Risk Factors and Dental Caries Incidence in Childhood Cancer Survivors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dental Examination
2.3. Medical Records Analysis and Questionnaire
2.4. Statistical Analysis
3. Results
3.1. Caries Frequency
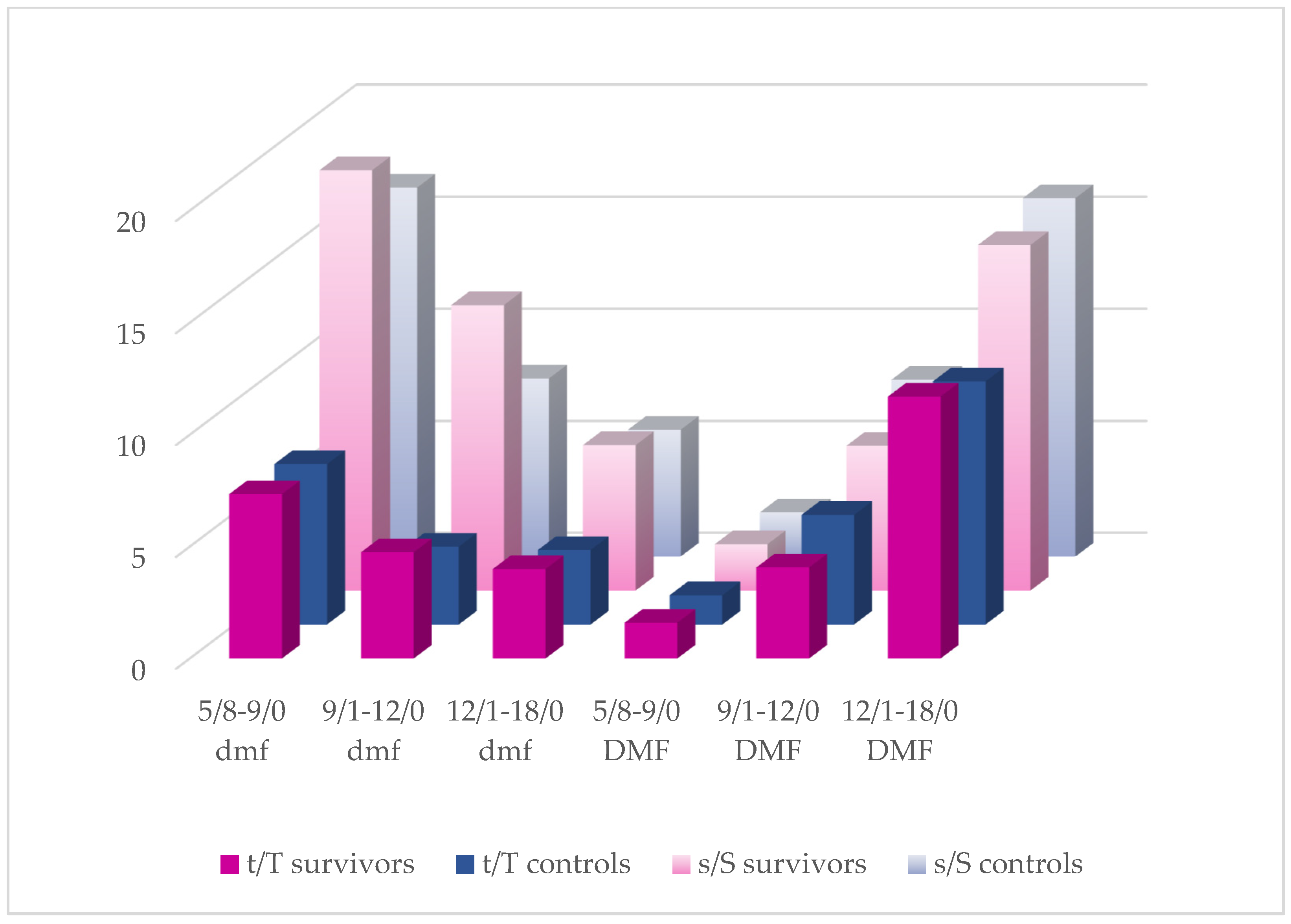
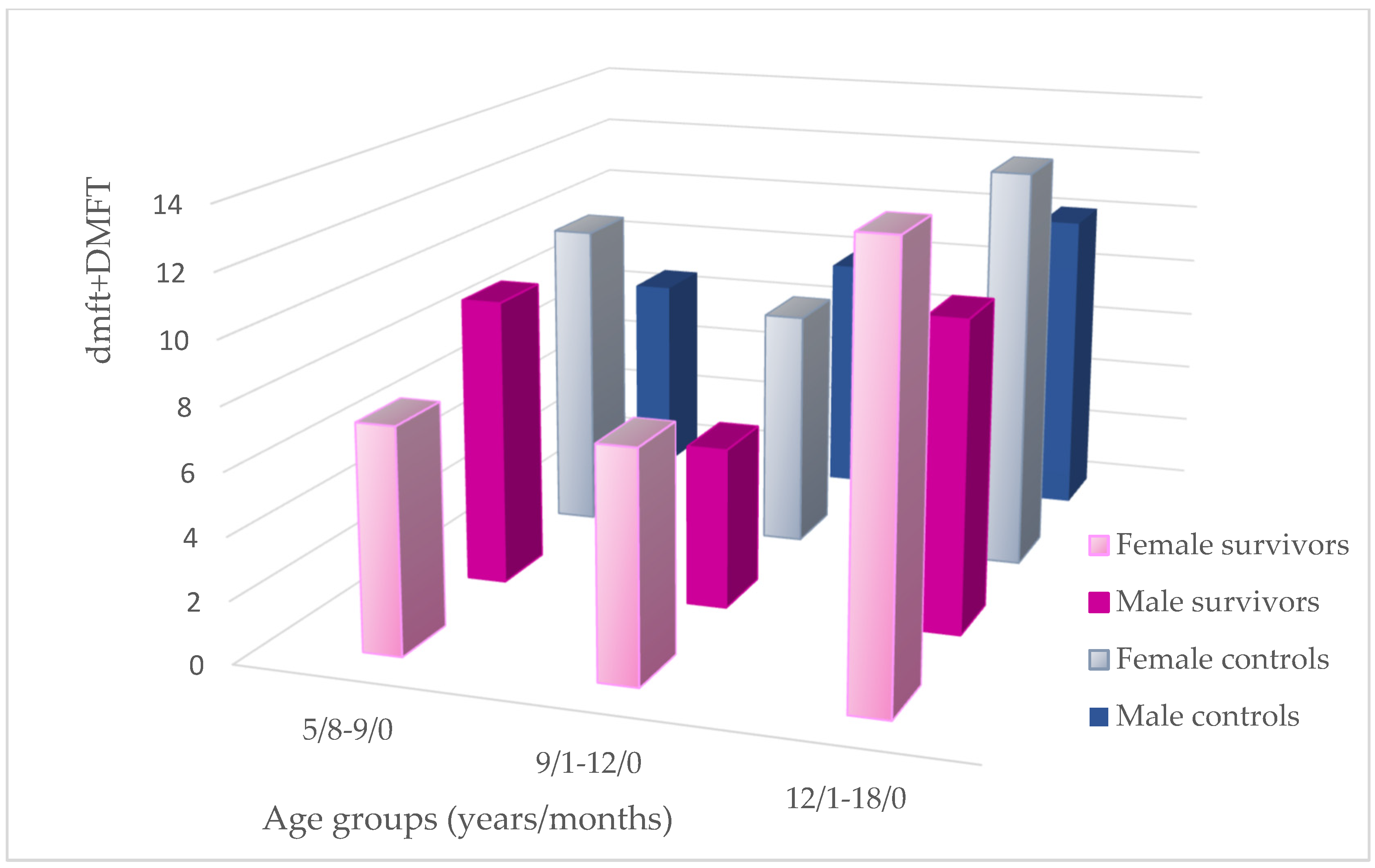
3.2. dmft(s)/DMFT(S) Analysis
| (a) | ||||||||
| Cancer Survivors N = 18 | Control Group N = 36 | U Mann–Whitney | ||||||
| Variable | Mean SD | Median (Range) | Median Rank | Mean SD | Median (Range) | Median Rank | p-Value | rg |
| dmft | 7.35 ±5.14 | 8 (0–17) | 28.15 | 7.17 ±4.15 | 7 (0–17) | 26.46 | 0.709 | 0.05 |
| ft | 1.06 ±1.43 | 1 (0–4) | 19.56 | 2.94 ±2.62 | 3 (0–8) | 30.51 | 0.013 | 0.34 |
| dmfs | 18.76 ±16.21 | 18 (0–57) | 24.53 | 16.47 ±13.15 | 13 (0–55) | 23.75 | 0.844 | 0.03 |
| fs | 2.47 ±3.63 | 1 (0–14) | 21.80 | 5.53 ±5.98 | 4 (0–25) | 25.03 | 0.292 | 0.15 |
| DMFT | 1.60 ±1.99 | 0 (0–6) | 28.18 | 1.31 ±1.59 | 0.5 (0–4) | 26.44 | 0.703 | 0.05 |
| FT | 0.13 ±0.34 | 0 (0–1) | 21.18 | 0.59 ±1.20 | 0 (0–4) | 29.75 | 0.055 | 0.26 |
| DMFS | 2.07 ±2.86 | 0 (0–10) | 23.97 | 1.97 ±2.84 | 0.5 (0–11) | 24.02 | 0.990 | <0.01 |
| FS | 0.40 ±1.02 | 0 (0–4) | 23.50 | 0.66 ±1.53 | 0 (0–6) | 24.23 | 0.811 | 0.03 |
| dmft + DMFT | 8.28 ±5.17 | 9 (0–18) | 27.78 | 8.33 ±4.58 | 8 (0–19) | 27.36 | 0.927 | 0.01 |
| dmfs + DMFS | 19.44 ±16.05 | 19.5 (0–58) | 28.22 | 18.14 ±13.93 | 15 (0–55) | 27.14 | 0.811 | 0.03 |
| PI | 1.058 ±0.60 | 0.997 (0.229–1.816) | 33.31 | 0.734 ±0.482 | 0.639 (0.000–1.674) | 24.60 | 0.055 | 0.26 |
| GI | 0.381 ±0.33 | 0.288 (0.000–0.955) | 27.31 | 0.402 ±0.351 | 0.309 (0.000–1.344) | 27.60 | 0.949 | <0.01 |
| Remission time (months) | 50.83 ±15.37 | 50 (24–85) | - | - | ||||
| No primary teeth—1patient No permanent teeth—3 patients | No primary teeth—0 patients No permanent teeth—4 patients | |||||||
| (b) | ||||||||
| Cancer Survivors N = 15 | Control Group N = 29 | U Mann–Whitney | ||||||
| Variable | Mean SD | Median (Range) | Median Rank | Mean SD | Median (Range) | Median Rank | p-Value | rg |
| dmft | 4.75 ±4.79 | 2 (0–12) | 16.50 | 3.48 ±2.14 | 3 (0–8) | 17.16 | 0.865 | 0.03 |
| ft | 0.00 ±0.00 | 0 (0–0) | 10.00 | 1.16 ±1.54 | 1 (0–6) | 19.24 | 0.008 | 0.46 |
| dmfs | 12.75 ±16.87 | 3.5 (0–50) | 15.13 | 7.96 ±5.37 | 8 (0–21) | 17.60 | 0.527 | 0.11 |
| fs | 0.00 ±0.00 | 0 (0–0) | 10.00 | 2.56 ±3.19 | 2 (0–12) | 19.24 | 0.009 | 0.46 |
| DMFT | 4.07 ±3.23 | 4 (0–14) | 20.10 | 4.90 ±3.33 | 4 (0–14) | 23.74 | 0.365 | 0.14 |
| FT | 0.73 ±1.18 | 0 (0–4) | 15.40 | 2.17 ±2.00 | 2 (0–8) | 26.17 | 0.006 | 0.41 |
| DMFS | 6.47 ±6.23 | 4 (0–21) | 20.27 | 7.90 ±7.28 | 6 (0–31) | 23.66 | 0.405 | 0.13 |
| FS | 1.40 ±1.67 | 0 (0–5) | 16.73 | 3.62 ±3.41 | 2 (0–12) | 25.48 | 0.028 | 0.33 |
| dmft + DMFT | 6.60 ±5.16 | 5 (0–16) | 18.57 | 7.90 ±3.13 | 7 (3–14) | 24.53 | 0.142 | 0.22 |
| dmfs + DMFS | 13.93 ±14.35 | 8 (0–56) | 19.20 | 14.83 ±7.90 | 13 (3–36) | 24.21 | 0.220 | 0.18 |
| PI | 1.214 ±0.639 | 1.087 (0.125–2.163) | 23.53 | 1.126 ± 0.577 | 1.208 (0–2.196) | 21.97 | 0.701 | 0.06 |
| GI | 0.708 ±0.435 | 0.595 (0–1.667) | 22.19 | 0.472 ± 0.379 | 0.388 (0–1.602) | 25.12 | 0.477 | 0.10 |
| Remission time (months) | 77.00 ±26.90 | 71 (24–118) | - | - | ||||
| No primary teeth—7 patients No permanent teeth—0 patients | No primary teeth—4 patients No permanent teeth—0 patients | |||||||
| (c) | ||||||||
| Cancer Survivors N = 7 | Control Group N = 15 | U Mann–Whitney | ||||||
| Variable | Mean SD | Median (Range) | Median Rank | Mean SD | Median (Range) | Median Rank | p-Value | rg |
