Therapeutic Potential of Prunus Species in Gastrointestinal Oncology
Simple Summary
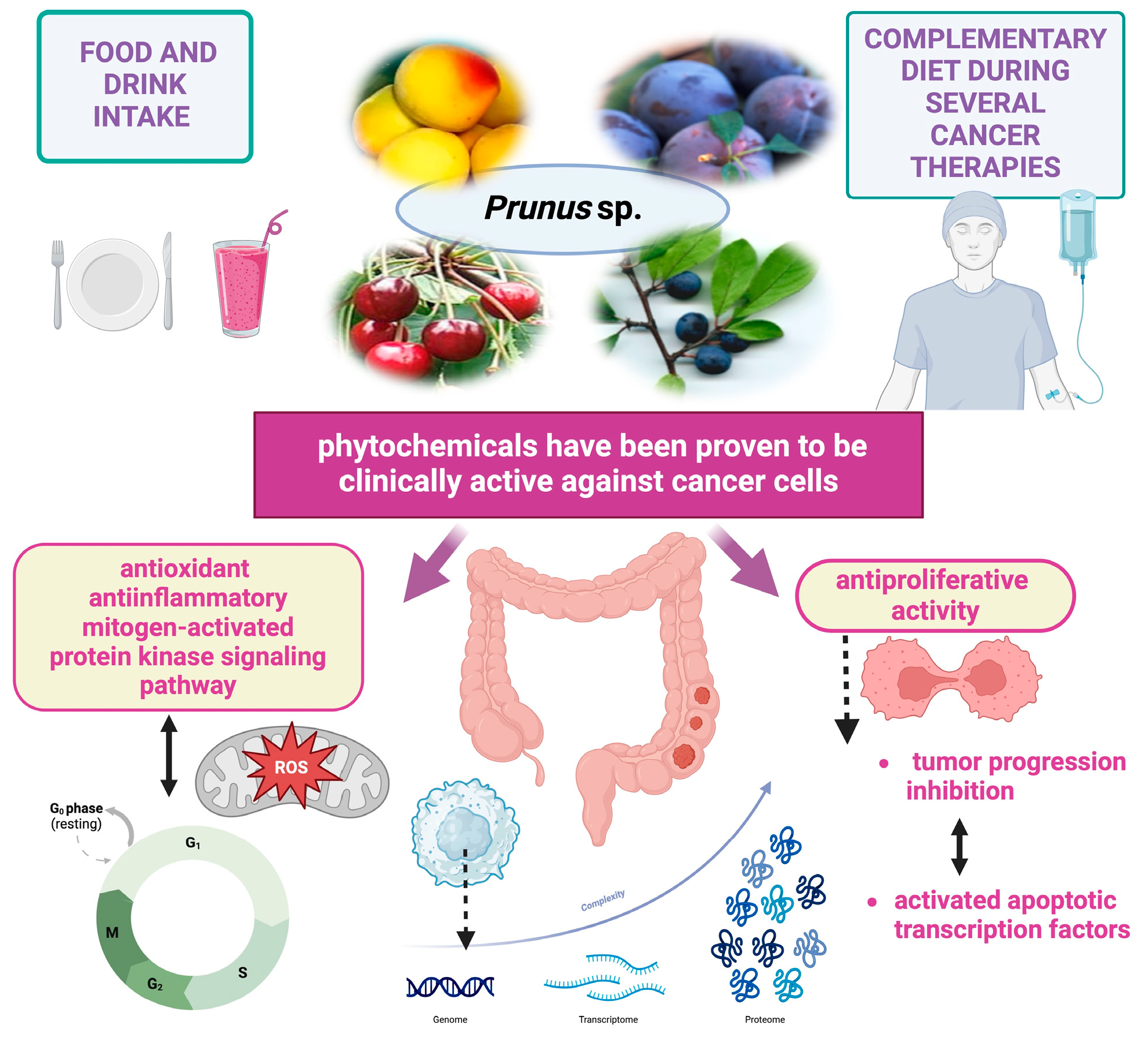
Abstract
1. Introduction
2. Materials and Methods
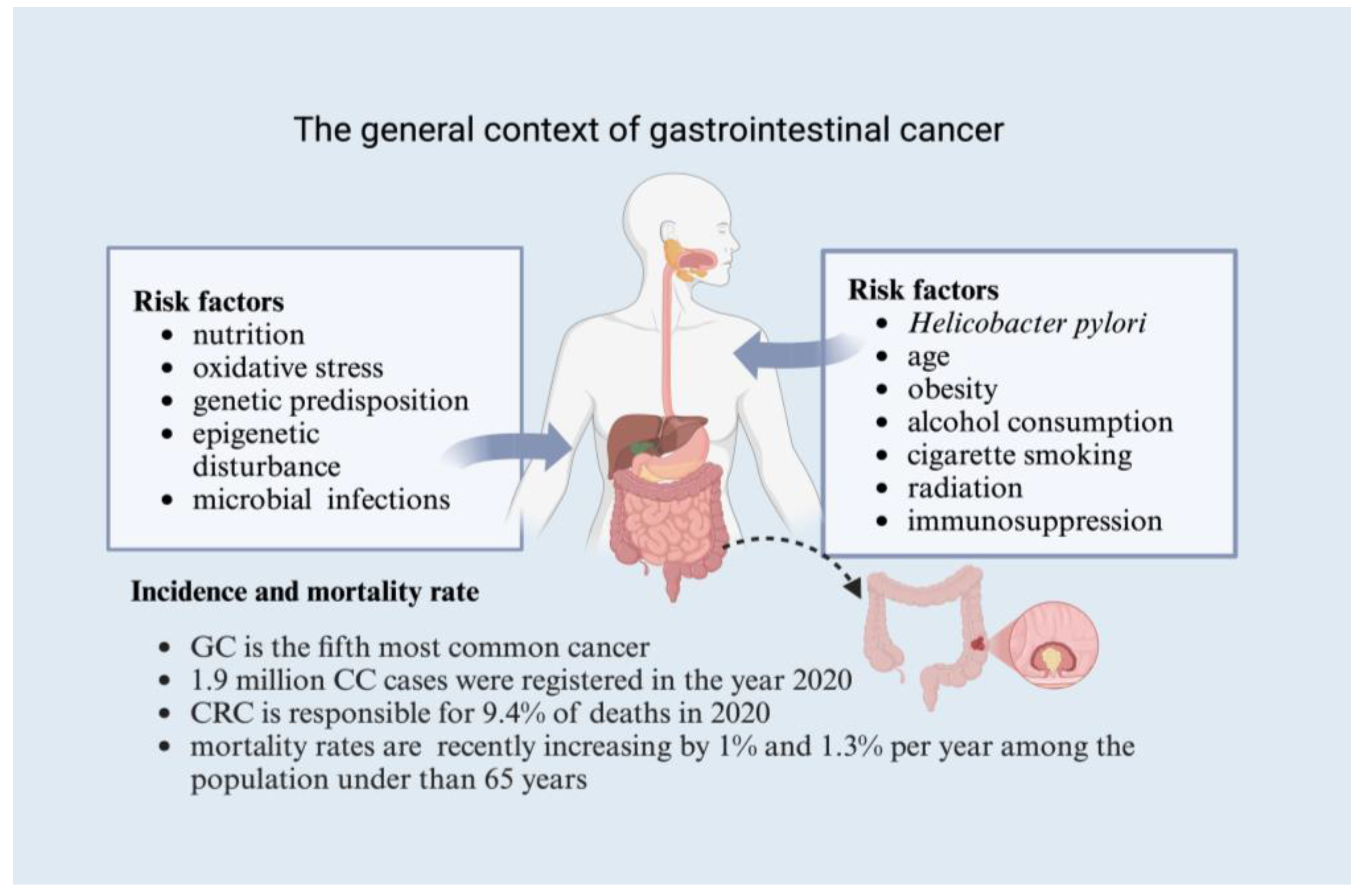
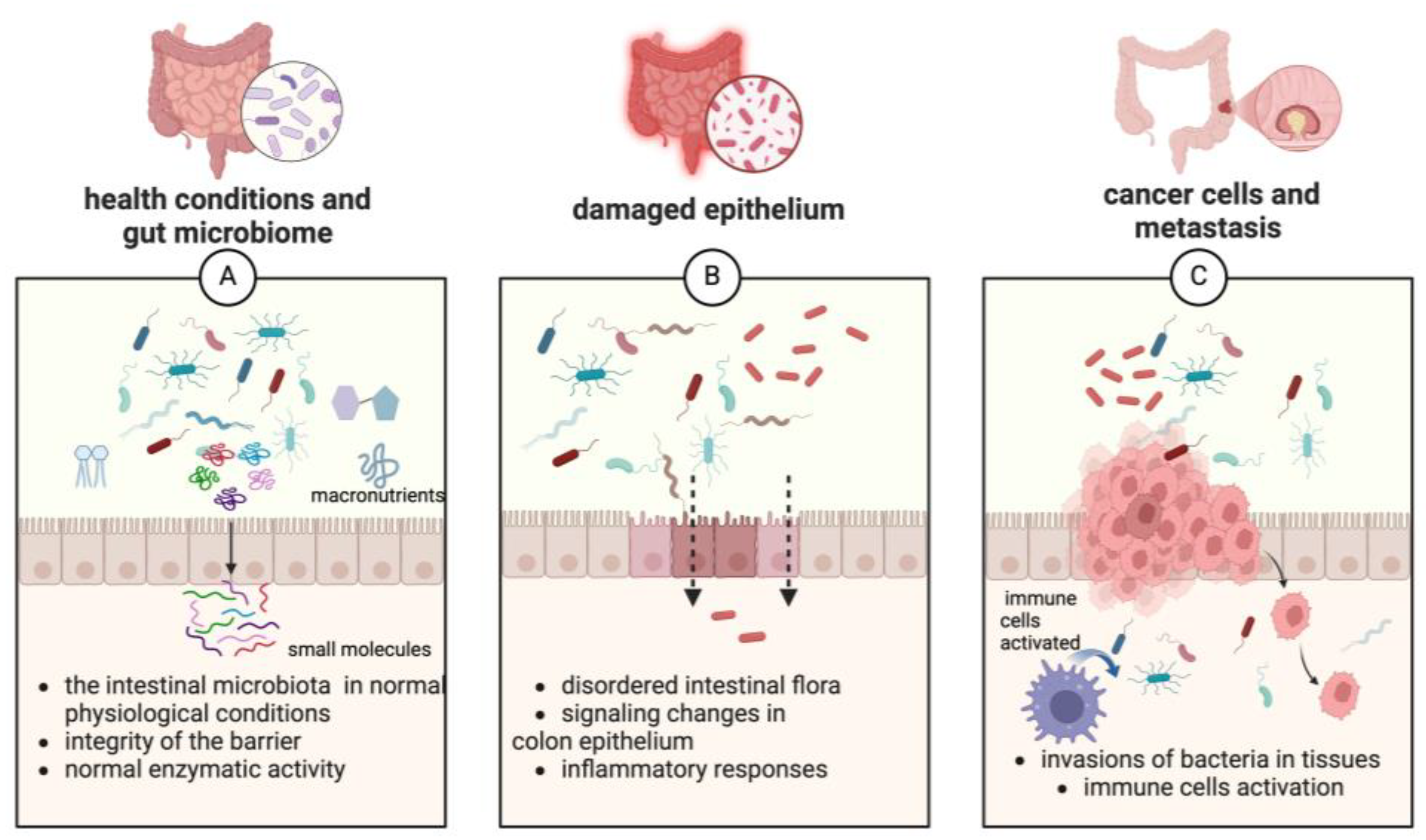
3. The Global Context of Gastrointestinal Cancer
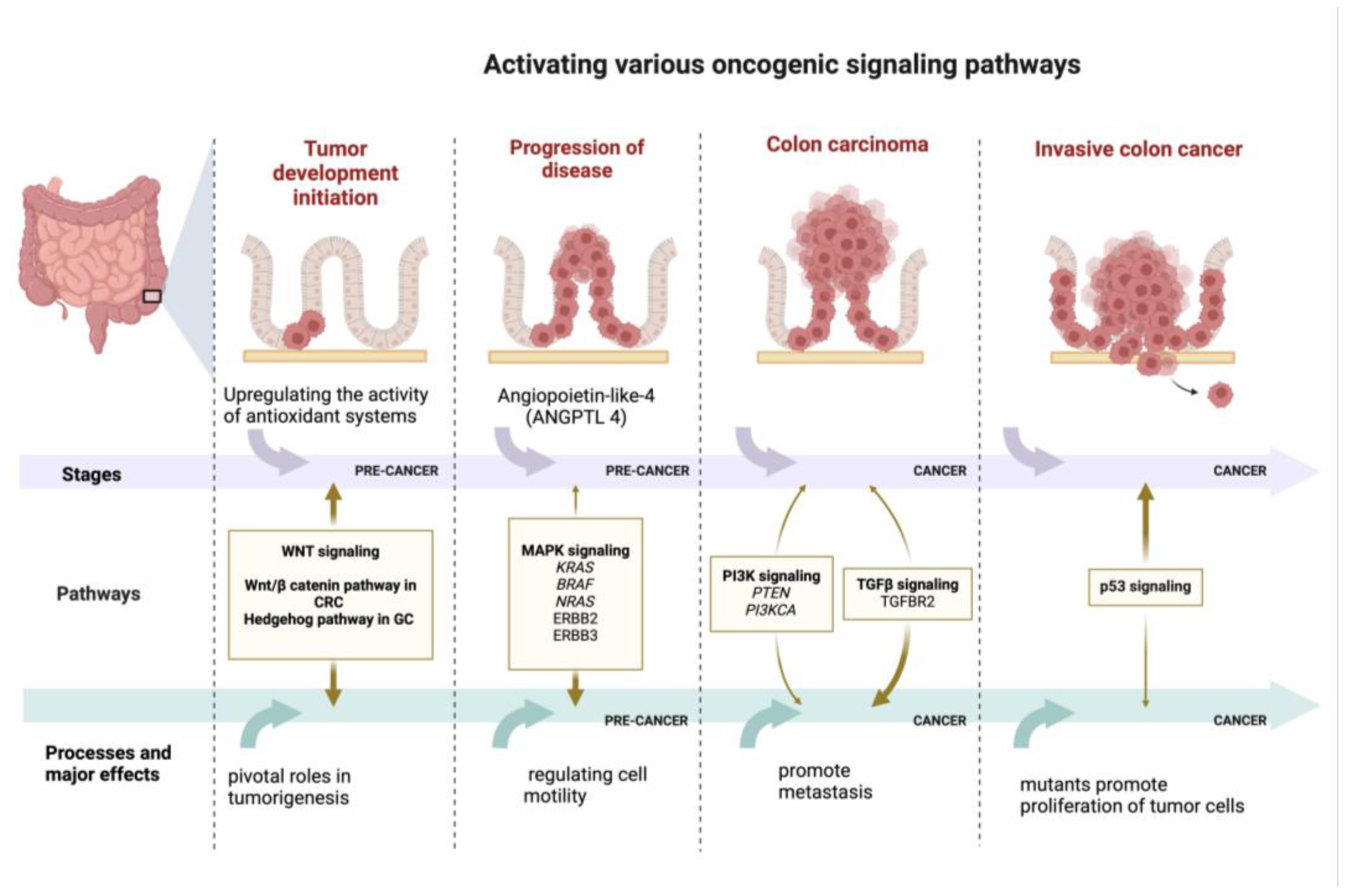
4. Key Signaling Pathways Associated with Gastrointestinal Cancer
- The phosphatidylinositol 3-kinase/protein kinase B/mechanistic target of rapamycin (PI3K/AKT/mTOR) signaling pathway is a highly relevant pathway for many pathological conditions, including cancer progression. It regulates the autophagy, apoptosis, and survival of various cancers, including malignant tumors of the gastrointestinal tract [10,71,72].
