Patients with Colorectal Cancer and BRAFV600E-Mutation in Argentina: A Real-World Study—The EMOGI-CRC01 Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Key Study Endpoints
2.3. Statistical Analyses
3. Results
3.1. Patient’s Characteristics
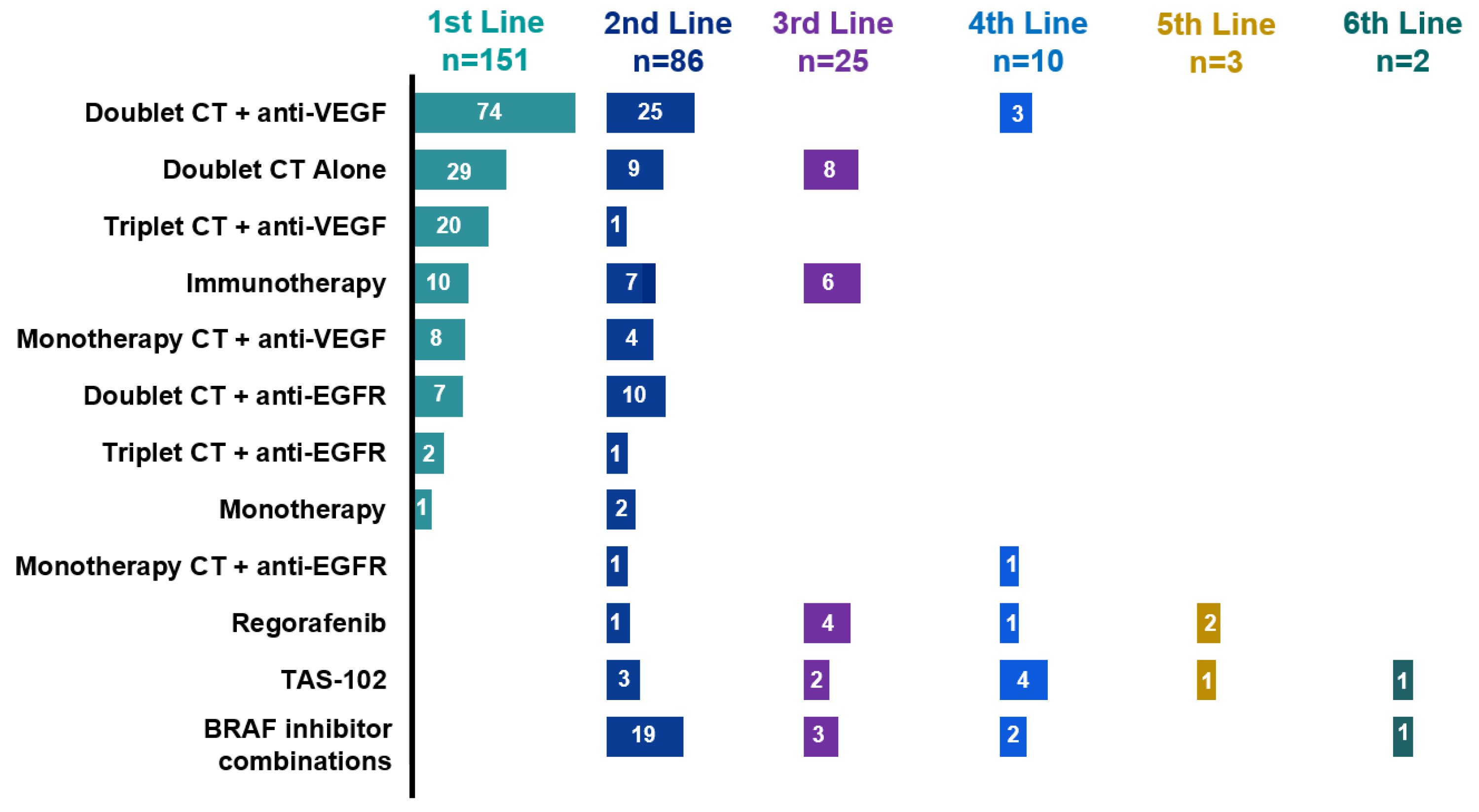
3.2. Treatment Sequences
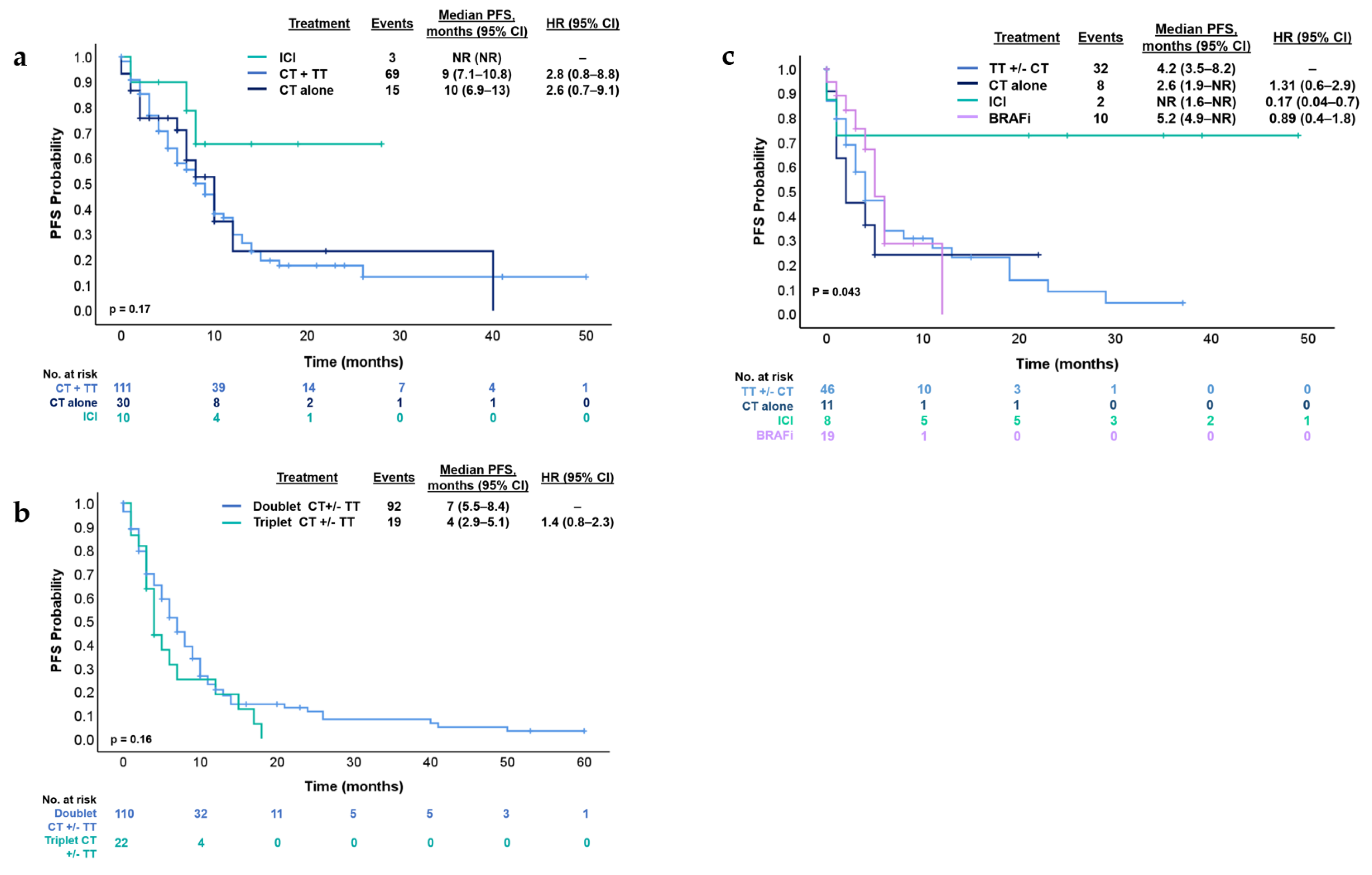
3.3. Treatment Strategies and Outcomes in First- and Second-Line Therapy
3.4. Immunotherapy and BRAF Inhibitors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Estadísticas—Incidencia. Argentina.gob.ar. 2019. Available online: https://www.argentina.gob.ar/salud/instituto-nacional-del-cancer/estadisticas/incidencia (accessed on 26 November 2024).
