Prognostic Value of Very Early Interim FDG PET/CT After Single Cycle of Chemotherapy for 10-Year Survival in Diffuse Large B-Cell Lymphoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. FDG PET/CT Acquisition
2.3. FDG PET/CT Image Analysis
2.4. Statistical Analysis
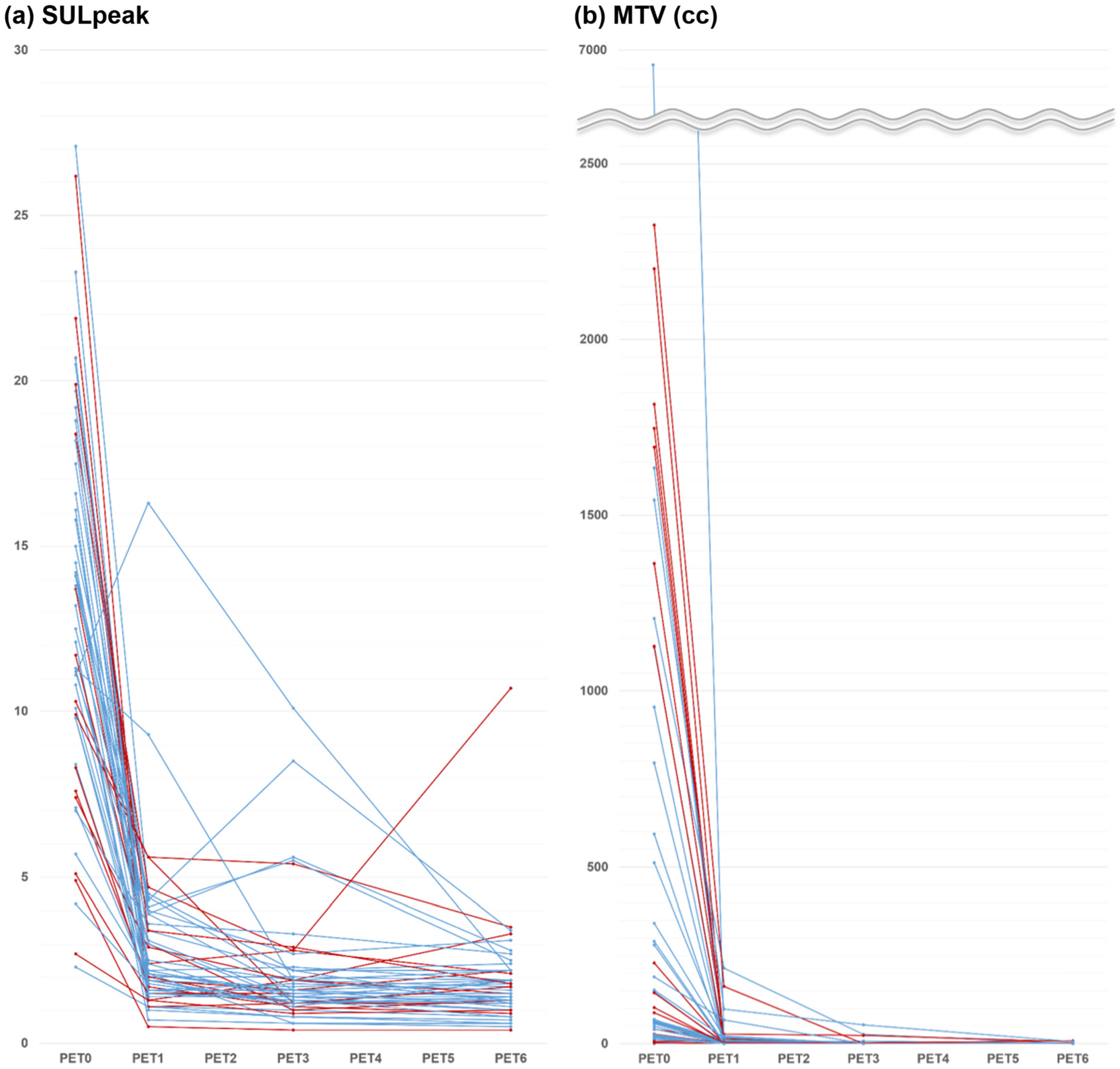
3. Results
3.1. Patient Characteristics
3.2. Early Interim Response
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mondello, P.; Mian, M. Frontline treatment of diffuse large B-cell lymphoma: Beyond R-CHOP. Hematol. Oncol. 2019, 37, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Spinner, M.A.; Advani, R.H. Current Frontline Treatment of Diffuse Large B-Cell Lymphoma. Oncology 2022, 36, 51–58. [Google Scholar]
- Ruppert, A.S.; Dixon, J.G.; Salles, G.; Wall, A.; Cunningham, D.; Poeschel, V.; Haioun, C.; Tilly, H.; Ghesquieres, H.; Ziepert, M.; et al. International prognostic indices in diffuse large B-cell lymphoma: A comparison of IPI, R-IPI, and NCCN-IPI. Blood 2020, 135, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Hodgkin Lymphoma (Version 1.2024). Available online: https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf (accessed on 12 October 2023).