- NF-kB signaling pathway. Persistent inflammation is a well-known mechanism that may lead to the onset of neoplastic processes and may also stimulate tumorigenesis by inducing DNA damage. In addition, cytokine-specific receptor-mediated signaling pathways are modulated by inflammatory processes and control some of the most vital aspects of tumor initiation and promotion in CRC, such as activating signal transducer and activator of transcription 3 (STAT3) through interleukin-6 (IL-6) and interleukin-11 (IL-11) signaling as well as tumor necrosis factor (TNF) receptor-mediated and interleukin-1 (IL-1) receptor-mediated NF-κB activation [4,73,74,75,76,77,78]. These processes include promoting cell proliferation (by regulating cyclin D, c-Myc, and IL-6, which regulate growth-promoting signals), inhibiting apoptosis (by inhibiting apoptotic genes including B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma extra-large (BclxL) transcription), promoting angiogenesis (inducing vascular endothelial growth factor (VEGF) expression), promoting tumor invasion (through E-selectin and matrix metalloproteinases (MMPs)), promoting epithelial–mesenchymal transition (EMT) and colon cancer stem cells (CSCs), and mediating tumor drug resistance [4,45,75,76,77,78,79,80]. It has been shown that the exposure of gastric epithelial cells to H. pylori infection causes the rapid activation of NF-κB, with the nuclear translocation of p50/RelA and p50/p50 dimers leading to potent messenger RNA (mRNA) accumulation for interleukin-8 (IL-8) in vitro [10].
- The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway is commonly activated by growth factors and cytokines, playing an important role in inflammation-driven colorectal cancer. It influences the tumor microenvironment (TME), angiogenesis, and the mechanisms that enable the cancer to evade immune system detection [4,73,74,75,76,77,78]. Studies using in vivo and in vitro models have demonstrated that JAK/STAT signaling is deregulated in malignant transformation and, therefore, may contribute significantly to the expansion of a variety of solid tumors and hematopoietic malignancies [81]. Specifically, in gastric tumor formation, the dysregulated activation of the JAK/STAT pathway has been implicated [10,81].
- The Wnt/β-catenin signaling pathway has an important function in regulating essential cellular processes like determining cell fate, adult homeostasis, organ development during embryogenesis, motility, polarity, and stem cell renewal. It is known that one of the driving forces in cancer is the impairment of the main physiological signaling pathways present in tumor cells caused by the presence of certain mutations [10,54,82]. Moreover, in a recent study, for the first time, it has been shown that the virulence factor FadA, from Fusobacterium nucleatum, interacts with E-cadherin, which is a cell surface molecule that mediates metastasis in CRC by activating an essential component of the Wnt/β-catenin signaling pathway, which is known to be the most damaged by mutations in CRC [63,83]
- Hippo signaling pathway. The crosstalk between Wnt and other pathways is significant for CRC pathogenesis. The synergism that possibly influences apoptosis and cell growth in CRC is expressed through the transcriptional regulation of Yes-associated protein (YAP), an effector of the Hippo pathway, by the β-catenin/T-cell factor 4 (TCF4) complex [4,73,74,75,76,77,78]. The dysregulation of Hippo pathway signaling in GC and other solid tumors contributes to unregulated cell division and the activation of metastasis [10].
- Notch signaling pathway. Notch can modulate the Wnt pathway signaling, demonstrating a complex relationship with Wnt. APC mutation disturbs Wnt signaling but activates Notch, a pathway that is important for colonic lesions early in tumorigenesis. Also, further interplay among the Wnt and Ras pathways causes APC mutations to stabilize Ras to enhance its oncogenic potential by modifying its proteasomal degradation [4,73,74,75,76,77,78]. Proliferation, tumor cell survival, and tumorigenesis in vivo are promoted by the activation of Notch signaling through several isoforms of hairy and enhancer of split 1 (HES1) found in different cellular contexts [10,82].
- Hh signaling pathway. Currently, Hh signaling is increasingly recognized for its putative oncogenic role in CRC pathology (Figure 3). It has emerged as a master regulator in cell proliferation, differentiation, and embryonic patterning [84]. The Hh family of proteins control numerous cellular processes in mammals—their roles include survival, apoptosis, proliferation, differentiation, invasion, and migration [4,73,74,75,76,77,78]. Hh signaling has been identified as a key factor in the formation and differentiation of gastric glands during embryonic development. In the adult stomach, the Hh pathway is a regulatory pathway that governs the differentiation of gastric epithelial cells and the maintenance of their maturation state, being indispensable for the physiology of the stomach. Gastric cancer cells exhibit both increased sonic hedgehog (SHH) expression and higher levels of Patched 1 (PTCH1) receptor. As a result, the overproduction of SHH activates Hh signaling, which in turn drives GC cell proliferation and progression [10,82].
- MAPK signaling pathway. Numerous studies have shown that the extracellular signal-regulated kinase (ERK)/MAPK pathways and downstream molecules (KRAS and NRAS: RAS family genes; Ras: small G-protein; BRAF: B-Raf proto-oncogene serine/threonine kinase; ERBB2 and ERBB3: ERBB epidermal growth factor receptor) [79,85,86] play a role in regulating cell motility in both gastric cancer (GC) and normal epithelial cells. Specifically, in GC, the ERK pathway modulates MMP activities, thereby influencing cell migration and tumor invasion. In addition, the angiopoietin protein-like-4 (ANGPTL4) induced following hypoxia exerts multiple influences on gastric scirrhous carcinoma neoplasia (Figure 3). Through the ANGPTL4-induced activation of the focal adhesion kinase (FAK)/Src/phosphoinositide 3-kinase (PI3K)-AKT/ERK signaling pathway, GC cells acquire anoikis resistance, which contributes to peritoneal metastasis [4,73,74,75,76,77,78].
- Transforming growth factor beta (TGF-β/Smad) signaling pathway. The TGF-b signaling pathway is an important modulator of intestinal homeostasis and inflammation; thus, the dysregulation of this pathway may be associated with carcinogenesis [87], related to the presence of inflammation in the gastrointestinal tract. In the early stages of neoplastic development, it acts as a tumor suppressor, but, in the later stages of the disease, it can shift its role to facilitate EMT and promote metastasis [4,10,73,74,75,76,77].
- TLRs signaling pathway. TLRs are type I transmembrane glycoproteins that exhibit a structure containing a repetitive sequence in the extracellular domain that is rich in leucine, a highly conserved homologous Toll/IL-1R domain (TIR) in the cytosolic region, and a transmembrane domain and a homologous Toll/IL-1R domain, with similarities with the signaling domain of IL-1R family members [10].
