Advancements in Surgical Management of Periacetabular Metastases: Emphasizing Minimally Invasive Techniques
Simple Summary
Abstract
1. Introduction
2. Necessity for Surgical Intervention: Clinical Manifestation of Periacetabular Metastatic Lesions
2.1. Ostealgia
2.2. Pathological Fractures and Acetabular Instability
2.3. Hypercalcemia
3. Rationale for Surgical Intervention: Biology of Osteolytic Metastases
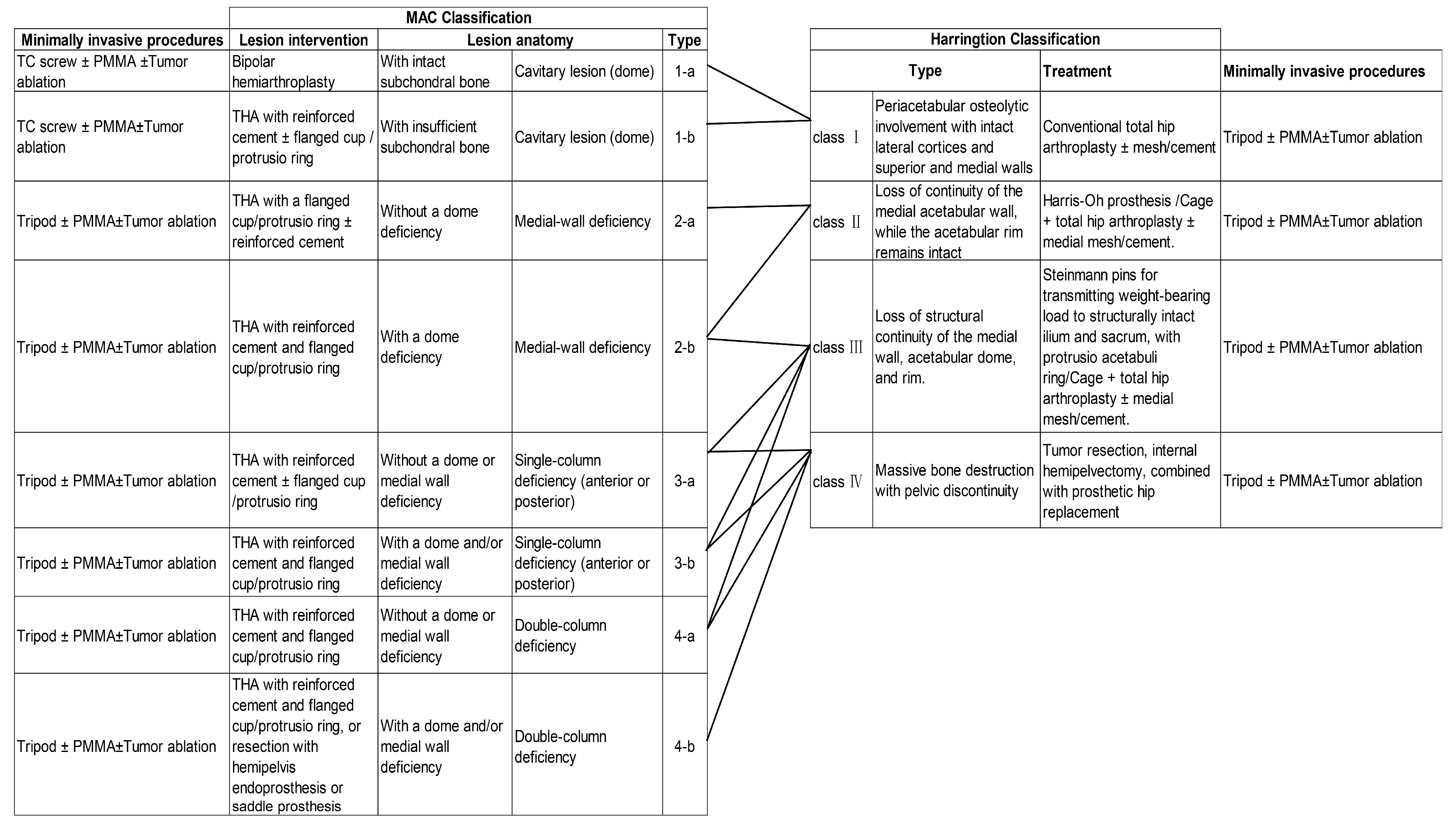
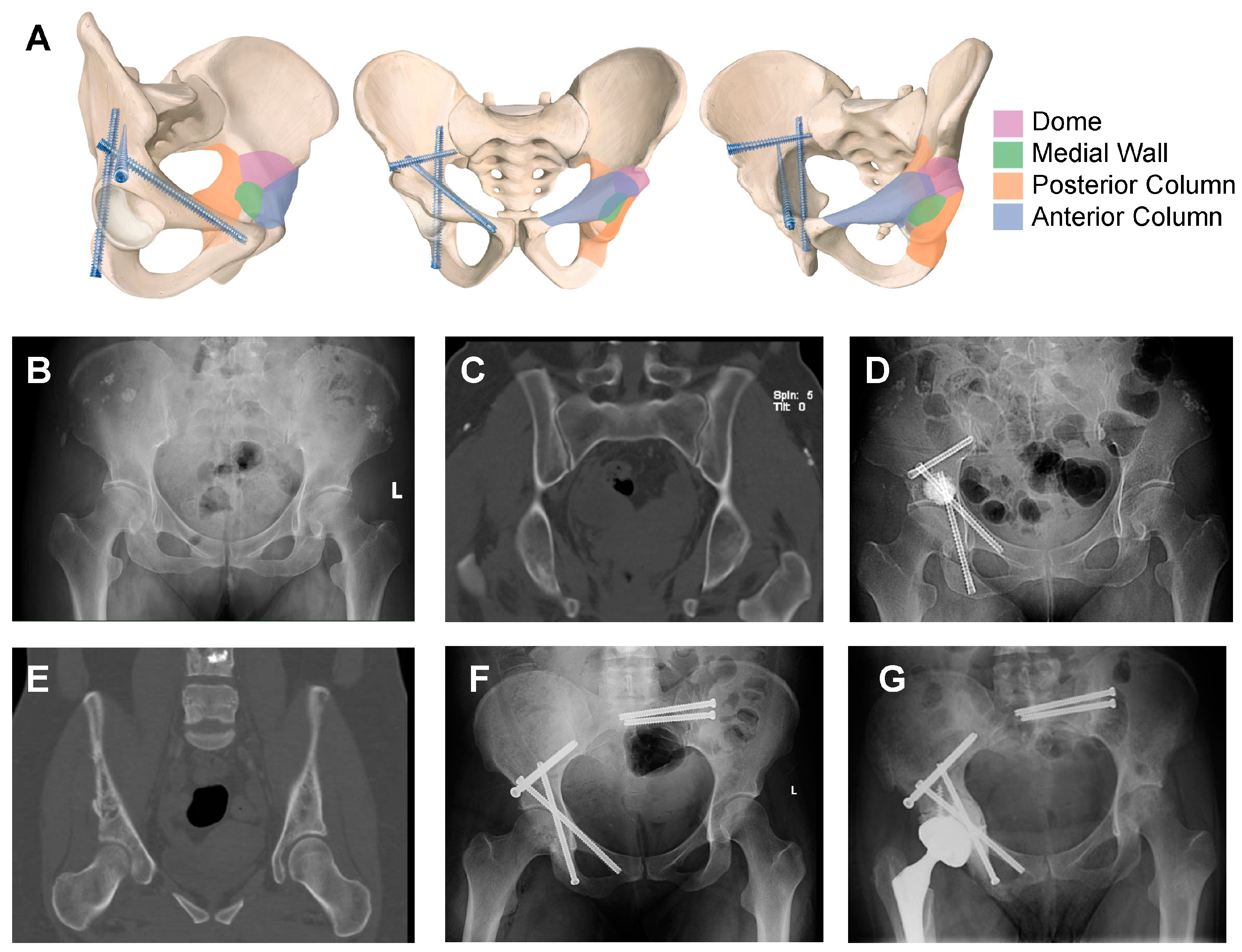
4. Planning for Surgical Intervention: Goals and Clinical Classification of Periacetabular Lesions
5. Execution of Surgical Intervention: Tumor Elimination and Structural Reinforcement
5.1. Open Surgery
5.2. Minimally Invasive Procedures
5.2.1. Tumor Elimination Techniques
Radiofrequency Ablation (RFA)
Cryoablation (CA)
Microwave Ablation (MWA)
High-Intensity Focused Ultrasound (HIFU)
Electroporation
5.2.2. Reconstruction Techniques
Kirschner Wire and Cannulated Screw Fixation
PMMA Bone Cement
5.3. Combination of Minimal Intervention Technique
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Boland, P.J.; Fabbri, N.; Healey, J.H. Rate and risk factors for wound complications after internal hemipelvectomy. Bone Joint J. 2020, 102-b, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.S.; Ziranu, A.; Piccioli, A.; Maccauro, G. Surgical treatment of acetabular metastasis. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3005–3010. [Google Scholar] [PubMed]
- Guzik, G. Treatment of metastatic lesions localized in the acetabulum. J. Orthop. Surg. Res. 2016, 11, 54. [Google Scholar] [CrossRef]
- Gillespie, E.F.; Yang, J.C.; Mathis, N.J.; Marine, C.B.; White, C.; Zhang, Z.; Barker, C.A.; Kotecha, R.; McIntosh, A.; Vaynrub, M.; et al. Prophylactic Radiation Therapy Versus Standard of Care for Patients With High-Risk Asymptomatic Bone Metastases: A Multicenter, Randomized Phase II Clinical Trial. J. Clin. Oncol. 2024, 42, 38–46. [Google Scholar] [CrossRef]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29 (Suppl. 4), iv166–iv191. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee, in WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents; World Health Organization: Geneva, Switzerland, 2018.