| dmft | 4.00 1 ±1.00 | 4 (3–5) | - | 3.33 ±0.94 | 4 (2–4) | - | - | - |
| ft | 0.50 1 ±0.50 | 0.5 (0–1) | - | 1.00 ±0.82 | 1 (0–2) | - | - | - |
| dmfs | 6.50 1 ±2.50 | 6.5 (4–9) | - | 5.67 ±2.05 | 6 (3–8) | - | - | - |
| fs | 0.50 1 ±0.50 | 0.5 (0–1) | - | 1.33 ±0.94 | 2 (0–2) | - | - | - |
| DMFT | 11.71 ±5.39 | 13 (4–19) | 11.93 | 10.87 ±4.72 | 13 (2–20) | 11.30 | 0.831 | 0.05 |
| FT | 1.71 ±2.31 | 1 (0–7) | 7.57 | 4.13 ±3.10 | 4 (1–12) | 13.33 | 0.050 | 0.42 |
| DMFS | 15.43 ±8.31 | 16 (4–29) | 11.14 | 16.00 ±7.55 | 16 (3–34) | 11.67 | 0.860 | 0.04 |
| FS | 2.57 ±2.56 | 1 (0–7) | 7.71 | 6.33 ±4.95 | 4 (1–18) | 13.27 | 0.060 | 0.40 |
| dmft + DMFT | 12.86 ±3.87 | 13 (7–19) | 12.29 | 11.53 ±4.05 | 13 (2–20) | 11.13 | 0.694 | 0.08 |
| dmfs + DMFS | 17.29 ±6.50 | 16 (8–29) | 11.00 | 17.13 ±6.68 | 17 (3–34) | 11.73 | 0.805 | 0.05 |
| PI | 1.193 ±0.645 | 1.509 (0.228–1.929) | 14.14 | 0.860 ±0.388 | 0.798 (0.313–1.556) | 10.27 | 0.192 | 0.28 |
| GI | 0.572 ±0.305 | 0.554 (0.141–1.130) | 13.43 | 0.423 ±0.304 | 0.339 (0.028–1.020) | 10.60 | 0.341 | 0.20 |
| Remission time (months) | 75.14 ±37.29 | 74 (24–133) | - | - | ||||
| No primary teeth—5 patients No permanent teeth—0 patients | No primary teeth—12 patients No permanent teeth—0 patients | |||||||
3.3. Comparison of Mean PI and GI Scores
3.4. dmft + DMFT and Different Risk Factors
| (a) | |||||||
| Variable | Categories N (%) (18 Survivors) | Spearman Correlation | Kruskall–Wallis/ Mann–Whitney | ||||
| Age Range 5/8–9/0 | rho | p-Value | p-Value | ||||
| Oral hygiene | Good | Fair | Poor | ||||
| 10 (55.56) | 8 (44.44) | 0 (0.00) | 0.61 | 0.008 | 0.068 b | ||
| Parental education | E/V | S | PS | ||||
| Women | 1 (5.55) | 7 (38.89) | 10 (55.56) | 0.11 | 0.652 | 0.892 a | |
| Men | 2 (11.11) | 11 (61.11) | 5 (27.78) | −0.19 | 0.452 | 0.488 a | |
| Parental employment | 0 | 1 | 2 | ||||
| 0 (0.00) | 9 (50.00) | 9 (50.00) | 0.18 | 0.469 | 0.452 b | ||
| Number of siblings | 0 | 1 | ≥2 | ||||
| 5 (27.78) | 11 (61.11) | 2 (11.11) | 0.48 | 0.044 | 0.138 a | ||
| Brushing frequency | 0 | 1 | ≥2 | ||||
| 1 (5.55) | 3 (16.67) | 14 (77.78) | −0.45 | 0.063 | 0.176 a | ||
| Diet | 0 | 1 * | |||||
| 11 (61.11) | 7 (38.89) | 0.67 | 0.002 | 0.006 b | |||
| Pregnancy | 0 | 1 | |||||
| 13 (72.22) | 5 (27.28) | 0.06 | 0.813 | 0.805 b | |||
| Oral hygiene problems during CT | 0 | 1 | |||||
| 12 (66.67) | 6 (33.33) | 0.26 | 0.294 | 0.280 b | |||
| (b) | |||||||
| Variable | Categories N (%) (15 Survivors) | Spearman Correlation | Kruskall–Wallis/ Mann–Whitney | ||||
| Age Range 9/1–12/0 | rho | p-Value | p-Value | ||||
| Oral hygiene | Good | Fair | Poor | ||||
| 5 (3.33) | 10 (66.67) | 0 (0.00) | 0.54 | 0.038 | 0.222 a | ||
| Parental education | E/V | S | PS | ||||
| Women | 4 (26.67) | 4 (26.67) | 7 (46.66) | −0.48 | 0.073 | 0.140 a | |
| Men | 4 (26.67) | 6 (40.00) | 5 (33.33) | −0.59 | 0.022 | 0.062 a | |
| Parental employment | 0 | 1 | 2 | ||||
| 0 (0.00) | 6 (40.00) | 9 (60.00) | −0.25 | 0.363 | 0.344 b | ||
| Number of siblings | 0 | 1 | ≥2 | ||||
| 1 (6.67) | 9 (60.00) | 5 (33.33) | 0.17 | 0.550 | 0.377 a | ||
| Brushing frequency | 0 | 1 | ≥2 | ||||
| 0 (0.00) | 1 (6.67) | 14 (93.33) | −0.31 | 0.260 | 0.245 a | ||
| Diet | 0 | 1 | |||||
| 13 (86.67) | 2 (13.33) | 0.25 | 0.368 | 0.349 b | |||
| Pregnancy | 0 | 1 | |||||
| 12 (80.00) | 3 (20.00) | 0.00 | 1.000 | 1.000 b | |||
| Oral hygiene problems during CT | 0 | 1 | |||||
| 11 (73.33) | 4 (26.67) | −0.21 | 0.452 | 0.432 b | |||
| (c) | |||||||