5. Therapeutic Strategies
6. The Role of Exploring Natural Therapies in Cancer
6.1. Natural Bioactive Compounds Used in Cancer Prevention and Therapy
6.2. Phytochemical Composition and Therapeutic Potential of Prunus Species
7. Mechanisms of Action of the Major Classes of Bioactive Compounds Found in Prunus Species Against Gastrointestinal Cancer
8. Preclinical Studies on Anticancer Implications of Prunus Species
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deep, A.; Kumar, D.; Bansal, N.; Narasimhan, B.; Marwaha, R.K.; Sharma, P.C. Understanding Mechanistic Aspects and Therapeutic Potential of Natural Substances as Anticancer Agents. Phytomedicine Plus 2023, 3, 100418. [Google Scholar] [CrossRef]
- Canga, I.; Vita, P.; Oliveira, A.I.; Castro, M.Á.; Pinho, C. In Vitro Cytotoxic Activity of African Plants: A Review. Molecules 2022, 27, 4989. [Google Scholar] [CrossRef] [PubMed]
- Omara, T.; Kiprop, A.K.; Ramkat, R.C.; Cherutoi, J.; Kagoya, S.; Moraa Nyangena, D.; Azeze Tebo, T.; Nteziyaremye, P.; Nyambura Karanja, L.; Jepchirchir, A.; et al. Medicinal Plants Used in Traditional Management of Cancer in Uganda: A Review of Ethnobotanical Surveys, Phytochemistry, and Anticancer Studies. Evid. Based Complement. Altern. Med. 2020, 2020, 3529081. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Mechchate, H.; Oumeslakht, L.; Zeouk, I.; Aboulaghras, S.; Balahbib, A.; Zengin, G.; Kamal, M.A.; Gallo, M.; Montesano, D.; et al. The Role of Epigenetic Modifications in Human Cancers and the Use of Natural Compounds as Epidrugs: Mechanistic Pathways and Pharmacodynamic Actions. Biomolecules 2022, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Abusaliya, A.; Ha, S.E.; Bhosale, P.B.; Kim, H.H.; Park, M.Y.; Vetrivel, P.; Kim, G.S. Glycosidic Flavonoids and Their Potential Applications in Cancer Research: A Review. Mol. Cell Toxicol. 2022, 18, 9–16. [Google Scholar] [CrossRef]
- Gaobotse, G.; Venkataraman, S.; Brown, P.D.; Masisi, K.; Kwape, T.E.; Nkwe, D.O.; Rantong, G.; Makhzoum, A. The Use of African Medicinal Plants in Cancer Management. Front. Pharmacol. 2023, 14, 1122388. [Google Scholar] [CrossRef]
- Bakrim, S.; El Omari, N.; El Hachlafi, N.; Bakri, Y.; Lee, L.H.; Bouyahya, A. Dietary Phenolic Compounds as Anticancer Natural Drugs: Recent Update on Molecular Mechanisms and Clinical Trials. Foods 2022, 11, 3323. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, Z.; Zhao, L.L.; Wang, W.; Gao, M.; Jia, X.; Ouyang, H.; He, J. Anti-Tumor Activities and Mechanisms of Traditional Chinese Medicines Formulas: A Review. Biomed. Pharmacother. 2020, 132, 110820. [Google Scholar] [CrossRef]
- Tesfaye, S.; Asres, K.; Lulekal, E.; Alebachew, Y.; Tewelde, E.; Kumarihamy, M.; Muhammad, I. Ethiopian Medicinal Plants Traditionally Used for the Treatment of Cancer, Part 2: A Review on Cytotoxic, Antiproliferative, and Antitumor Phytochemicals, and Future Perspective. Molecules 2020, 25, 4032. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Li, J.; Zhang, J.; Liu, D. Regulatory Effect of Traditional Chinese Medicines on Signaling Pathways of Process from Chronic Atrophic Gastritis to Gastric Cancer. Chin. Herb. Med. 2022, 14, 5–19. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Costa, A.R.; Flores-Félix, J.D.; Falcão, A.; Alves, G.; Silva, L.R. Anti-Inflammatory and Antiproliferative Properties of Sweet Cherry Phenolic-Rich Extracts. Molecules 2022, 27, 268. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting Apoptosis in Cancer Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Dogan, E.; Kara, H.G.; Kosova, B.; Cetintas, V.B. Targeting Apoptosis to Overcome Chemotherapy Resistance. In Metastasis; Exon Publications: Brisbane, QLD, Australia, 2022; pp. 163–180. [Google Scholar]
- Brodzicka, A.; Galanty, A.; Paśko, P. Modulation of Multidrug Resistance Transporters by Food Components and Dietary Supplements: Implications for Cancer Therapy Efficacy and Safety. Curr. Issues Mol. Biol. 2024, 46, 9686–9706. [Google Scholar] [CrossRef]
- Bajpai, P.; Usmani, S.; Kumar, R.; Prakash, O. Recent Advances in Anticancer Approach of Traditional Medicinal Plants: A Novel Strategy for Cancer Chemotherapy. Intell. Pharm. 2024, 2, 291–304. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Jahan, S.; Singh, R.; Saxena, J.; Ashraf, S.A.; Khan, A.; Choudhary, R.K.; Balakrishnan, S.; Badraoui, R.; Bardakci, F.; et al. Plants in Anticancer Drug Discovery: From Molecular Mechanism to Chemoprevention. Biomed. Res. Int. 2022, 2022, 5425485. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Daoud, S.; Mahmod, A.I.; Hamed, R.A.; Awajan, D.; Abuarab, S.F.; Odeh, L.H.; Khater, S.; Al Kury, L.T. Plants as a Source of Anticancer Agents: From Bench to Bedside. Molecules 2022, 27, 4818. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Wang, F.-X.; Jia, K.-K.; Kong, L.-D. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Jiménez-Garcia, M.; Capó, X.; Sönmez Gürer, E.; Sharopov, F.; Rachel, T.Y.L.; Ntieche Woutouoba, D.; Rescigno, A.; Peddio, S.; Zucca, P.; et al. Anticancer Properties of Bromelain: State-of-the-Art and Recent Trends. Front. Oncol. 2023, 12, 1068778. [Google Scholar] [CrossRef]
- Iancu, I.M.; Schröder, V.; Apetroaei, M.-R.; Crețu, R.M.; Mireșan, H.; Honcea, A.; Iancu, V.; Bucur, L.A.; Mitea, G.; Atodiresei-Pavalache, G. Biocompatibility of Membranes Based on a Mixture of Chitosan and Lythri Herba Aqueous Extract. Appl. Sci. 2023, 13, 8023. [Google Scholar] [CrossRef]
- Sianipar, N.F.; Muflikhati, Z.; Mangindaan, D.; Assidqi, K. Anticancer Potential of Tocopherols-Containing Plants and Semi-Synthetic Tocopherols. Plants 2024, 13, 2994. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, R.; Zhao, Z.; Xie, T.; Sui, X. Harnessing Phytochemicals: Innovative Strategies to Enhance Cancer Immunotherapy. Drug Resist. Updates 2025, 79, 101206. [Google Scholar] [CrossRef] [PubMed]
- Rizeq, B.; Gupta, I.; Ilesanmi, J.; AlSafran, M.; Rahman, M.M.; Ouhtit, A. The Power of Phytochemicals Combination in Cancer Chemoprevention. J. Cancer 2020, 11, 4521–4533. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Knaze, V.; Zamora-Ros, R. Polyphenols. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 512–521. [Google Scholar] [CrossRef]
- Samuel, H.S.; Akpanke, D.; Gideon, O.; Bulus, E.O.; Fatima, M.; Matilda, M.I. Antioxidant and Phytochemical Classification of Medicinal Plants Used in the Treatment of Cancer Disease. J. Chem. Lett. 2024, 5, 108–119. [Google Scholar] [CrossRef]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in Cancer Prevention: New Insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Mitra, S.; Tareq, A.M.; Das, R.; Emran, T.B.; Nainu, F.; Chakraborty, A.J.; Ahmad, I.; Tallei, T.E.; Idris, A.M.; Simal-Gandara, J. Polyphenols: A First Evidence in the Synergism and Bioactivities. Food Rev. Int. 2023, 39, 4419–4441. [Google Scholar] [CrossRef]