- Rasmusson, E.; Nilsson, P.; Kjellén, E.; Gunnlaugsson, A. Long-Term Risk of Hip Complications After Radiation Therapy for Prostate Cancer: A Dose-Response Study. Adv. Radiat. Oncol. 2021, 6, 100571. [Google Scholar] [CrossRef]
- Higham, C.E.; Faithfull, S. Bone Health and Pelvic Radiotherapy. Clin. Oncol. (R Coll Radiol) 2015, 27, 668–678. [Google Scholar] [CrossRef]
- Wang, K.; Tepper, J.E. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12 Pt 2, 6243s–6249s. [Google Scholar] [CrossRef]
- Lindsay, A.D. Skeletal metastatic disease of the acetabulum: Historical and evolving techniques for management. Ann. Jt. 2022, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Papalexis, N.; Parmeggiani, A.; Peta, G.; Spinnato, P.; Miceli, M.; Facchini, G. Minimally Invasive Interventional Procedures for Metastatic Bone Disease: A Comprehensive Review. Curr. Oncol. 2022, 29, 4155–4177. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, J.; Mizera, M.M.; Tarpada, S.P.; Seetharaman, M.; Sen, M.K.; Hoang, B.; Geller, D.S. A graphic guide to the percutaneous tripod acetabular reconstruction for metastatic cancer. J. Surg. Oncol. 2021, 123, 1316–1327. [Google Scholar] [CrossRef]
- Clohisy, D.R.; Mantyh, P.W. Bone cancer pain. Cancer 2003, 97 (Suppl. 3), 866–873. [Google Scholar] [CrossRef]
- Chau, M.M.; Clohisy, D.R. Chapter 57—Mechanisms and Management of Bone Cancer Pain. In Bone Sarcomas and Bone Metastases—From Bench to Bedside, 3rd ed.; Heymann, D., Ed.; Academic Press: New York, NY, USA, 2022; pp. 853–861. [Google Scholar]
- Herget, G.; Saravi, B.; Schwarzkopf, E.; Wigand, M.; Südkamp, N.; Schmal, H.; Uhl, M.; Lang, G. Clinicopathologic characteristics, metastasis-free survival, and skeletal-related events in 628 patients with skeletal metastases in a tertiary orthopedic and trauma center. World J. Surg. Oncol. 2021, 19, 62. [Google Scholar] [CrossRef]
- Hashimoto, K.; Shinyashiki, Y.; Ohtani, K.; Kakinoki, R.; Akagi, M. How proximal femur fracture patients aged 65 and older fare in survival and cause of death 5+ years after surgery: A long-term follow-up. Medicine 2023, 102, e33863. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Park, S.H.; Eber, M.R.; Peters, C.M.; Shiozawa, Y. Skeletal complications in cancer patients with bone metastases. Int. J. Urol. 2016, 23, 825–832. [Google Scholar] [CrossRef]
- Zagzag, J.; Hu, M.I.; Fisher, S.B.; Perrier, N.D. Hypercalcemia and cancer: Differential diagnosis and treatment. CA Cancer J. Clin. 2018, 68, 377–386. [Google Scholar] [CrossRef]
- Guise, T.A.; Wysolmerski, J.J. Cancer-Associated Hypercalcemia. N. Engl. J. Med. 2022, 386, 1443–1451. [Google Scholar] [CrossRef]
- Lipton, A.; Uzzo, R.; Amato, R.J.; Ellis, G.K.; Hakimian, B.; Roodman, G.D.; Smith, M.R. The science and practice of bone health in oncology: Managing bone loss and metastasis in patients with solid tumors. J. Natl. Compr. Canc. Netw. 2009, 7 (Suppl. 7), S1–S29, quiz S30. [Google Scholar] [CrossRef]
