Assessment of the Mechanisms of Action of Eribulin in Patients with Advanced Liposarcoma Through the Evaluation of Radiological, Functional, and Tissue Responses: A Prospective Monocentric Study (Malibu Study)
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Objectives
2.2. Study Design
- Histological diagnosis of liposarcoma
- Advanced stage (unresectable or metastatic) disease treated with at least two previous lines of chemotherapy (or ineligible for anthracycline and treated with at least a previous line of chemotherapy)
- Age ≥ 18 years
- Ability to give informed consent
- Prior eribulin treatment
- Patients unsuitable for biopsy due to medical conditions
- biopsy site (precisely identified in patients who had CT scan-guided biopsies)
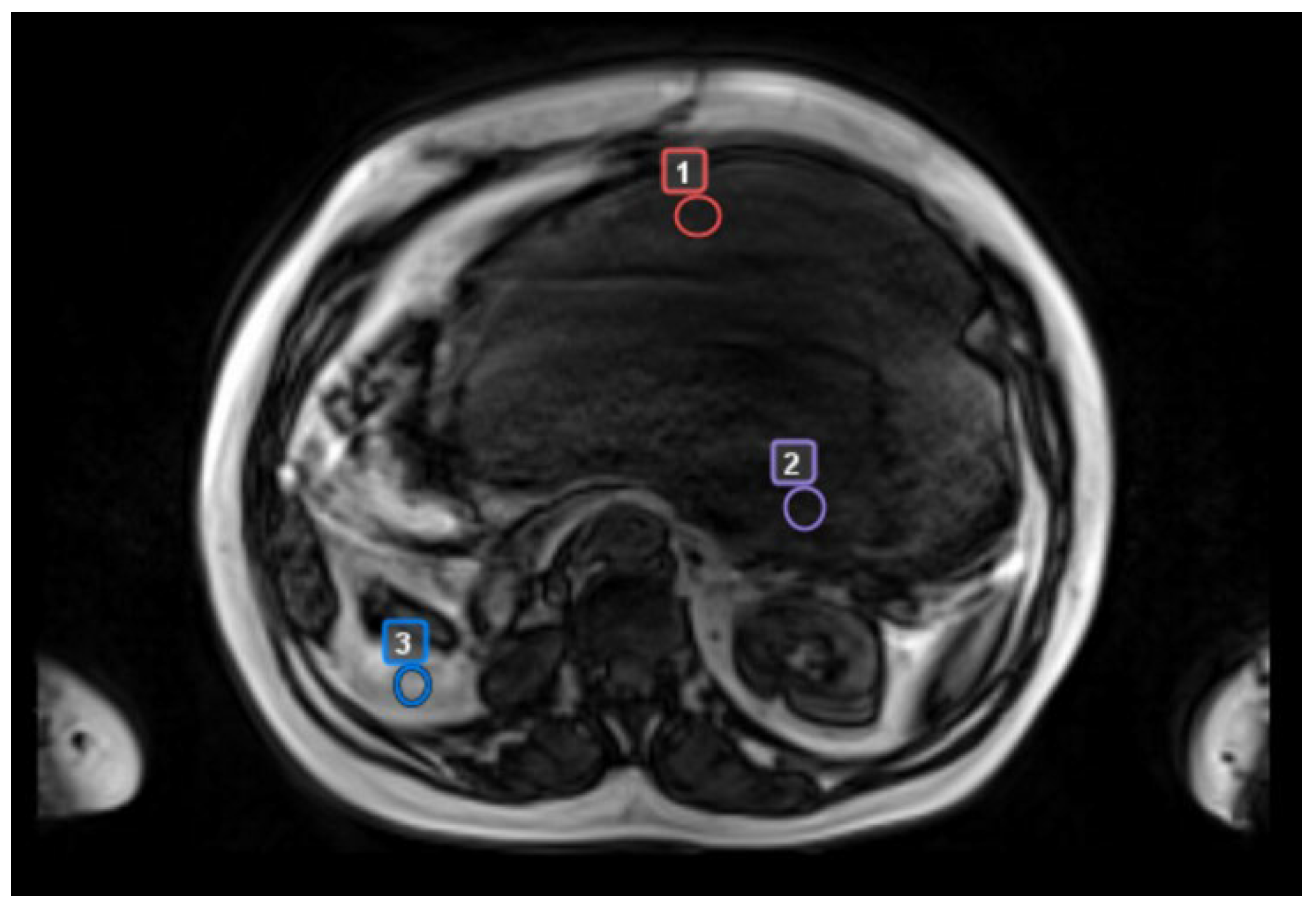
