Simple Summary
Lung cancer is a deadly malignancy and new treatments are needed to improve survival rates. Many lung tumours exhibit a feature called chromosomal instability, which is characterized by ongoing acquisition of genetic alterations that promote tumour aggression. Interestingly, chromosomal instability is a “double-edged sword”. A moderate level can support tumour growth and progression but a high level causes cell death. Therefore, chromosomal instability represents a weakness in cancer cells that could be exploited to kill lung tumours. Kinesins are a family of proteins with several members having key roles in chromosome segregation during mitosis. Because of this function, inhibiting mitotic kinesins can amplify chromosomal instability. This review summarizes the potential of targeting kinesins to elevate chromosomal instability to lethal levels as a strategy for killing chromosomally unstable lung cancers.
Abstract
New therapeutic approaches that antagonize tumour-promoting phenotypes in lung cancer are needed to improve patient outcomes. Chromosomal instability (CIN) is a hallmark of lung cancer characterized by the ongoing acquisition of genetic alterations that include the gain and loss of whole chromosomes or segments of chromosomes as well as chromosomal rearrangements during cell division. Although it provides genetic diversity that fuels tumour evolution and enables the acquisition of aggressive phenotypes like immune evasion, metastasis, and drug resistance, too much CIN can be lethal because it creates genetic imbalances that disrupt essential genes and induce severe proteotoxic and metabolic stress. As such, sustaining advantageous levels of CIN that are compatible with survival is a fine balance in cancer cells, and potentiating CIN to levels that exceed a tolerable threshold is a promising treatment strategy for inherently unstable tumours like lung cancer. Kinesins are a superfamily of motor proteins with many members having functions in mitosis that are critical for the correct segregation of chromosomes and, consequently, maintaining genomic integrity. Accordingly, inhibition of such kinesins has been shown to exacerbate CIN. Therefore, inhibiting mitotic kinesins represents a promising strategy for amplifying CIN to lethal levels in vulnerable cancer cells. In this review, we describe the concept of CIN as a therapeutic vulnerability and comprehensively summarize studies reporting the clinical and functional relevance of kinesins in lung cancer, with the goal of outlining how kinesin inhibition, or “targeting kinesins”, holds great potential as an effective strategy for treating lung cancer.
1. Introduction
1.1. Lung Cancer Background
Worldwide, lung cancer was responsible for nearly 2.5 million new cancer cases and over 1.8 million deaths in 2022 [1]. This indicates the immense burden lung cancer imposes on patients and families affected and the associated toll on global economies and healthcare systems. Global lung cancer incidence mirrors the prevalence of tobacco smoking in many countries [2], although up to 25% of lung cancers arise in non-smokers [3]. Despite advances in early detection due to the implementation of screening programs and translation of new treatment strategies, the 5-year survival rate for lung cancer patients remains between 10% and 20% in most countries [2]. The two major histological types of lung cancer are non-small cell lung cancer (NSCLC; ~85%) and small cell lung cancer (SCLC; ~15%) [4]. NSCLC is further subdivided into 3 subtypes with different histological and molecular features, including lung adenocarcinoma (LUAD), squamous cell carcinoma (LUSC), and large cell carcinoma, of which LUAD is the most commonly diagnosed (~40%) [5]. Lung cancer therapy is dependent on the histological subtype and stage of the tumour, as well as the presence or absence of response-predictive DNA or protein biomarkers, including oncogenic mutations and PD-L1 expression. Standard of care treatments include surgery for early-stage tumours, radiation, chemotherapy, immunotherapy, or combinations of these modalities, as well as targeted therapies that antagonize oncogenic drivers such as EGFR and KRAS inhibitors [6]. Although the therapeutic landscape has greatly expanded over the past two decades, treatments are rarely curative, and the fact remains that lung cancer patients exhibit some of the poorest survival outcomes of all cancer patients [1]. This emphasizes the need for innovative treatment strategies to improve survival rates.
