Immunological Network Signature of Naïve Non-Oncogene-Addicted Non-Small Cell Lung Cancer Patients Treated with Anti-PD1 Therapy: A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Characteristics
2.2. PBMC and Serum Collection
2.3. Flow Cytometry
2.4. Cytokine and Chemokine Evaluation
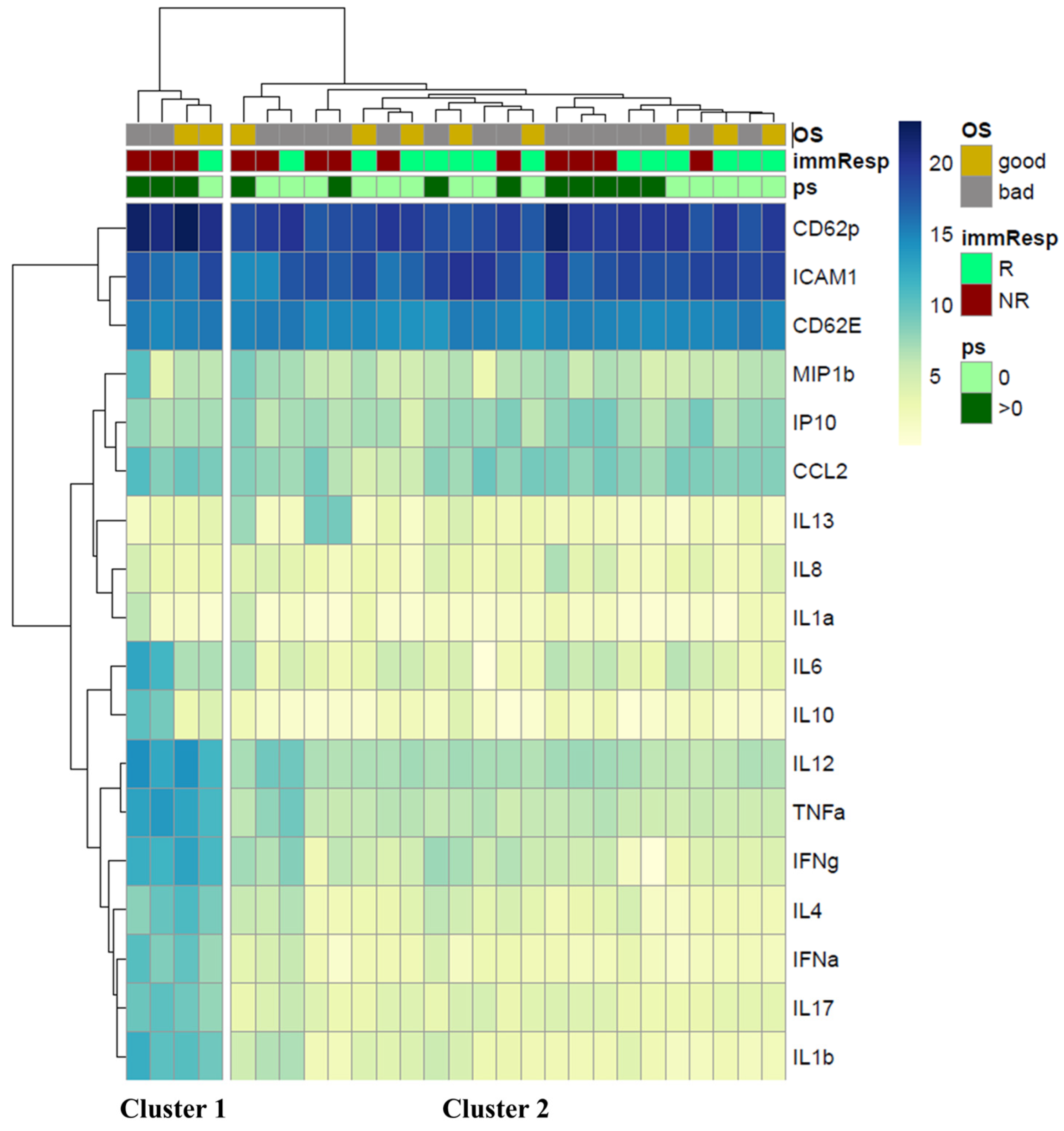
2.5. Hierarchical Clustering of Circulant Molecules’ Expression Profile
2.6. Differential Correlation Analysis of Multiple Clinical Conditions
3. Results
3.1. Patients’ Characteristics
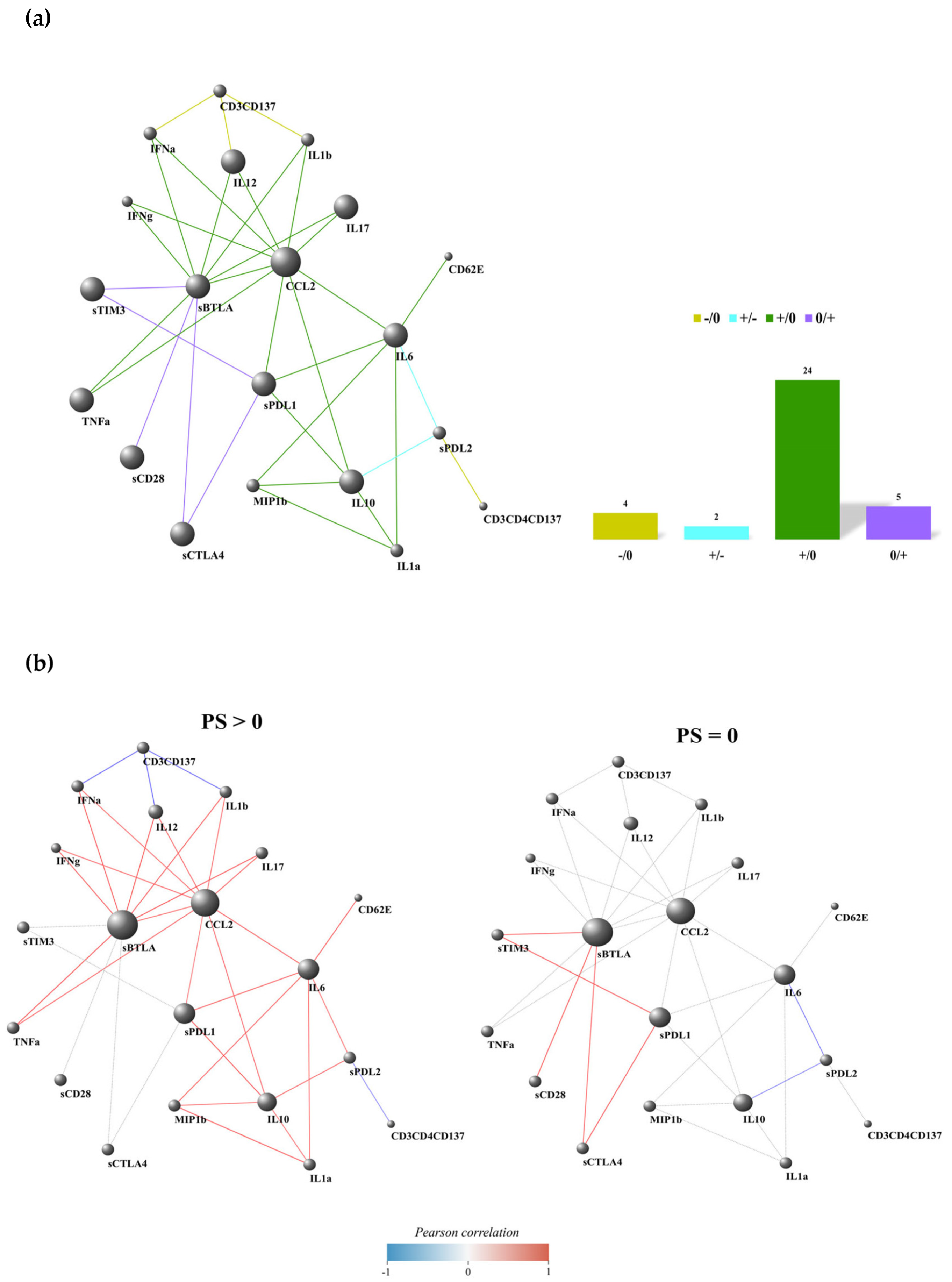
3.2. Non-Responding Patients with PS > 0 Showed an Immunosuppressive Soluble Checkpoint Signature
3.3. Non-Responding Patients Showed a Pronounced Inflammatory Network
3.4. PD-L2-IL6 and PD-L2-IL10 Connections Were Inversely Correlated in Patients with PS > 0 and PS = 0
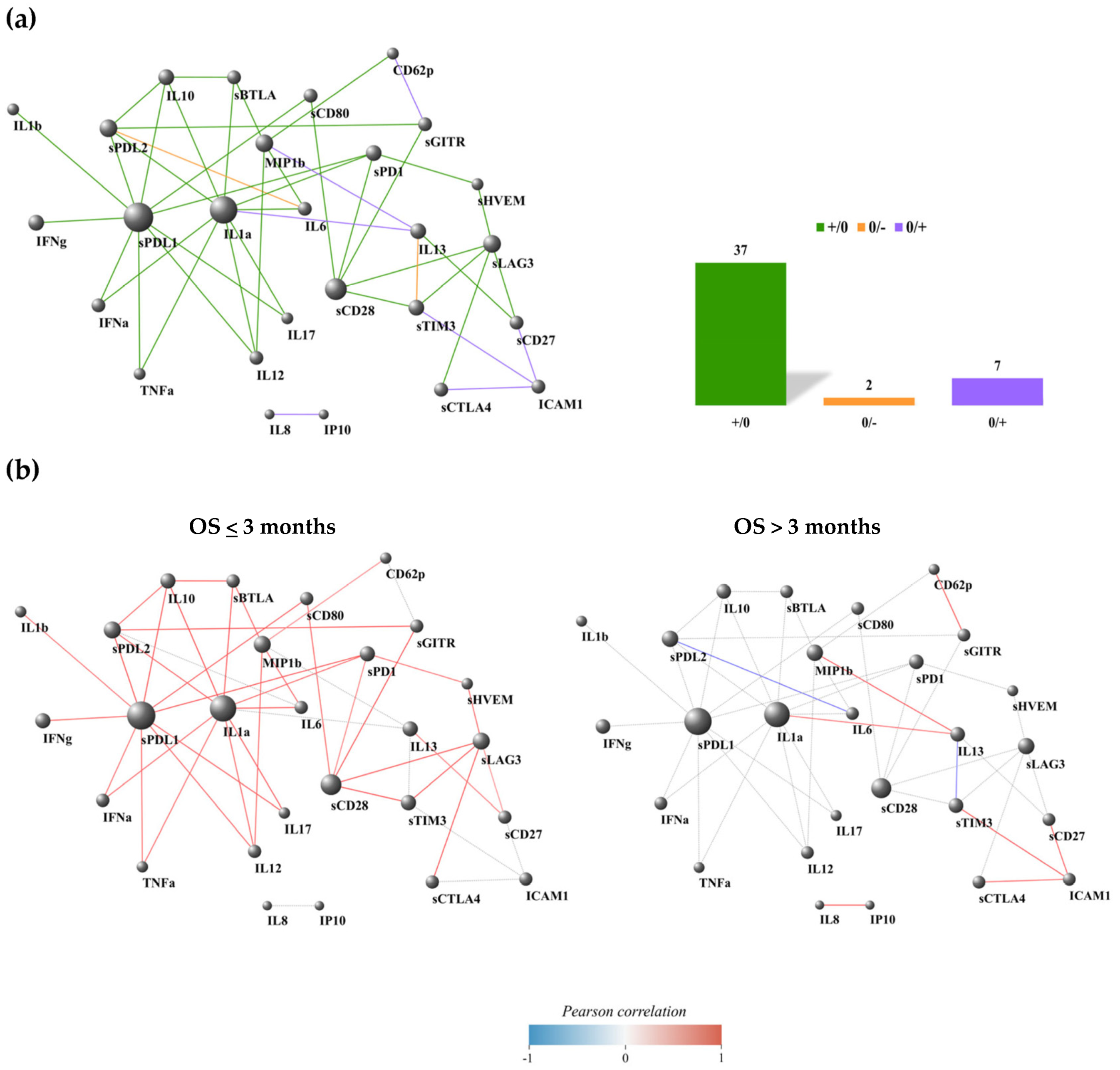
3.5. Most of the PD-L1 Connections Were Interrupted in Patients with a Favorable Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSCLC | Non-small cell lung cancer |
| PS | Performance status |
| OS | Overall survival |
| TPS | Tumor proportional score |
| IDO | Indoleamine 2,3-deoxygenase |
| FoxO3 | Forkhead Box O3 |
| EOCG | Eastern Cooperative Oncology Group |
| FDR | False discovery rate |
| DGCA | Different gene correlation |
References
- Liu, J.; Zhong, Y.; Peng, S.; Zhou, X.; Gan, X. Efficacy and safety of PD1/PD-L1 blockades versus docetaxel in patients with pretreated advanced non-small-cell lung cancer: A meta-analysis. Onco Targets Ther. 2018, 11, 8623–8632. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 78, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Vinay, D.S.; Kwon, B.S. 4-1BB signaling beyond T cells. Cell Mol. Immunol. 2011, 8, 281–284. [Google Scholar] [CrossRef]
- Cannons, J.L.; Lau, P.; Ghumman, B.; DeBenedette, M.A.; Yagita, H.; Okumura, K.; Watts, T.H. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J. Immunol. 2001, 167, 1313–1324. [Google Scholar] [CrossRef]
- Menk, A.V.; Scharping, N.E.; Rivadeneira, D.B.; Calderon, M.J.; Watson, M.J.; Dunstane, D.; Watkins, S.C.; Delgoffe, G.M. 4-1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J. Exp. Med. 2018, 215, 1091–1100. [Google Scholar] [CrossRef]