- “worst” tumor area with radiologic features suggestive of the most aggressive biology (reduction in ADC maps, high contrast enhancement, low T2 signal)
- “best” tumor area with the least aggressive radiologic features (lack of one or more aggressive features)
- a control area, identified as normal adipose tissue located in the same body region but separate from tumor tissue
3. Results
3.1. Patients’ Characteristics
3.2. Clinical Evaluation
3.3. Radiological Evaluation
3.3.1. RECIST Assessment (CT Scan)
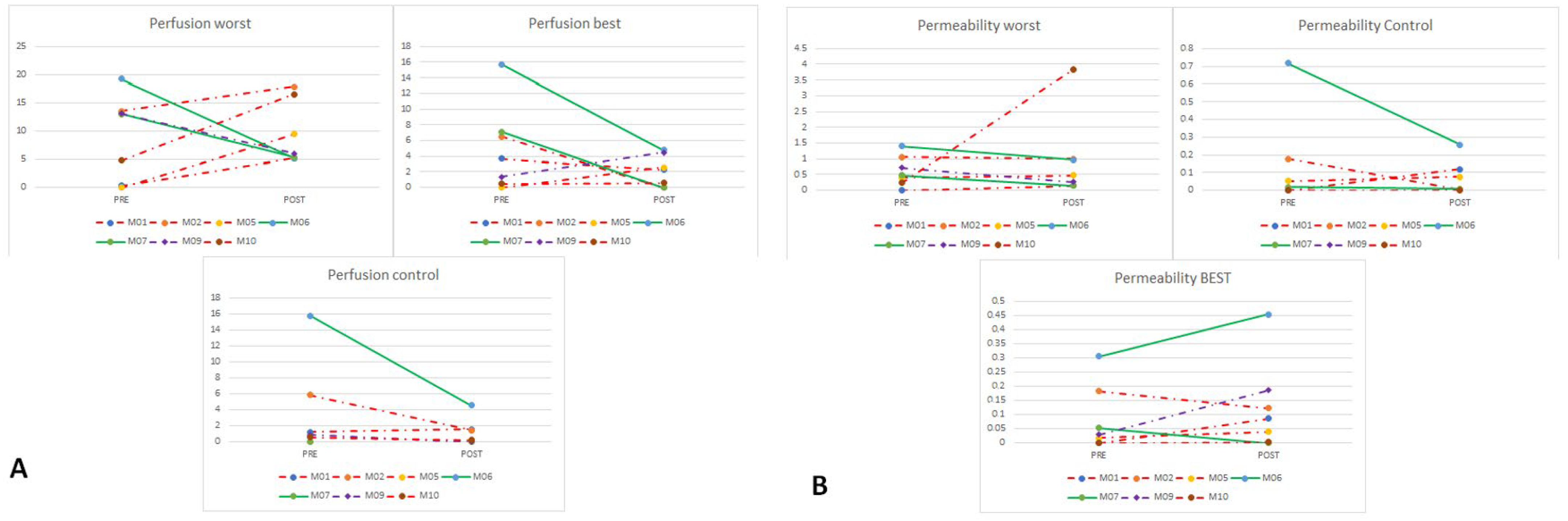
3.3.2. DCE-MRI Assessment
3.4. Pathology Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gronchi, A.; Miah, A.B.; Dei Tos, A.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours. In WHO Classification of Tumours Series, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020; Volume 3, Available online: https://publications.Iarc.fr/588 (accessed on 31 July 2024).
