Evaluating Sorafenib (SORA-2) as Second-Line Treatment for Unresectable Hepatocellular Carcinoma: A European Retrospective Multicenter Study †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Therapy Scheme
2.4. Statistical Analysis
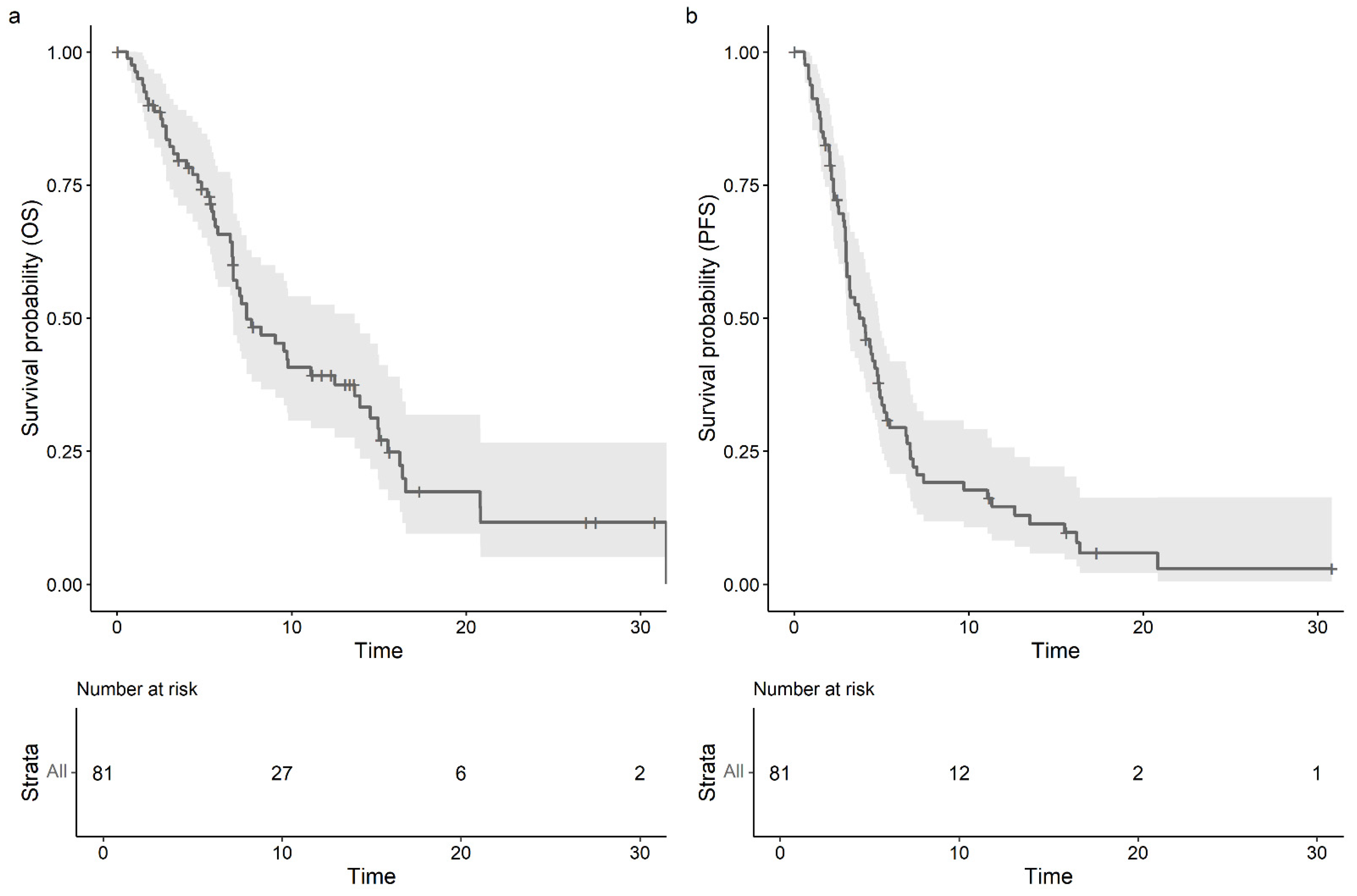
3. Results
3.1. Patient and Therapy Characteristics
3.2. Effectiveness
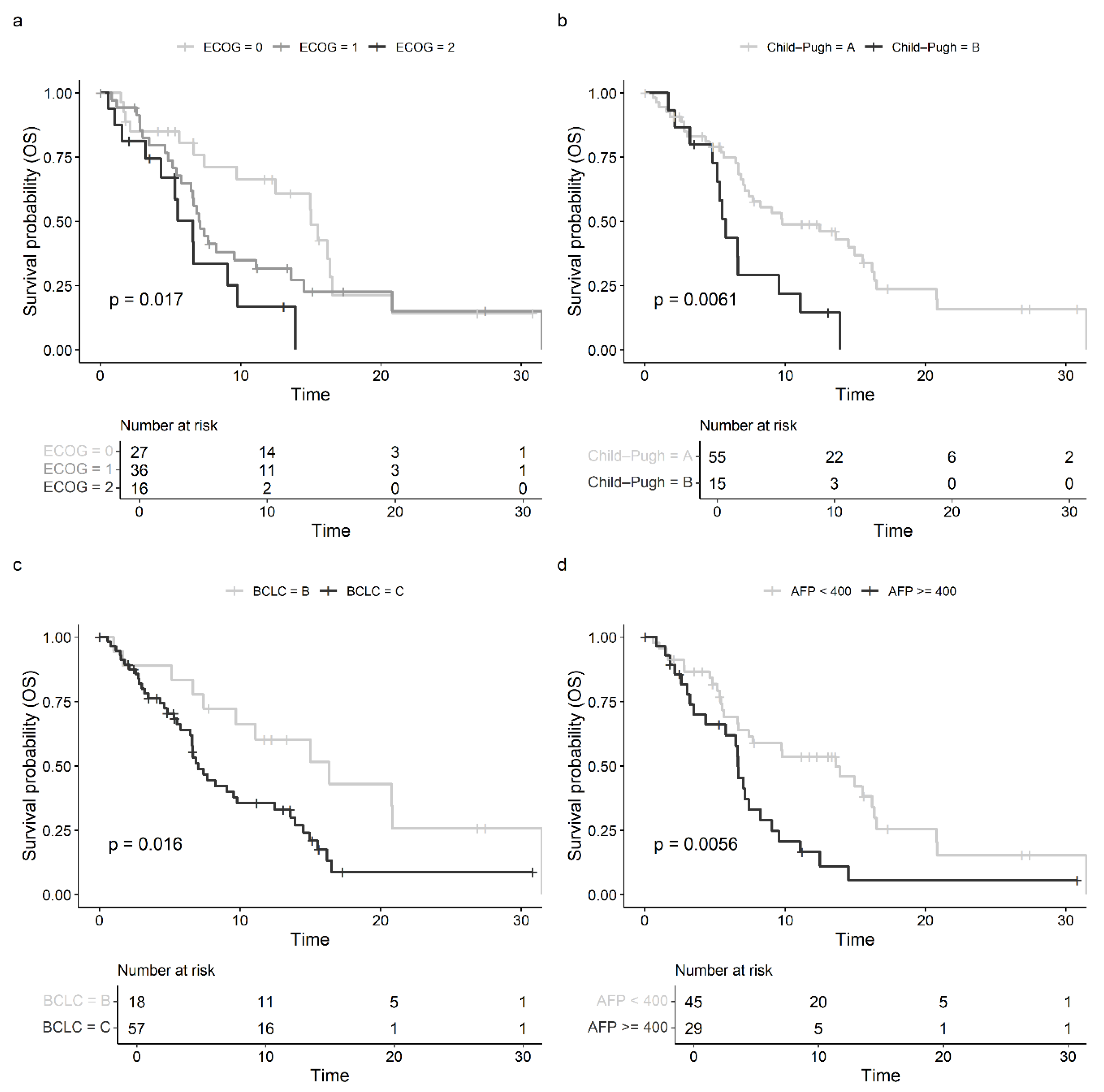
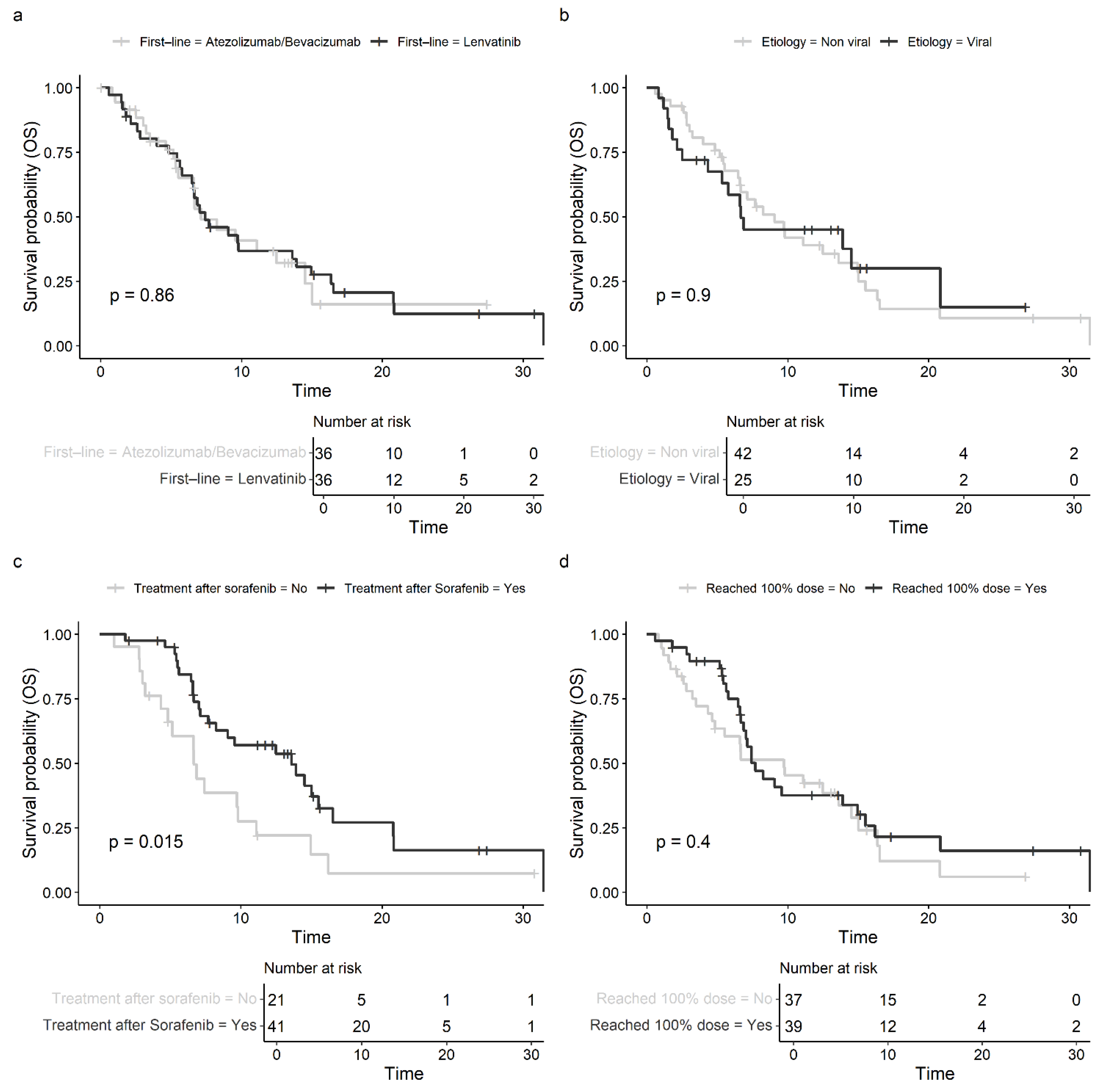
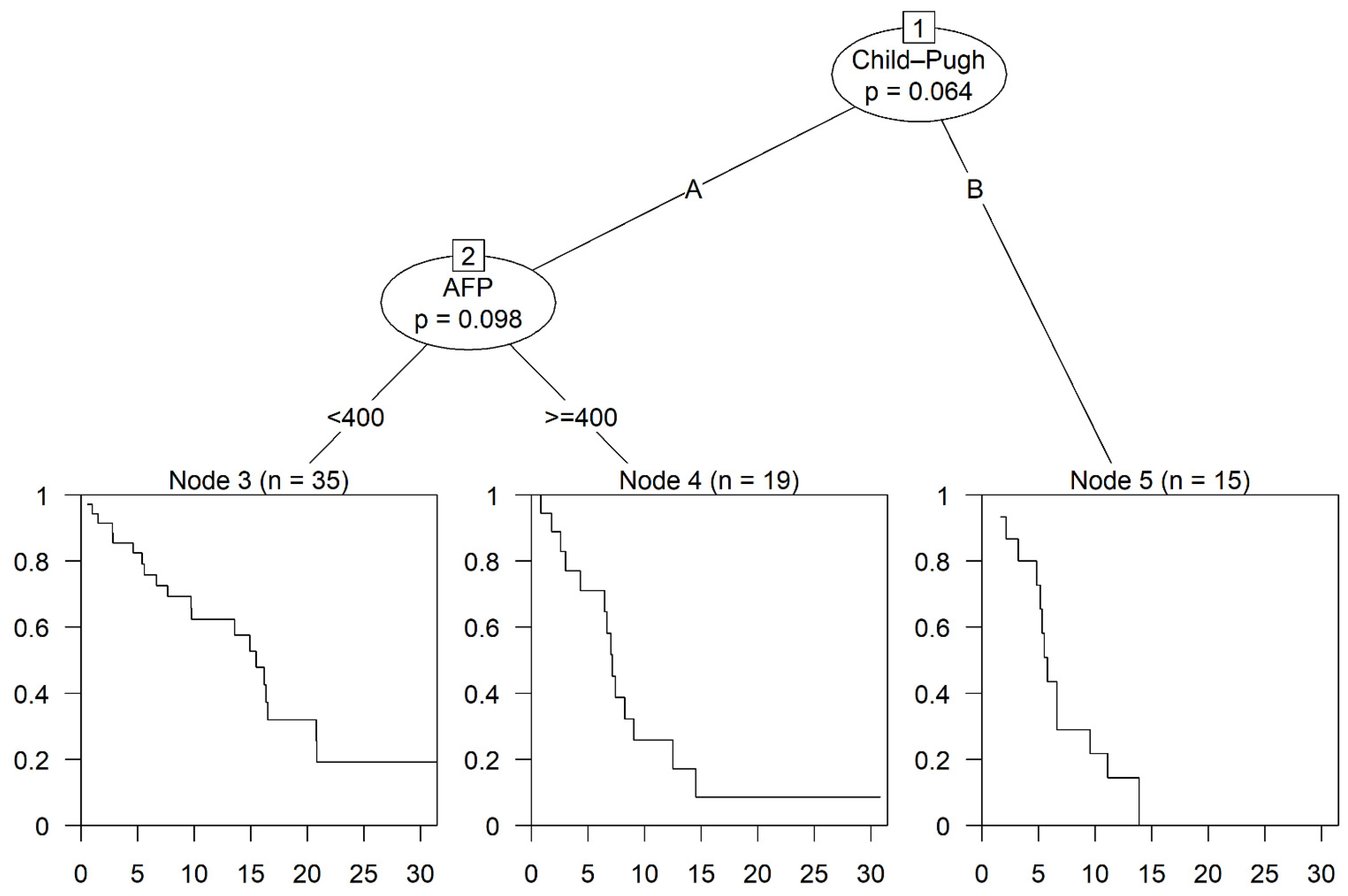
3.3. Prognostic Factors and Subgroup Analysis
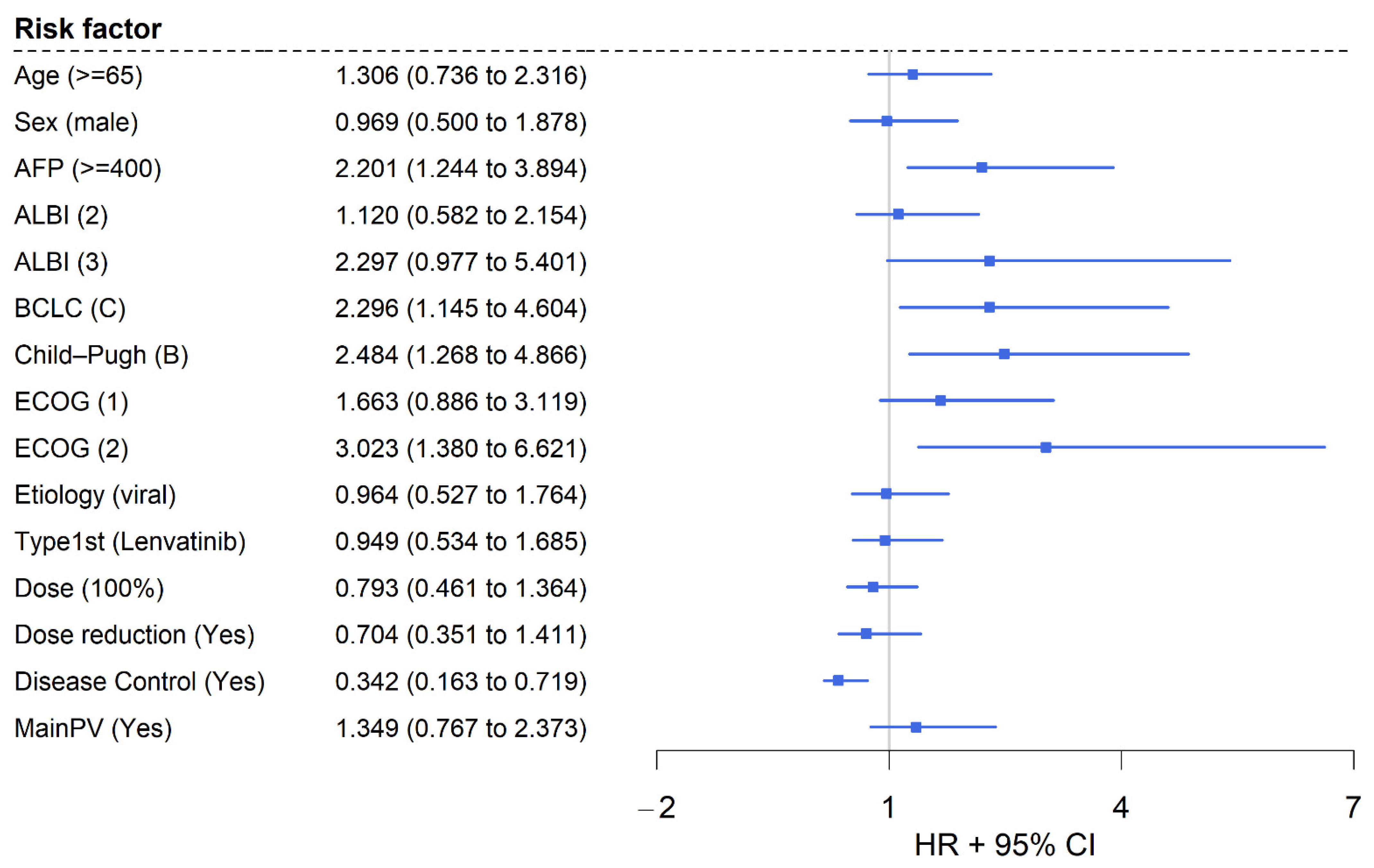
3.4. Multivariable Analysis
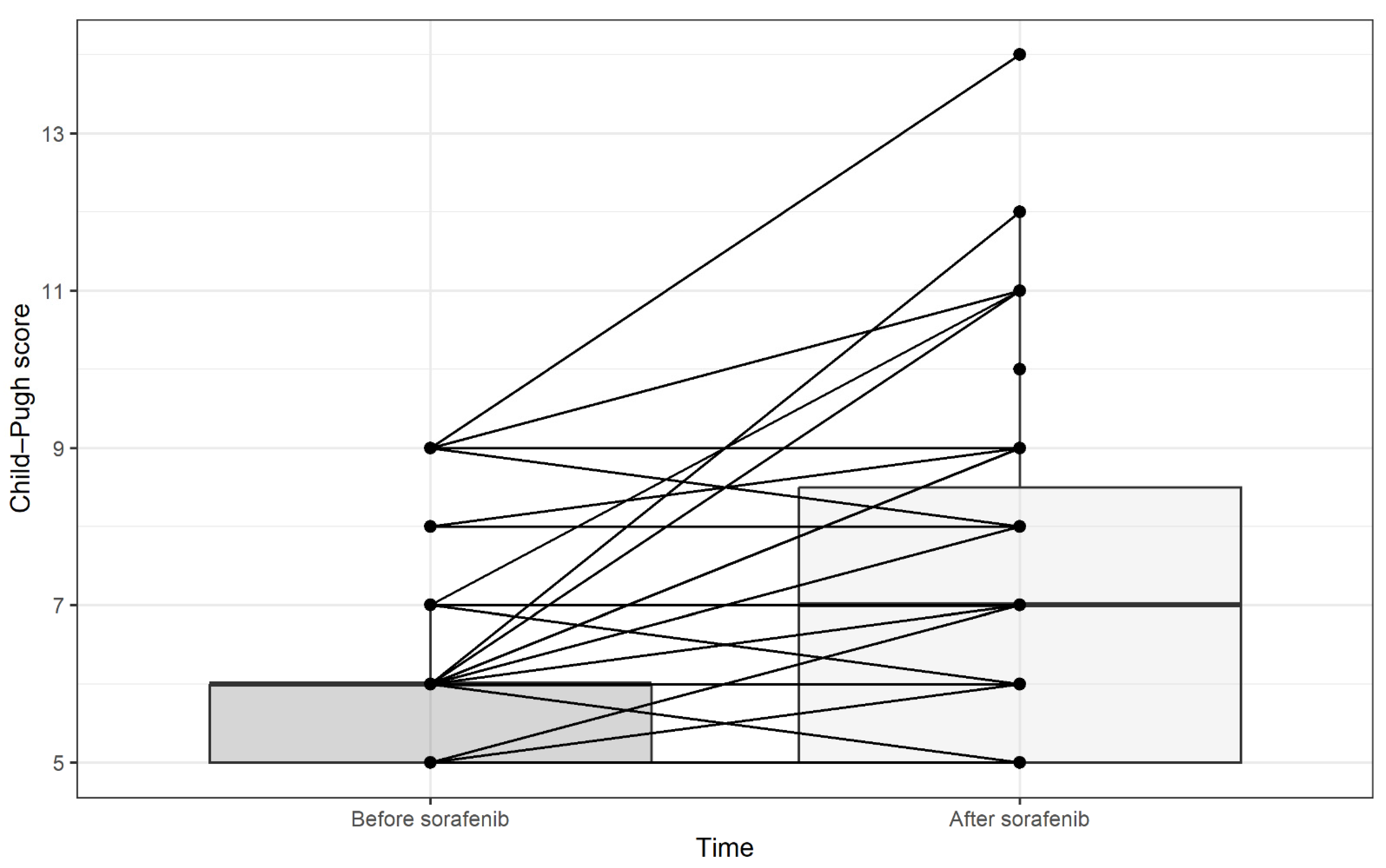
3.5. Toxicity
4. Discussion
5. Conclusions
Published Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2L | second-line therapy |
| ALBI | Albumin-Bilirubin Index |
| AFP | alpha-fetoprotein |
| ASH | alcoholic steatohepatitis |
| BCLC | Barcelona Clinic of Liver Cancer |
| CP | Child–Pugh |
| CI | confidence interval |
| CR | complete response |
| CT | computed tomography |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DCR | disease control rate |
| ECOG | Eastern Cooperative Oncology Group |
| EMA | European Medicines Agency |
| HCC | hepatocellular carcinoma |
| ICI | immune checkpoint inhibitor |
| MASH | Metabolically Associated Steatohepatitis |
| MASLD | Metabolically Associated Steatotic Liver Disease |
| MRI | magnetic resonance imaging |
| MWA | microwave ablation |
| ORR | overall response rate |
| OS | overall survival |
| PD | progressive disease |
| PFS | progression-free survival |
| PR | partial response |
| RFA | radiofrequency ablation |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| SD | stable disease |
| TARE | transarterial radioembolization |
| TKI | tyrosine kinase inhibitor |
| VEGF | vascular endothelial growth factor |
References
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Martinelli, E. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Maida, M.; Orlando, E.; Cammà, C.; Cabibbo, G. Staging systems of hepatocellular carcinoma: A review of literature. World J. Gastroenterol. 2014, 20, 4141–4150. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.C.; Szklaruk, J.; Kaseb, A.O.; Hassabo, H.M.; Elsayes, K.M. TNM/Okuda/Barcelona/UNOS/CLIP International Multidisciplinary Classification of Hepatocellular Carcinoma: Concepts, perspectives, and radiologic implications. Abdom. Imaging 2014, 39, 1070–1087. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef]