- Aznar, M.A.; Labiano, S.; Diaz-Lagares, A.; Molina, C.; Garasa, S.; Azpilikueta, A.; Etxeberria, I.; Sanchez-Paulete, A.R.; Korman, A.J.; Esteller, M.; et al. CD137 (4-1BB) Costimulation Modifies DNA Methylation in CD8(+) T Cell-Relevant Genes. Cancer Immunol. Res. 2018, 6, 69–78. [Google Scholar] [CrossRef]
- Ye, Q.; Song, D.G.; Poussin, M.; Yamamoto, T.; Best, A.; Li, C.; Coukos, G.; Powell, D.J., Jr. CD137 accurately identifies and enriches for naturally occurring tumor reactive T cells in tumor. Clin. Cancer Res. 2014, 20, 44–55. [Google Scholar] [CrossRef]
- Gelibter, A.; Asquino, A.; Strigari, L.; Zizzari, I.G.; Tuosto, L.; Scirocchi, F.; Pace, A.; Siringo, M.; Tramontano, E.; Bianchini, S.; et al. CD137(+) and regulatory T cells as independent prognostic factors of survival in advanced non-oncogene addicted NSCLC patients treated with immunotherapy as first-line. J. Transl. Med. 2024, 22, 329. [Google Scholar] [CrossRef] [PubMed]
- Gelibter, A.; Tuosto, L.; Asquino, A.; Siringo, M.; Sabatini, A.; Zizzari, I.G.; Pace, A.; Scirocchi, F.; Valentino, F.; Bianchini, S.; et al. Anti-PD1 therapies induce an early expansion of Ki67+CD8+ T cells in metastatic non-oncogene addicted NSCLC patients. Front. Immunol. 2024, 15, 1483182. [Google Scholar]
- Khan, M.; Arooj, S.; Wang, H. Soluble B7-CD28 Family Inhibitory Immune Checkpoint Proteins and Anti-Cancer Immunotherapy. Front. Immunol. 2021, 12, 651634. [Google Scholar] [CrossRef] [PubMed]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The importance of exosomal PD-L1 in tumour immune evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef]
- Hayashi, H.; Chamoto, K.; Hatae, R.; Kurosaki, T.; Togashi, Y.; Fukoka, K.; Goto, M.; Chiba, T.; Tomida, S.; Ota, T.; et al. Soluble immune checkpoint factors reflect exhaustion of antitumor immunity and response to PD-1 blockade. J. Clin. Investig. 2024, 134, e168318. [Google Scholar] [CrossRef]