- Ducimetière, F.; Lurkin, A.; Ranchère-Vince, D.; Decouvelaere, A.-V.; Péoc’H, M.; Istier, L.; Chalabreysse, P.; Muller, C.; Alberti, L.; Bringuier, P.-P.; et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS ONE 2011, 6, e20294. [Google Scholar] [CrossRef] [PubMed]
- Schöffski, P. Established and experimental systemic treatment options for advanced liposarcoma. Oncol. Res. Treat. 2022, 45, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.T.J.; Thway, K.; Huang, P.H.; Jones, R.L. Clinical and molecular spectrum of liposarcoma. J. Clin. Oncol. 2018, 36, 151–159. [Google Scholar] [CrossRef]
- Sciot, R.; Gerosa, C.; Faa, G. (Eds.) Adipocytic, Vascular and Skeletal Muscle Tumors: A Practical Diagnostic Approach; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Sleijfer, S.; Seynaeve, C.; Verweij, J. Using single-agent therapy in adult patients with advanced soft tissue sarcoma can still be considered standard care. Oncologist 2005, 10, 833–841. [Google Scholar] [CrossRef]
- Santoro, A.; Tursz, T.; Mouridsen, H.; Verweij, J.; Steward, W.; Somers, R.; Buesa, J.; Casali, P.; Spooner, D.; Rankin, E. Doxorubicin vs. CYVADIC vs. doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: A randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J. Clin. Oncol. 1995, 13, 1537–1545. [Google Scholar] [CrossRef]
- Borden, E.C.; Amato, D.A.; Rosenbaum, C.; Enterline, H.T.; Shiraki, M.J.; Creech, R.H.; Lerner, H.J.; Carbone, P.P. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J. Clin. Oncol. 1987, 5, 840–850. [Google Scholar] [CrossRef]
- Langmans, C.; Cornillie, J.; van Cann, T.; Wozniak, A.; Hompes, D.; Sciot, R.; Debiec-Rychter, M.; Vandenbempt, I.; Schöffski, P. Retrospective Analysis of Patients with Advanced Liposarcoma in a Tertiary Referral Center. Oncol. Res. Treat. 2019, 42, 396–404. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: Results of a phase III randomized multicenter clinical trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin vs. dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- D’Incalci, M.; Galmarini, C.M. A review of trabectedin (ET-743): A unique mechanism of action. Mol. Cancer Ther. 2010, 9, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Dybdal-Hargreaves, N.F.; Risinger, A.L.; Mooberry, S.L. Eribulin mesylate: Mechanism of action of a unique microtubule-targeting agent. Clin. Cancer Res. 2015, 21, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, P.; Deb, B.; Kumar, P. Multifarious targets beyond microtubules-role of eribulin in cancer therapy. Front. Biosci. (School Ed.) 2021, 13, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; O’Shaughnessy, J.; Loesch, D.; Blum, J.L.; Vahdat, L.T.; Petrakova, K.; Chollet, P.; Manikas, A.; Diéras, V.; Delozier, T.; et al. Eribulin monotherapy vs. treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet 2011, 377, 914–923. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Trojani, M.; Contesso, G.; Coindre, J.M.; Rouesse, J.; Bui, N.B.; De Mascarel, A.; Goussot, J.F.; David, M.; Bonichon, F.; Lagarde, C. Soft-tissue sarcomas of adults—Study of pathological prognostic variables and definition of a histopathological grading system. Int. J. Cancer 1984, 33, 37–42. [Google Scholar] [CrossRef]