| Variable | Categories N (%) (7 Survivors) | Spearman Correlation | Kruskall–Wallis/ Mann–Whitney | ||||
| Age Range 12/1–18/0 | rho | p-Value | p-Value | ||||
| Oral hygiene | Good | Fair | Poor | ||||
| 2 (28.57) | 5 (71.43) | 0 (0.00) | 0.20 | 0.670 | 0.241 b | ||
| Parental education | E/V | S | PS | ||||
| Women | 1 (14.29) | 4 (57.14) | 2 (28.57) | −0.26 | 0.571 | 0.600 a | |
| Men | 3 (42.86) | 3 (42.86) | 1 (14.28) | −0.43 | 0.338 | 0.559 a | |
| Parental employment | 0 | 1 | 2 | ||||
| 0 (0.00) | 5 (71.43) | 2 (28.57) | 0.32 | 0.485 | 0.434 b | ||
| Number of siblings | 0 | 1 | ≥2 | ||||
| 3 (42.86) | 3 (42.86) | 1 (14.28) | 0.35 | 0.441 | 0.312 a | ||
| Brushing frequency | 0 | 1 | ≥2 | ||||
| 2 (28.57) | 2 (28.57) | 3 (42.86) | −0.19 | 0.682 | 0.506 a | ||
| Diet | 0 | 1 | |||||
| 2 (28.57) | 5 (71.43) | −0.16 | 0.733 | 0.696 b | |||
| Pregnancy | 0 | 1 | |||||
| 6 (85.72) | 1 (14.28) | −0.62 | 0.139 | 0.130 b | |||
| Oral hygiene problems during CT | 0 | 1 | |||||
| 5 (71.43) | 2 (28.57) | 0.16 | 0,733 | 0.696 b | |||
| (a) | ||||||||
| Variable | Categories N (%) (36 Controls) | Spearman Correlation | Kruskall–Wallis/ Mann–Whitney | |||||
| Age Range 5/8–9/0 | rho | p-Value | p-Value | |||||
| Oral hygiene | Good | Fair | Poor | |||||
| 23 (63.89) | 13 (36.11) | 0 (0.00) | 0.21 | 0.229 | 0.740 b | |||
| Parental education | E/V | S | PS | |||||
| Women | 8 (22.22) | 22 (61.11) | 6 (16.67) | 0.12 | 0.505 | 0.794 a | ||
| Men | 16 (44.44) | 16 (44.44) | 4 (11.12) | −0.09 | 0.610 | 0.874 a | ||
| Parental employment | 0 | 1 | 2 | |||||
| 2 (5.55) | 15 (41.67) | 19 (52.78) | −0.26 | 0.123 | 0.167 a | |||
| Number of siblings | 0 | 1 | ≥2 | |||||
| 8 (22.22) | 17 (47.22) | 11 (30.56) | 0.27 | 0.113 | 0.067 a | |||
| Brushing frequency | 0 | 1 | ≥2 | |||||
| 7 (19.44) | 2 (5.56) | 27 (75.00) | 0.13 | 0.448 | 0.739 a | |||
| Diet | 0 | 1 * | ||||||
| 17 (47.22) | 19 (52.78) | 0.35 | 0.036 | 0.038 b | ||||
| Pregnancy | 0 | 1 | ||||||
| 29 (80.56) | 7 (19.44) | 0.05 | 0.753 | 0.748 b | ||||
| (b) | ||||||||
| Variable | Categories N (%) (29 Controls) | Spearman Correlation | Kruskall–Wallis/ Mann–Whitney | |||||
| Age Range 9/1–12/0 | rho | p-Value | p-Value | |||||
| Oral hygiene | Good | Fair | Poor | |||||
| 11 (37.93) | 15 (51.72) | 3 (10.35) | 0.12 | 0.549 | 0.186 a | |||
| Parental education | E/V* | S | PS | |||||
| Women | 6 (20.69)* | 16 (55.17) | 7 (24.14) | −0.55 | 0.002 | 0.014 a | ||
| Men | 14 (48.28) | 13 (44.82) | 2 (6.90) | −0.26 | 0.167 | 0.335 a | ||
| Parental employment | 0 | 1 | 2 | |||||
| 2 (6.90) | 9 (31.03) | 18 (62.07) | −0.35 | 0.061 | 0.070 a | |||
| Number of siblings | 0 | 1 | ≥2 | |||||
| 2 (6.90) | 16 (55.17) | 11 (37.93) | 0.41 | 0.028 | 0.061 a | |||
| Brushing frequency | 0 | 1 | ≥2 | |||||
| 6 (20.69) | 5 (17.24) | 18 (62.07) | −0.26 | 0.172 | 0.356 a | |||
| Diet | 0 | 1 | ||||||
| 16 (55.17) | 13 (44.83) | −0.04 | 0.846 | 0.842 b | ||||
| Pregnancy | 0 | 1 | ||||||
| 22 (75.86) | 7 (24.14) | −0.10 | 0.616 | 0.607 b | ||||
| (c) | ||||||||
| Variable | Categories N (%) (15 Controls) | Spearman Correlation | Kruskall–Wallis/ Mann–Whitney | |||||
| Age Range 12/1–18/0 | rho | p-Value | p-Value | |||||
| Oral hygiene | Good | Fair | Poor | |||||
| 9 (60.00) | 6 (40.00) | 0 (0.00) | 0.41 | 0.129 | 0.188 b | |||
| Parental education | E/V | S | PS | |||||
| Women | 3 (20.00) | 7 (46.67) | 5 (33.33) | −0.16 | 0.582 | 0.722 a | ||
| Men | 7 (46.67) | 5 (33.33) | 3 (20.00) | −0.06 | 0.846 | 0.972 a | ||
| Parental employment | 0 | 1 | 2 | |||||