- Kluska, M.; Woźniak, K. Natural Polyphenols as Modulators of Etoposide Anti-Cancer Activity. Int. J. Mol. Sci. 2021, 22, 6602. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary Polyphenols in Chemoprevention and Synergistic Effect in Cancer: Clinical Evidences and Molecular Mechanisms of Action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.H.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Hwang, K.H.; Moon, Y.G.; Heo, J.D.; Seong, J.K.; Ahn, M.; et al. Potential Anticancer Effects of Isoflavone Prunetin and Prunetin Glycoside on Apoptosis Mechanisms. Int. J. Mol. Sci. 2024, 25, 11713. [Google Scholar] [CrossRef] [PubMed]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-Inflammatory and Anti-Allergic Potential of Dietary Flavonoids: A Review. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Y. The Potential Benefits of Polyphenols for Corneal Diseases. Biomed. Pharmacother. 2023, 169, 115862. [Google Scholar] [CrossRef]
- Bahrin, A.A.; Moshawih, S.; Dhaliwal, J.S.; Kanakal, M.M.; Khan, A.; Lee, K.S.; Goh, B.H.; Goh, H.P.; Kifli, N.; Ming, L.C. Cancer Protective Effects of Plums: A Systematic Review. Biomed. Pharmacother. 2022, 146, 112568. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lucero-Prisno, D.E.; Zhang, L.; Xu, W.; Wong, S.H.; Ng, S.C.; Wong, M.C.S. Updated Epidemiology of Gastrointestinal Cancers in East Asia. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 271–287. [Google Scholar] [CrossRef]
- Jardim, S.R.; de Souza, L.M.P.; de Souza, H.S.P. The Rise of Gastrointestinal Cancers as a Global Phenomenon: Unhealthy Behavior or Progress? Int. J. Environ. Res. Public Health 2023, 20, 3640. [Google Scholar] [CrossRef]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, L.; He, X.; Luo, Y. Gastrointestinal Cancers in China, the USA, and Europe. Gastroenterol. Rep. 2021, 9, 91–104. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, R.; Li, J.; Zeng, H.; Li, L.; Chen, R.; Sun, K.; Han, B.; Bray, F.; Wei, W.; et al. Global, Regional, and National Lifetime Risks of Developing and Dying from Gastrointestinal Cancers in 185 Countries: A Population-Based Systematic Analysis of GLOBOCAN. Lancet Gastroenterol. Hepatol. 2024, 9, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.; Cagir, B. Colon Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Guan, W.L.; He, Y.; Xu, R.H. Gastric Cancer Treatment: Recent Progress and Future Perspectives. J. Hematol. Oncol. 2023, 16, 57. [Google Scholar] [CrossRef]
- Zhou, M.; Li, J.; Luo, D.; Zhang, H.; Yu, Z.; Chen, Y.; Li, Q.; Liang, F.; Chen, R. Network Pharmacology and Molecular Docking–Based Investigation: Prunus mume Against Colorectal Cancer via Silencing RelA Expression. Front. Pharmacol. 2021, 12, 761980. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Badgwell, B.D. Current Treatment and Recent Progress in Gastric Cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef]
- Karakaş, N.; Okur, M.E.; Öztunç, N.; Polat, D.Ç.; Karadağ, A.E. Laurocerasus Officinalis Roem. Fruit Extract Induces Cell Death through Caspase Mediated Apoptosis in Gastric Cancer Cell Lines. Turk. J. Biochem. 2021, 46, 213–221. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Waldum, H.; Fossmark, R. Gastritis, Gastric Polyps and Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 6548. [Google Scholar] [CrossRef]
- Ahmed, M. Colon Cancer: A Clinician’s Perspective in 2019. Gastroenterol. Res. 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Condello, M.; Meschini, S. Role of Natural Antioxidant Products in Colorectal Cancer Disease: A Focus on a Natural Compound Derived from Prunus Spinosa, Trigno Ecotype. Cells 2021, 10, 3326. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut Microbiota in Colorectal Cancer: Biological Role and Therapeutic Opportunities. Cancers 2023, 15, 866. [Google Scholar] [CrossRef]
- Aghabozorgi, A.S.; Bahreyni, A.; Soleimani, A.; Bahrami, A.; Khazaei, M.; Ferns, G.A.; Avan, A.; Hassanian, S.M. Role of Adenomatous Polyposis Coli (APC) Gene Mutations in the Pathogenesis of Colorectal Cancer; Current Status and Perspectives. Biochimie 2019, 157, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt Signaling in Colorectal Cancer: Pathogenic Role and Therapeutic Target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef]
- Jalili, F.; Hajizadeh, M.; Mehrabani, S.; Ghoreishy, S.M.; MacIsaac, F. The Association between Neighborhood Socioeconomic Status and the Risk of Incidence and Mortality of Colorectal Cancer: A Systematic Review and Meta-Analysis of 1,678,582 Participants. Cancer Epidemiol. 2024, 91, 102598. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Reichmann, R.; Kaaks, R.; Jenab, M.; Bueno-de-Mesquita, H.B.; Dahm, C.C.; Eriksen, A.K.; Tjønneland, A.; Artaud, F.; Boutron-Ruault, M.-C.; et al. Development and Validation of a Lifestyle-Based Model for Colorectal Cancer Risk Prediction: The LiFeCRC Score. BMC Med. 2021, 19, 1. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Rodríguez-Barranco, M.; Ching-López, A.; Petrova, D.; Molina-Montes, E.; Amiano, P.; Barricarte-Gurrea, A.; Chirlaque, M.-D.; Agudo, A.; Sánchez, M.-J. Evidence Update on the Relationship between Diet and the Most Common Cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: A Systematic Review. Nutrients 2021, 13, 3582. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, F.; Tan, P.; Huang, H.; Wang, Z.; Xie, J.; Wang, L.; Liu, D.; Hu, Z. The Interactions between Traditional Chinese Medicine and Gut Microbiota in Cancers: Current Status and Future Perspectives. Pharmacol. Res. 2024, 203, 107148. [Google Scholar] [CrossRef]
- Walrath, T.; Dyamenahalli, K.U.; Hulsebus, H.J.; McCullough, R.L.; Idrovo, J.-P.; Boe, D.M.; McMahan, R.H.; Kovacs, E.J. Age-Related Changes in Intestinal Immunity and the Microbiome. J. Leukoc. Biol. 2021, 109, 1045–1061. [Google Scholar] [CrossRef]
- Pellanda, P.; Ghosh, T.S.; O’Toole, P.W. Understanding the Impact of Age-Related Changes in the Gut Microbiome on Chronic Diseases and the Prospect of Elderly-Specific Dietary Interventions. Curr. Opin. Biotechnol. 2021, 70, 48–55. [Google Scholar] [CrossRef]
- Burns, M.B.; Lynch, J.; Starr, T.K.; Knights, D.; Blekhman, R. Virulence Genes Are a Signature of the Microbiome in the Colorectal Tumor Microenvironment. Genome Med. 2015, 7, 55. [Google Scholar] [CrossRef]
- Ahmad Kendong, S.M.; Raja Ali, R.A.; Nawawi, K.N.M.; Ahmad, H.F.; Mokhtar, N.M. Gut Dysbiosis and Intestinal Barrier Dysfunction: Potential Explanation for Early-Onset Colorectal Cancer. Front. Cell Infect. Microbiol. 2021, 11, 744606. [Google Scholar] [CrossRef] [PubMed]