- Galle, P.R.; Decaens, T.; Kudo, M.; Qin, S.; Fonseca, L.; Sangro, B.; Karachiwala, H.; Park, J.-W.; Gane, E.; Pinter, M.; et al. Nivolumab (NIVO) plus ipilimumab (IPI) vs lenvatinib (LEN) or sorafenib (SOR) as first-line treatment for unresectable hepatocellular carcinoma (uHCC): First results from CheckMate 9DW. J. Clin. Oncol. 2024, 42 (Suppl. S17), LBA4008. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Kudo, M.; Meyer, T.; Bai, Y.; Guo, Y.; Meng, Z.; Satoh, T.; Marino, D.; Assenat, E.; Li, S.; et al. Tislelizumab vs Sorafenib as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Pillai, A.; Marron, T.U.; Fessas, P.; Saeed, A.; Jun, T.; Dharmapuri, S.; Szafron, D.; Naqash, A.R.; Gampa, A.; et al. Patterns and outcomes of subsequent therapy after immune checkpoint inhibitor discontinuation in HCC. Hepatol. Commun. 2022, 6, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, F.; Brandi, G.; Garuti, F.; Barbera, M.A.; Tortora, R.; Casadei Gardini, A.; Granito, A.; Tovoli, F.; De Lorenzo, S.; Inghilesi, A.L.; et al. Metronomic capecitabine as second-line treatment for hepatocellular carcinoma after sorafenib discontinuation. J. Cancer Res. Clin. Oncol. 2018, 144, 403–414. [Google Scholar] [CrossRef]
- Persano, M.; Rimini, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Presa, J.; et al. Sequential therapies after atezolizumab plus bevacizumab or lenvatinib first-line treatments in hepatocellular carcinoma patients. Eur. J. Cancer 2023, 189, 112933. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Merle, P.; Kudo, M.; Edeline, J.; Bouattour, M.; Cheng, A.L.; Chan, S.L.; Yau, T.; Garrido, M.; Knox, J.; Daniele, B.; et al. Pembrolizumab as Second-Line Therapy for Advanced Hepatocellular Carcinoma: Longer Term Follow-Up from the Phase 3 KEYNOTE-240 Trial. Liver Cancer 2023, 12, 309–320. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Kim, J.H.; Ryu, M.H.; Park, S.R.; Lee, D.; Kim, K.M.; Shim, J.H.; Lim, Y.S.; Lee, H.C.; Lee, J.; et al. Clinical Outcomes with Multikinase Inhibitors after Progression on First-Line Atezolizumab plus Bevacizumab in Patients with Advanced Hepatocellular Carcinoma: A Multinational Multicenter Retrospective Study. Liver Cancer 2021, 10, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Feng, Y.H.; Yen, C.J.; Chen, S.C.; Lin, Y.T.; Lu, L.C.; Hsu, C.H.; Cheng, A.L.; Shao, Y.Y. Prognosis and treatment pattern of advanced hepatocellular carcinoma after failure of first-line atezolizumab and bevacizumab treatment. Hepatol. Int. 2022, 16, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Talbot, T.; D’Alessio, A.; Pinter, M.; Balcar, L.; Scheiner, B.; Marron, T.U.; Jun, T.; Dharmapuri, S.; Ang, C.; Saeed, A.; et al. Progression patterns and therapeutic sequencing following immune checkpoint inhibition for hepatocellular carcinoma: An international observational study. Liver Int. 2023, 43, 695–707. [Google Scholar] [CrossRef]
- Chan, S.L.; Ryoo, B.Y.; Mo, F.; Chan, L.L.; Cheon, J.; Li, L.; Wong, K.H.; Yim, N.; Kim, H.; Yoo, C. Multicentre phase II trial of cabozantinib in patients with hepatocellular carcinoma after immune checkpoint inhibitor treatment. J. Hepatol. 2024, 81, 258–264. [Google Scholar] [CrossRef]
- El-Khoueiry, A.; Kim, T.-Y.; Blanc, J.-F.; Rosmorduc, O.; Decaens, T.; Mathurin, P.; Merle, P.; Dayyani, F.; Masi, G.; Galle, P.; et al. International, open-label phase 2 study of regorafenib plus pembrolizumab in patients with advanced hepatocellular carcinoma (HCC) previously treated with immune checkpoint inhibitors (ICI). J. Clin. Oncol. 2024, 42, 4007. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.S.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Goh, M.J.; Kang, W.; Kim, S.U. Sorafenib versus nivolumab after lenvatinib treatment failure in patients with advanced hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2023, 35, 191–197. [Google Scholar] [CrossRef]
- Lee, C.-K.; Yoo, C.; Hong, J.Y.; Park, S.J.; Kim, J.W.; Tai, D.W.M.; Kim, H.; Korphaisarn, K.; Tanasanvimon, S.; Chen, S.-C.; et al. Real-World Study of Systemic Treatment after First-Line Atezolizumab plus Bevacizumab for Hepatocellular Carcinoma in Asia-Pacific Countries. Liver Cancer 2024, 1–15. [Google Scholar] [CrossRef]
