Immunoscore Predicted by Dynamic Contrast-Enhanced Computed Tomography Can Be a Non-Invasive Biomarker for Immunotherapy Susceptibility of Hepatocellular Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
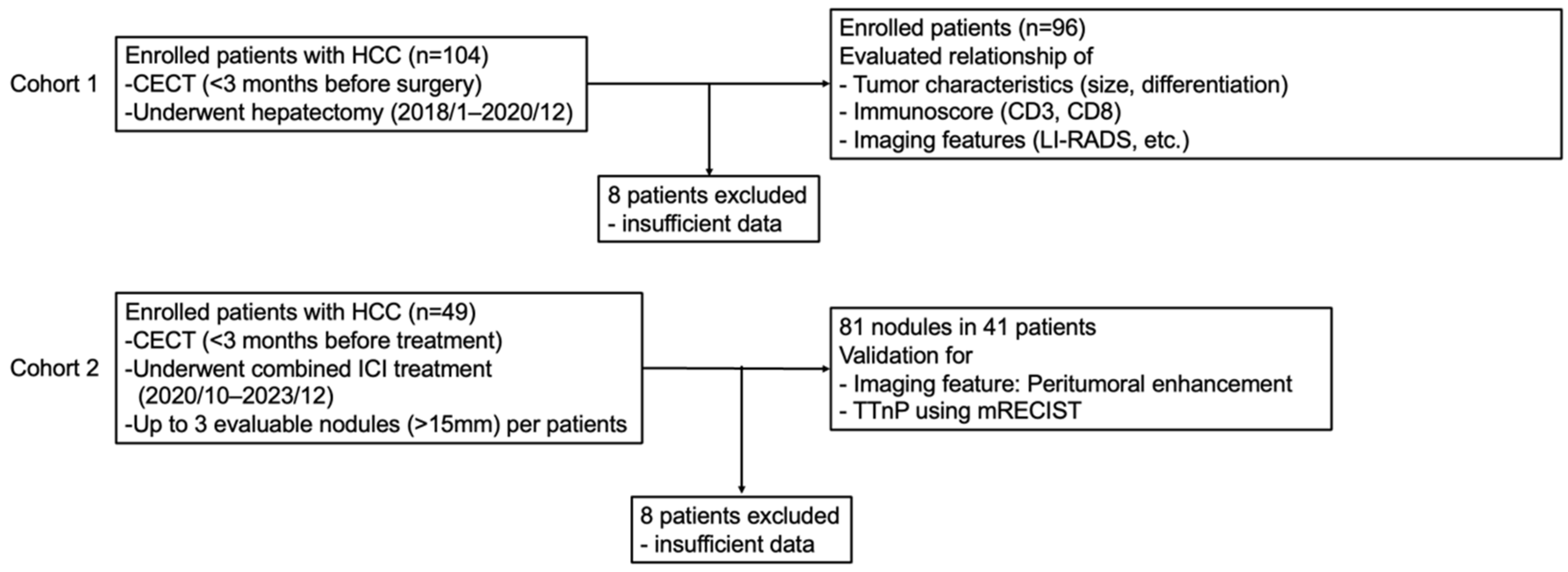
2.1. Study Design
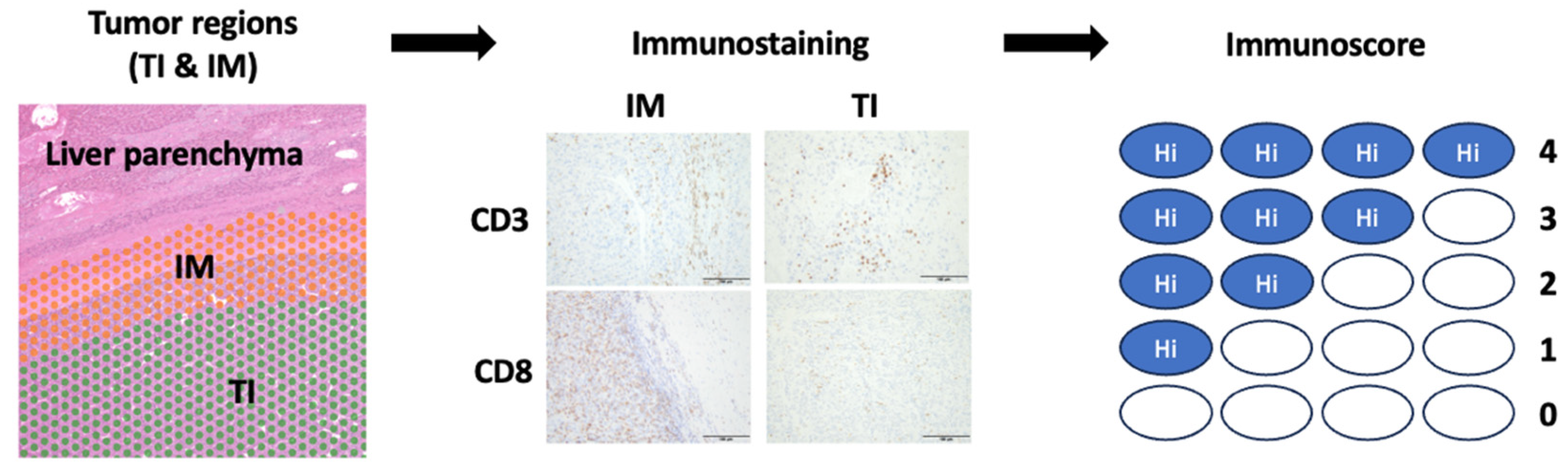
2.2. Histological Analysis
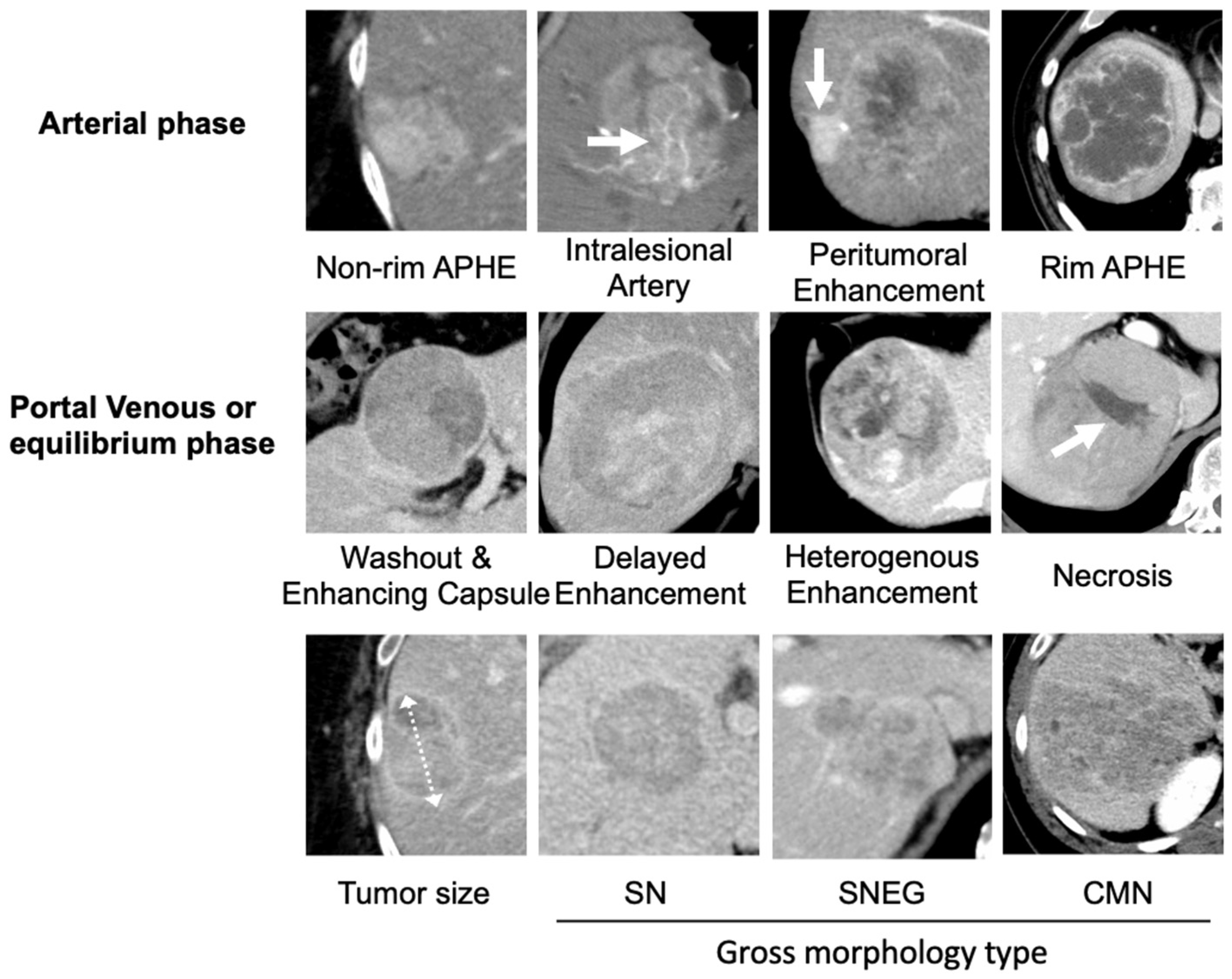
2.3. Image Analysis
2.4. Statistical Analyses
3. Results
3.1. Cohort 1
3.1.1. Characteristics
3.1.2. Peritumoral Enhancement of CECT Findings Could Predict Immunoscore
3.2. Cohort 2
Susceptibility to Combined Immunotherapy of Nodules with Identified CECT Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC | hepatocellular carcinoma |
| CECT | contrast-enhanced computed tomography |
| PD-L1 | programmed death-ligand 1 |
| TTnP | Time to nodular progression |
| FFPE | formalin-fixed embedded |
| TI | tumor interior |
| SN | simple nodular |
| SNEG | single nodular type with extranodular growth |
| CMN | confluent multinodular |
| LI-RADS | Liver Imaging Reporting and Data System |
| non-rim APHE | non-rim arterial phase hyperenhancement |
| ICI | immune checkpoint inhibitor |
| EOB-MRI | gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging |
References
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Gabrielson, A.; Wu, Y.; Wang, H.; Jiang, J.; Kallakury, B.; Gatalica, Z.; Reddy, S.; Kleiner, D.; Fishbein, T.; Johnson, L.; et al. Intratumoral CD3 and CD8 T-cell densities associated with relapse-free survival in HCC. Cancer Immunol. Res. 2016, 4, 419–430. [Google Scholar] [CrossRef]
- Liu, W.R.; Tian, M.X.; Tang, Z.; Fang, Y.; Zhou, Y.F.; Song, S.S.; Jiang, X.F.; Wang, H.; Tao, C.Y.; Zhou, P.Y.; et al. Nine-factor-based immunohistochemistry classifier predicts recurrence for early-stage hepatocellular carcinoma after curative resection. Br. J. Cancer 2020, 123, 92–100. [Google Scholar] [CrossRef]
- Lehrich, B.M.; Zhang, J.; Monga, S.P.; Dhanasekaran, R. Battle of the biopsies: Role of tissue and liquid biopsy in hepatocellular carcinoma. J. Hepatol. 2024, 80, 515–530. [Google Scholar] [CrossRef]