- Cancer of the Colon and Rectum—Cancer Stat Facts. SEER n.d. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 26 November 2024).
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B., 3rd; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 678–700. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, A.R.; Hamilton, S.R.; Allegra, C.J.; Grody, W.; Cushman-Vokoun, A.M.; Funkhouser, W.K.; Kopetz, S.E.; Lieu, C.; Lindor, N.M.; Minsky, B.D.; et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 1453–1486. [Google Scholar] [CrossRef]
- Caputo, F.; Santini, C.; Bardasi, C.; Cerma, K.; Casadei-Gardini, A.; Spallanzani, A.; Andrikou, K.; Cascinu, S.; Gelsomino, F. BRAF-Mutated Colorectal Cancer: Clinical and Molecular Insights. Int. J. Mol. Sci. 2019, 20, 5369. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef]
- Tabernero, J.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef]
- Belló, M.; Becerril-Montekio, V. The health system of Argentina. Salud Publica Mex. 2011, 53 (Suppl. 2), S96–S108. [Google Scholar]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B Methodol. 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Yaeger, R.; Shi, Q.; Dueck, A.C.; Dib, E.G.; Kazmi, S.M.A.; Alese, O.B.; Krishnamurthi, S.S.; Nixon, A.B.; Shergill, A.; O’Reilly, E.M.; et al. A randomized trial of consolidation-targeted adjuvant therapy with encorafenib and cetuximab versus usual care for patients with stage II/III BRAF V600E colon cancer: Alliance for Clinical Trials in Oncology A022004. J. Clin. Oncol. 2023, 41, TPS3641. [Google Scholar] [CrossRef]
- AIO-Studien-gGmbH. Neoadjuvant Encorafenib, Binimetinib and Cetuximab for Patients with BRAF V600E Mutat-ed/pMMR Localized Colorectal Cancer. Clinicaltrials.gov; Report No.: NCT05510895. 2023. Available online: https://clinicaltrials.gov/study/NCT05510895 (accessed on 15 March 2024).
- Federation Francophone de Cancerologie Digestive. Combination of Encorafenib Plus Cetuximab in a Neoadjuvant Setting in Patients with Braf v600e-mutates Localised Colon or Upper Rectum Cancer (Neoraf Study). Clinicaltrials.gov. Report No.: NCT05706779. 2023. Available online: https://clinicaltrials.gov/study/NCT05706779 (accessed on 17 March 2024).