- Tovoli, F.; Pallotta, D.P.; Vivaldi, C.; Campani, C.; Federico, P.; Palloni, A.; Dalbeni, A.; Soldà, C.; Lani, L.; Svegliati-Baroni, G.; et al. Suboptimal outcomes of sorafenib as a second-line treatment after atezolizumab-bevacizumab for unresectable hepatocellular carcinoma. Dig. Liver Dis. 2024, 56, 2079–2084. [Google Scholar] [CrossRef]
- Health UDo, SH. Common Terminology Criteria for Adverse Events (CTCAE). 2017. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 10 March 2025).
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, H.J.; Han, K.H.; Han, S.Y.; Heo, J.; Woo, H.Y.; Um, S.H.; Kim, Y.H.; Kweon, Y.O.; Lim, H.Y.; et al. Real-Life Experience of Sorafenib Treatment for Hepatocellular Carcinoma in Korea: From GIDEON Data. Cancer Res. Treat 2016, 48, 1243–1252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelley, R.K.; Rimassa, L.; Cheng, A.L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kudo, M.; Venook, A.P.; Ye, S.L.; Bronowicki, J.P.; Chen, X.P.; Dagher, L.; Furuse, J.; Geschwind, J.H.; de Guevara, L.L.; et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J. Hepatol. 2016, 65, 1140–1147. [Google Scholar] [CrossRef]
- Chon, Y.E.; Kim, D.Y.; Kim, M.N.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Ahn, S.H.; Ha, Y.; Lee, J.H.; Lee, K.S.; et al. Sorafenib vs. Lenvatinib in advanced hepatocellular carcinoma after atezolizumab/bevacizumab failure: A real-world study. Clin. Mol. Hepatol. 2024, 30, 345–359. [Google Scholar] [CrossRef]
- Wu, M.; Fulgenzi, C.A.M.; D’Alessio, A.; Cortellini, A.; Celsa, C.; Manfredi, G.F.; Stefanini, B.; Wu, Y.L.; Huang, Y.H.; Saeed, A.; et al. Second-line treatment patterns and outcomes in advanced HCC after progression on atezolizumab/bevacizumab. JHEP Rep. 2025, 7, 101232. [Google Scholar] [CrossRef]
- Finn, R.; Kudo, M.; Klumpen, H.J.; Lim, H.; Merle, P.; Ikeda, M.; Masi, G.; Frenette, C.; Kim, Y.; Gerolami, R.; et al. Regorafenib in patients with unresectable hepatocellular carcinoma (uHCC) in routine clinical practice: Exploratory analysis of overall survival (OS) in the prospective, observational REFINE study. J. Clin. Oncol. 2022, 40, 433. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Finn, R.; Gu, K.; Chen, X.; Merle, P.; Lee, K.