- Aoki, T.; Nishida, N.; Ueshima, K.; Morita, M.; Chishina, H.; Takita, M.; Hagiwara, S.; Ida, H.; Minami, Y.; Yamada, A.; et al. Higher enhancement intrahepatic nodules on the hepatobiliary phase of GD-EOB-DTPA-enhanced MRI as a poor responsive marker of anti-PD-1/PD-l1 monotherapy for unresectable hepatocellular carcinoma. Liver Cancer 2021, 10, 615–628. [Google Scholar] [CrossRef]
- Murai, H.; Kodama, T.; Maesaka, K.; Tange, S.; Motooka, D.; Suzuki, Y.; Shigematsu, Y.; Inamura, K.; Mise, Y.; Saiura, A.; et al. Multiomics identifies the link between intratumor steatosis and the exhausted tumor immune microenvironment in hepatocellular carcinoma. Hepatology 2023, 77, 77–91. [Google Scholar] [CrossRef]
- Kunichika, H.; Minamiguchi, K.; Tachiiri, T.; Shimizu, K.; Taiji, R.; Yamada, A.; Nakano, R.; Irizato, M.; Yamauchi, S.; Marugami, A.; et al. Prediction of efficacy for Atezolizumab/Bevacizumab in unresectable hepatocellular carcinoma with hepatobiliary-phase gadolinium ethoxybenzyl-diethylenetriaminepentaacetic acid MRI. Cancers 2024, 16, 2275. [Google Scholar] [CrossRef]
- Ueshima, E.; Sofue, K.; Kodama, T.; Yamamoto, S.; Komatsu, M.; Komatsu, S.; Ishihara, N.; Umeno, A.; Yamaguchi, T.; Hori, M.; et al. Gadoxetic acid-enhanced magnetic resonance imaging features can predict immune-excluded phenotype of hepatocellular carcinoma. Liver Cancer 2024, 1–15. [Google Scholar] [CrossRef]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) version 2018: Imaging of hepatocellular carcinoma in at-risk patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, H.; Zhao, H.; Jiang, Y.; Liu, Q.; Chen, Q.; Wang, W.; Rong, P. Preoperative CT for characterization of aggressive macrotrabecular-massive subtype and vessels that encapsulate tumor clusters pattern in hepatocellular carcinoma. Radiology 2021, 300, 219–229. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.H.; Lee, J.E.; Sinn, D.H.; Park, C.K. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J. Hepatol. 2017, 67, 526–534. [Google Scholar] [CrossRef]