| 1 (6.67) | 5 (33.33) | 9 (60.00) | −0.36 | 0.193 | 0.386 a | |||
| Number of siblings | 0 | 1 | ≥2 | |||||
| 1 (6.67) | 8 (53.33) | 6 (40.00) | 0.36 | 0.191 | 0.215 a | |||
| Brushing frequency | 0 | 1 | ≥2 | |||||
| 2 (13.33) | 3 (20.00) | 10 (66.67) | −0.16 | 0.570 | 0.829 a | |||
| Diet | 0 | 1 | ||||||
| 6 (40.00) | 9 (60.00) | 0.29 | 0.299 | 0.282 b | ||||
| Pregnancy | 0 | 1 | ||||||
| 14 (93.33) | 1 (6.67) | −0.44 | 0.101 | 0.100 b | ||||
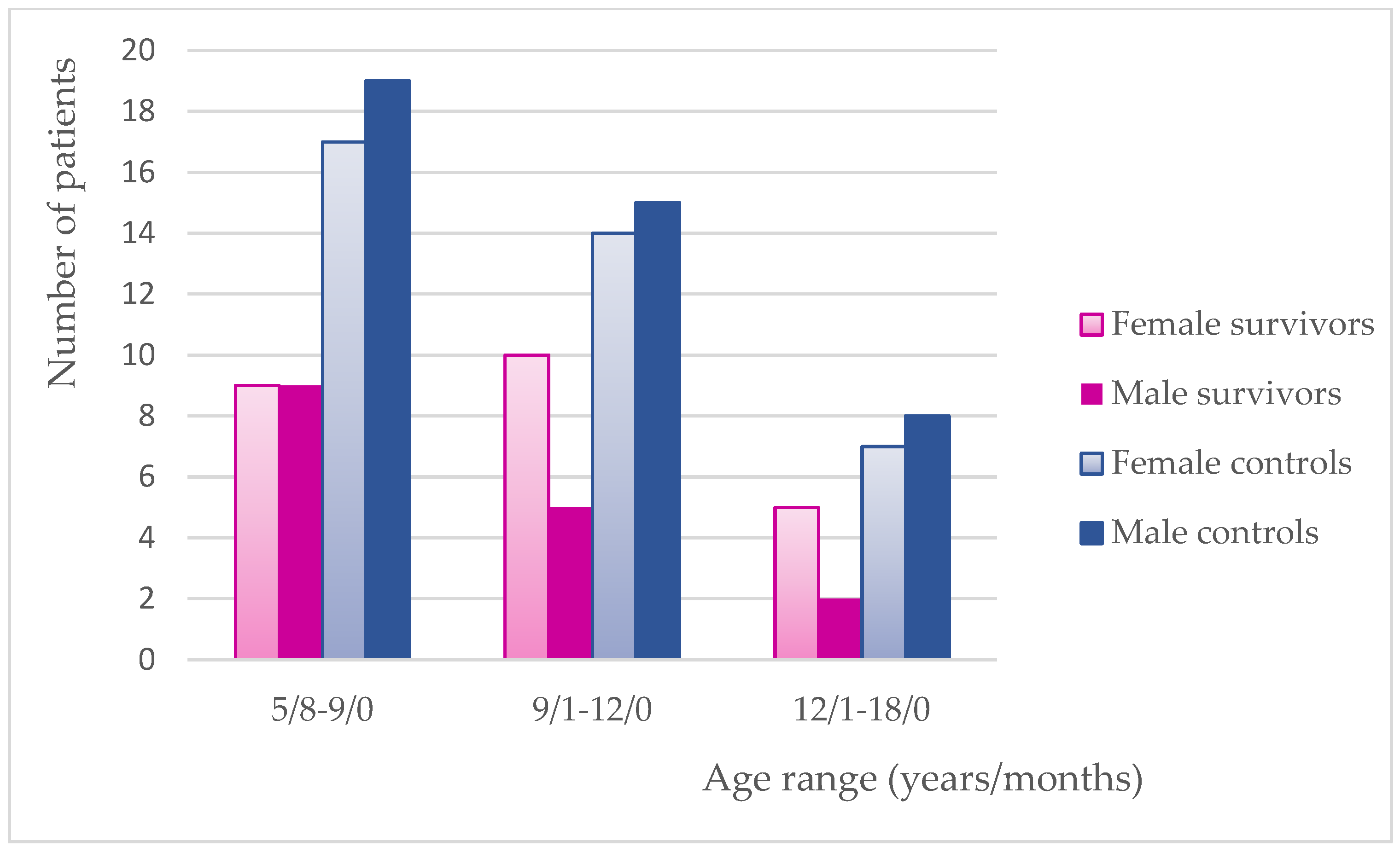
3.5. Gender Distribution in This Study
4. Discussion
4.1. DMFT Analysis
4.2. Oral Hygiene: PI and Tooth Brushing Frequency
4.3. Socioeconomic Status: Education Level, Employment, and Number of Siblings
4.4. Dietary Habits
4.5. Pregnancy Complications
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Chemotherapy |
| RT | Radiotherapy |
| dmft/s | decayed, missing, filled tooth/surface (primary teeth) |
| DMFT/S | Decayed, Missing, Filled Tooth/Surface (permanent teeth) |
| ft/s | filled tooth/surface (primary teeth) |
| FT/S | Filled Tooth/Surface (permanent teeth) |
| PI | Plaque Index |
| GI | Gingival Index |
| E | Elementary (education) |
| V | Vocational (education) |
| S | Secondary (education) |
| PS | Postsecondary (education) |
| Rho | Spearman’s correlation coefficient |
| PTB | Preterm birth |
| LBW | Low birth weight |
| SGA | Small-for-gestational age |
References
- Seremidi, K.; Kavvadia, K.; Kattamis, A.; Polychronopoulou, A. Dental caries and dental developmental defects as adverse effects of antineoplastic treatment in childhood cancer survivors. Eur. Arch. Paediatr. Dent. 2023, 24, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Patni, T.; Lee, C.; Li, Y.; Kaste, S.; Zhu, L.; Sun, R.; Hudson, M.M.; Ness, K.K.; Ana Neumann, A.; Robison, L.L. Factors for poor oral health in long-term childhood cancer survivors. BMC Oral. Health 2023, 23, 73. [Google Scholar] [CrossRef]
- Jodłowska, A.; Postek-Stefańska, L. Tooth abnormalities and their age-dependent occurrence in leukemia survivors. Cancers 2023, 15, 5420. [Google Scholar] [CrossRef] [PubMed]
- Stolze, J.; Vlaanderen, K.C.E.; Holtbach, F.C.E.D.; Teepen, J.C.; Kremer, L.C.M.; Loonen, J.J.; van Dulmen-den Broeder, E.; van den Heuvel-Eibrink, M.M.; Helena, J.H.; van der Pal, H.J.H.; et al. Long-term effects of childhood cancer treatment on dentition and oral health: A dentist survey study from the DCCSS LATER 2 Study. Cancers 2021, 13, 5264. [Google Scholar] [CrossRef] [PubMed]
- Çetiner, D.; Çetiner, S.; Uraz, A.; Alpaslan, G.H.; Alpaslan, C.; Memikoğlu, T.U.T.; Karadeniz, C. Oral and dental alterations and growth disruption following chemotherapy in long-term survivors of childhood malignancies. Support. Care Cancer 2019, 27, 1891–1899. [Google Scholar] [CrossRef]
- ul-Ain, Q.; Sahar; Malik, A.M. Prevalence of dental caries among school going children in mixed dentition stage six to fifteen years of age in the suburbs schools of Islamabad, Pakistan. JKCD 2019, 9, 29–33. [Google Scholar]
- Epstein, J.B.; Thariat, J.; Bensadoun, R.J.; Barasch, A.; Murphy, B.A.; Kolnick, L.; Popplewell, L.; Maghami, E. Oral complications of cancer and cancer therapy. From cancer treatment to survivorship. CA Cancer J. Clin. 2012, 62, 400–422. [Google Scholar] [CrossRef]
- Lauritano, D.; Petruzzi, M. Decayed, missing and filled teeth index and dental anomalies in long-term survivors leukaemic children: A prospective controlled study. Med. Oral. Patol. Oral. Cir. Bucal 2012, 17, 977–980. [Google Scholar] [CrossRef]