- Fellows, R.C.; Chun, S.K.; Larson, N.; Fortin, B.M.; Mahieu, A.L.; Song, W.A.; Seldin, M.M.; Pannunzio, N.R.; Masri, S. Disruption of the Intestinal Clock Drives Dysbiosis and Impaired Barrier Function in Colorectal Cancer. Sci. Adv. 2024, 10, eado1458. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Ma, X.; Xu, M.; Li, J. Potential of Natural Products and Gut Microbiome in Tumor Immunotherapy. Chin. Med. 2024, 19, 161. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Docherty, S.L.; Porter, L.S.; Bailey, D.E. Common and Co-Occurring Symptoms Experienced by Patients with Gastric Cancer. Oncol. Nurs. Forum 2020, 47, 187–202. [Google Scholar] [CrossRef]
- Adam, M.; Chang, G.J.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.A.; Deming, D.; Garrido-Laguna, I.; Grem, J.L.; Harmath, C.; Randolph Hecht, J.; et al. NCCN Guidelines Version 6.2024 Colon Cancer Continue NCCN Guidelines Panel Disclosures; NCCN: Plymouth Meeting, PA, USA, 2025. [Google Scholar]
- Wang, K.; Diao, M.; Yang, Z.; Liu, M.; Salvador, J.T. Identification of Subgroups of Patients with Gastrointestinal Cancers Based on Symptom Severity and Frequency: A Latent Profile and Latent Class Analysis. Eur. J. Oncol. Nurs. 2024, 68, 102479. [Google Scholar] [CrossRef] [PubMed]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Harsha, C.; Banik, K.; Ang, H.L.; Girisa, S.; Vikkurthi, R.; Parama, D.; Rana, V.; Shabnam, B.; Khatoon, E.; Kumar, A.P.; et al. Targeting Akt/Mtor in Oral Cancer: Mechanisms and Advances in Clinical Trials. Int. J. Mol. Sci. 2020, 21, 3285. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, L.; Kim, E.; Yi, J.; Huang, H.; Kim, H.; Raza, M.A.; Park, S.; Jang, S.; Kim, K.; et al. Rhein Induces Oral Cancer Cell Apoptosis and ROS via Suppresse AKT/MTOR Signaling Pathway In Vitro and In Vivo. Int. J. Mol. Sci. 2023, 24, 8507. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.-Q.; Luo, Q.; Wang, L.; Song, G.-B.; Sheng, J.-P.; Xu, B. Signaling Pathways Involved in Colorectal Cancer: Pathogenesis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Ahmad, R.; Singh, J.K.; Wunnava, A.; Al-Obeed, O.; Abdulla, M.; Srivastava, S.K. Emerging Trends in Colorectal Cancer: Dysregulated Signaling Pathways (Review). Int. J. Mol. Med. 2021, 47, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Ha, J.; Kim, J.; Cho, Y.; Ahn, J.; Cheon, C.; Kim, S.H.; Ko, S.G.; Kim, B. Natural Products for Pancreatic Cancer Treatment: From Traditional Medicine to Modern Drug Discovery. Nutrients 2021, 13, 3801. [Google Scholar] [CrossRef]
- Won, Y.S.; Kim, J.H.; Lizardo, R.C.M.; Min, H.J.; Cho, H.D.; Hong, S.M.; Seo, K. Il The Flavonol Isoquercitrin Promotes Mitochondrial-Dependent Apoptosis in Sk-Mel-2 Melanoma Cell via the Pi3k/Akt/Mtor Pathway. Nutrients 2020, 12, 3683. [Google Scholar] [CrossRef] [PubMed]
- Abusaliya, A.; Jeong, S.H.; Bhosale, P.B.; Kim, H.H.; Park, M.Y.; Kim, E.; Won, C.K.; Park, K.I.; Heo, J.D.; Kim, H.W.; et al. Mechanistic Action of Cell Cycle Arrest and Intrinsic Apoptosis via Inhibiting Akt/MTOR and Activation of P38-MAPK Signaling Pathways in Hep3B Liver Cancer Cells by Prunetrin—A Flavonoid with Therapeutic Potential. Nutrients 2023, 15, 3407. [Google Scholar] [CrossRef]
- Malki, A.; Elruz, R.A.; Gupta, I.; Allouch, A.; Vranic, S.; Al Moustafa, A.E. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int. J. Mol. Sci. 2021, 22, 130. [Google Scholar] [CrossRef]
- Stefani, C.; Miricescu, D.; Stanescu-Spinu, I.I.; Nica, R.I.; Greabu, M.; Totan, A.R.; Jinga, M. Growth Factors, Pi3k/Akt/Mtor and Mapk Signaling Pathways in Colorectal Cancer Pathogenesis: Where Are We Now? Int. J. Mol. Sci. 2021, 22, 10260. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Chen, T.; Cheng, X.; Xiao, H.; Meng, X.; Jiang, Y. Inhibition and Potential Treatment of Colorectal Cancer by Natural Compounds via Various Signaling Pathways. Front. Oncol. 2022, 12, 956793. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Seo, J.; Lee, M.; Kim, C.; Kim, E.; Yoon, G.; Cho, S.; Cho, Y.S.; Choi, H.W.; Shim, J.; et al. Licochalcone C Induced Apoptosis in Human Oral Squamous Cell Carcinoma Cells by Regulation of the JAK2/STAT3 Signaling Pathway. J. Cell Biochem. 2018, 119, 10118–10130. [Google Scholar] [CrossRef]
- Mericli, F.; Becer, E.; Kabadayi, H.; Hanoglu, A.; Hanoglu, D.Y.; Yavuz, D.O.; Ozek, T.; Vatansever, S. Fatty Acid Composition and Anticancer Activity in Colon Carcinoma Cell Lines of Prunus Dulcis Seed Oil. Pharm. Biol. 2017, 55, 1239–1248. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-Catenin Signaling in Cancers and Targeted Therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- Sigulinsky, C.L.; Li, X.; Levine, E.M. Expression of Sonic Hedgehog and Pathway Components in the Embryonic Mouse Head: Anatomical Relationships between Regulators of Positive and Negative Feedback. BMC Res. Notes 2021, 14, 300. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Śmiech, M.; Leszczyński, P.; Kono, H.; Wardell, C.; Taniguchi, H. Emerging BRAF Mutations in Cancer Progression and Their Possible Effects on Transcriptional Networks. Genes 2020, 11, 1342. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, H.; Xu, J.; Li, C.; Zhang, Y.; Wang, G.; Liu, Y.; Cai, S.; Fang, W.; Li, J.; et al. TGFBR2 Mutation Predicts Resistance to Immune Checkpoint Inhibitors in Patients with Non-Small Cell Lung Cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211038477. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive Oxygen Species in Cancer: Current Findings and Future Directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Rogoff, H.A. Implications of Reactive Oxygen Species on Cancer Formation and Its Treatment. Semin. Oncol. 2021, 48, 238–245. [Google Scholar] [CrossRef]
- Cuevas-Cianca, S.I.; Romero-Castillo, C.; Gálvez-Romero, J.L.; Juárez, Z.N.; Hernández, L.R. Antioxidant and Anti-Inflammatory Compounds from Edible Plants with Anti-Cancer Activity and Their Potential Use as Drugs. Molecules 2023, 28, 1488. [Google Scholar] [CrossRef]
- Powell, E.; Piwnica-Worms, D.; Piwnica-Worms, H. Contribution of P53 to Metastasis. Cancer Discov. 2014, 4, 405–414. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic Signaling Pathways and Anti-Angiogenic Therapy for Cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Singh, P.; Lim, B. Targeting Apoptosis in Cancer. Curr. Oncol. Rep. 2022, 24, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, S.; Zamanian, M.Y.; Ivraghi, M.S.; Golmohammadi, M.; Modanloo, M.; Kamiab, Z.; Pourhosseini, S.M.E.; Heidari, M.; Bazmandegan, G. A Comprehensive View on the Apigenin Impact on Colorectal Cancer: Focusing on Cellular and Molecular Mechanisms. Food Sci. Nutr. 2023, 11, 6789–6801. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Peterson, C.Y.; Sriram, D.; Mahipal, A. Early Stage Colon Cancer: Current Treatment Standards, Evolving Paradigms, and Future Directions. World J. Gastrointest. Oncol. 2020, 12, 808–832. [Google Scholar] [CrossRef]