- Bläker, H.; Alwers, E.; Arnold, A.; Herpel, E.; Tagscherer, K.E.; Roth, W.; Jansen, L.; Walter, V.; Kloor, M.; Chang-Claude, J.; et al. The Association Between Mutations in BRAF and Colorectal Cancer-Specific Survival Depends on Microsatellite Status and Tumor Stage. Clin. Gastroenterol. Hepatol. 2019, 17, 455–462.e6. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Kopetz, S.; Tie, J.; Gibbs, P.; Jiang, Z.Q.; Lieu, C.H.; Agarwal, A.; Maru, D.M.; Sieber, O.; Desai, J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011, 117, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Venderbosch, S.; Nagtegaal, I.D.; Maughan, T.S.; Smith, C.G.; Cheadle, J.P.; Fisher, D.; Kaplan, R.; Quirke, P.; Seymour, M.T.; Richman, S.D.; et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 2014, 20, 5322–5330. [Google Scholar] [CrossRef]
- Rosenbaum, M.W.; Bledsoe, J.R.; Morales-Oyarvide, V.; Huynh, T.G.; Mino-Kenudson, M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod. Pathol. 2016, 29, 1104–1112. [Google Scholar] [CrossRef]
- Feng, D.; Qin, B.; Pal, K.; Sun, L.; Dutta, S.; Dong, H.; Liu, X.; Mukhopadhyay, D.; Huang, S.; Sinicrope, F.A. BRAFV600E-induced, tumor intrinsic PD-L1 can regulate chemotherapy-induced apoptosis in human colon cancer cells and in tumor xenografts. Oncogene 2019, 38, 6752–6766. [Google Scholar] [CrossRef]
- Martinelli, E.; Cremolini, C.; Mazard, T.; Vidal, J.; Virchow, I.; Tougeron, D.; Cuyle, P.J.; Chibaudel, B.; Kim, S.; Ghanem, I.; et al. Real-world first-line treatment of patients with BRAFV600E-mutant metastatic colorectal cancer: The CAPSTAN CRC study. ESMO Open 2022, 7, 100603. [Google Scholar] [CrossRef]
- Xu, T.; Li, J.; Wang, Z.; Zhang, X.; Zhou, J.; Lu, Z.; Shen, L.; Wang, X. Real-world treatment and outcomes of patients with metastatic BRAF mutant colorectal cancer. Cancer Med. 2023, 12, 10473–10484. [Google Scholar] [CrossRef]
- O’Connor, J.; Torrecillas-Torres, L.; Alvarado, F.; Colombero, C.; Sasse, A. Biomarkers and treatment characteristics in metastatic colorectal cancer RASwt patients in Latin America. Gac. Mex. Oncol. 2023, 22, 10022. [Google Scholar] [CrossRef]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lonardi, S.; Masi, G.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Spadi, R.; Zaniboni, A.; et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: Results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann. Oncol. 2015, 26, 1188–1194. [Google Scholar] [CrossRef]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- Ros, J.; Baraibar, I.; Sardo, E.; Mulet, N.; Salvà, F.; Argilés, G.; Martini, G.; Ciardiello, D.; Cuadra, J.L.; Tabernero, J.; et al. BRAF, MEK and EGFR inhibition as treatment strategies in BRAF V600E metastatic colorectal cancer. Ther. Adv. Med. Oncol. 2021, 13, 1758835921992974. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Lang, I.; Folprecht, G.; Nowacki, M.; Barone, C.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Celik, I.; Kohne, C. Cetuximab plus FOLFIRI: Final data from the CRYSTAL study on the association of KRAS and BRAF biomarker status with treatment outcome. J. Clin. Oncol. 2010, 28, 3570. [Google Scholar] [CrossRef]