- Zizzari, I.G.; Di Filippo, A.; Scirocchi, F.; Di Pietro, F.R.; Rahimi, H.; Ugolini, A.; Scagnoli, S.; Vernocchi, P.; Del Chierico, F.; Putignani, L.; et al. Soluble Immune Checkpoints, Gut Metabolites and Performance Status as Parameters of Response to Nivolumab Treatment in NSCLC Patients. J. Pers. Med. 2020, 10, 208. [Google Scholar] [CrossRef]
- Mohammadzadeh, S.; Andalib, A.; Khanahmad, H.; Esmaeil, N. Human recombinant soluble PD1 can interference in T cells and Treg cells function in response to MDA-MB-231 cancer cell line. Am. J. Clin. Exp. Immunol. 2023, 12, 11–23. [Google Scholar]
- Kakoulidou, M.; Giscombe, R.; Zhao, X.; Lefvert, A.K.; Wang, X. Human Soluble CD80 is generated by alternative splicing, and recombinant soluble CD80 binds to CD28 and CD152 influencing T-cell activation. Scand. J. Immunol. 2007, 66, 529–537. [Google Scholar] [CrossRef]
- Lin, Z.; Tang, Y.; Chen, Z.; Li, S.; Xu, X.; Hou, X.; Chen, Z.; Wen, J.; Zeng, W.; Meng, X.; et al. Soluble CD80 oral delivery by recombinant Lactococcus suppresses tumor growth by enhancing antitumor immunity. Bioeng. Transl. Med. 2023, 8, e10533. [Google Scholar] [CrossRef]
- Himuro, H.; Nakahara, Y.; Igarashi, Y.; Kouro, T.; Higashijima, N.; Matsuo, N.; Murakami, S.; Wei, F.; Horaguchi, S.; Tsuji, K.; et al. Clinical roles of soluble PD-1 and PD-L1 in plasma of NSCLC patients treated with immune checkpoint inhibitors. Cancer Immunol. Immunother. 2023, 72, 2829–2840. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.R.; Thakkar, K.N.; Qian, J.; Kariolis, M.S.; Huang, W.; Nandagopal, S.; Yang, T.T.C.; Diep, A.N.; Cherf, G.M.; Xu, Y.; et al. Neutralization of PD-L2 is Essential for Overcoming Immune Checkpoint Blockade Resistance in Ovarian Cancer. Clin. Cancer Res. 2021, 27, 4435–4448. [Google Scholar] [CrossRef] [PubMed]
- Scirocchi, F.; Strigari, L.; Di Filippo, A.; Napoletano, C.; Pace, A.; Rahimi, H.; Botticelli, A.; Rughetti, A.; Nuti, M.; Zizzari, I.G. Soluble PD-L1 as a Prognostic Factor for Immunotherapy Treatment in Solid Tumors: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 14496. [Google Scholar] [CrossRef]
- Li, R.; Liang, H.; Shang, Y.; Yang, Z.; Wang, K.; Yang, D.; Bao, J.; Xi, W.; Zhou, D.; Ni, W.; et al. Characteristics of Soluble PD-L1 and PD-1 Expression and Their Correlations with Immune Status and Prognosis in Advanced Lung Cancer. Asia Pac. J. Clin. Oncol. 2025. [Google Scholar] [CrossRef]