- Li, M.G.; Zhang, Y.N.; Hu, Y.Y.; Li, L.; Lyu, H.L. Preoperative prediction of microvascular invasion classification in hepatocellular carcinoma based on clinical features and MRI parameters. Oncol. Lett. 2024, 28, 310. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, S.; Zou, W.; Zhao, X.; Chen, H.; He, R.; Jia, N.; Song, K.; Liu, W.; Wang, P. Advancing microvascular invasion diagnosis: A multi-center investigation of novel MRI-based models for precise detection and classification in early-stage small hepatocellular carcinoma (≤3 cm). Abdom. Radiol. 2024, 28. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Matsui, O.; Kobayashi, S.; Sanada, J.; Kouda, W.; Ryu, Y.; Kozaka, K.; Kitao, A.; Nakamura, K.; Gabata, T. Hepatocelluar nodules in liver cirrhosis: Hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom. Imaging 2011, 36, 264–272. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, J.M.; Sirlin, C.B. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: Part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 2014, 273, 30–50. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Moretto, R.; Rossini, D.; Catteau, A.; Antoniotti, C.; Giordano, M.; Boccaccino, A.; Ugolini, C.; Proietti, A.; Conca, V.; Kassambara, A.; et al. Dissecting tumor lymphocyte infiltration to predict benefit from immune-checkpoint inhibitors in metastatic colorectal cancer: Lessons from the AtezoT RIBE study. J. Immunother. Cancer 2023, 11, e006633. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Kelly, C.; Hay, J.; Sansom, O.; Maka, N.; Oien, K.; Iveson, T.; Saunders, M.; Kerr, R.; Tomlinson, I.; et al. Prognostic and Predictive Value of Immunoscore in Stage III Colorectal Cancer: Pooled Analysis of Cases From the SCOT and IDEA-HORG Studies. J. Clin. Oncol. 2024, 42, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Åkerla, J.; Helminen, O.; Väyrynen, J.P.; Parkkinen, A.; Järvenpää, H.; Böhm, J.; Ahtiainen, M.; Seikkula, H. CD3+ and CD8+ T cell-based immune cell score as a prognostic factor in clear-cell renal cell carcinoma. Acta Oncol. 2024, 63, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Miyake, T.; Maehira, H.; Shiohara, M.; Iida, H.; Nitta, N.; Tani, M. Contribution of Immunoscore to Survival Prediction in Pancreatic Ductal Adenocarcinoma. Anticancer. Res. 2024, 44, 4483–4492. [Google Scholar] [CrossRef]