- Trotman, J.; Barrington, S.F. The role of PET in first-line treatment of Hodgkin lymphoma. Lancet Haematol. 2021, 8, e67–e79. [Google Scholar] [CrossRef] [PubMed]
- B-Cell Lymphomas (Version 6.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf (accessed on 10 October 2023).
- Ghielmini, M.; Vitolo, U.; Kimby, E.; Montoto, S.; Walewski, J.; Pfreundschuh, M.; Federico, M.; Hoskin, P.; McNamara, C.; Caligaris-Cappio, F.; et al. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: Diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL). Ann. Oncol. 2013, 24, 561–576. [Google Scholar] [CrossRef]
- Yamane, T.; Daimaru, O.; Ito, S.; Yoshiya, K.; Nagata, T.; Ito, S.; Uchida, H. Decreased 18F-FDG uptake 1 day after initiation of chemotherapy for malignant lymphomas. J. Nucl. Med. 2004, 45, 1838–1842. [Google Scholar]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), 122S–150S. [Google Scholar] [CrossRef]
- Park, H.L.; Han, E.J.; O, J.H.; Choi, B.O.; Park, G.; Jung, S.E.; Yahng, S.A.; Eom, K.S.; Cho, S.G.; on behalf of Catholic University Lymphoma Group. Early Interim Chemotherapy Response Evaluation by F-18 FDG PET/CT in Diffuse Large B Cell Lymphoma. Diagnostics 2020, 10, 1002. [Google Scholar] [CrossRef]
- O, J.H.; Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef]
- Dang, C.; Gilewski, T.A.; Surbone, A.; Norton, L. Growth Curve Analysis. In Holland-Frei Cancer Medicine, 6th ed.; BC Decker: Hamilton, ON, USA, 2003. [Google Scholar]
- Al Tabaa, Y.; Bailly, C.; Kanoun, S. FDG-PET/CT in Lymphoma: Where Do We Go Now? Cancers 2021, 13, 5222. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.S.; Dehdashti, F.; Wahl, R.L. FDG PET/CT-based Response Assessment in Malignancies. Radiographics 2023, 43, e220122. [Google Scholar] [CrossRef]
- Burggraaff, C.N.; de Jong, A.; Hoekstra, O.S.; Hoetjes, N.J.; Nievelstein, R.A.J.; Jansma, E.P.; Heymans, M.W.; de Vet, H.C.W.; Zijlstra, J.M. Predictive value of interim positron emission tomography in diffuse large B-cell lymphoma: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 65–79. [Google Scholar] [CrossRef]
- Mikhaeel, N.G.; Cunningham, D.; Counsell, N.; McMillan, A.; Radford, J.A.; Ardeshna, K.M.; Lawrie, A.; Smith, P.; Clifton-Hadley, L.; O’Doherty, M.J.; et al. FDG-PET/CT after two cycles of R-CHOP in DLBCL predicts complete remission but has limited value in identifying patients with poor outcome—Final result of a UK National Cancer Research Institute prospective study. Br. J. Haematol. 2021, 192, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Mamot, C.; Klingbiel, D.; Hitz, F.; Renner, C.; Pabst, T.; Driessen, C.; Mey, U.; Pless, M.; Bargetzi, M.; Krasniqi, F.; et al. Final Results of a Prospective Evaluation of the Predictive Value of Interim Positron Emission Tomography in Patients with Diffuse Large B-Cell Lymphoma Treated with R-CHOP-14 (SAKK 38/07). J. Clin. Oncol. 2015, 33, 2523–2529. [Google Scholar] [CrossRef]