- Selvaggi, G.; Scagliotti, G.V. Management of bone metastases in cancer: A review. Crit. Rev. Oncol. Hematol. 2005, 56, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Maffulli, N.; Trivellas, A.; Eschweiler, J.; Tingart, M.; Driessen, A. Bone metastases: A comprehensive review of the literature. Mol. Biol. Rep. 2020, 47, 6337–6345. [Google Scholar] [CrossRef]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Goncalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar]
- Prantl, L.; Muehlberg, F.; Navone, N.M.; Song, Y.H.; Vykoukal, J.; Logothetis, C.J.; Alt, E.U. Adipose tissue-derived stem cells promote prostate tumor growth. Prostate 2010, 70, 1709–1715. [Google Scholar] [CrossRef]
- Harrington, K.D. The management of acetabular insufficiency secondary to metastatic malignant disease. J. Bone Jt. Surg. Am. 1981, 63, 653–664. [Google Scholar] [CrossRef]
- Issack, P.S.; Kotwal, S.Y.; Lane, J.M. Management of metastatic bone disease of the acetabulum. J. Am. Acad. Orthop. Surg. 2013, 21, 685–695. [Google Scholar] [CrossRef]
- Marco, R.A.; Sheth, D.S.; Boland, P.J.; Wunder, J.S.; Siegel, J.A.; Healey, J.H. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J. Bone Jt. Surg. Am. 2000, 82, 642–651. [Google Scholar] [CrossRef]
- Guo, W.; Li, D.; Tang, X.; Yang, Y.; Ji, T. Reconstruction with modular hemipelvic prostheses for periacetabular tumor. Clin. Orthop. Relat. Res. 2007, 461, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Ogura, K.; Christ, A.; Bartelstein, M.; Kenan, S.; Fabbri, N.; Healey, J. Periacetabular reconstruction following limb-salvage surgery for pelvic sarcomas. J. Bone Oncol. 2021, 31, 100396. [Google Scholar] [CrossRef] [PubMed]
- Abudu, A.; Grimer, R.J.; Cannon, S.R.; Carter, S.R.; Sneath, R.S. Reconstruction of the hemipelvis after the excision of malignant tumours. Complications and functional outcome of prostheses. J. Bone Jt. Surg. Br. 1997, 79, 773–779. [Google Scholar] [CrossRef]
- Jansen, J.A.; van de Sande, M.A.; Dijkstra, P.D. Poor long-term clinical results of saddle prosthesis after resection of periacetabular tumors. Clin. Orthop. Relat. Res. 2013, 471, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Aljassir, F.; Beadel, G.P.; Turcotte, R.E.; Griffin, A.M.; Bell, R.S.; Wunder, J.S.; Isler, M.H. Outcome after pelvic sarcoma resection reconstructed with saddle prosthesis. Clin. Orthop. Relat. Res. 2005, 438, 36–41. [Google Scholar] [CrossRef]
- Charles, T.; Ameye, L.; Gebhart, M. Surgical treatment for periacetabular metastatic lesions. Eur. J. Surg. Oncol. 2017, 43, 1727–1732. [Google Scholar] [CrossRef]
- Lavignac, P.; Prieur, J.; Fabre, T.; Descamps, J.; Niglis, L.; Carlier, C.; Bouthors, C.; Baron-Trocellier, T.; Sailhan, F.; Bonnevialle, P. Surgical treatment of peri-acetabular metastatic disease: Retrospective, multicentre study of 91 THA cases. Orthop. Traumatol. Surg. Res. 2020, 106, 1025–1032. [Google Scholar] [CrossRef]