- Brummel, K.; Eerkens, A.L.; de Bruyn, M.; Nijman, H.W. Tumour-infiltrating lymphocytes: From prognosis to treatment selection. Br. J. Cancer 2023, 128, 451–458. [Google Scholar] [CrossRef]
- Aizaz, M.; Khan, A.S.; Khan, M.; Musazade, E.; Yang, G. Advancements in tumor-infiltrating lymphocytes: Historical insights, contemporary milestones, and future directions in oncology therapy. Crit. Rev. Oncol. Hematol. 2024, 202, 104471. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Y.M.; Liu, Z.Y.; Zhang, X.; Zhou, Y.; Jiang, J.L.; Zhu, P.; Yang, X.M.; Tang, J.; Chen, Z.N. Prognostic Landscape of Tumor-Infiltrating T and B Cells in Human Cancer. Front. Immunol. 2022, 12, 731329. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Bibeau, F.; Greillier, L.; Fumet, J.D.; Ilie, A.; Monville, F.; Laugé, C.; Catteau, A.; Boquet, I.; Majdi, A.; et al. Immunoscore immune checkpoint using spatial quantitative analysis of CD8 and PD-L1 markers is predictive of the efficacy of anti- PD1/PD-L1 immunotherapy in non-small cell lung cancer. EBiomedicine 2023, 92, 104633. [Google Scholar] [CrossRef]
- Antoniotti, C.; Carullo, M.; Rossini, D.; Pietrantonio, F.; Salvatore, L.; Lonardi, S.; Tamberi, S.; Sciortino, C.; Conca, V.; Calegari, M.A.; et al. Liver metastases do not predict resistance to the addition of atezolizumab to first-line FOLFOXIRI plus bevacizumab in proficient MMR metastatic colorectal cancer: A secondary analysis of the AtezoTRIBE study. ESMO Open 2025, 10, 104135. [Google Scholar] [CrossRef]
- Melero, I.; Yau, T.; Kang, Y.K.; Kim, T.Y.; Santoro, A.; Sangro, B.; Kudo, M.; Hou, M.M.; Matilla, A.; Tovoli, F.; et al. Nivolumab plus ipilimumab combination therapy in patients with advanced hepatocellular carcinoma previously treated with sorafenib: 5-year results from CheckMate 040. Ann. Oncol. 2024, 35, 537–548. [Google Scholar] [CrossRef]

| Patient Characteristics | n = 96 |
|---|---|
| Age [range, SD] (years) | 72 [33–91, 9.9] |
| Sex (female/male) | 20/76 |
| Etiology (HBV/HCV/NBNC) | 21/27/48 |
| Alcohol usage | 36 |
| BMI [range, SD] (kg/m2) | 23.2 [16.1–39.0, 4.1] |