1.2. Chromosomal Instability in Cancer
Genomic instability is a hallmark of many malignancies characterized by the ongoing acquisition of DNA alterations, including mutations, translocations, insertions, deletions, and gains and losses of chromosomal segments and/or entire chromosomes during cell division [7,8]. Several mechanisms have been identified to cause genomic instability, including compromised DNA damage response and repair pathways, chromosome segregation errors driven by defective cell cycle checkpoints, telomere shortening, and centrosome amplification, among others [9,10]. It can be broadly categorized into nucleotide instability that induces single nucleotide variants (i.e., mutations), microsatellite instability that drives expansion and/or contraction of short repeat sequences, and chromosomal instability (CIN, the type relevant for this review), which causes structural and/or numerical changes in chromosomes. Chromosomal alterations and aneuploidies induced by CIN provide genetic diversity that fuels tumour evolution and adaptation [8,10]. As a result, CIN is considered an enabling feature of cancer cells because it allows them to acquire additional malignant phenotypes like drug resistance, immune evasion, and the ability to metastasize [8,10,11]. In recognition of its multifaceted contributions to tumour progression, targeting CIN has emerged as an attractive therapeutic paradigm in oncology [9,12].
Lung cancers are among the most genomically unstable tumour types, often exhibiting high copy number alteration burdens caused by instability at the chromosomal level [13,14,15]. Thus, targeting CIN could be an effective treatment approach for lung cancer patients. Herein, we describe the concept of CIN as an actionable vulnerability in lung cancer. We then introduce the kinesin family of motor proteins and comprehensively summarize evidence implicating specific kinesins as promising targets whose inhibition could be leveraged to kill genomically unstable lung tumours. Finally, we offer our perspective on challenges that must be addressed to realize the therapeutic potential of targeting kinesins in lung and other cancers.
2. Exploiting Chromosomal Instability (CIN) for Cancer Therapy
2.1. CIN: A Vulnerability in Cancer Cells
A low to moderate level of CIN is advantageous for tumour progression, but excessive instability causes severe proteotoxic, metabolic, replication, and mitotic stress that can be fatal [10]. As a result, CIN in cancer has been described as a “double-edged sword”, requiring tumour cells to navigate a fine balance between the benefits of tumour-promoting genetic diversity and the consequences of exceeding a tolerable threshold [10,16]. However, the recurrence of CIN in cancer genomes suggests its benefits outweigh the costs, rendering tumours vulnerable to additional instability [9,10]. As such, unstable cancer cells must rely on tolerance mechanisms to preserve some level of stability to mitigate CIN’s potentially lethal consequences. Based on this premise, inhibition of such mechanisms could be leveraged to amplify instability to lethal levels. Since non-malignant cells are euploid, genomically stable, and have functional checkpoints in place to maintain genomic integrity and guard against the proliferation of abnormal cells, this treatment approach could preferentially target cancer cells. As described in Section 3, kinesins facilitate accurate chromosome segregation during mitosis, making their function critical for maintaining genome stability. Therefore, kinesins represent targets for exacerbating CIN to induce lethality in unstable cancer cells. Below, we briefly mention distinct strategies that demonstrate the concept of targeting CIN before focusing the remainder of this review on kinesins and their therapeutic potential in lung cancer.
2.2. Strategies for Targeting CIN in Cancer
Several approaches for exploiting CIN have been proposed, such as inhibiting proteins involved in genome maintenance or proteins that mitigate stress induced by CIN (e.g., proteotoxic and metabolic stress). Examples of the latter include potentiating CIN-associated stress with inhibitors of heat shock proteins like HSP90 or agonists of AMPK [8]. An approach illustrating the former involves abrogating the spindle assembly checkpoint (SAC) by inhibiting MPS1 (also known as TTK), the kinase that activates the SAC. Briefly, MPS1 inhibition forces mitotic progression before chromosomes are properly attached to the mitotic spindle, resulting in lethal chromosome segregation errors and mitotic catastrophe [17]. Other cell cycle kinases like PLK1, PLK4, AURKA, AURKB, and WEE1 that regulate processes critical for faithful chromosome segregation such as mitotic entry, centrosome duplication, spindle assembly, and spindle-kinetochore attachments can also be targeted to induce lethality by potentiating CIN [9,10,12]. The therapeutic efficacy of PARP inhibitors in ovarian and triple-negative breast cancer patients whose tumours harbour BRCA1 or BRCA2 mutations that promote CIN illustrates the potential for similar synthetic lethal strategies to be effective in the clinic [18]. Given their influence on CIN, kinesin proteins are also attractive targets in this regard.