- Pregno, P.; Chiappella, A.; Bello, M.; Botto, B.; Ferrero, S.; Franceschetti, S.; Giunta, F.; Ladetto, M.; Limerutti, G.; Menga, M.; et al. Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood 2012, 119, 2066–2073. [Google Scholar] [CrossRef]
- Duhrsen, U.; Muller, S.; Hertenstein, B.; Thomssen, H.; Kotzerke, J.; Mesters, R.; Berdel, W.E.; Franzius, C.; Kroschinsky, F.; Weckesser, M.; et al. Positron Emission Tomography-Guided Therapy of Aggressive Non-Hodgkin Lymphomas (PETAL): A Multicenter, Randomized Phase III Trial. J. Clin. Oncol. 2018, 36, 2024–2034. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Kreissl, M.C.; Su, L.; Wu, Z.; Hacker, M.; Liu, J.; Zhang, X.; Bo, Y.; Zhang, H.; Li, X.; et al. Prognostic analysis of interim (18)F-FDG PET/CT in patients with diffuse large B cell lymphoma after one cycle versus two cycles of chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 478–488. [Google Scholar] [CrossRef]
- Kostakoglu, L.; Goldsmith, S.J.; Leonard, J.P.; Christos, P.; Furman, R.R.; Atasever, T.; Chandramouly, A.; Verma, S.; Kothari, P.; Coleman, M. FDG-PET after 1 cycle of therapy predicts outcome in diffuse large cell lymphoma and classic Hodgkin disease. Cancer 2006, 107, 2678–2687. [Google Scholar] [CrossRef]
- Kostakoglu, L.; Coleman, M.; Leonard, J.P.; Kuji, I.; Zoe, H.; Goldsmith, S.J. PET predicts prognosis after 1 cycle of chemotherapy in aggressive lymphoma and Hodgkin’s disease. J. Nucl. Med. 2002, 43, 1018–1027. [Google Scholar]
- Mylam, K.J.; Kostakoglu, L.; Hutchings, M.; Coleman, M.; Lamonica, D.; Czuczman, M.S.; Diehl, L.F.; Nielsen, A.L.; Jensen, P.; Loft, A.; et al. (18)F-fluorodeoxyglucose-positron emission tomography/computed tomography after one cycle of chemotherapy in patients with diffuse large B-cell lymphoma: Results of a Nordic/US intergroup study. Leuk. Lymphoma 2015, 56, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Eertink, J.J.; Burggraaff, C.N.; Heymans, M.W.; Duhrsen, U.; Huttmann, A.; Schmitz, C.; Muller, S.; Lugtenburg, P.J.; Barrington, S.F.; Mikhaeel, N.G.; et al. Optimal timing and criteria of interim PET in DLBCL: A comparative study of 1692 patients. Blood Adv. 2021, 5, 2375–2384. [Google Scholar] [CrossRef]
- Horvat, M.; Zadnik, V.; Juznic Setina, T.; Boltezar, L.; Pahole Golicnik, J.; Novakovic, S.; Jezersek Novakovic, B. Diffuse large B-cell lymphoma: 10 years’ real-world clinical experience with rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisolone. Oncol. Lett. 2018, 15, 3602–3609. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Zhong, Q.Z.; Wu, T.; Wu, Y.P.; Yang, Y.; Chen, B.; Jing, H.; Tang, Y.; Jin, J.; et al. Decreased lymphoma-related deaths and improved long-term relative survival with radiotherapy for early-stage diffuse large B-cell lymphoma in the rituximab era. Radiother. Oncol. 2023, 188, 109902. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.F.; Berger, F.; Chassagne-Clement, C.; Ffrench, M.; Callet-Bauchu, E.; Sebban, C.; Ghesquieres, H.; Broussais-Guillaumot, F.; Salles, G.; Coiffier, B. Lymphoma recurrence 5 years or later following diffuse large B-cell lymphoma: Clinical characteristics and outcome. J. Clin. Oncol. 2010, 28, 2094–2100. [Google Scholar] [CrossRef]
