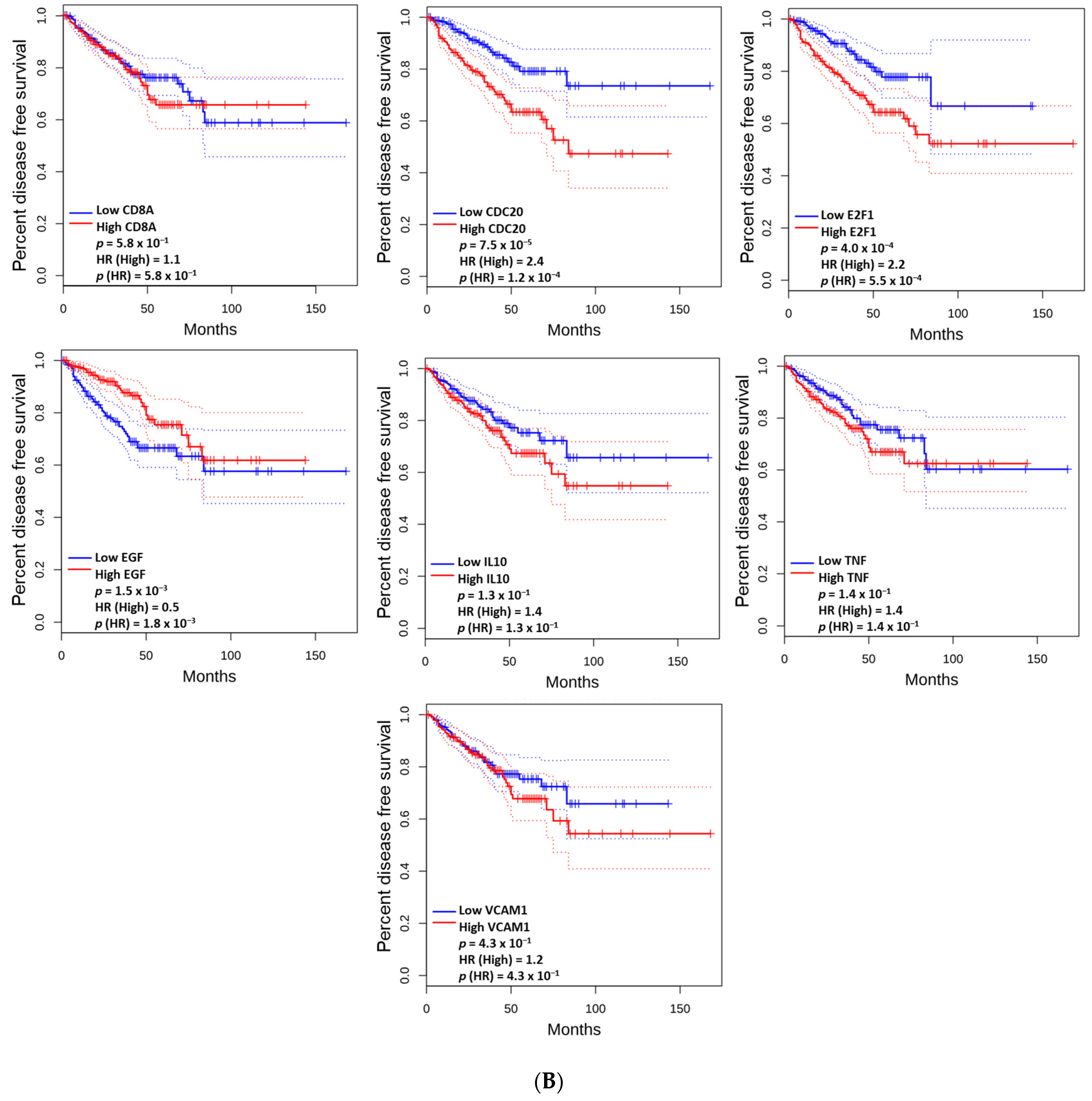
3.5. The Prognostic Value of the Altered and Hub Genes
3.6. Functional Enrichment Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Gleason, D.F.; Mellinger, G.T. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J. Urol. 1974, 111, 58–64. [Google Scholar] [CrossRef]
- Ham, W.S.; Chalfin, H.J.; Feng, Z.; Trock, B.J.; Epstein, J.I.; Cheung, C.; Humphreys, E.; Partin, A.W.; Han, M. New Prostate Cancer Grading System Predicts Long-term Survival Following Surgery for Gleason Score 8-10 Prostate Cancer. Eur. Urol. 2017, 71, 907–912. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Rojas, A.M.; Bownes, P.J.; Lowe, G.J.; Ostler, P.J.; Bryant, L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother. Oncol. 2012, 103, 217–222. [Google Scholar] [CrossRef]
- Dayes, I.S.; Parpia, S.; Gilbert, J.; Julian, J.A.; Davis, I.R.; Levine, M.N.; Sathya, J. Long-Term Results of a Randomized Trial Comparing Iridium Implant Plus External Beam Radiation Therapy With External Beam Radiation Therapy Alone in Node-Negative Locally Advanced Cancer of the Prostate. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 90–93. [Google Scholar] [CrossRef]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar] [CrossRef]
- Kishan, A.U.; Cook, R.R.; Ciezki, J.P.; Ross, A.E.; Pomerantz, M.M.; Nguyen, P.L.; Shaikh, T.; Tran, P.T.; Sandler, K.A.; Stock, R.G.; et al. Radical Prostatectomy, External Beam Radiotherapy, or External Beam Radiotherapy With Brachytherapy Boost and Disease Progression and Mortality in Patients With Gleason Score 9–10 Prostate Cancer. JAMA 2018, 319, 896–905. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [CrossRef]
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar]
- de Bruijn, I.; Kundra, R.; Mastrogiacomo, B.; Tran, T.N.; Sikina, L.; Mazor, T.; Li, X.; Ochoa, A.; Zhao, G.; Lai, B.; et al. Analysis and Visualization of Longitudinal Genomic and Clinical Data from the AACR Project GENIE Biopharma Collaborative in cBioPortal. Cancer Res. 2023, 83, 3861–3867. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Sandler, K.A.; Cook, R.R.; Ciezki, J.P.; Ross, A.E.; Pomerantz, M.M.; Nguyen, P.L.; Shaikh, T.; Tran, P.T.; Stock, R.G.; Merrick, G.S.; et al. Clinical Outcomes for Patients With Gleason Score 10 Prostate Adenocarcinoma: Results From a Multi-institutional Consortium Study. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 883–888. [Google Scholar] [CrossRef]
- Kneebone, A.; Fraser-Browne, C.; Duchesne, G.M.; Fisher, R.; Frydenberg, M.; Herschtal, A.; Williams, S.G.; Brown, C.; Delprado, W.; Haworth, A.; et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): A randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020, 21, 1331–1340. [Google Scholar] [CrossRef]