-H.; Bouattour, M.; Cao, P.; Wang, W.; Cheng, A.-L.; Zhu, L.; et al. Abstract CT222: Pembrolizumab (pembro) for previously treated advanced hepatocellular carcinoma (aHCC): Meta-analysis of the phase 3 KEYNOTE-240 and KEYNOTE-394 studies. Cancer Res. 2022, 82, CT222. [Google Scholar] [CrossRef]
- Qin, H.N.; Ning, Z.; Sun, R.; Jin, C.X.; Guo, X.; Wang, A.M.; Liu, J.W. Lenvatinib as second-line treatment in patients with unresectable hepatocellular carcinoma: A retrospective analysis. Front. Oncol. 2022, 12, 1003426. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, C.C.; Liu, Y.W.; Li, W.F.; Chen, Y.H. Clinical impact of lenvatinib in patients with unresectable hepatocellular carcinoma who received sorafenib. PeerJ 2020, 8, e10382. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.; Shimose, S.; Kim, H.D.; Niizeki, T.; Ryu, M.H.; Shirono, T.; Ryoo, B.Y.; Iwamoto, H.; Yoo, C. Lenvatinib versus sorafenib as second-line therapy following progression on atezolizumab-bevacizumab in patients with unresectable hepatocellular carcinoma: A multicenter retrospective study from Korea and Japan. J. Cancer Res. Clin. Oncol. 2025, 151, 52. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.J.H.; Tang, A.S.P.; Lim, W.H.; Ng, C.H.; Nah, B.; Fu, C.; Xiao, J.; Koh, B.; Tay, P.W.L.; Tan, E.X.; et al. Survival Trends in Sorafenib for Advanced Hepatocellular Carcinoma: A Reconstructed Individual Patient Data Meta-Analysis of Randomized Trials. Liver Cancer 2023, 12, 445–456. [Google Scholar] [CrossRef]
- Pressiani, T.; Boni, C.; Rimassa, L.; Labianca, R.; Fagiuoli, S.; Salvagni, S.; Ferrari, D.; Cortesi, E.; Porta, C.; Mucciarini, C.; et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: A prospective feasibility analysis. Ann. Oncol. 2013, 24, 406–411. [Google Scholar] [CrossRef]
- Bruix, J.; Cheng, A.L.; Meinhardt, G.; Nakajima, K.; De Sanctis, Y.; Llovet, J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J. Hepatol. 2017, 67, 999–1008. [Google Scholar] [CrossRef]
- Scheiner, B.; Pomej, K.; Kirstein, M.M.; Hucke, F.; Finkelmeier, F.; Waidmann, O.; Himmelsbach, V.; Schulze, K.; von Felden, J.; Fründt, T.W.; et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy—Development and validation of the CRAFITY score. J. Hepatol. 2022, 76, 353–363. [Google Scholar] [CrossRef]