- Andrzejczak, A.; Karabon, L. BTLA biology in cancer: From bench discoveries to clinical potentials. Biomark. Res. 2024, 12, 8. [Google Scholar] [CrossRef]
- Ward, F.J.; Dahal, L.N.; Wijesekera, S.K.; Abdul-Jawad, S.K.; Kaewarpai, T.; Xu, H.; Vickers, M.A.; Barker, R.N. The soluble isoform of CTLA-4 as a regulator of T-cell responses. Eur. J. Immunol. 2013, 43, 1274–1285. [Google Scholar] [CrossRef]
- Grohmann, U.; Orabona, C.; Fallarino, F.; Vacca, C.; Calcinaro, F.; Falorni, A.; Candeloro, P.; Belladonna, M.L.; Bianchi, R.; Fioretti, M.C.; et al. CTLA-4-ig regulates tryptophan catabolism invivo. Nat. Immunol. 2002, 3, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Dejean, A.S.; Beisner, D.R.; Ch’en, I.L.; Kerdiles, Y.M.; Babour, A.; Arden, K.C.; Castrillon, D.H.; DePinho, R.A.; Hedrick, S.M. Transcription factorFoxo3 controls the magnitude of T cell immune responses bymodulating the function of dendritic cells. Nat. Immunol. 2009, 10, 504–513. [Google Scholar] [CrossRef]
- Esen, F.; Cikman, D.I.; Engin, A.; Turna, A.; Batur, S.; Oz, B.; Turna, H.Z.; Deniz, G.; Aktas Cetin, E. Functional and phenotypic changes in natural killer cells expressing immune checkpoint receptors PD-1, CTLA-4, LAG-3, and TIGIT in non-small cell lung cancer: The comparative analysis of tumor microenvironment, peripheral venous blood, and tumor-draining veins. Immunol. Res. 2024, 73, 18. [Google Scholar]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Liu, Q.; Shaibu, Z.; Xu, A.; Yang, F.; Cao, R.; Yang, F. Predictive value of serum cytokines in patients with non-small-cell lung cancer receiving anti-PD-1 blockade therapy: A meta-analysis. Clin. Exp. Med. 2025, 25, 59. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Ouyang, Y.; Feng, X.; Yu, C.; Zhang, H.; Chen, S.; Liu, Z.; Wang, B.; Li, X.; Liu, Z.; et al. IL-10RA promotes lung cancer cell proliferation by increasing fatty acid oxidation via STAT3 signaling pathway. Pulm. Pharmacol. Ther. 2025, 88, 102344. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, C.; Jiang, H.; Chu, T.; Zhong, R.; Zhang, X.; Shen, Y.; Han, B. Prognostic role of serum cytokines level in non-small cell lung cancer patients with anti-PD-1 and chemotherapy combined treatment. Front. Immunol. 2024, 15, 1430301. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- McKenzie, A.T.; Katsyv, I.; Song, W.M.; Wang, M.; Zhang, B. DGCA: A comprehensive R package for Differential Gene Correlation Analysis. BMC Syst. Biol. 2016, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Bonett, D.A.; Wright, T.A. Sample size requirements for estimating Pearson, Kendall, and Spearman correlations. Psychometrika 2000, 65, 23–28. [Google Scholar] [CrossRef]
- Haile, S.T.; Horn, L.A.; Ostrand-Rosenberg, S. A soluble form of CD80 enhances antitumor immunity by neutralizing programmed death ligand-1 and simultaneously providing costimulatio. Cancer Immunol. Res. 2014, 2, 610–615. [Google Scholar] [CrossRef]
- Botticelli, A.; Zizzari, I.G.; Scagnoli, S.; Pomati, G.; Strigari, L.; Cirillo, A.; Cerbelli, B.; Di Filippo, A.; Napoletano, C.; Scirocchi, F.; et al. The Role of Soluble LAG3 and Soluble Immune Checkpoints Profile in Advanced Head and Neck Cancer: A Pilot Study. J. Pers. Med. 2021, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, F.; Moradi, A.; Sadeghirad, H.; Kulasinghe, A. Tissue biomarkers of immune checkpoint inhibitor therapy. Immunol. Cell Biol. 2024, 102, 179–193. [Google Scholar] [CrossRef]
- Mezi, S.; Pomati, G.; Fiscon, G.; Amirhassankhani, S.; Zizzari, I.G.; Napoletano, C.; Rughetti, A.; Rossi, E.; Schinzari, G.; Tortora, G.; et al. A network approach to define the predictive role of immune profile on tumor response and toxicity of anti PD-1 single agent immunotherapy in patients with solid tumors. Front. Immunol. 2023, 14, 1199089. [Google Scholar] [CrossRef]
- Cheng, C.C.; Chang, J.; Ho, A.S.; Sie, Z.L.; Peng, C.L.; Wang, C.L.; Dev, K.; Chang, C.C. Tumor-intrinsic IFNalpha and CXCL10 are critical for immunotherapeutic efficacy by recruiting and activating T lymphocytes in tumor microenvironment. Cancer Immunol. Immunother. 2024, 73, 175. [Google Scholar] [CrossRef] [PubMed]