- Schoder, H.; Polley, M.C.; Knopp, M.V.; Hall, N.; Kostakoglu, L.; Zhang, J.; Higley, H.R.; Kelloff, G.; Liu, H.; Zelenetz, A.D.; et al. Prognostic value of interim FDG-PET in diffuse large cell lymphoma: Results from the CALGB 50303 Clinical Trial. Blood 2020, 135, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Kostakoglu, L.; Nowakowski, G.S. End-of-Treatment PET/Computed Tomography Response in Diffuse Large B-Cell Lymphoma. PET Clin. 2019, 14, 307–315. [Google Scholar] [CrossRef]
- Lin, C.; Itti, E.; Haioun, C.; Petegnief, Y.; Luciani, A.; Dupuis, J.; Paone, G.; Talbot, J.N.; Rahmouni, A.; Meignan, M. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J. Nucl. Med. 2007, 48, 1626–1632. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Song, Y.; Meng, X.; Cao, Z.; Zhao, W.; Zhang, Y.; Guo, R.; Zhou, X.; Yang, Z.; Li, N. Harmonization of standard uptake values across different positron emission tomography/computed tomography systems and different reconstruction algorithms: Validation in oncology patients. EJNMMI Phys. 2023, 10, 19. [Google Scholar] [CrossRef]
- Jiang, C.; Ding, C.; Xu, J.; Teng, Y.; Chen, J.; Wang, Z.; Zhou, Z. Will Baseline Total Lesion Glycolysis Play a Role in Improving the Prognostic Value of the NCCN-IPI in Primary Gastric Diffuse Large B-Cell Lymphoma Patients Treated with the R-CHOP Regimen? Clin. Nucl. Med. 2021, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]

| Variables | No. of Patients | |
|---|---|---|
| Age (years) | Mean ± SD (range) | 55 ± 15 (21–81) |
| ≤65 | 30 (59%) | |
| >65 | 21 (41%) | |
| Sex | Male | 31 (61%) |
| Female | 20 (39%) | |
| ECOG PS | 0–1 | 40 (78%) |
| 2–4 | 11 (22%) | |
| LDH level | Normal | 25 (49%) |
| Elevated | 26 (51%) | |
| Extranodal involvement | 0–1 | 22 (43%) |
| ≥2 | 29 (57%) | |
| Ann Arbor stage | I | 5 (10%) |
| II | 16 (31%) | |
| III | 12 (24%) | |
| IV | 18 (35%) | |
| IPI score | 0 | 6 (12%) |
| 1 | 11 (21%) | |
| 2 | 10 (20%) | |
| 3 | 13 (25%) | |
| 4 | 8 (16%) | |
| 5 | 3 (6%) | |
| Histologic subtype | GCB | 20 (39%) |
| Non-GCB | 25 (49%) | |
| Unknown | 6 (12%) |
| Variables | PFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (≤65 y vs. >65 y) | 3.35 (1.206–9.303) | 0.020 * | 2.872 (1.018–8.104) | 0.046 * |
| Sex (male vs. female) | 0.404 (0.131–1.248) | 0.115 | 0.549 (0.175–1.725) | 0.304 |
| ECOG PS (0–1 vs. 2–5) | 1.456 (0.466–4.547) | 0.518 | 1.461 (0.465–4.593) | 0.516 |
| LDH (normal vs. elevated) | 3.978 (1.263–12.532) | 0.018 * | 3.335 (1.056–10.527) | 0.040 * |