| Diabetes mellitus | 31 |
| Liver cirrhosis | 15 |
| Child–Pugh (A/B/C) | 82/12/2 |
| BCLC stage (A/B/C) | 49/30/17 |
| Number of tumors [range, SD], | 1 [1–10] |
| AFP [range, SD], (ng/mL) | 9 [1.5–533,413, 65,978] |
| DCP [range, SD], (AU/L) | 219 [15–473,139, 58,368] |
| Differentiation (wel/mod/por) | 9/69/18 |
| IHC Findings | n = 96 |
| TI CD3 | 58 [3–921] |
| TI CD8 | 14 [0–585] |
| IM CD3 | 96 [8–950] |
| IM CD8 | 21 [0–671] |
| Immunoscore (0/1/2/3/4-points) | 41/10/22/16/7 |
| CT Findings | n = 96 |
| Tumor size [range, SD] (mm) | 57 [15–160, 38] |
| Gross morphology (SN/SNEG/CM) | 53/28/15 |
| Non-rim APHE | 80 |
| Washout | 93 |
| Enhancing capsule | 78 |
| Rim APHE | 15 |
| Peritumoral enhancement | 40 |
| Delayed enhancement | 18 |
| Heterogenous enhancement | 69 |
| Intralesional artery | 48 |
| Necrosis | 34 |
| Variables | Estimate | Standard Error | 95% CI | |t| | p-Value | VIF |
|---|---|---|---|---|---|---|
| Univariate | ||||||
| Tumor size (mm) | 0.034 | 0.037 | 0.107 to 0.039 | 0.932 | 0.354 | - |
| Gross morphology (SN vs. SNEG/CM) | −0.547 | 0.318 | −1.177 to 0.084 | 1.720 | 0.089 | - |
| Nonrim APHE | −0.642 | 0.362 | 1.360 to 0.077 | 1.774 | 0.079 | - |
| Washout | 0.705 | 0.503 | −0.294 to 1.703 | 1.401 | 0.165 | - |
| Enhancing capsule | 0.590 | 0.355 | −0.114 to 1.294 | 1.663 | 0.100 | - |
| Rim APHE | −1.002 | 0.373 | −1.742 to −0.263 | 2.690 | 0.009 ** | - |
| Peritumoral enhancement | −1.076 | 0.274 | −1.621 to −0.532 | 3.928 | <0.001 ** | - |
| Delayed enhancement | 0.528 | 0.383 | −0.232 to 1.289 | 1.380 | 0.171 | - |
| Heterogenous enhancement | 0.047 | 0.294 | −0.536 to 0.630 | 0.162 | 0.872 | - |
| Intralesional artery | 0.128 | 0.281 | −0.430 to 0.686 | 0.456 | 0.650 | - |
| Necrosis | −0.250 | 0.297 | 0.839 to 0.339 | 0.842 | 0.402 | - |
| Multivariate | ||||||
| Intercept | 2.323 | 0.332 | 1.664 to 2.981 | 7.004 | <0.001 | |
| Rim APHE | −0.432 | 0.407 | −1.240 to 0.375 | 1.063 | 0.290 | 1.289 |
| Peritumoral enhancement | −0.920 | 0.311 | −1.537 to −0.302 | 2.958 | 0.004 ** | 1.289 |
| Patient Characteristics | n = 40 (Patients) |
|---|---|
| Age [range, SD], (years) | 70 [48–83, 8] |