- El Dika, I.; Capanu, M.; Chou, J.F.; Harding, J.J.; Ly, M.; Hrabovsky, A.D.; Do, R.K.G.; Shia, J.; Millang, B.; Ma, J.; et al. Phase II trial of sorafenib and doxorubicin in patients with advanced hepatocellular carcinoma after disease progression on sorafenib. Cancer Med. 2020, 9, 7453–7459. [Google Scholar] [CrossRef]
- Bitzer, M.; Horger, M.; Giannini, E.G.; Ganten, T.M.; Wörns, M.A.; Siveke, J.T.; Dollinger, M.M.; Gerken, G.; Scheulen, M.E.; Wege, H.; et al. Resminostat plus sorafenib as second-line therapy of advanced hepatocellular carcinoma—The SHELTER study. J. Hepatol. 2016, 65, 280–288. [Google Scholar] [CrossRef]
- Xu, L.; Che, X. Sorafenib combined with tarexib for first-line treatment of unresectable hepatocellular carcinoma and its predictive role and correlation with PD-L1 CTCs. Front. Oncol. 2024, 14, 1478596. [Google Scholar] [CrossRef]
- Cabibbo, G.; Reig, M.; Celsa, C.; Torres, F.; Battaglia, S.; Enea, M.; Rizzo, G.E.M.; Petta, S.; Calvaruso, V.; Di Marco, V.; et al. First-Line Immune Checkpoint Inhibitor-Based Sequential Therapies for Advanced Hepatocellular Carcinoma: Rationale for Future Trials. Liver Cancer 2022, 11, 75–84. [Google Scholar] [CrossRef]
- Möhring, C.; Berger, M.; Sadeghlar, F.; Berres, M.L.; Radu, I.P.; Roderburg, C.; Vogel, A.; Sinner, F.; Pirozzi, A.; Weinmann, A.; et al. 191P Evaluating sorafenib as second-line treatment for advanced hepatocellular carcinoma: SORA-2, an European retrospective multicenter study. Ann. Oncol. 2024, 35, S86. [Google Scholar] [CrossRef]
| Overall (N = 81) | |
|---|---|
| Gender | |
| Female | 17 (21.0%) |
| Male | 64 (79.0%) |
| Age | |
| Median [Min, Max] | 67.8 [42.1, 83.7] |
| ECOG status before sorafenib | |
| 0 | 27 (33.3%) |
| 1 | 36 (44.4%) |
| 2 | 16 (19.8%) |
| Missing | 2 (2.5%) |
| Etiology of cirrhosis | |
| Non-viral | 41 (50.6%) |
| Viral | 25 (30.9%) |
| Other | 1 (1.2%) |
| Missing | 14 (17.3%) |
| Non-viral | |
| ASH | 28 (34.6%) |
| MASLD/MASH | 13 (16.0%) |
| Viral | |
| Hepatitis B | 7 (8.6%) |
| Hepatitis C | 18 (22.2%) |
| Child–Pugh stage and score before sorafenib | |