- Funahashi, Y.; Okamoto, K.; Adachi, Y.; Semba, T.; Uesugi, M.; Ozawa, Y.; Tohyama, O.; Uehara, T.; Kimura, T.; Watanabe, H.; et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. 2014, 105, 1334–1342. [Google Scholar] [CrossRef]
- Kawano, S.; Asano, M.; Adachi, Y.; Matsui, J. Antimitotic and Non-mitotic Effects of Eribulin Mesilate in Soft Tissue Sarcoma. Anticancer Res. 2016, 36, 1553–1561. [Google Scholar]
- Nagy, J.A.; Chang, S.-H.; Dvorak, A.M.; Dvorak, H.F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Chouaib, S.; Noman, M.Z.; Kosmatopoulos, K.; Curran, M.A. Hypoxic stress: Obstacles and opportunities for innovative immunotherapy of cancer. Oncogene 2017, 36, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kruczynski, A.; Poli, M.; Dossi, R.; Chazottes, E.; Berrichon, G.; Ricome, C.; Giavazzi, R.; Hill, B.T.; Taraboletti, G. Anti-angiogenic, vascular-disrupting and anti-metastatic activities of vinflunine, the latest vinca alkaloid in clinical development. Eur. J. Cancer 2006, 42, 2821–2832. [Google Scholar] [CrossRef]
- Hill, S.; Lonergan, S.; Denekamp, J.; Chaplin, D. Vinca alkaloids: Anti-vascular effects in a murine tumour. Eur. J. Cancer 1993, 29A, 1320–1324. [Google Scholar] [CrossRef]
- van der Graaf, W.T.; Blay, J.-Y.; Chawla, S.P.; Kim, D.-W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef]
- Sleijfer, S.; Ray-Coquard, I.; Papai, Z.; Le Cesne, A.; Scurr, M.; Schöffski, P.; Collin, F.; Pandite, L.; Marreaud, S.; De Brauwer, A.; et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J. Clin. Oncol. 2009, 27, 3126–3132. [Google Scholar] [CrossRef]
- Chamberlain, F.E.; Wilding, C.; Jones, R.L.; Huang, P. Pazopanib in patients with advanced intermediate-grade or high-grade liposarcoma. Expert Opin. Investig. Drugs 2019, 28, 505–511. [Google Scholar] [CrossRef]
- Samuels, B.L.; Chawla, S.P.; Somaiah, N.; Staddon, A.P.; Skubitz, K.M.; Milhem, M.M.; Kaiser, P.E.; Portnoy, D.C.; Priebat, D.A.; Walker, M.S.; et al. Results of a prospective phase 2 study of pazopanib in patients with advanced intermediate-grade or high-grade liposarcoma. Cancer 2017, 123, 4640–4647. [Google Scholar] [CrossRef]
- Valverde, C.M.; Martin Broto, J.; Lopez-Martin, J.A.; Romagosa, C.; Sancho Marquez, M.P.; Carrasco, J.A.; Poveda, A.; Bauer, S.; Martinez-Trufero, J.; Cruz, J.; et al. Phase II clinical trial evaluating the activity and tolerability of pazopanib in patients (pts) with advanced and/or metastatic liposarcoma (LPS): A joint Spanish Sarcoma Group (GEIS) and German Interdisciplinary Sarcoma Group (GISG) Study—NCT01692496. J. Clin. Oncol. 2016, 34 (Suppl. S15), 11039. [Google Scholar] [CrossRef]