- Belardelli, F.; Ferrantini, M.; Proietti, E.; Kirkwood, J.M. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor. Rev. 2002, 13, 119–134. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Wu, F.; Xu, J.; Huang, Q.; Han, J.; Duan, L.; Fan, J.; Lv, Z.; Guo, M.; Hu, G.; Chen, L.; et al. The Role of Interleukin-17 in Lung Cancer. Mediators Inflamm. 2016, 2016, 8494079. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. IL-13 effector functions. Annu. Rev. Immunol. 2003, 21, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, I.G.; Di Filippo, A.; Botticelli, A.; Strigari, L.; Pernazza, A.; Rullo, E.; Pignataro, M.G.; Ugolini, A.; Scirocchi, F.; Di Pietro, F.R.; et al. Circulating CD137+ T Cells Correlate with Improved Response to Anti-PD1 Immunotherapy in Patients with Cancer. Clin. Cancer Res. 2022, 28, 1027–1037. [Google Scholar] [CrossRef]
- Cirillo, A.; Zizzari, I.G.; Botticelli, A.; Strigari, L.; Rahimi, H.; Scagnoli, S.; Scirocchi, F.; Pernazza, A.; Pace, A.; Cerbelli, B.; et al. Circulating CD137(+) T Cell Levels Are Correlated with Response to Pembrolizumab Treatment in Advanced Head and Neck Cancer Patients. Int. J. Mol. Sci. 2023, 24, 7114. [Google Scholar] [CrossRef]
- Hao, Q.; Vadgama, J.V.; Wang, P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun. Signal 2020, 18, 82. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, Y.; Che, X.; Zhang, M.; Li, Z.; Li, C.; Wang, S.; Wen, T.; Hou, K.; Shao, X.; et al. Anti-PD-1 Therapy Response Predicted by the Combination of Exosomal PD-L1 and CD28. Front. Oncol. 2020, 10, 760. [Google Scholar]


| Tot | N°27 (100%) |
|---|---|
| Sex | |
| Male | 18 (67%) |
| Female | 9 (33%) |
| AgeMedian range | 48–84 |
| ≤75 | 22 (81) |
| >75 | 5 (19) |
| Histotype | |
| Squamous | 6 (22) |
| Adenocarcinoma | 21 (78) |
| TPS | |
| <50% | 16 (59) |
| ≥50% | 11 (41) |
| EOCG Performance Status | |
| 0 | 15 (55) |
| >0 | 12 (45) |
| Overall Survival | |
| ≤3 months | 6 (22) |
| >3 months | 21 (78) |
| Smoking Status | |
| Current | 13 (48) |
| Former | 7 (26) |
| Non-smoker | 7 (26) |
| Response to immunotherapy | |
| Yes | 14 (52) |
| No | 13 (48) |