- Stintzing, S.; Miller-Phillips, L.; Modest, D.P.; Fischer von Weikersthal, L.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Kahl, C.; et al. Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: Analysis of the FIRE-3 (AIO KRK-0306) study. Eur. J. Cancer 2017, 79, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Arnold, D.; Cervantes, A.; Stintzing, S.; Van Cutsem, E.; Tabernero, J.; Taieb, J.; Wasan, H.; Ciardiello, F. European expert panel consensus on the clinical management of BRAFV600E-mutant metastatic colorectal cancer. Cancer Treat. Rev. 2023, 115, 102541. [Google Scholar] [CrossRef]
- Peixoto, R.D.; Chakhtoura, J.J.A.; Aguilar-Ponce, J.L.; Garcia-Rivello, H.; Jansen, A.M.; Parra Medina, R.; Ramos-Esquivel, A.; Stefani, S.D. BRAF Testing in Melanoma and Colorectal Cancer in Latin America: Challenges and Opportunities. Cureus 2022, 14, e31972. [Google Scholar] [CrossRef]
- Loupakis, F.; Intini, R.; Cremolini, C.; Orlandi, A.; Sartore-Bianchi, A.; Pietrantonio, F.; Pella, N.; Spallanzani, A.; Dell’Aquila, E.; Scartozzi, M.; et al. A validated prognostic classifier forV600EBRAF-mutated metastatic colorectal cancer: The ‘BRAF BeCool’ study. Eur. J. Cancer 2019, 118, 121–130. [Google Scholar] [CrossRef]
- Ros, J.; Rodríguez-Castells, M.; Saoudi, N.; Baraibar, I.; Salva, F.; Tabernero, J.; Élez, E. Treatment of BRAF-V600E mutant metastatic colorectal cancer: New insights and biomarkers. Expert Rev. Anticancer Ther. 2023, 23, 797–806. [Google Scholar] [CrossRef]
- Bottos, A.; Martini, M.; Di Nicolantonio, F.; Comunanza, V.; Maione, F.; Minassi, A.; Appendino, G.; Bussolino, F.; Bardelli, A. Targeting oncogenic serine/threonine-protein kinase BRAF in cancer cells inhibits angiogenesis and abrogates hypoxia. Proc. Natl. Acad. Sci. USA 2012, 109, E353–E359. [Google Scholar] [CrossRef]
- Quintanilha, J.C.F.; Graf, R.P.; Oxnard, G.R. BRAF V600E and RNF43 Co-mutations Predict Patient Outcomes with Targeted Therapies in Real-World Cases of Colorectal Cancer. Oncologist 2023, 28, e171–e174. [Google Scholar] [CrossRef]
- Elez, E.; Ros, J.; Fernández, J.; Villacampa, G.; Moreno-Cárdenas, A.B.; Arenillas, C.; Bernatowicz, K.; Comas, R.; Li, S.; Kodack, D.P.; et al. RNF43 mutations predict response to anti-BRAF/EGFR combinatory therapies in BRAFV600E metastatic colorectal cancer. Nat. Med. 2022, 28, 2162–2170. [Google Scholar] [CrossRef]