- Grünwald, V.; Karch, A.; Schuler, M.; Schöffski, P.; Kopp, H.-G.; Bauer, S.; Kasper, B.; Lindner, L.H.; Chemnitz, J.-M.; Crysandt, M.; et al. Randomized Comparison of Pazopanib and Doxorubicin as First-Line Treatment in Patients with Metastatic Soft Tissue Sarcoma Age 60 Years or Older: Results of a German Intergroup Study. J. Clin. Oncol. 2020, 38, 3555–3564. [Google Scholar] [CrossRef]
- Gavert, N.; Ben-Ze’ev, A. Epithelial–mesenchymal transition and the invasive potential of tumors. Trends Mol. Med. 2008, 14, 199–209. [Google Scholar] [CrossRef]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Ozawa, Y.; Kimura, T.; Sato, Y.; Kuznetsov, G.; Xu, S.; Uesugi, M.; Agoulnik, S.; Taylor, N.; Funahashi, Y.; et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial–mesenchymal transition (EMT) to mesenchymal–epithelial transition (MET) states. Br. J. Cancer 2014, 110, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Escudero, J.; Heredia-Soto, V.; Wang, Y.; Ruiz, P.; Hu, Y.; Gallego, A.; Pozo-Kreilinger, J.J.; Martinez-Marin, V.; Berjon, A.; Ortiz-Cruz, E.; et al. Eribulin activity in soft tissue sarcoma monolayer and three-dimensional cell line models: Could the combination with other drugs improve its antitumoral effect? Cancer Cell Int. 2021, 21, 646. [Google Scholar] [CrossRef] [PubMed]


| Patient ID | Age at Diagnosis | Gender | Histology | Primary | Stage at Diagnosis | Grade (FNCLCC Grading System) at Diagnosis | Disease Sites at Enrollment | Prior Treatments | Time (Months) from Diagnosis to Eribulin Treatment |
|---|---|---|---|---|---|---|---|---|---|
| M01 | 60 | F | DDLPS (spindle cells in myxoid stroma) | retroperitoneum | III | G2 or G3 * | abdomen, soft tissues | EI × 2/trabe × 9 | 22 |
| M02 | 44 | F | DDLPS (lipoblastic differentiation) | retroperitoneum | III | G3 | abdomen | AI × 3/trabe × 10 | 94 |
| M03 | 59 | M | WDLPS/ DDLPS (focal hypercellularity) | retroperitoneum | III | G2 or G3 * | abdomen | trabe × 4 | 91 |
| M04 | 71 | M | DDLPS (spindle and pleomorphic cells in myxoid stroma) | retroperitoneum | III | G3 | abdomen, bone, lung | A × 5/trabe × 4 | 90 |
| M05 | 34 | F | DDLPS (spindle and pleomorphic cells) | extremities | III | G2 | lung, soft tissues | AI × 6/ifo × 3/trabe × 7 | 40 |
| M06 | 42 | F | DDLPS (spindle and pleomorphic cells) | retroperitoneum | III | G3 | abdomen | trabe × 4/ifo × 2 | 64 |
| M07 | 28 | F | MLPS (high grade) | extremities | III | G3 | abdomen, bone | AI × 5/trabe × 22/ ifo × 3/gem × 3 | 128 |
| M08 | 80 | M | DDLPS (spindle and pleomorphic cells in myxoid stroma) | mediastinum | III | G3 | lung, liver | ifo × 6/trabe × 4 | 18 |
| M09 | 67 | M | MLPS (high grade) | extremities | III | G3 | bone, soft tissues | trabe × 40/ifo × 3 | 70 |
| M10 | 42 | M | DDLPS (myxofibrosarcoma-like component) | retroperitoneum | III | G3 | abdomen | trabe × 8/ifo × 6 | 123 |
| M11 | 63 | M | DDLPS (spindle and pleomorphic cells) | retroperitoneum | III | G3 | lung | A × 3/ifo × 5 | 49 |