- Parker, C.C.; Clarke, N.W.; Cook, A.D.; Kynaston, H.G.; Petersen, P.M.; Catton, C.; Cross, W.; Logue, J.; Parulekar, W.; Payne, H.; et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): A randomised, controlled phase 3 trial. Lancet 2020, 396, 1413–1421. [Google Scholar] [CrossRef]
- Sargos, P.; Chabaud, S.; Latorzeff, I.; Magne, N.; Benyoucef, A.; Supiot, S.; Pasquier, D.; Abdiche, M.S.; Gilliot, O.; Graff-Cailleaud, P.; et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): A randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1341–1352. [Google Scholar] [CrossRef]
- Vale, C.L.; Fisher, D.; Kneebone, A.; Parker, C.; Pearse, M.; Richaud, P.; Sargos, P.; Sydes, M.R.; Brawley, C.; Brihoum, M.; et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: A prospectively planned systematic review and meta-analysis of aggregate data. Lancet 2020, 396, 1422–1431. [Google Scholar] [CrossRef]
- Tilki, D.; Chen, M.H.; Wu, J.; Huland, H.; Graefen, M.; Wiegel, T.; Bohmer, D.; Mohamad, O.; Cowan, J.E.; Feng, F.Y.; et al. Adjuvant Versus Early Salvage Radiation Therapy for Men at High Risk for Recurrence Following Radical Prostatectomy for Prostate Cancer and the Risk of Death. J. Clin. Oncol. 2021, 39, 2284–2293. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, C.; Fu, Q.; Li, T.; Zhang, X.; Zhu, C.; Qin, X.; Wu, B. Pan-Cancer Analysis of Oncogenic Role of RAD54L and Experimental Validation in Hepatocellular Carcinoma. J. Inflamm. Res. 2023, 16, 3997–4017. [Google Scholar] [CrossRef]
- Tundidor, I.; Seijo-Vila, M.; Blasco-Benito, S.; Rubert-Hernández, M.; Adámez, S.; Andradas, C.; Manzano, S.; Álvarez-López, I.; Sarasqueta, C.; Villa-Morales, M.; et al. Identification of fatty acid amide hydrolase as a metastasis suppressor in breast cancer. Nat. Commun. 2023, 14, 3130. [Google Scholar] [CrossRef]
- Haag, T.; Herkt, C.E.; Walesch, S.K.; Richter, A.M.; Dammann, R.H. The apoptosis associated tyrosine kinase gene is frequently hypermethylated in human cancer and is regulated by epigenetic mechanisms. Genes Cancer 2014, 5, 365–374. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, Y.; Jiang, P.; Fu, Z.; Liu, Y. High MAST2 mRNA expression and its role in diagnosis and prognosis of liver cancer. Sci. Rep. 2019, 9, 19865. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Niu, D.; Chen, Y.; Mi, H.; Mo, Z.; Pang, G. The epiphany derived from T-cell-inflamed profiles: Pan-cancer characterization of CD8A as a biomarker spanning clinical relevance, cancer prognosis, immunosuppressive environment, and treatment responses. Front. Genet. 2022, 13, 974416. [Google Scholar] [CrossRef]