| Clinical Variables | Cluster 1 | Cluster 2 | Cluster 3 | p-Value Fisher Test | |
|---|---|---|---|---|---|
| Overall survival | Good | 2 | 10 | 3 | NS |
| Bad | 1 | 5 | 4 | ||
| Response to therapy | R | 3 | 6 | 3 | NS |
| NR | 0 | 9 | 4 | ||
| Performance Status | 0 | 0 | 8 | 6 | p < 0.0005 |
| >0 | 3 | 7 | 1 | ||
| Clinical Variables | Cluster 1 | Cluster 2 | p-Value Fisher Test | |
|---|---|---|---|---|
| Overall Survival | Good | 15 | 2 | NS |
| Bad | 8 | 2 | ||
| Response to therapy | R | 10 | 3 | NS |
| NR | 13 | 1 | ||
| Performance Status | 0 | 3 | 1 | NS |
| >0 | 9 | 14 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibilio, P.; Zizzari, I.G.; Gelibter, A.; Siringo, M.; Tuosto, L.; Pace, A.; Asquino, A.; Valentino, F.; Sabatini, A.; Petti, M.; et al. Immunological Network Signature of Naïve Non-Oncogene-Addicted Non-Small Cell Lung Cancer Patients Treated with Anti-PD1 Therapy: A Pilot Study. Cancers 2025, 17, 922. https://doi.org/10.3390/cancers17060922
Sibilio P, Zizzari IG, Gelibter A, Siringo M, Tuosto L, Pace A, Asquino A, Valentino F, Sabatini A, Petti M, et al. Immunological Network Signature of Naïve Non-Oncogene-Addicted Non-Small Cell Lung Cancer Patients Treated with Anti-PD1 Therapy: A Pilot Study. Cancers. 2025; 17(6):922. https://doi.org/10.3390/cancers17060922
Chicago/Turabian StyleSibilio, Pasquale, Ilaria Grazia Zizzari, Alain Gelibter, Marco Siringo, Lucrezia Tuosto, Angelica Pace, Angela Asquino, Flavio Valentino, Arianna Sabatini, Manuela Petti, and et al. 2025. "Immunological Network Signature of Naïve Non-Oncogene-Addicted Non-Small Cell Lung Cancer Patients Treated with Anti-PD1 Therapy: A Pilot Study" Cancers 17, no. 6: 922. https://doi.org/10.3390/cancers17060922
APA StyleSibilio, P., Zizzari, I. G., Gelibter, A., Siringo, M., Tuosto, L., Pace, A., Asquino, A., Valentino, F., Sabatini, A., Petti, M., Bellati, F., Santini, D., Nuti, M., Farina, L., Rughetti, A., & Napoletano, C. (2025). Immunological Network Signature of Naïve Non-Oncogene-Addicted Non-Small Cell Lung Cancer Patients Treated with Anti-PD1 Therapy: A Pilot Study. Cancers, 17(6), 922. https://doi.org/10.3390/cancers17060922