| Patient ID | Histology | Target Lesions Site | Pre-Treatment General Assessment | Pre-Treatment Perfusion | Pre-Treatment Permeability | Post-Treatment General Assessment | Post-Treatment Perfusion | Post-Treatment Permeability |
|---|---|---|---|---|---|---|---|---|
| M01 | DDLPS | abdomen | one worst nodule in bulky mass | high in worst nodule | high in worst nodule | PD (worst nodule no more detectable, overall increased bulky mass) | significantly higher | stable |
| M02 | DDLPS | abdomen | homogeneous mass | high in 2 regions | high in 2 regions | PD (1 nodule increased, 1 nodule stable) | higher | significantly lower in stable nodule, remains high in increased nodule |
| M03 | WDLPS/ DDLPS | abdomen | homogeneous multifocal masses | low | low | SD | stable (low) | stable (low) |
| M05 | DDLPS | thigh | all high-grade nodule | high in rim, low in center (necrosis) | NA | PD (overall dimension) | stable (high in rim, low in center—necrosis) | high (no comparison available) |
| M06 | DDLPS | abdomen | one worst nodule, multifocal disease | very high | very high | PR | significantly lower | significantly lower |
| M07 | MLPS | abdomen | homogeneous nodules | low | low | SD | lower | lower |
| M09 | MLPS | trunk | homogeneous nodules | high in rim, low in center (necrosis) | low | SD | lower | lower |
| M10 | DDLPS | abdomen | two worst nodules in bulky mass | high in 2 worst nodules, low in center (necrosis) | high in 2 worst nodules rims | PD (increased worst nodules dimension) | high in 2 worst nodules rims, low in the rest of the mass | stable (high in 2 worst nodules rims, low in the rest of the mass) |
| Patient ID | Histology | Pre-Treatment Assessment | Post-Treatment Assessment |
|---|---|---|---|
| M01 | DDLPS | Myxoid stroma, low cellularity, high vascularization | Significant cellularity reduction |
| M02 | DDLPS | Many blood vessels, uneven cellularity, pleomorphic cells | Significantly less vascularized |
| M03 | WDLPS/DDLPS | Adipose tissue with slightly higher cellularity (WDLPS) | No significant variations |
| M05 | DDLPS | High cellularity | Lower cellularity, more blood vessels, necrosis |
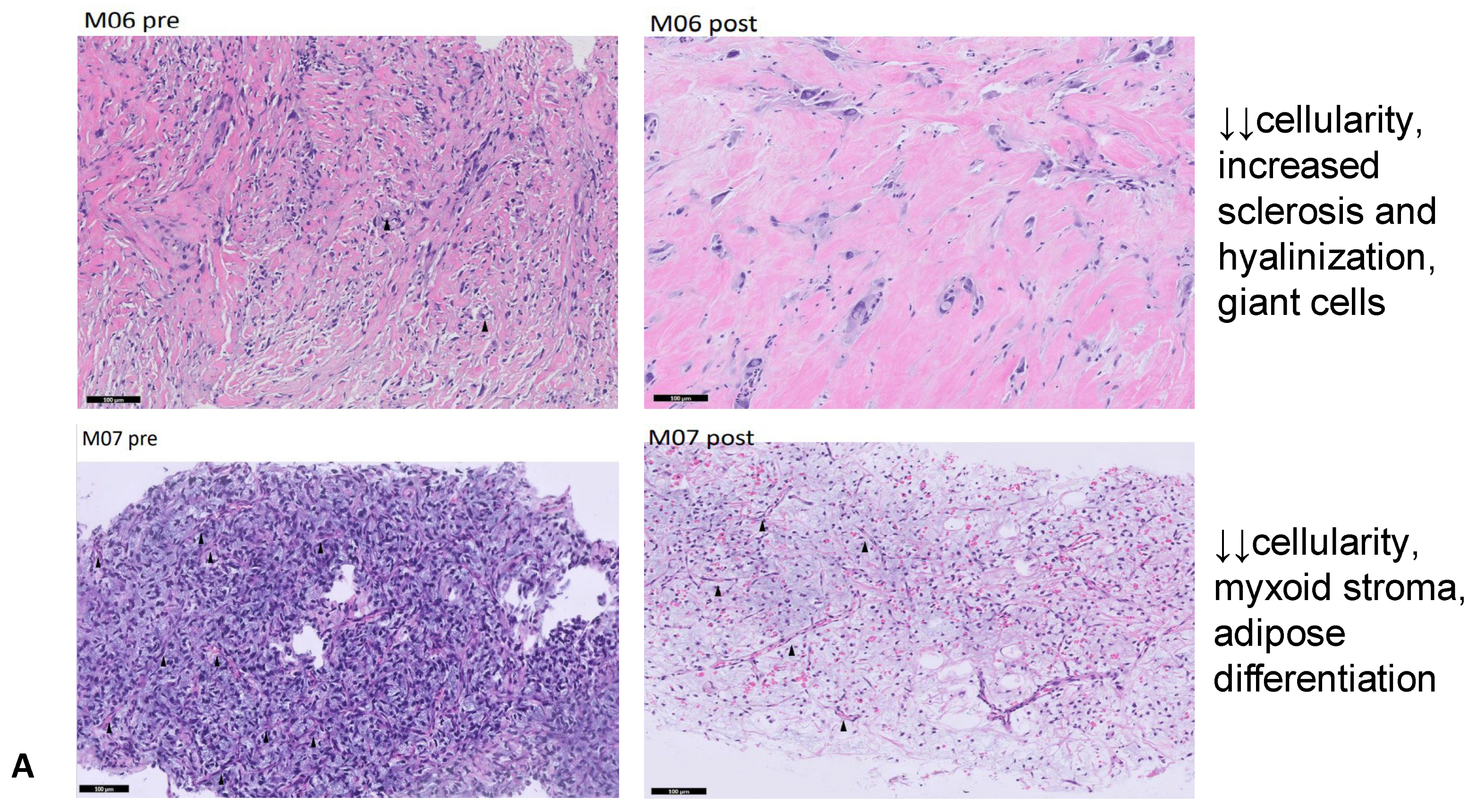
| M06 | DDLPS | High cellularity, scarce pleomorphism | Significant cellularity reduction, increased sclerosis and hyalinization, giant cells |
| M07 | MLPS | High cellularity, many blood vessels | Significant cellularity reduction, more myxoid stroma, lipoblasts and signs of adipose differentiation |
| M09 | MLPS | Homogeneous MLPS | Increased sclerosis and hyalinization and signs of adipose differentiation |
| M10 | DDLPS | High necrosis, no lipoblasts | No necrosis, signs of adipose differentiation |
| Patient ID | Histology | Protocol Completion | Total Eribulin Cycles | RECIST Response (Target Lesions) | DCE-MRI Response | Histology Changes | Concordance Between RECIST, MRI and Histology |
|---|---|---|---|---|---|---|---|
| M01 | DDLPS | yes | 4 | PD (29 × 24 cm vs. 19 × 15 cm) | PD | ↓↓ cellularity | 67% |
| M02 | DDLPS | yes | 4 | PD (7 × 6.7 cm vs. 5.8 × 3.5 cm, 6 × 5 cm vs. 3.8 × 3.5 cm) | PD | ↓↓ vascularization | 67% |