| A | 55 (67.9%) |
| 5 | 31 (38.3%) |
| 6 | 24 (29.6%) |
| B | 15 (18.5%) |
| 7 | 8 (9.9%) |
| 8 | 3 (3.7%) |
| 9 | 4 (4.9%) |
| Missing | 11 (13.6%) |
| ALBI-Score before sorafenib | |
| 1 | 22 (27.2%) |
| 2 | 39 (48.1%) |
| 3 | 11 (13.6%) |
| Missing | 9 (11.1%) |
| BCLC before sorafenib | |
| A | 1 (1.2%) |
| B | 18 (22.2%) |
| C | 57 (70.4%) |
| D | 1 (1.2%) |
| Missing | 4 (4.9%) |
| Macrovascular invasion before sorafenib | 44 (54.3%) |
| Thereof main portal vein invasion | 25 (69.4%) |
| Extrahepatic tumor spread | 47 (58.0%) |
| AFP at before sorafenib | |
| <400 ng/mL | 45 (55.6%) |
| ≥400 ng/mL | 29 (35.8%) |
| Missing | 7 (8.6%) |
| Previous surgical and local therapy | |
| TACE | 35 (43.2%) |
| RFA | 6 (7.4%) |
| MWA | 11 (13.6%) |
| TARE | 6 (7.4%) |
| Resection | 27 (33.3%) |
| Systemic first-line therapy | |
| Atezolizumab/bevacizumab | 35 (43.2%) |
| Lenvatinib | 36 (44.4%) |
| Clinical trial with ICI | 10 (12.3%) |
| Overall (N = 81) | |
|---|---|
| Patients reaching 100% sorafenib dose | 39 (48.1%) |
| Patients requiring sorafenib dose reduction | 31 (38.3%) |
| Reasons for sorafenib treatment cessation | |
| Death | 9 (11.1%) |
| Patient wish | 2 (2.5%) |
| Progress | 32 (39.5%) |
| Toxicity | 23 (28.4%) |
| Missing | 15 (18.5%) |
| Treatment of HCC after sorafenib | 41 (50.6%) |
| Number of subsequent treatments | |
| 1 subsequent treatment | 38 (46.9%) |
| 2 subsequent treatments | 11 (26.8%) |
| 3 subsequent treatments | 4 (9.8%) |
| Subsequent Treatments | |
| Systemic third-line treatment | 38 (46.9%) |
| Atezolizumab/bevacizumab | 2 (5.0%) |
| Cabozantinib | 26 (65.0%) |
| Lenvatinib | 2 (5.0%) |
| Nivolumab/ipilimumab | 2 (5.0%) |
| Ramucirumab | 2 (5.0%) |
| Regorafenib | 4 (10.0%) |
| MWA | 1 (2.4%) |
| RFA | 1 (2.4%) |
| Stereotactic body radiotherapy | 1 (2.4%) |
| TARE | 1 (2.4%) |
| Missing information about further treatment | 2 (4.8%) |
| Therapy response | N = 55 |
| Complete response (CR) | 0 (0%) |
| Partial response (PR) | 3 (5.5%) |
| Stable disease (SD) | 20 (36.4%) |
| Progressive disease (PD) | 32 (58.2%) |
| Objective response rate (ORR = CR + PR) | 3 (5.5%) |
| Disease control rate (DCR = CR + PR + SD) | 23 (41.8%) |
| Adverse Event CTCAE Grade 3–5 | N = 81 |