3. Kinesins: Classification, Structure, and General Functions
Kinesins are a superfamily of unidirectional motor proteins that bind and move along microtubules powered by ATP hydrolysis [19,20]. To date, 45 kinesin genes have been identified in the human genome. Kinesin proteins are broadly classified into families defined based on structural similarity in their motor domains [19,20]. They can also be subdivided into three distinct groups—N-, M-, and C-type kinesins—based on the location of their motor domain. N-kinesins contain a motor domain in the N-terminal region and are plus-end-directed, travelling towards the rapidly growing plus end of microtubules. C-kinesins have a motor domain in the C-terminal region and are minus-end-directed, travelling towards the slower-growing end of microtubules anchored to the centrosome. The motor domain of M-kinesins is located in the middle of the protein, and these kinesins are non-motile.
Structurally, all kinesins contain a motor domain, a coiled-coil stalk, and a tail domain [20,21]. The highly conserved motor domain, also referred to as the head, contains binding sites for ATP and microtubules. Kinesin motor domains exhibit 30–60% homology in their amino acid sequences [21]. In contrast, the tail domains of kinesins are highly variable and dictate the binding specificity of kinesins to diverse cargo such as intracellular vesicles, organelles, or macromolecules [20,21]. In addition to direct binding mediated by the tail domain, scaffold and adapter proteins can also facilitate kinesin recognition and binding to cargo [20]. Inversely, cargo unloading is commonly mediated by Ca2+ signalling, Rab GTPase activity, and phosphorylation of kinesins [20]. The stalk domains of kinesins are also variable, with the coiled-coil structure enabling homo- and heterodimerization with other family members [21].
Kinesins generally serve two functions in the cell, being involved in mitosis and/or intracellular transport. As a result, kinesins can also be classified as mitotic and non-mitotic. They have been implicated in organismal development, morphogenesis, neuronal function, and various pathologies [19,20]. Mitotic kinesins have diverse roles in mitosis, including spindle assembly, bipolar spindle formation, spindle-kinetochore attachments, chromosome congression and alignment, chromosome segregation, and cytokinesis (Figure 1, Table 1) [19,21]. They carry out these functions by leveraging their motor activity, microtubule binding, stabilization, and depolymerization abilities to (i) transport chromosomes along microtubules, (ii) exert forces on microtubules and alter their length to influence spindle assembly, positioning, and chromosome alignment, (iii) transport and organize microtubules and proteins to the spindle midzone for cytokinesis, and (iv) attach kinetochores to K-fibres, tethering chromosomes to the mitotic spindle. Given that these functions are critical for accurate chromosome segregation during cell division, many mitotic kinesins contribute to maintaining genomic fidelity during mitosis, and their loss of function enhances CIN. Accordingly, mitotic kinesins could be targeted to kill genomically unstable tumours (Figure 2). Supporting this concept, several mitotic kinesins have been reported as vulnerabilities and therapeutic targets in lung cancers with CIN, as discussed below. Although our review focuses on kinesins as targets for exacerbating CIN to lethal levels in cancer cells, it is also important to recognize that some of the therapeutic effects associated with kinesin inhibition, particularly for non-mitotic kinesins (described in Section 5), are likely attributable to their functions in intracellular transport (Table 1).