- Patel, T.H.; Cecchini, M. Targeted Therapies in Advanced Gastric Cancer. Curr. Treat. Options Oncol. 2020, 21, 70. [Google Scholar] [CrossRef]
- Hossain, S.; Yousaf, M.; Liu, Y.; Chang, D.; Zhou, X. An Overview of the Evidence and Mechanism of Drug–Herb Interactions Between Propolis and Pharmaceutical Drugs. Front. Pharmacol. 2022, 13, 876183. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic Colorectal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Shinji, S.; Yamada, T.; Matsuda, A.; Sonoda, H.; Ohta, R.; Iwai, T.; Takeda, K.; Yonaga, K.; Masuda, Y.; Yoshida, H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J. Nippon. Med. Sch. 2022, 89, 246–254. [Google Scholar] [CrossRef]
- Ohishi, T.; Kaneko, M.K.; Yoshida, Y.; Takashima, A.; Kato, Y.; Kawada, M. Current Targeted Therapy for Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 1702. [Google Scholar] [CrossRef]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B., III; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. 2022, 41, 678–700. [Google Scholar] [CrossRef]
- Winters, D.A.; Soukup, T.; Sevdalis, N.; Green, J.S.A.; Lamb, B.W. The Cancer Multidisciplinary Team Meeting: In Need of Change? History, Challenges and Future Perspectives. BJU Int. 2021, 128, 271–279. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Muskała, M.; Merecz-Sadowska, A.; Sikora, J.; Picot, L.; Sitarek, P. Anti-Inflammatory and Anticancer Effects of Anthocyanins in In Vitro and In Vivo Studies. Antioxidants 2024, 13, 1143. [Google Scholar] [CrossRef] [PubMed]
- Shalayel, M.H.F.; Al-Mazaideh, G.M.; Alanezi, A.A.; Almuqati, A.F.; Alotaibi, M. Diosgenin and Monohydroxy Spirostanol from Prunus Amygdalus Var Amara Seeds as Potential Suppressors of EGFR and HER2 Tyrosine Kinases: A Computational Approach. Pharmaceuticals 2023, 16, 704. [Google Scholar] [CrossRef] [PubMed]
- Petran, M.; Dragoș, D.; Stoian, I.; Vlad, A.; Gilca, M. Current Use of Medicinal Plants for Children’s Diseases among Mothers in Southern Romania. Front. Pharmacol. 2024, 15, 1377341. [Google Scholar] [CrossRef]
- Talib, W.H.; Baban, M.M.; Bulbul, M.F.; Al-Zaidaneen, E.; Allan, A.; Al-Rousan, E.W.; Ahmad, R.H.Y.; Alshaeri, H.K.; Alasmari, M.M.; Law, D. Natural Products and Altered Metabolism in Cancer: Therapeutic Targets and Mechanisms of Action. Int. J. Mol. Sci. 2024, 25, 9593. [Google Scholar] [CrossRef]
- Kasapoğlu, K.N.; Altin, G.; Farooqi, A.A.; Salehi, B.; Özçelik, B.; Setzer, W.N.; Sharifi-Rad, J. Anti-Proliferative, Genotoxic and Cytotoxic Effects of Phytochemicals Isolated from Anatolian Medicinal Plants. Cell Mol. Biol. 2020, 66, 145–159. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, W.; Jahan, I.; Zhai, R.; Yao, K.; Yan, J.; Li, P. The Cytotoxic Activity and Metabolic Profiling of Hyptis rhomboidea Mart. et Gal. Molecules 2024, 29, 4216. [Google Scholar] [CrossRef]
- Mahmood, M.A.; Abd, A.H.; Kadhim, E.J. Assessing the Cytotoxicity of Phenolic and Terpene Fractions Extracted from Iraqi Prunus Arabica on AMJ13 and SK-GT-4 Human Cancer Cell Lines. F1000Resarch 2023, 12, 433. [Google Scholar] [CrossRef]
- Meschini, S.; Pellegrini, E.; Condello, M.; Occhionero, G.; Delfine, S.; Condello, G.; Mastrodonato, F. Cytotoxic and Apoptotic Activities of Prunus Spinosa Trigno Ecotype Extract on Human Cancer Cells. Molecules 2017, 22, 1578. [Google Scholar] [CrossRef]
- ALI, S.; Munazir, M. Medicinal Plants Used in the Management of Cancer and Other Diseases in Swat District, Pakistan. Asian J. Nat. Prod. Biochem. 2024, 22, 8–18. [Google Scholar] [CrossRef]
- Calvo-Castro, L.A.; Irías-Mata, A.; Cano-Contreras, D.; Arnáez-Serrano, E.; Chacón-Cerdas, R.; Starbird-Pérez, R.; Morales-Sánchez, J.; Centeno-Cerdas, C. Self-Emulsifying Micellization of Crude Extracts from Apple (Malus domestica cv. Anna), Plum (Prunus domestica cv. Satsuma), and Guava (Psidium guajava L.) Fruits. Molecules 2023, 28, 1297. [Google Scholar] [CrossRef]
- Sharma, E.; Attri, D.C.; Sati, P.; Dhyani, P.; Szopa, A.; Sharifi-Rad, J.; Hano, C.; Calina, D.; Cho, W.C. Recent Updates on Anticancer Mechanisms of Polyphenols. Front. Cell Dev. Biol. 2022, 10, 1005910. [Google Scholar] [CrossRef] [PubMed]
- Gökalp, F.D.; Qipa, E. Cytotoxic and Apoptotic Effects of Prunus spinosa Fruit Extract on HT-29 Colon Cancer Line. Int. J. Plant Based Pharm. 2024, 4, 64–70. [Google Scholar] [CrossRef]
- Mitea, G.; Schröder, V.; Iancu, I.M.; Mireșan, H.; Iancu, V.; Bucur, L.A.; Badea, F.C. Molecular Targets of Plant-Derived Bioactive Compounds in Oral Squamous Cell Carcinoma. Cancers 2024, 16, 3612. [Google Scholar] [CrossRef]
- Mitea, G.; Iancu, I.M.; Blebea, N.M.; Bucur, L.A.; Schroder, V.; Badea, V.; Badea, C.F.; Nuca, C.; Radu, M.D.; Iancu, V. Cotinine and IL-6—Biomarkers for Estimating Pharmacological Processes in Various Forms of Periodontitis. In Proceedings of the 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 18–19 November 2021; pp. 1–4. [Google Scholar]
- Fonseca, L.R.S.; Silva, G.R.; Luís, Â.; Cardoso, H.J.; Correia, S.; Vaz, C.V.; Duarte, A.P.; Socorro, S. Sweet Cherries as Anti-Cancer Agents: From Bioactive Compounds to Function. Molecules 2021, 26, 2941. [Google Scholar] [CrossRef]
- Kitic, D.; Miladinovic, B.; Randjelovic, M.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Seidel, V. Anticancer Potential and Other Pharmacological Properties of Prunus armeniaca L.: An Updated Overview. Plants 2022, 11, 1885. [Google Scholar] [CrossRef] [PubMed]
- Rubegeta, E.; Makolo, F.; Kamatou, G.; Enslin, G.; Chaudhary, S.; Sandasi, M.; Cunningham, A.B.; Viljoen, A. The African Cherry: A Review of the Botany, Traditional Uses, Phytochemistry, and Biological Activities of Prunus Africana (Hook.f.) Kalkman. J. Ethnopharmacol. 2023, 305, 116004. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hoyos, M.; Arnáez-Serrano, E.; Quesada-Mora, S.; Azofeifa-Cordero, G.; Wilhelm-Romero, K.; Quirós-Fallas, M.I.; Alvarado-Corella, D.; Vargas-Huertas, F.; Sánchez-Kopper, A. Hrms Characterization, Antioxidant and Cytotoxic Activities of Polyphenols in Malus Domestica Cultivars from Costa Rica. Molecules 2021, 26, 7367. [Google Scholar] [CrossRef]
- Gegiu, G.; Bucur, L.; Popescu, A.; Radu, M.-D.; Mihai, S.; Badea, V. Studies on the Phytochemical Composition and Antioxidant Activity of a Prunus spinosa L. Aqueous Extract. Rev. Chim. 2020, 71, 80–84. [Google Scholar] [CrossRef]
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Plum (Prunus Domestica): Morphology, Taxonomy, Composition and Health Benefits. In Fruits Grown in Highland Regions of the Himalayas; Springer International Publishing: Cham, Switzerland, 2021; pp. 169–179. [Google Scholar]