- He, W.; Meng, J. CDC20: A novel therapeutic target in cancer. Am. J. Transl. Res. 2023, 15, 678–693. [Google Scholar]
- Zhang, Q.; Huang, H.; Liu, A.; Li, J.; Liu, C.; Sun, B.; Chen, L.; Gao, Y.; Xu, D.; Su, C. Cell division cycle 20 (CDC20) drives prostate cancer progression via stabilization of β-catenin in cancer stem-like cells. EBioMedicine 2019, 42, 397–407. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, C.; Zhu, Y.; Wu, Q.; Jiang, Y.; Huang, Y.; Hu, Y. Key Genes And Pathways Controlled By E2F1 In Human Castration-Resistant Prostate Cancer Cells. Onco Targets Ther. 2019, 12, 8961–8976. [Google Scholar] [CrossRef]
- Xu, H.; Xu, K.; He, H.H.; Zang, C.; Chen, C.H.; Chen, Y.; Qin, Q.; Wang, S.; Wang, C.; Hu, S.; et al. Integrative Analysis Reveals the Transcriptional Collaboration between EZH2 and E2F1 in the Regulation of Cancer-Related Gene Expression. Mol. Cancer Res. 2016, 14, 163–172. [Google Scholar] [CrossRef]
- Pützer, B.M.; Engelmann, D. E2F1 apoptosis counterattacked: Evil strikes back. Trends Mol. Med. 2013, 19, 89–98. [Google Scholar] [CrossRef]
- Liang, Y.X.; Lu, J.M.; Mo, R.J.; He, H.C.; Xie, J.; Jiang, F.N.; Lin, Z.Y.; Chen, Y.R.; Wu, Y.D.; Luo, H.W.; et al. E2F1 promotes tumor cell invasion and migration through regulating CD147 in prostate cancer. Int. J. Oncol. 2016, 48, 1650–1658. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef]
- Dennis, K.L.; Blatner, N.R.; Gounari, F.; Khazaie, K. Current status of interleukin-10 and regulatory T-cells in cancer. Curr. Opin. Oncol. 2013, 25, 637–645. [Google Scholar] [CrossRef]
- Bakir, W.A.; Gaidan, H.A.; Al-Kaabi, M.M. Immunohistochemical expression of interlukin10 (IL10) and heat shock protein-90 (HSP-90) in prostatic carcinoma. Indian J. Pathol. Microbiol. 2020, 63, 230–234. [Google Scholar] [CrossRef]
- Schlesinger, M.; Bendas, G. Vascular cell adhesion molecule-1 (VCAM-1)--an increasing insight into its role in tumorigenicity and metastasis. Int. J. Cancer 2015, 136, 2504–2514. [Google Scholar] [CrossRef]
- VanHeyst, K.A.; Choi, S.H.; Kingsley, D.T.; Huang, A.Y. Ectopic Tumor VCAM-1 Expression in Cancer Metastasis and Therapy Resistance. Cells 2022, 11, 3922. [Google Scholar] [CrossRef]
- Sethi, G.; Sung, B.; Aggarwal, B.B. TNF: A master switch for inflammation to cancer. Front. Biosci. 2008, 13, 5094–5107. [Google Scholar] [CrossRef]
- Wajant, H. The role of TNF in cancer. Results Probl. Cell Differ. 2009, 49, 1–15. [Google Scholar] [CrossRef]


| Gleason Score 8–9 (n = 179) | Gleason Score 10 (n = 13) | p | |
|---|---|---|---|
| Age (years), median (IQR) | 63 (57, 66.5) | 65 (60, 68) | 0.193 |
| Baseline PSA, ng/mL, median (IQR) | 8.9 (5.35, 14.8) | 14.2 (8.2, 64.1) | 0.038 |