- Hutton, A.; Bradwell, M.; English, M.; Chapple, I. The oral health needs of children after treatment for a solid tumour or lymphoma. Int. J. Paediatr. Dent. 2010, 20, 15–23. [Google Scholar] [CrossRef]
- Alberth, M.; Kovalecz, G.; Nemes, J.; Ma´th, J.; Kiss, C.; Ma´rton, I.J. Oral health of long-term childhood cancer survivors. Pediatr. Blood Cancer 2004, 43, 88–90. [Google Scholar] [CrossRef]
- Guagnano, R.; Romano, F.; Berger, M.; Fagioli, F.; Vallone, V.; Bello, L.; Vitale, M.C.; Defabianis, P. Long-term effect of anticancer therapy on dentition of Italian children in remission from malignant disease: A cross-sectional study. Eur. J. Paediatr. Dent. 2022, 23, 131–136. [Google Scholar] [CrossRef]
- Dens, F.; Boute, P.; Otten, J.; Vinckier, F.; Declerck, D. Dental caries, gingival health, and oral hygiene of long term survivors of paediatric malignant diseases. Arch. Dis. Child. 1995, 72, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, G.; Wu, R.; Yu, G. Oral health status and oral habits of children and adolescents with hemophilia: A report from the children’s hemophilia comprehensive care center of China. Eur. J. Pediatr. 2024, 183, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, M.S.; Su1, S.; Crespo, C.J.; Hung, M. Men and oral health: A review of sex and gender differences. Am. J. Mens. Health 2021, 15, 15579883211016361. [Google Scholar] [CrossRef] [PubMed]
- Proc, P.; Szczepańska, J.; Herud, A.; Zubowska, M.; Fendler, W.; Młynarski, W. Dental caries among childhood cancer survivors. Medicine 2019, 98, e14279. [Google Scholar] [CrossRef]
- Abbass, M.M.S.; Mahmoud, S.A.; Moshy, S.E.; Rady, D.; AbuBakr, N.; Radwan, I.A.; Ahmed, A.; Abdou, A.; Al-Jawaldeh, A. The prevalence of dental caries among Egyptian children and adolescences and its association with age, socioeconomic status, dietary habits and other risk factors. A cross-sectional study. F1000Research 2019, 8, 8. [Google Scholar] [CrossRef]
- Mejia, G.C.; Elani, H.W.; Harper, S.; Thomson, W.M.; Ju, X.; Kawachi, I.; Kaufman, J.S.; Jamieson, L.M. Socioeconomic status, oral health and dental disease in Australia, Canada, New Zealand and the United States. BMC Oral. Health 2018, 18, 176. [Google Scholar] [CrossRef]
- Kumar, S.; Tadakamadla, J.; Kroon, J.; Johnson, N.W. Impact of parent-related factors on dental caries in the permanent dentition of 6–12-year-old children: A systematic review. J. Dent. 2016, 46, 1–11. [Google Scholar] [CrossRef]
- Corrêa-Faria, P.; Paixão-Gonçalves, S.; Paiva, S.M.; Pordeus, I.A. Incidence of dental caries in primary dentition and risk factors: A longitudinal study. Braz. Oral. Res. 2016, 30, e59. [Google Scholar] [CrossRef]
- Mejia, G.; Jamieson, L.M.; Ha, D.; Spencer, A.J. Greater inequalities in dental treatment than in disease experience. J. Dent. Res. 2014, 93, 966–971. [Google Scholar] [CrossRef]
- Busenhart, D.M.; Erb, J.; Rigakos, G.; Eliades, T.; Papageorgiou, S.N. Adverse effects of chemotherapy on the teeth and surrounding tissues of children with cancer: A systematic review with meta-analysis. Oral. Oncol. 2018, 83, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, O.; Hermann, P.; Kivovics, P.; Garami, M. Long-term effects of chemotherapy on dental status of children cancer survivors. Pediatr. Hematol. Oncol. 2013, 30, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Çubukçu, C.E.; Sevinir, B. Dental health of long-term childhood survivors who had oral supervision during treatment: A case-control study. Pediatr. Hematol. Oncol. 2008, 25, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Pajari, U.; Yliniemi, R.; Möttönen, M. The risk of dental caries in childhood cancer is not high in the teeth are caries-free at diagnosis. Pediatr. Hematol. Oncol. 2001, 18, 181–185. [Google Scholar] [CrossRef]