- Plaud, A.; Gaillard, J.; Gouin, F.; Le Thuaut, A.; Ageneau, P.; Berchoud, J.; Fouasson-Chailloux, A.; Crenn, V. Functional and Survival Outcomes of Patients following the Harrington Procedure for Complex Acetabular Metastatic Lesions. Curr. Oncol. 2022, 29, 5875–5890. [Google Scholar] [CrossRef]
- Xu, S.; Guo, Z.; Shen, Q.; Peng, Y.; Li, J.; Li, S.; He, P.; Jiang, Z.; Que, Y.; Cao, K.; et al. Reconstruction of Tumor-Induced Pelvic Defects With Customized, Three-Dimensional Printed Prostheses. Front. Oncol. 2022, 12, 935059. [Google Scholar] [CrossRef]
- Traub, F.; Andreou, D.; Niethard, M.; Tiedke, C.; Werner, M.; Tunn, P.U. Biological reconstruction following the resection of malignant bone tumors of the pelvis. Sarcoma 2013, 2013, 745360. [Google Scholar] [CrossRef]
- Scoccianti, G.; Scanferla, R.; Scorianz, M.; Frenos, F.; Sacchetti, F.; Muratori, F.; Campanacci, D.A. Surgical treatment for pelvic bone metastatic disease from renal cell carcinoma. J. Surg. Oncol. 2023, 128, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Rowell, P.; Lowe, M.; Sommerville, S.; Dickinson, I. Is an Acetabular Cage and Cement Fixation Sufficiently Durable for the Treatment of Destructive Acetabular Metastases? Clin. Orthop. Relat. Res. 2019, 477, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Yang, Y.; Tang, X.; Liang, H.; Yan, T.; Yang, R.; Guo, W. 3D-Printed Modular Hemipelvic Endoprosthetic Reconstruction Following Periacetabular Tumor Resection: Early Results of 80 Consecutive Cases. J. Bone Jt. Surg. Am. 2020, 102, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Amouyel, T.; Vieillard, M.H.; Duhamel, A.; Maynou, C.; Duterque-Coquillaud, M.; Dumont, C. Is surgery without curettage effective for periacetabular Metastasis? Insights from a survival study of 93 patients. J. Bone Oncol. 2024, 49, 100643. [Google Scholar] [CrossRef]
- Nayar, S.K.; Kostakos, T.A.; Savvidou, O.; Vlasis, K.; Papagelopoulos, P.J. Outcomes of Hip Reconstruction for Metastatic Acetabular Lesions: A Scoping Review of the Literature. Curr. Oncol. 2022, 29, 3849–3859. [Google Scholar] [CrossRef]
- Krishnan, C.K.; Han, I.; Kim, H.S. Outcome after Surgery for Metastases to the Pelvic Bone: A Single Institutional Experience. Clin. Orthop. Surg. 2017, 9, 116–125. [Google Scholar] [CrossRef]
- Ji, T.; Guo, W.; Yang, R.L.; Tang, X.D.; Wang, Y.F. Modular hemipelvic endoprosthesis reconstruction--experience in 100 patients with mid-term follow-up results. Eur. J. Surg. Oncol. 2013, 39, 53–60. [Google Scholar] [CrossRef]
- Moynagh, M.R.; Kurup, A.N.; Callstrom, M.R. Thermal Ablation of Bone Metastases. Semin. Interv. Radiol. 2018, 35, 299–308. [Google Scholar]
- Shah, D.R.; Green, S.; Elliot, A.; McGahan, J.P.; Khatri, V.P. Current oncologic applications of radiofrequency ablation therapies. World J. Gastrointest. Oncol. 2013, 5, 71–80. [Google Scholar] [CrossRef]