| Extranodal involvement (<2 vs. ≥2) | 1.671 (0.615–4.538) | 0.314 | 1.795 (0.612–5.265) | 0.287 |
| Stage (I–II vs. III–IV) | 3.284 (1.041–10.365) | 0.043 * | 3.785 (1.056–13.572) | 0.041 * |
| IPI score (0–2 vs. 3–5) | 3.271 (1.124–9.518) | 0.030 * | 2.792 (0.952–8.188) | 0.062 |
| PFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Adjusted for IPI | Univariate | Adjusted for IPI | ||||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| PET1 | DS | 1.26 (0.48–3.31) | 0.634 | 1.60 (0.58–4.46) | 0.368 | ||||
| SULpeak | 0.91 (0.67–1.22) | 0.516 | 0.96 (0.74–1.25) | 0.762 | |||||
| MTV | 1.00 (0.99–1.01) | 0.840 | 1.00 (0.99–1.01) | 0.762 | |||||
| %ΔSUL1 | 1.00 (0.98–1.03) | 0.767 | 1.00 (0.98–1.02) | 0.891 | |||||
| %ΔMTV1 | 0.99 (0.97–1.01) | 0.434 | 0.99 (0.96–1.01) | 0.259 | |||||
| PET6 | DS | 2.90 (1.00–8.39) | 0.049 * | – | 3.34 (1.13–9.82) | 0.029 * | 3.34 (1.13–9.82) | 0.029 * | |
| SULpeak | 1.42 (1.06–1.91) | 0.019 * | 1.34 (1.00–1.82) | 0.054 | 1.65 (1.14–2.39) | 0.008 * | 1.65 (1.14–2.39) | 0.008 * | |
| MTV | 1.33 (1.07–1.66) | 0.011 * | 1.33 (1.07–1.66) | 0.011 * | 1.37 (1.09–1.71) | 0.006 * | 1.37 (1.09–1.71) | 0.006 * | |
| %ΔSUL6 | 0.97 (0.93–1.01) | 0.113 | 0.95 (0.91–1.00) | 0.029 * | 0.94 (0.90–0.99) | 0.014 * | |||
| %ΔMTV6 | 0.37 (0.16–0.86) | 0.021 * | – | 0.23 (0.09–0.61) | 0.003 * | 0.24 (0.09–0.61) | 0.003 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, E.J.; Park, H.L.; Yahng, S.-A.; Min, G.-J.; Choi, B.-O.; Park, G.; O, J.H.; Cho, S.-G., on behalf of Catholic University Lymphoma Group. Prognostic Value of Very Early Interim FDG PET/CT After Single Cycle of Chemotherapy for 10-Year Survival in Diffuse Large B-Cell Lymphoma. Cancers 2025, 17, 926. https://doi.org/10.3390/cancers17060926
Han EJ, Park HL, Yahng S-A, Min G-J, Choi B-O, Park G, O JH, Cho S-G on behalf of Catholic University Lymphoma Group. Prognostic Value of Very Early Interim FDG PET/CT After Single Cycle of Chemotherapy for 10-Year Survival in Diffuse Large B-Cell Lymphoma. Cancers. 2025; 17(6):926. https://doi.org/10.3390/cancers17060926
Chicago/Turabian StyleHan, Eun Ji, Hye Lim Park, Seung-Ah Yahng, Gi-June Min, Byung-Ock Choi, Gyeongsin Park, Joo Hyun O, and Seok-Goo Cho on behalf of Catholic University Lymphoma Group. 2025. "Prognostic Value of Very Early Interim FDG PET/CT After Single Cycle of Chemotherapy for 10-Year Survival in Diffuse Large B-Cell Lymphoma" Cancers 17, no. 6: 926. https://doi.org/10.3390/cancers17060926
APA StyleHan, E. J., Park, H. L., Yahng, S.-A., Min, G.-J., Choi, B.-O., Park, G., O, J. H., & Cho, S.-G., on behalf of Catholic University Lymphoma Group. (2025). Prognostic Value of Very Early Interim FDG PET/CT After Single Cycle of Chemotherapy for 10-Year Survival in Diffuse Large B-Cell Lymphoma. Cancers, 17(6), 926. https://doi.org/10.3390/cancers17060926