- Mishra, S.; Vyas, S. Therapeutic and pharmacological potential of Prunus domestica: A Comprehensive Review. Int. J. Pharm. Sci. Res. 2021, 12, 3034. [Google Scholar] [CrossRef]
- Forni, C.; Rossi, M.; Borromeo, I.; Feriotto, G.; Platamone, G.; Tabolacci, C.; Mischiati, C.; Beninati, S. Flavonoids: A Myth or a Reality for Cancer Therapy? Molecules 2021, 26, 3583. [Google Scholar] [CrossRef]
- Nabende, P.; Karanja, S.; Mwatha, J.; Wachira, S. Anti-Proliferative Activity of Prunus Africana, Warburgia Stuhlmannii and Maytenus Senegalensis Extracts in Breast and Colon Cancer Cell Lines. Eur. J. Med. Plants 2015, 5, 366–376. [Google Scholar] [CrossRef]
- Gong, X.P.; Tang, Y.; Song, Y.Y.; Du, G.; Li, J. Comprehensive Review of Phytochemical Constituents, Pharmacological Properties, and Clinical Applications of Prunus mume. Front. Pharmacol. 2021, 12, 679378. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Sawada, T.; Okada, T.; Ohsawa, T.; Adachi, M.; Keiichi, K. New Anti-Proliferative Agent, MK615, from Japanese Apricot “Prunus Mume” Induces Striking Autophagy in Colon Cancer Cells in Vitro. World J. Gastroenterol. 2007, 13, 6512–6517. [Google Scholar] [PubMed]
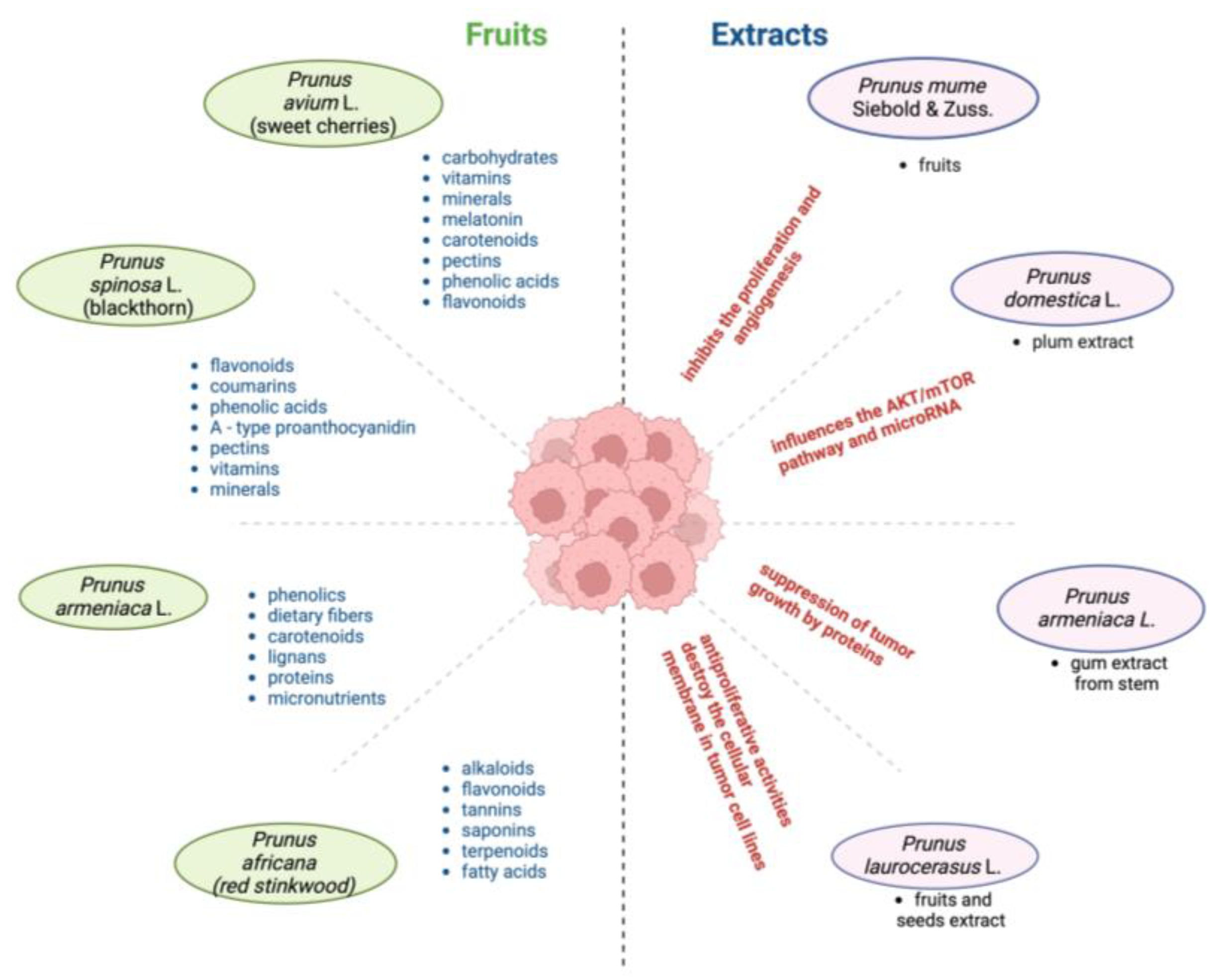
| Prunus Species | Phytochemical Composition | Therapeutic Potential | Applications | References |
|---|---|---|---|---|
| Prunus avium L. | Water (>80%), carbohydrates (≈16%), fat (0.2%), melatonin (≈1586 ng/100 g), anthocyanins (1734 mg/100 g), other flavonoids (396 mg/100 g), phenolic acids (162 mg/100 g) | Antioxidant, anti-inflammatory | Reducing oxidative stress, inflammation, and potentially cancer-related signaling pathways | [11,118] |
| Prunus spinosa L. | Flavone/ols compounds (64.62 ± 0.58 mg/100 g of dry weight), phenolic acid compounds (38.36 ± 0.19 mg/100 g), and anthocyanins (0.63 µg/100 g) | Antioxidant, antibacterial, astringent, diuretic | Treatment of gastrointestinal issues, diuretic and purgative properties | [53] |
| Prunus laurocerasus L. | Phenolic acids (vanillic acid, caffeic acid, chlorogenic acid, gallic acid (GAE)), flavonoids (quercetin (QE), anthocyanins, tannins), and cyanogenic glycosides Total flavonoid content mgQE/100 g extract: 502.10 ± 6.85 mg QE/100 g Total phenolic content mg GAE/100 g extract: 461.31 ± 4.98 mg GAE/100 g | Antioxidant, protective against gastric cancer, antiproliferative, antispasmodic, diuretic, antitussive | Treatment of kidney stones, stomach ulcers, bronchitis, and eczemas; antiproliferative on tumor cells | [48,108] |
| Prunus armeniaca L. | Dietary fiber, fats, proteins, sugars, vitamins, carotenoids, phenolics, lignans, volatile compounds, cyanogenic glycosides (amygdalin up to 4.9%) | Anticancer, anti-inflammatory, hepatoprotective | Treatment of gynecological, respiratory, and digestive disorders; anticancer signaling pathways | [112,119] |
| Prunus africana (Hook.f.) Kalkman | Phytosterols (1.5–2.5% of the dry weight of the bark), phenols (3 and 7 mg GAE/g extract), triterpenes (0.5–3.5% of the dry weight of the bark), fatty acids, and long-chain fatty alcohols | Anti-inflammatory, analgesic, antimicrobial, antioxidant, antiviral, antimutagenic, anti-asthmatic, antiandrogenic | Used for cancer treatment by limiting tumor growth and metastasis | [29,120] |
| Prunus dulcis (Mill) D.A. Webb | Almond seeds contain fixed oils (38.8%), phenolic compounds, minerals, vitamins | Anti-inflammatory, immunostimulant, antiproliferative | Treatment of IBS, constipation, and cancer; chemopreventive properties | [82] |
| Prunus domestica L. | Phenolic acids (gallic acid 0.81 µg/mg extract in plum native extract), flavonoids (quercetin 0.55 µg/mg extract in plum native extract), anthocyanins | Antioxidant, anticancer | Prevention of CRC; reduces oxidative damage, supports cancer drug synergism | [37,121] |
| Scientific Name of the Plant | Cancer Type | Model | Mechanism of Action | Target | References |
|---|---|---|---|---|---|
| Prunus domestica L. | CC | Caco-2 cells | Decreases proinflammatory markers (NF-κB, Cox-2, iNOS), modulates AKT/mTOR and miRNA pathways | AKT/mTOR pathway, miR-143 | [37] |
| Prunus avium L. | CC | Caco-2 cells | Influences the p38-MAPK signaling pathway | Cancer signaling pathways | [11,118] |
| Prunus mume Siebold and Zucc. extract | CRC | SW480, COLO, WiDr (in vitro); CRC model mice (in vivo) | Inhibits RelA, Bcl2, caspase 3; promotes Bax, cleaved caspase 3, and EGFR | RelA, Bcl2, EGFR | [45,80,127] |
| Prunus amygdalus L. var. amara (almond) oil | CC | Colo-320 and Colo-741 cells, in vivo animal studies | Decreases Ki-67 expression, caspase-independent apoptosis | Ki-67, caspases | [82,105] |
| Prunus spinosa L. ethanolic and aqueous extract | CRC | GLC, COLO320 cell lines | Cytotoxic effects, suppresses cancer growth | Colorectal cancer cells | [115] |
| Prunus spinosa L. extract (Trigno M) | CC, CRC | HCT116 cell line; colon cancer xenografts in mice | Delayed tumor progression and decreased tumor necrosis | Cancer signaling pathways | [53] |
| Prunus domestica L. methanolic extract | CC | Colon-26 cells, SW1116, HT29, Caco-2 cells | Significant growth inhibition and apoptosis induction | Cancer cell proteins, mitochondrial activity | [37] |
| Prunus armeniaca L. methanolic extract | CC | HCT-116 colon cells, Caco-2 cells | Inhibits growth in a dose-dependent manner, high antiproliferative activity | Cancer signaling pathways | [119] |
| Prunus laurocerasus L. methanolic extract | GC | AGS and MKN-45 cells | Induces significant cell death while preserving human fibroblasts | Cancer signaling pathways | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitea, G.; Iancu, I.M.; Schröder, V.; Roșca, A.C.; Iancu, V.; Crețu, R.-M.; Mireșan, H. Therapeutic Potential of Prunus Species in Gastrointestinal Oncology. Cancers 2025, 17, 938. https://doi.org/10.3390/cancers17060938
Mitea G, Iancu IM, Schröder V, Roșca AC, Iancu V, Crețu R-M, Mireșan H. Therapeutic Potential of Prunus Species in Gastrointestinal Oncology. Cancers. 2025; 17(6):938. https://doi.org/10.3390/cancers17060938
Chicago/Turabian StyleMitea, Gabriela, Irina Mihaela Iancu, Verginica Schröder, Adrian Cosmin Roșca, Valeriu Iancu, Ruxandra-Mihaela Crețu, and Horațiu Mireșan. 2025. "Therapeutic Potential of Prunus Species in Gastrointestinal Oncology" Cancers 17, no. 6: 938. https://doi.org/10.3390/cancers17060938
APA StyleMitea, G., Iancu, I. M., Schröder, V., Roșca, A. C., Iancu, V., Crețu, R.-M., & Mireșan, H. (2025). Therapeutic Potential of Prunus Species in Gastrointestinal Oncology. Cancers, 17(6), 938. https://doi.org/10.3390/cancers17060938