| M03 | WDLPS/ DDLPS | yes | 3 years | SD (6.5 × 4.8 cm vs. 7.5 × 4.8 cm) | SD | none | 100% |
| M04 | DDLPS | no | 4 | PD (10 × 8 cm vs. 8.3 × 3.5 cm) | NA | NA | NA |
| M05 | DDLPS | yes | 4 | PD (9.6 × 5.4 cm vs. 6.5 × 4.3 cm) | PD | ↓ cellularity, ↑ vascularization, necrosis | 100% |
| M06 | DDLPS | yes | 9 | SD (2.8 × 2 cm vs. 2.5 × 1.8 cm, 4.5 × 4.3 cm vs. 4.9 × 4.8 cm) | PR | ↓↓ cellularity, increased sclerosis and hyalinization, giant cells | 100% |
| M07 | MLPS | yes | 8 | PR (8.7 × 5 cm vs. 13.9 × 64, 6.3 × 4.4 cm vs. 7.8 × 7.5 cm) | SD | ↓↓ cellularity, myxoid stroma, adipose differentiation | 100% |
| M08 | DDLPS | no | 2 | PD (new multiple lung and mediastinum lesions, increased dimension of prior lesions) | NA | NA | NA |
| M09 | MLPS | yes | 4 | PD (new abdominal lesion, SD in target lesions: 9.5 × 8.2 cm vs. 9.8 × 7.8 cm, 5.8 × 3.9 cm vs. 5.6 × 4.2 cm) | SD | Increased sclerosis and hyalinization, adipose differentiation | 67% |
| M10 | DDLPS | yes | 3 | PD (9.8 × 5.3 cm vs. 6.9 × 4.9 cm, 7.9 × 4.6 cm vs. 6.5 × 4.9 cm) | PD | adipose differentiation | 67% |
| M11 | DDLPS | no | 1 | PD (new clinically significant pleural effusion) | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grimaudo, M.S.; D’Orazio, F.; Renne, S.L.; D’Incalci, M.; Maki, R.G.; Colombo, P.; Balzarini, L.; Laffi, A.; Santoro, A.; Bertuzzi, A.F. Assessment of the Mechanisms of Action of Eribulin in Patients with Advanced Liposarcoma Through the Evaluation of Radiological, Functional, and Tissue Responses: A Prospective Monocentric Study (Malibu Study). Cancers 2025, 17, 976. https://doi.org/10.3390/cancers17060976
Grimaudo MS, D’Orazio F, Renne SL, D’Incalci M, Maki RG, Colombo P, Balzarini L, Laffi A, Santoro A, Bertuzzi AF. Assessment of the Mechanisms of Action of Eribulin in Patients with Advanced Liposarcoma Through the Evaluation of Radiological, Functional, and Tissue Responses: A Prospective Monocentric Study (Malibu Study). Cancers. 2025; 17(6):976. https://doi.org/10.3390/cancers17060976
Chicago/Turabian StyleGrimaudo, Maria Susanna, Federico D’Orazio, Salvatore Lorenzo Renne, Maurizio D’Incalci, Robert G. Maki, Piergiuseppe Colombo, Luca Balzarini, Alice Laffi, Armando Santoro, and Alexia Francesca Bertuzzi. 2025. "Assessment of the Mechanisms of Action of Eribulin in Patients with Advanced Liposarcoma Through the Evaluation of Radiological, Functional, and Tissue Responses: A Prospective Monocentric Study (Malibu Study)" Cancers 17, no. 6: 976. https://doi.org/10.3390/cancers17060976
APA StyleGrimaudo, M. S., D’Orazio, F., Renne, S. L., D’Incalci, M., Maki, R. G., Colombo, P., Balzarini, L., Laffi, A., Santoro, A., & Bertuzzi, A. F. (2025). Assessment of the Mechanisms of Action of Eribulin in Patients with Advanced Liposarcoma Through the Evaluation of Radiological, Functional, and Tissue Responses: A Prospective Monocentric Study (Malibu Study). Cancers, 17(6), 976. https://doi.org/10.3390/cancers17060976