- Ros, J.; Matito, J.; Villacampa, G.; Comas, R.; Garcia, A.; Martini, G.; Baraibar, I.; Saoudi, N.; Salvà, F.; Martin, Á.; et al. Plasmatic BRAF-V600E allele fraction as a prognostic factor in metastatic colorectal cancer treated with BRAF combinatorial treatments. Ann. Oncol. 2023, 34, 543–552. [Google Scholar] [CrossRef]

| Variable | Total = 161 n | (%) |
|---|---|---|
| Median age, years | 58.3 (47–69) | |
| Sex | ||
| Female | 96 | 59.6% |
| Male | 65 | 40.4% |
| ECOG * | ||
| 0–1 | 135 | 85.7 |
| ≥2 | 23 | 14.3 |
| Tumor location | ||
| Right | 114 | 70.8% |
| Left | 33 | 20.5% |
| Rectum | 14 | 8.7 |
| Stage at diagnosis | ||
| I | 1 | 0.6% |
| II | 12 | 7.5% |
| III | 33 | 20.5% |
| IV | 115 | 71.4% |
| Histology | ||
| Adenocarcinoma | 112 | 69.5 |
| Mucinous | 43 | 26.8 |
| Unknown | 6 | 3.7 |
| Number of metastasis site | ||
| One | 69 | 42.8 |
| Two or more | 78 | 48.5 |
| Unknown | 14 | 8.7 |
| Site of metastasis | ||
| Liver | 90 | 55.9% |
| Nodes | 82 | 50.9% |
| Lung | 19 | 11.8% |
| Peritoneum | 37 | 23% |
| Type of testing | ||
| Primary tumor | 144 | 89.5 |
| Metastasis | 8 | 4.9 |
| Liquid biopsy | 9 | 5.6 |
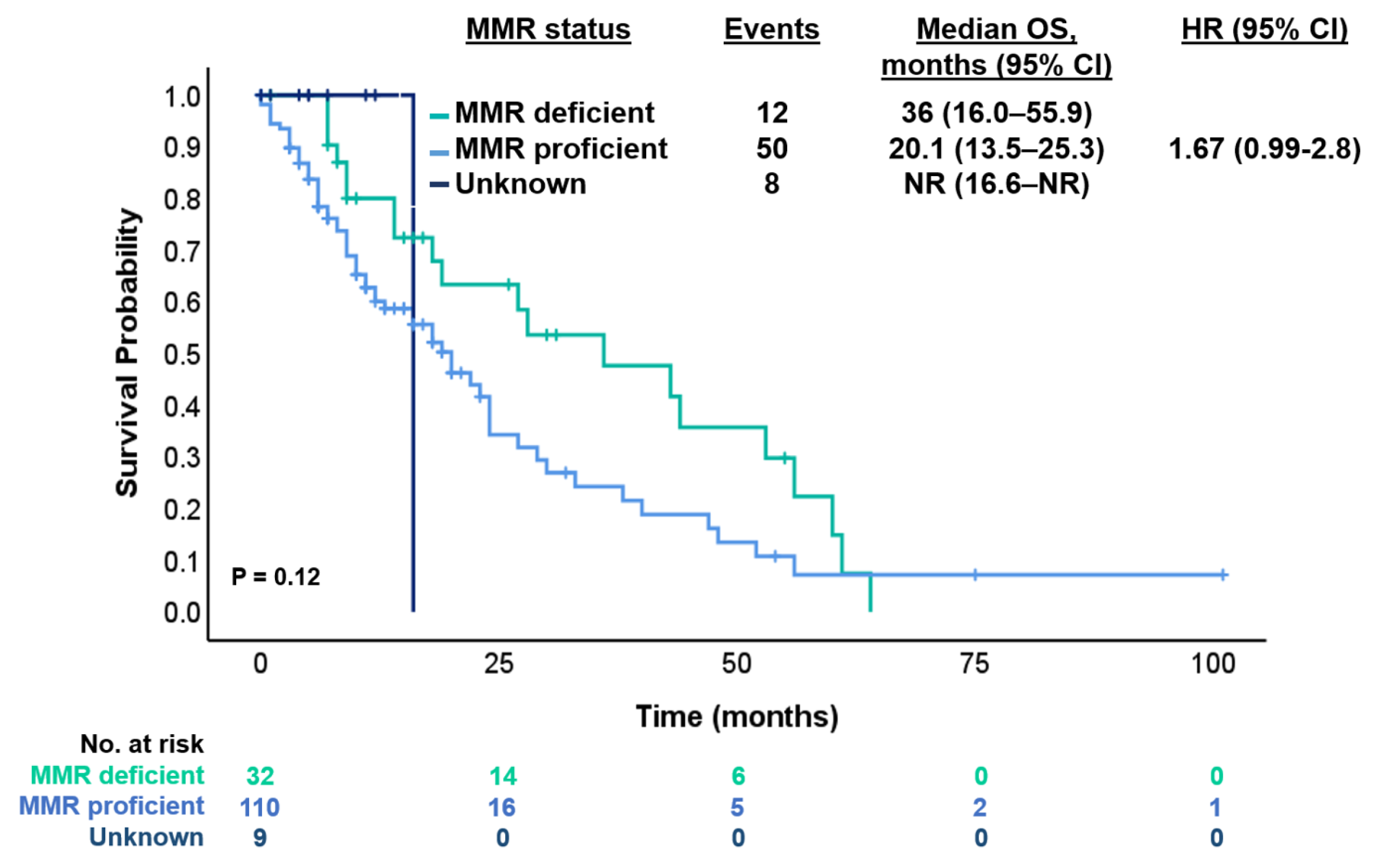
| Status MMR ** | ||
| MMR-deficient | 35 | 21.7% |
| MMR-proficient | 117 | 72.7% |
| Unknown | 9 | 5.6% |
| Status RAS | ||
| Mutated | 3 | 1.2% |
| Wild-type | 134 | 83.8% |
| Unknown | 24 | 15% |
| Status BRAF | ||
| Before election 1st line | 56 | 34.7% |
| Before election 2nd line | 80 | 49.7% |
| After election 2nd or more line | 25 | 15.6% |
| Surgery | ||
| Curative | 77 | 47.8% |
| Palliative | 22 | 13.6% |
| No | 62 | 72% |
| Lines of treatment received | ||
| First | 151 | 93.8% |
| Second | 86 | 53.4% |
| Third | 25 | 15.5% |
| Forth | 10 | 6.2% |
| Fifth | 3 | 1.8% |
| Sixth | 2 | 1.2% |
| VARIABLE | Progression or Death | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Yes n = 87 (%) | No n = 64 (%) | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age at diagnosis | ||||||
| ≤50 years | 27 (62.8) | 16 (37.2) | - | - | ||
| 50–70 years | 44 (56.4) | 34 (43–6) | 0.71 (0.44–1.16) | 0.18 | 0.76 (0.45–1.27) | 0.29 |
| >70 years | 16 (53.4) | 14 (46.6) | 0.58 (0.31–1.08) | 0.08 | 0.74 (0.37–1.43) | 0.36 |
| Gender | ||||||
| Female | 49 (55) | 40 (45) | - | - | ||
| Male | 38 (61.3) | 24 (38.7) | 1.57 (1.01–2.4) | 0.04 | 1.55 (0.98–2.45) | 0.06 |
| Stage at diagnosis | / | / | ||||
| I | 1 (100) | - | - | |||
| II | 7 (58.3) | 5 (41.7) | 0.15 (0.01–0.99) | 0.08 | ||
| III | 18 (54.5) | 15 (45.5) | 0.12 (0.01–0.72) | 0.04 | ||
| IV | 61 (58.1) | 44 (41.9) | 0.14 (0.01–0.86) | 0.05 | ||
| Tumor location | ||||||