- Cazzato, R.L.; De Marini, P.; Leonard-Lorant, I.; Dalili, D.; Koch, G.; Autrusseau, P.A.; Mayer, T.; Weiss, J.; Auloge, P.; Garnon, J.; et al. Percutaneous thermal ablation of sacral metastases: Assessment of pain relief and local tumor control. Diagn. Interv. Imaging 2021, 102, 355–361. [Google Scholar] [CrossRef]
- Tomasian, A.; Jennings, J.W. Percutaneous minimally invasive thermal ablation for management of osseous metastases: Recent advances. Int. J. Hyperth. 2019, 36, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, H.; Xu, H.R.; Liu, J.Z.; Pan, J.; Zhai, H.Z.; Lu, C.Y.; Zhao, X.; Chen, Y.Q.; Zhou, L.L.; et al. Analgesia of percutaneous thermal ablation plus cementoplasty for cancer bone metastases. J. Bone Oncol. 2019, 19, 100266. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, R.L.; Garnon, J.; Ramamurthy, N.; Koch, G.; Tsoumakidou, G.; Caudrelier, J.; Arrigoni, F.; Zugaro, L.; Barile, A.; Masciocchi, C.; et al. Percutaneous image-guided cryoablation: Current applications and results in the oncologic field. Med. Oncol. 2016, 33, 140. [Google Scholar] [CrossRef] [PubMed]
- Callstrom, M.R.; Dupuy, D.E.; Solomon, S.B.; Beres, R.A.; Littrup, P.J.; Davis, K.W.; Paz-Fumagalli, R.; Hoffman, C.; Atwell, T.D.; Charboneau, J.W.; et al. Percutaneous image-guided cryoablation of painful metastases involving bone: Multicenter trial. Cancer 2013, 119, 1033–1041. [Google Scholar] [CrossRef]
- Gallusser, N.; Goetti, P.; Becce, F.; Vauclair, F.; Rüdiger, H.A.; Bize, P.E.; Cherix, S. Percutaneous image-guided cryoablation of painful bone metastases: A single institution experience. Orthop. Traumatol. Surg. Res. 2019, 105, 369–374. [Google Scholar] [CrossRef]
- Arrigoni, F.; Bianchi, G.; Formiconi, F.; Palumbo, P.; Zugaro, L.; Gravina, G.L.; Barile, A.; Masciocchi, C. CT-guided cryoablation for management of bone metastases: A single center experience and review of the literature. Radiol. Med. 2022, 127, 199–205. [Google Scholar] [CrossRef]
- Brace, C.L. Microwave ablation technology: What every user should know. Curr. Probl. Diagn. Radiol. 2009, 38, 61–67. [Google Scholar] [CrossRef]
- de Baere, T. New techniques of tumor ablation (microwaves, electroporation). J. Radiol. 2011, 92, 789–795. [Google Scholar] [CrossRef]
- Pusceddu, C.; Sotgia, B.; Fele, R.M.; Melis, L. Treatment of bone metastases with microwave thermal ablation. J. Vasc. Interv. Radiol. 2013, 24, 229–233. [Google Scholar] [CrossRef]
- Cazzato, R.L.; de Rubeis, G.; de Marini, P.; Dalili, D.; Koch, G.; Auloge, P.; Garnon, J.; Gangi, A. Percutaneous microwave ablation of bone tumors: A systematic review. Eur. Radiol. 2021, 31, 3530–3541. [Google Scholar] [CrossRef]
- Scipione, R.; Anzidei, M.; Bazzocchi, A.; Gagliardo, C.; Catalano, C.; Napoli, A. HIFU for Bone Metastases and other Musculoskeletal Applications. Semin. Interv. Radiol. 2018, 35, 261–267. [Google Scholar]