- Duggal, M.S.; Curzon, M.E.J.; Bailey, C.C.; Lewis, I.J.; Prendergast, M. Dental parameters in the long term survivors of childhood cancer compared with siblings. Oral. Oncol. 1997, 33, 348–353. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Doan, H.T.P.; Hoang, H.T.; Huynh, N.C.N.; Hsu, M.L.; Wong, M.C.M. Developing and assessing the efficiency of VOSER software in recording dental caries according to WHO’s criteria 2013. J. Dent. Sci. 2023, 18, 1822–1829. [Google Scholar] [CrossRef]
- Silness, J.; Loe, H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odont Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Loe, H.; Silness, J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odont Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Honkala, E.; Runnel, R.; Honkala, S.; Olak, J.; Vahlberg, T.; Saag, M.; Mäkinen, K.K. Measuring Dental Caries in the Mixed Dentition by ICDAS. Int. J. Dent. 2011, 2011, 150424. [Google Scholar] [CrossRef]
- Luiz, R.R.; Pires dos Santos, A.P.; Souza Goncalves, L.; Nadanovsky, P. The ageless DMFT index (A-DMFT): A proposal for a unified DMFT index for all ages and dentitions. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Grieshaber, A.; Haschemi, A.A.; Waltimo, T.; Bornstein, M.M.; Kulik, E.M. Caries status of first-born child is a predictor for caries experiencein younger siblings. Clin. Oral. Investig. 2022, 26, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Defabianis, P.; Bocca, N.; Romano, F. Prevalence and association of dental anomalies and tooth decay in Italian childhood cancer survivors. J. Clin. Pediatr. Dent. 2023, 47, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Bhat, M.; Choudhary, H.; Joshi, V.; Walia, S.S.; Soni, R.K. Prevalence of dental caries with salivary assessment in six to twelve years old school-going children in Shahpura Tehsil, Jaipur. Cureus 2022, 14, e27802. [Google Scholar] [CrossRef] [PubMed]
- Olczak-Kowalczyk, D.; Krasuska-Sławińska, E.; Brożyna, A.; Turska-Szybka, A.; Dembowska-Bagińska, B. Dental caries in children and adolescents during and after antineoplastic chemotherapy. J. Clin. Pediatr. Dent. 2018, 42, 225–230. [Google Scholar] [CrossRef]
- Maguire, A.; Craft, A.W.; Evans, R.G.B.; Amineddine, H.; Kernahan, J.; Macleod, R.I.; Murray, J.J.; Welbury, L.L. The long-term effects of treatment on the dental condition of children surviving malignant disease. Cancer 1987, 60, 2570–2575. [Google Scholar] [CrossRef]
- Avsar, A.; Darka, O.; Pinarli, G. Long-term effects of chemotherapy on caries formation, dental development and salivary factors in childhood cancer survivors. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2007, 104, 781–789. [Google Scholar] [CrossRef]
- Oguz, A.; Cetiner, S.; Karadeniz, C.; Alpaslan, G.; Alpaslan, C.; Pinarli, G. Long-term effects of chemotherapy on orodental structures in children with non-Hodgkin’s lymphoma. Eur. J. Oral. Sci. 2004, 112, 8–11. [Google Scholar] [CrossRef]
- Vera-Virrueta, C.G.; Sansores-Ambrosio, F.; Casanova-Rosado, J.F.; Minaya-Sánchez, M.I.; Casanova-Rosado, A.J.; Casanova-Sarmiento, J.A.; Guadarrama-Reyes, S.C.; de la Rosa-Santillana, R.; Medina-Solís, C.E.; Maupomé, G. Experience, prevalence, and severity of dental caries in Mexican preschool and school-aged children. Cureus 2023, 15, e51079. [Google Scholar] [CrossRef]