| Sex (female/male) | 8/32 |
| Etiology (HBV/HCV/NBNC) | 7/11/22 |
| Child–Pugh (A/B/C) | 34/5/1 |
| BCLC stage (A/B/C) | 0/21/19 |
| Number of tumors enrolled [range, SD], | 2 [1–3] |
| ICI treatment (Ate + Bev/Dur + Tre) | 26/14 |
| CT Findings | n = 81 (Nodules) |
| Tumor size [range, SD], (mm) | 39.7 [11–157, 35] |
| Gross morphology (SN/SNEG/CM) | 39/25/17 |
| Peritumoral enhancement | 27 |
| Treatment Effect | n = 81 (Nodules) |
| Best response CR/PR/SD/PD | 7/22/38/14 |
| TTnP median | 294 days |
| Uncensored/censored | 41/40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueshima, E.; Sofue, K.; Komatsu, S.; Ishihara, N.; Komatsu, M.; Umeno, A.; Nishiuchi, K.; Kozuki, R.; Yamaguchi, T.; Matsuura, T.; et al. Immunoscore Predicted by Dynamic Contrast-Enhanced Computed Tomography Can Be a Non-Invasive Biomarker for Immunotherapy Susceptibility of Hepatocellular Carcinoma. Cancers 2025, 17, 948. https://doi.org/10.3390/cancers17060948
Ueshima E, Sofue K, Komatsu S, Ishihara N, Komatsu M, Umeno A, Nishiuchi K, Kozuki R, Yamaguchi T, Matsuura T, et al. Immunoscore Predicted by Dynamic Contrast-Enhanced Computed Tomography Can Be a Non-Invasive Biomarker for Immunotherapy Susceptibility of Hepatocellular Carcinoma. Cancers. 2025; 17(6):948. https://doi.org/10.3390/cancers17060948
Chicago/Turabian StyleUeshima, Eisuke, Keitaro Sofue, Shohei Komatsu, Nobuaki Ishihara, Masato Komatsu, Akihiro Umeno, Kentaro Nishiuchi, Ryohei Kozuki, Takeru Yamaguchi, Takanori Matsuura, and et al. 2025. "Immunoscore Predicted by Dynamic Contrast-Enhanced Computed Tomography Can Be a Non-Invasive Biomarker for Immunotherapy Susceptibility of Hepatocellular Carcinoma" Cancers 17, no. 6: 948. https://doi.org/10.3390/cancers17060948
APA StyleUeshima, E., Sofue, K., Komatsu, S., Ishihara, N., Komatsu, M., Umeno, A., Nishiuchi, K., Kozuki, R., Yamaguchi, T., Matsuura, T., Tada, T., & Murakami, T. (2025). Immunoscore Predicted by Dynamic Contrast-Enhanced Computed Tomography Can Be a Non-Invasive Biomarker for Immunotherapy Susceptibility of Hepatocellular Carcinoma. Cancers, 17(6), 948. https://doi.org/10.3390/cancers17060948