- Knavel, E.M.; Brace, C.L. Tumor ablation: Common modalities and general practices. Tech. Vasc. Interv. Radiol. 2013, 16, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Napoli, A.; Anzidei, M.; Marincola, B.C.; Brachetti, G.; Ciolina, F.; Cartocci, G.; Marsecano, C.; Zaccagna, F.; Marchetti, L.; Cortesi, E.; et al. Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound. Invest. Radiol. 2013, 48, 351–358. [Google Scholar] [CrossRef]
- Tasu, J.P.; Tougeron, D.; Rols, M.P. Irreversible electroporation and electrochemotherapy in oncology: State of the art. Diagn. Interv. Imaging 2022, 103, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; van den Tol, P.M.; Davalos, R.V.; Rubinsky, B.; de Gruijl, T.D.; et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef]
- Yang, R.; Goch, A.; Murphy, D.; Wang, J.; Charubhumi, V.; Fox, J.; Sen, M.; Hoang, B.; Geller, D. A Novel Tripod Percutaneous Reconstruction Technique in Periacetabular Lesions Caused by Metastatic Cancer. J. Bone Jt. Surg. Am. 2020, 102, 592–599. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Z.; Liang, H.; Zhang, R.; Liu, X.; Zhang, J.; Singh, S.; Guo, W.; Yan, T.; Hoang, B.H.; et al. Artificial intelligence assisted preoperative planning and 3D-printing guiding frame for percutaneous screw reconstruction in periacetabular metastatic cancer patients. Front. Bioeng. Biotechnol. 2024, 12, 1404937. [Google Scholar] [CrossRef]
- Park, J.W.; Lim, H.J.; Kang, H.G.; Kim, J.H.; Kim, H.S. Percutaneous Cementoplasty for the Pelvis in Bone Metastasis: 12-Year Experience. Ann. Surg. Oncol. 2022, 29, 1413–1422. [Google Scholar] [CrossRef]
- Cotten, A.; Demondion, X.; Boutry, N.; Cortet, B.; Chastanet, P.; Duquesnoy, B.; Leblond, D. Therapeutic percutaneous injections in the treatment of malignant acetabular osteolyses. Radiographics 1999, 19, 647–653. [Google Scholar] [CrossRef]
- Maccauro, G.; Liuzza, F.; Scaramuzzo, L.; Milani, A.; Muratori, F.; Rossi, B.; Waide, V.; Logroscino, G.; Logroscino, C.A.; Maffulli, N. Percutaneous acetabuloplasty for metastatic acetabular lesions. BMC Musculoskelet Disord 2008, 9, 66. [Google Scholar] [CrossRef]
- Colman, M.W.; Karim, S.M.; Hirsch, J.A.; Yoo, A.J.; Schwab, J.H.; Hornicek, F.J.; Raskin, K.A. Percutaneous Acetabuloplasty Compared with Open Reconstruction for Extensive Periacetabular Carcinoma Metastases. J. Arthroplast. 2015, 30, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Cotten, A.; Deprez, X.; Migaud, H.; Chabanne, B.; Duquesnoy, B.; Chastanet, P. Malignant acetabular osteolyses: Percutaneous injection of acrylic bone cement. Radiology 1995, 197, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Ibe, I.; Dussik, C.M.; Callan, A.K.; Barr, J.; Lee, F.Y. Emerging Minimally Invasive Percutaneous Procedures for Periacetabular Osteolytic Metastases. J. Bone Jt. Surg. Am. 2023, 105, 479–489. [Google Scholar] [CrossRef]
- Morris, M.T.; Alder, K.D.; Moushey, A.; Munger, A.M.; Milligan, K.; Toombs, C.; Conway, D.; Lee, I.; Chen, F.; Tommasini, S.M.; et al. Biomechanical restoration of metastatic cancer-induced peri-acetabular bone defects by ablation-osteoplasty-reinforcement-internal fixation technique (AORIF): To screw or not to screw? Clin. Biomech. 2022, 92, 105565. [Google Scholar] [CrossRef] [PubMed]