- Cordova Maciel, J.C.; de Castro Jr, C.G.; Brunetto, A.L.; Di Leone, L.P.; Dias da Silveira, H.E. Oral health and dental anomalies in patients treated for leukemia in childhood and adolescence. Pediatr. Blood Cancer 2009, 53, 361–365. [Google Scholar] [CrossRef]
- Marec-Berard, P.; Chaux-Bodard, A.G.; Lagrange, H.; Gourmet, R.; Bergeron, C. Long-term effects of chemotherapy on dental status in children treated for nephroblastoma. Pediatr. Hematol. 2005, 22, 581–588. [Google Scholar] [CrossRef]
- Haidar, Z.S. Radiation-induced salivary gland damage/dysfunction in head and neck cancer: Nano-bioengineering strategies and artificial intelligence for prevention, therapy and reparation. J. Radiol. Oncol. 2022, 6, 027–044. [Google Scholar] [CrossRef]
- Gawade, P.L.; Hudson, M.M.; Kaste, S.C.; Neglia, J.P.; Constine, L.S.; Robison, L.L.; Ness, K.K. A systematic review of dental late effects in survivors of childhood cancer. Pediatr. Blood Cancer 2014, 61, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, M.; Vieira, A.R. Explaining gender differences in caries: A multifactorial approach to a multifactorial disease. Int. J. Dent. 2010, 2010, 649643. [Google Scholar] [CrossRef]
- Lam, P.P.Y.; Chua, H.; Ekambaram, M.; Lo, E.C.M.; Yiu, C.K.Y. Risk predictors of early childhood caries increment—A systematic review and meta-analysis. J. Evid. Based Dent. Pract. 2022, 22, 101732. [Google Scholar] [CrossRef]
- Chrisopoulos, S.; AlKhtib, A.O.; Al Darwish, M.S.; Mohamed, H.G.S.; Tintu Mathew, T.; Ghanim Ali Al Mannai, G.A.A.; Abdulmalik, M.; Al Thani, M.; de Vries, J.; Loc Giang Do, L.G.; et al. Correlates of childhood caries: A study in Qatar. Int. J. Paediatr. Dent. 2024, 34, 179–189. [Google Scholar] [CrossRef]
- Moimaz, S.A.S.; Fadel, C.B.; Lolli, L.Z.; Garbin, C.A.S.; Garbin, A.J.I.; Saliba, N.A. Social aspects of dental caries in the context of mother-child pairs. J. Appl. Oral. Sci. 2014, 22, 73–78. [Google Scholar] [CrossRef]
- Schwendicke, F.; Dörfer, C.; Schlattmann, P.; Page, L.F.; Thomson, W.; Paris, S. Socioeconomic inequality and caries: A systematic re- view and meta-analysis. J. Dent. Res. 2015, 94, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Aldossri, M.; Farmer, J.; Gomaa, N.; Quiñonez, C.; Ravaghi, V. Changes in income-related inequalities in oral health status in Ontario, Canada. Community Dent. Oral. Epidemiol. 2021, 49, 110–118. [Google Scholar] [CrossRef]
- Liu, M.; Yun, Q.; Zhao, M.; Chen, W.; Zhang, H.; Hou, W.; Chang, C. Association of siblings’ presence and oral health-related quality of life among children: A cross-sectional study. BMC Oral. Health 2021, 21, 153. [Google Scholar] [CrossRef]
- Aydinoglu, S.; Arslan, I. Are anxiety and the presence of siblings risk factors for dental neglect and oral health status in children? Arch Pediatr. 2021, 28, 123–128. [Google Scholar] [CrossRef]
- Bilal, S.; Abdulla, A.M.; Andiesta, N.S.; Babar, M.G.; Pau, A. Role of family functioning and health-related quality of life in pre-school children with dental caries: A cross-sectional study. Health Qual. Life Outcomes 2021, 19, 192. [Google Scholar] [CrossRef] [PubMed]
- Virkkala, V.F.; Eloranta, A.; Suominen, A.L.; Vierola, A.; Ikävalko, T.; Väistö, J.; Mikkonen, S.; Methuen, M.; Schwab, U.; Viljakainen, H.T.; et al. Associations of diet quality, food consumption, eating frequency and eating behavior with dental caries experience in Finnish children: A 2-year longitudinal study. Br. J. Nutr. 2023, 129, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatric Dentistry. Policy on dietary recommendations for infants, children, and adolescents. In The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2024; pp. 109–113. [Google Scholar]