| Prostatectomy tumor stage, No. (%) | 0.200 | ||
| T2 | 27 (15%) | 0 (0%) | |
| T3a or higher | 152 (85%) | 13 (100%) | |
| Prostatectomy margin status, No. (%) | <0.001 | ||
| Negative | 90 (50%) | 0 (0%) | |
| Positive | 89 (50%) | 13 (100%) | |
| Prostatectomy nodal status, No. (%) | 0.400 | ||
| Negative (N0) | 123 (69%) | 7 (54%) | |
| Positive (N1) | 56 (31%) | 6 (46%) | |
| * Follow-up (month), median (IQR) | 37.87 (16.53, 64.37) | 43.40 (19.30, 70.23) | 0.002 |
| Covariates | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | AHR | 95% CI | p | |
| Age (years) | 1.004 | 0.966–1.044 | 0.825 | 1.003 | 0.996–0.965 | 0.860 |
| Baseline PSA, ng/mL | ||||||
| <4 | 0.939 | 0.407–2.170 | 0.884 | 1.058 | 0.443–2.528 | 0.898 |
| 4–10 | Reference | Reference | Reference | Reference | ||
| >10 | 1.122 | 0.890–1.901 | 0.667 | 0.934 | 0.532–1.638 | 0.813 |
| Prostatectomy tumor stage | ||||||
| T2 | Reference | Reference | Reference | Reference | ||
| T3a or higher | 2.535 | 0.919–6.991 | 0.072 | 2.213 | 0.793–6.175 | 0.129 |
| Prostatectomy margin status | ||||||
| Negative | Reference | Reference | Reference | Reference | ||
| Positive | 1.489 | 0.894–2.483 | 0.126 | 1.206 | 0.658–2.217 | 0.545 |
| Prostatectomy nodal status | ||||||
| Negative (N0) | Reference | Reference | Reference | Reference | ||
| Positive (N1) | 1.227 | 0.724–2.080 | 0.446 | 1.153 | 0.657–2.023 | 0.619 |
| Prostatectomy Gleason score | ||||||
| 8–9 | Reference | Reference | Reference | Reference | ||
| 10 | 3.148 | 1.485–6.676 | 0.002 | 2.669 | 1.183–6.023 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.W.; Tuac, Y.; Li, S.; Leeman, J.E.; King, M.T.; Orio, P.F.; Nguyen, P.L.; D’Amico, A.V.; Aktan, C.; Sayan, M. Clinical Outcomes and Genomic Alterations in Gleason Score 10 Prostate Cancer. Cancers 2025, 17, 1055. https://doi.org/10.3390/cancers17071055
Chen LW, Tuac Y, Li S, Leeman JE, King MT, Orio PF, Nguyen PL, D’Amico AV, Aktan C, Sayan M. Clinical Outcomes and Genomic Alterations in Gleason Score 10 Prostate Cancer. Cancers. 2025; 17(7):1055. https://doi.org/10.3390/cancers17071055
Chicago/Turabian StyleChen, Luke W., Yetkin Tuac, Sophia Li, Jonathan E. Leeman, Martin T. King, Peter F. Orio, Paul L. Nguyen, Anthony V. D’Amico, Cagdas Aktan, and Mutlay Sayan. 2025. "Clinical Outcomes and Genomic Alterations in Gleason Score 10 Prostate Cancer" Cancers 17, no. 7: 1055. https://doi.org/10.3390/cancers17071055
APA StyleChen, L. W., Tuac, Y., Li, S., Leeman, J. E., King, M. T., Orio, P. F., Nguyen, P. L., D’Amico, A. V., Aktan, C., & Sayan, M. (2025). Clinical Outcomes and Genomic Alterations in Gleason Score 10 Prostate Cancer. Cancers, 17(7), 1055. https://doi.org/10.3390/cancers17071055