|---|---|
| No | 28 (34.6%) |
| Yes | 41 (50.6%) |
| Missing | 12 (14.8%) |
| Adverse Events | N = 69 |
| Hand-foot skin reaction | 10 (14.4%) |
| Liver dysfunction | 9 (13.0%) |
| Diarrhea | 8 (11.6%) |
| Fatigue | 5 (7.2%) |
| Rash | 4 (5.8%) |
| Nausea | 2 (2.9%) |
| Arterial hypertension | 2 (2.9%) |
| Renal insufficiency | 2 (2.9%) |
| Cornea implant rejection | 1 (1.4%) |
| Bleeding | 1 (1.4%) |
| Mucositis | 1 (1.4%) |
| Myocardial infarction | 1 (1.4%) |
| Tremor | 1 (1.4%) |
| Facial paresis | 1 (1.4%) |
| Voice changes | 1 (1.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möhring, C.; Berger, M.; Sadeghlar, F.; Zhou, X.; Zhou, T.; Monin, M.B.; Shmanko, K.; Welland, S.; Sinner, F.; Schwacha-Eipper, B.; et al. Evaluating Sorafenib (SORA-2) as Second-Line Treatment for Unresectable Hepatocellular Carcinoma: A European Retrospective Multicenter Study. Cancers 2025, 17, 972. https://doi.org/10.3390/cancers17060972
Möhring C, Berger M, Sadeghlar F, Zhou X, Zhou T, Monin MB, Shmanko K, Welland S, Sinner F, Schwacha-Eipper B, et al. Evaluating Sorafenib (SORA-2) as Second-Line Treatment for Unresectable Hepatocellular Carcinoma: A European Retrospective Multicenter Study. Cancers. 2025; 17(6):972. https://doi.org/10.3390/cancers17060972
Chicago/Turabian StyleMöhring, Christian, Moritz Berger, Farsaneh Sadeghlar, Xin Zhou, Taotao Zhou, Malte Benedikt Monin, Kateryna Shmanko, Sabrina Welland, Friedrich Sinner, Birgit Schwacha-Eipper, and et al. 2025. "Evaluating Sorafenib (SORA-2) as Second-Line Treatment for Unresectable Hepatocellular Carcinoma: A European Retrospective Multicenter Study" Cancers 17, no. 6: 972. https://doi.org/10.3390/cancers17060972
APA StyleMöhring, C., Berger, M., Sadeghlar, F., Zhou, X., Zhou, T., Monin, M. B., Shmanko, K., Welland, S., Sinner, F., Schwacha-Eipper, B., Bauer, U., Roderburg, C., Pirozzi, A., Ben Khaled, N., Schrammen, P., Balcar, L., Pinter, M., Ettrich, T. J., Saborowski, A., ... Gonzalez-Carmona, M. A. (2025). Evaluating Sorafenib (SORA-2) as Second-Line Treatment for Unresectable Hepatocellular Carcinoma: A European Retrospective Multicenter Study. Cancers, 17(6), 972. https://doi.org/10.3390/cancers17060972