- Araneta KT, S.; Rizkallah, M.; Boucher, L.M.; Turcotte, R.E.; Aoude, A. Joint-sparing reconstruction for extensive periacetabular metastases: Literature review and a novel minimally invasive surgical technique. J. Bone Oncol. 2022, 34, 100428. [Google Scholar] [CrossRef]
- English, D.I.; Lea, W.B.; King, D.M.; Tutton, S.M.; Neilson, J.C. Minimally Invasive Stabilization with or without Ablation for Metastatic Periacetabular Tumors. J. Bone Jt. Surg. Am. 2021, 103, 1184–1192. [Google Scholar] [CrossRef]
- Dussik, C.M.; Toombs, C.; Alder, K.D.; Yu, K.E.; Berson, E.R.; Ibe, I.K.; Li, F.; Lindskog, D.M.; Friedlaender, G.E.; Latich, I.; et al. Percutaneous Ablation, Osteoplasty, Reinforcement, and Internal Fixation for Pain and Ambulatory Function in Periacetabular Osteolytic Malignancies. Radiology 2023, 307, e221401. [Google Scholar] [CrossRef]
- Yang, R.; Singh, S.; Falk, A.; Wang, J.; Thornhill, B.; Fox, J.; Sen, M.; Hoang, B.; Geller, D.S. Percutaneous Screw Stabilization of Non-Periacetabular Pelvic Lesions Caused by Metastatic Cancer and Multiple Myeloma. J. Bone Joint Surg. Am. 2022, 104, 577–585. [Google Scholar] [CrossRef]

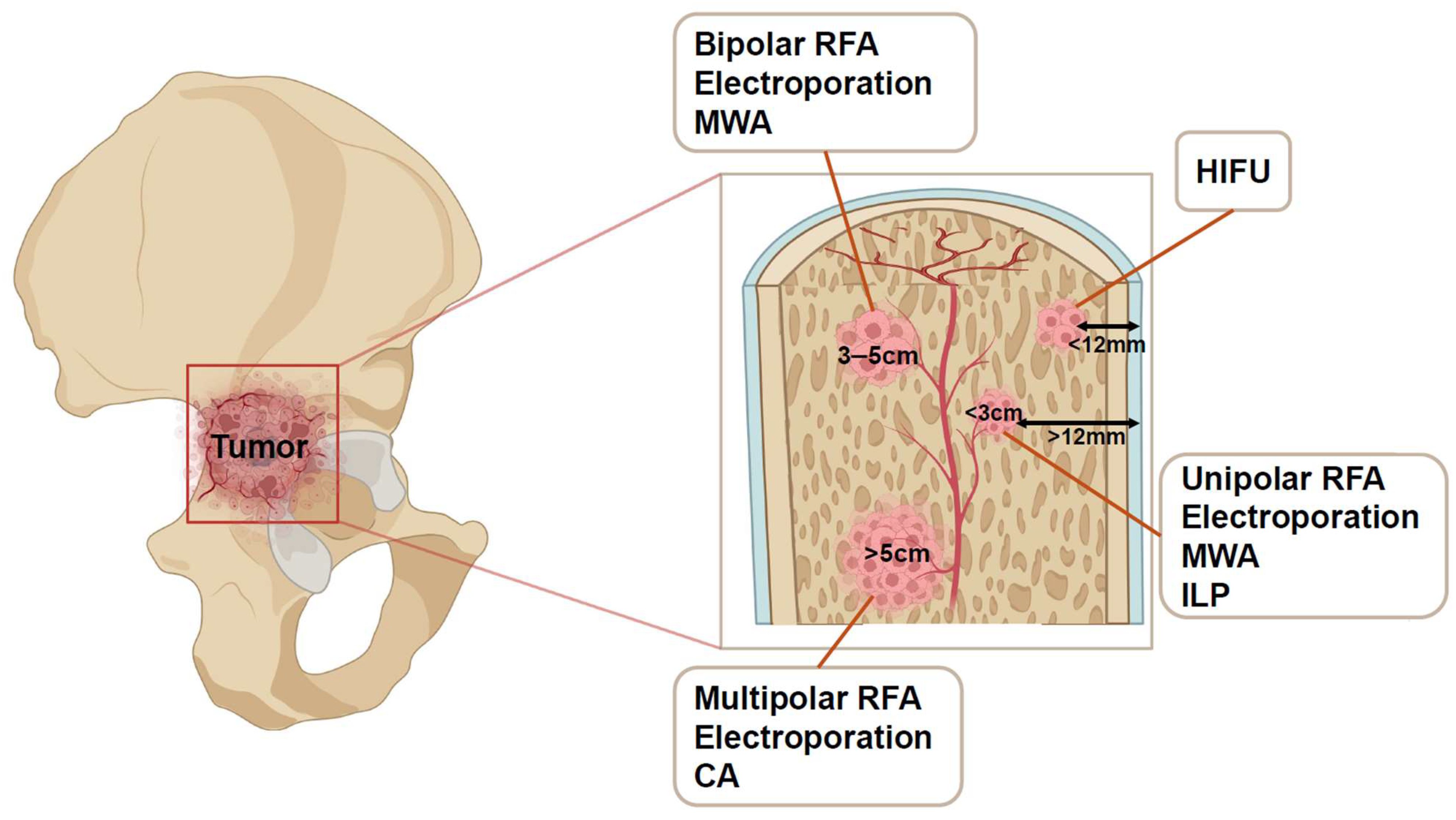
| Tumor Size | Ablation | |
|---|---|---|
| Tumor < 3 cm | Single, recurring, multiple (<5), tumors | RFA/MWA |
| Tumor adjacent to neurovascular bundle | Bipolar RFA/Electroporation | |
| 3 cm < Tumor < 5 cm | Bipolar RFA/Electroporation/MWA | |
| Tumor > 5 cm | Multipolar RFA/Electroporation/CA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, J.; Qi, F.; Liang, H.; Liu, X.; Zhao, Z.; Chen, L.; Zhang, R.; Yang, R.Y.; Goker, B.; Singh, S.; et al. Advancements in Surgical Management of Periacetabular Metastases: Emphasizing Minimally Invasive Techniques. Cancers 2025, 17, 1015. https://doi.org/10.3390/cancers17061015
Guan J, Qi F, Liang H, Liu X, Zhao Z, Chen L, Zhang R, Yang RY, Goker B, Singh S, et al. Advancements in Surgical Management of Periacetabular Metastases: Emphasizing Minimally Invasive Techniques. Cancers. 2025; 17(6):1015. https://doi.org/10.3390/cancers17061015
Chicago/Turabian StyleGuan, Jian, Feiyang Qi, Haijie Liang, Xingyu Liu, Zhiqing Zhao, Linxi Chen, Ranxin Zhang, Ryan Y. Yang, Barlas Goker, Swapnil Singh, and et al. 2025. "Advancements in Surgical Management of Periacetabular Metastases: Emphasizing Minimally Invasive Techniques" Cancers 17, no. 6: 1015. https://doi.org/10.3390/cancers17061015
APA StyleGuan, J., Qi, F., Liang, H., Liu, X., Zhao, Z., Chen, L., Zhang, R., Yang, R. Y., Goker, B., Singh, S., Hoang, B. H., Geller, D. S., Wang, J., & Yang, R. (2025). Advancements in Surgical Management of Periacetabular Metastases: Emphasizing Minimally Invasive Techniques. Cancers, 17(6), 1015. https://doi.org/10.3390/cancers17061015