- Nirunsittirat, A.; Pitiphat, W.; McKinney, C.M.; DeRouen, T.A.; Chansamak, N.; Angwaravong, O.; Patcharanuchat, P.; Pimpak, T. Adverse birth outcomes and childhood caries: A cohort study. Community Dent. Oral. Epidemiol. 2016, 44, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Keiko Tanaka, K.; Miyake, Y. Low birth weight, preterm birth or small-for-gestational-age are not associated with dental caries in young Japanese children. BMC Oral. Health 2014, 14, 38. [Google Scholar]
- Cho, G.J.; Kim, S.Y.; Lee, H.C.; Kim, H.Y.; Lee, K.M.; Han, S.W.; Oh, M.J. Association between dental caries and adverse pregnancy outcomes. Sci. Rep. 2020, 10, 5309. [Google Scholar] [CrossRef]
- van der Tas, J.T.; Wolvius, E.B.; Kragt, L.; Rivadeneira, F.; Moll, H.A.; Steegers, E.A.P.; Schalekamp-Timmermans, S. Caries experience among children born after a complicated pregnancy. Community Dent. Oral. Epidemiol. 2021, 49, 225–231. [Google Scholar] [CrossRef]
- Gravina, D.B.; Cruvinel, V.R.; Azevedo, T.D.; Toledo, O.A.; Bezerra, A.C. Prevalence of dental caries in children born prematurely or at full term. Braz. Oral. Res. 2006, 20, 353–357. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, D.; Lin, H.; Tao, Y. The association between low birth weight and/or preterm birth and dental caries—A systematic review and meta-analysis. Int. J. Dent. Hyg. 2023, 21, 599–610. [Google Scholar] [CrossRef]
- Occhi-Alexandre, I.G.P.; Cruz, P.V.; Bendo, C.B.; Paiva, S.M.; Isabela Almeida Pordeus, I.A.; Martins, C.C. Prevalence of dental caries in preschool children born preterm and/or with low birth weight: A systematic review with meta-analysis of prevalence data. Int. J. Paediatr. Dent. 2020, 30, 265–275. [Google Scholar] [CrossRef]
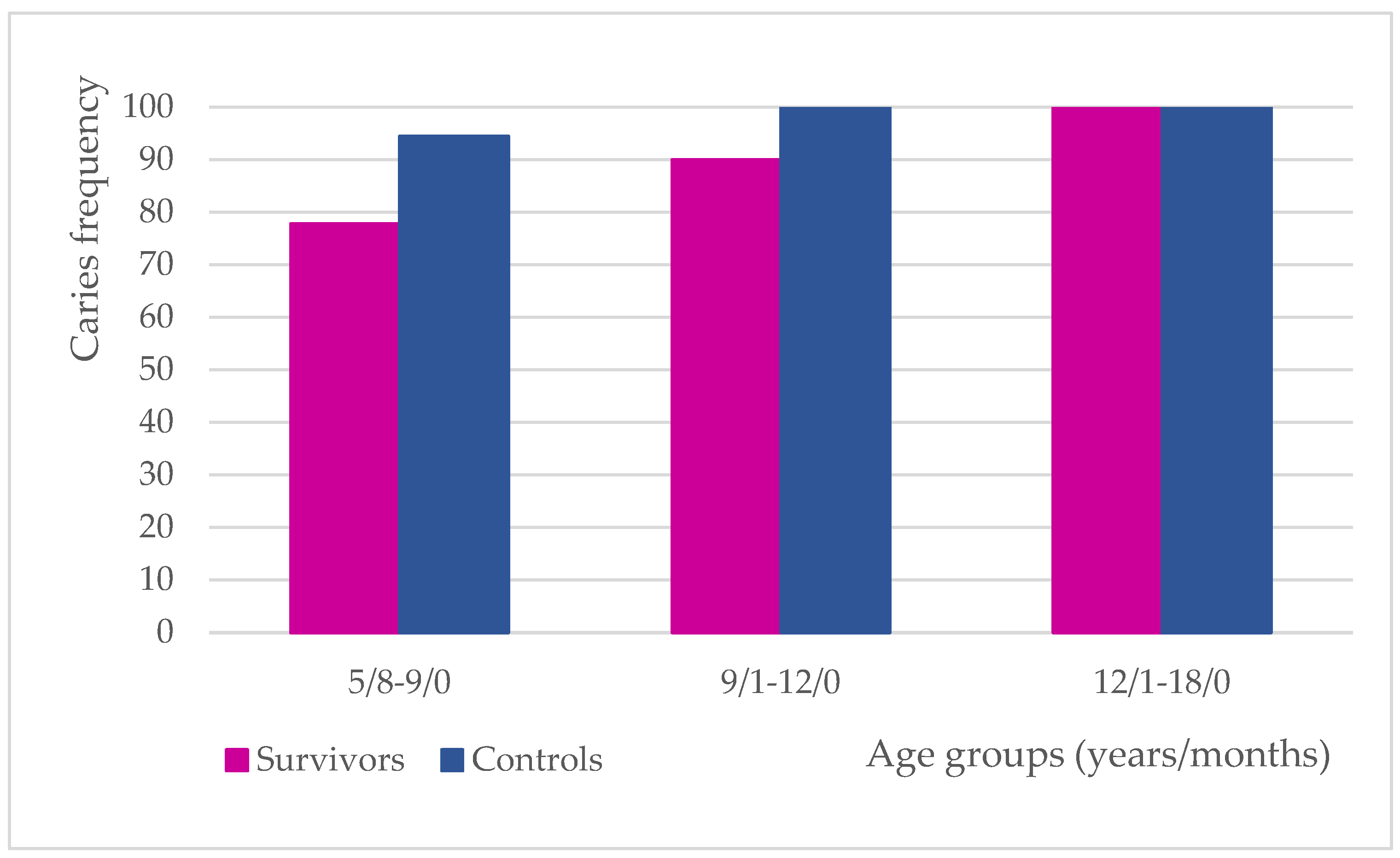
| Number of Survivors (%) | ||
|---|---|---|
| Age at cancer diagnosis (years/months) | 0–5 | 34 (85.00) |
| 5/1–10 | 6 (15.00) | |
| Type of cancer diagnosis | Solid tumors 1 | 31 (77.50) |
| Hematological cancers 2 | 9 (22.50) | |
| Type of anticancer treatment | Chemotherapy 3 | 40 (100.00) |
| Surgery | 29 (72.50) | |
| Radiotherapy | 13 (32.50) | |
| Duration of chemotherapy (weeks) | 0–24 | 6 (15.00) |
| 25–50 | 14 (35.00) | |
| 51–75 | 7 (17.50) | |
| 76–100 | 6 (15.00) | |
| 100–125 | 7 (17.50) | |
| Age at dental examination (years/months) | 5/8–9/0 | 18 (45.00) |
| 9/1–12/0 | 15 (37.50) | |
| 12/1–18/0 | 7 (17.50) | |
| Gender | Female | 24 (60.00) |
| Male | 16 (40.00) | |
| Problems during chemotherapy | Oral health 4 | 24 (60.00) |
| Oral hygiene | 12 (30.00) | |
| Cariogenic diet | 4 (10.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jodłowska, A.; Ilczuk-Rypuła, D. Risk Factors and Dental Caries Incidence in Childhood Cancer Survivors. Cancers 2025, 17, 1003. https://doi.org/10.3390/cancers17061003
Jodłowska A, Ilczuk-Rypuła D. Risk Factors and Dental Caries Incidence in Childhood Cancer Survivors. Cancers. 2025; 17(6):1003. https://doi.org/10.3390/cancers17061003
Chicago/Turabian StyleJodłowska, Anna, and Danuta Ilczuk-Rypuła. 2025. "Risk Factors and Dental Caries Incidence in Childhood Cancer Survivors" Cancers 17, no. 6: 1003. https://doi.org/10.3390/cancers17061003
APA StyleJodłowska, A., & Ilczuk-Rypuła, D. (2025). Risk Factors and Dental Caries Incidence in Childhood Cancer Survivors. Cancers, 17(6), 1003. https://doi.org/10.3390/cancers17061003