| Left | 61 (57.5) | 45 (42.5) | - | - | ||
| Right | 14 (45.2) | 17 (54.8) | 1.12 (0.62–2.01) | 0.71 | 0.96 (0.53–1.77) | 0.09 |
| Rectum | 12 (85.7) | 2 (14.3) | 2.1 (1.12–3.9) | 0.01 | 1.67 (0.37–3.3) | 0.14 |
| Histology | / | / | ||||
| Adenocarcinoma | 60 (54.6) | 50 (45.4) | - | |||
| Mucinous | 23 (65.7) | 12 (34.3) | 0.76 (0.26–2.22) | 0.61 | ||
| Diagnosis of the metastatic setting | / | / | ||||
| Synchronous | 66 (62.3) | 40 (37.7) | 0.94 (0.13–8.87) | 0.96 | ||
| Metachronous | 21 (46.7) | 24 (53.3) | 0.65 (0.09–4.84) | 0.67 | ||
| Liver metastasis | / | / | ||||
| No | 34 (50.7) | 33 (49.3) | - | - | ||
| Yes | 53 (63.1) | 31 (36.9) | 1.18 (0.77–2.72) | 0.45 | ||
| Peritoneum metastasis | ||||||
| No | 65 (55.1) | 53 (44.9) | - | - | ||
| Yes | 20 (64.5) | 11 (35.5) | 1.64 (0.99–2.72) | 0.05 | 1.92 (1.12–3.28) | 0.01 |
| MMR status | / | / | ||||
| pMMR | 72 (60.5) | 47 (39.5) | - | - | ||
| dMMR | 15 (46.9) | 17 (53.1) | 0.65 (0.38–1.16) | 0.15 | ||
| Surgery primary tumor | 1.79 (1.11–2.88) | 0.01 | ||||
| No | 72 (60.5) | 46 (45.5) | 1.8 (1.15–2.8) | 0.009 | ||
| Yes | 15 (46.9) | 18 (36) | - | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catani, G.; Kim, S.; Waisberg, F.; Enrico, D.; Luca, R.; Esteso, F.; Bruno, L.; Rodríguez, A.; Bortz, M.; Freile, B.; et al. Patients with Colorectal Cancer and BRAFV600E-Mutation in Argentina: A Real-World Study—The EMOGI-CRC01 Study. Cancers 2025, 17, 1007. https://doi.org/10.3390/cancers17061007
Catani G, Kim S, Waisberg F, Enrico D, Luca R, Esteso F, Bruno L, Rodríguez A, Bortz M, Freile B, et al. Patients with Colorectal Cancer and BRAFV600E-Mutation in Argentina: A Real-World Study—The EMOGI-CRC01 Study. Cancers. 2025; 17(6):1007. https://doi.org/10.3390/cancers17061007
Chicago/Turabian StyleCatani, Greta, Stefano Kim, Federico Waisberg, Diego Enrico, Romina Luca, Federico Esteso, Luisina Bruno, Andrés Rodríguez, Marcos Bortz, Berenice Freile, and et al. 2025. "Patients with Colorectal Cancer and BRAFV600E-Mutation in Argentina: A Real-World Study—The EMOGI-CRC01 Study" Cancers 17, no. 6: 1007. https://doi.org/10.3390/cancers17061007
APA StyleCatani, G., Kim, S., Waisberg, F., Enrico, D., Luca, R., Esteso, F., Bruno, L., Rodríguez, A., Bortz, M., Freile, B., Chacón, M., Oviedo Albor, A. I., Méndez, G., Slutsky, E., Baiud, M. C., Llanos, R., Solonyezny, A., Basbus, L., Arroyo, G., ... O’Connor, J. M. (2025). Patients with Colorectal Cancer and BRAFV600E-Mutation in Argentina: A Real-World Study—The EMOGI-CRC01 Study. Cancers, 17(6), 1007. https://doi.org/10.3390/cancers17061007