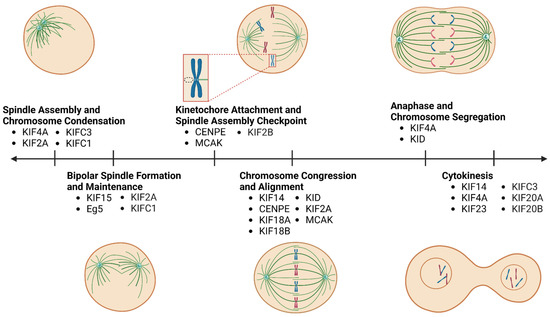
Figure 1.
Timeline indicating the roles of kinesins in mitosis. Kinesins are involved in several critical processes during mitosis, including spindle formation, chromosome condensation, pole separation, chromosome attachment to the spindle, chromosome congression, alignment, and segregation, as well as cytokinesis. Mitotic kinesins contribute to these processes by transporting chromosomes, positioning spindle poles and microtubules, exerting push and pull forces on spindle microtubules by “walking” along them, regulating microtubule dynamics, and attaching kinetochores to K-fibres of the spindle. Note, increments between ticks of the timeline do not represent relative time intervals between subsequent stages of mitosis.

Table 1.
Kinesins relevant in lung cancer.
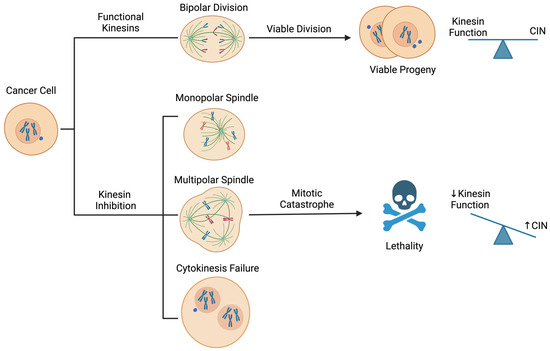
Figure 2.
Therapeutic concept for targeting kinesins to exploit chromosomal instability (CIN) in cancer cells. Inhibition of kinesin function induces mitotic stresses, including but not limited to monopolar and multipolar spindle formation and cytokinesis failure. These abnormalities can cause severe chromosome segregation errors and aneuploidies that potentiate CIN beyond tolerable levels, leading to mitotic catastrophe and cell death immediately or upon subsequent cell divisions. Blue dots outside the nucleus indicate micronuclei.
4. Kinesins in Lung Cancer
In this section we present the findings of studies that have identified kinesins as clinically or functionally significant in lung cancer with respect to patient characteristics and prognosis, and/or their involvement in lung tumour biology and progression. We also discuss kinesin-targeted inhibitors where relevant. Although we focus on mitotic kinesins because of their relevance for exploiting CIN, we also describe non-mitotic kinesins with reported significance in lung cancer.
4.1. Kinesin-3 Family: KIF14
KIF14 regulates cytokinesis and also has roles in the congression and alignment of chromosomes during mitosis [19]. KIF14 depletion causes chromosomal misalignment and cytokinesis failure, producing multinucleated cells that ultimately undergo cell death [19]. This is likely because tetraploid cells produced by cytokinesis failure contain extra centrosomes that induce multipolar cell divisions, resulting in segregation errors and aneuploidies that are often lethal immediately or upon subsequent attempts to divide (Figure 2) [8,22].
Transcriptomic analyses identified KIF14 as a prognostic gene whose high expression was correlated with shorter survival times in lung cancer patients [23]. KIF14 was also identified as part of a multigene expression signature predictive of poor overall survival in LUAD patients, further supporting its prognostic relevance [24,25]. Elevated KIF14 mRNA expression was also an indicator of poor outcomes in independent NSCLC cohorts, with higher expression observed in LUSC compared to LUAD tumours [26,27,28,29]. Consistently, Ling et al. identified that KIF14 mRNA expression was significantly higher in LUSC but not LUAD tumours compared to normal lung samples [23]. Conversely, Hung et al. used immunohistochemistry (IHC) to evaluate KIF14 protein expression in LUAD and found that it was downregulated in tumours compared to bronchial epithelial cells and that low KIF14 expression measured by qRT-PCR was associated with worse overall survival in LUAD patients [30]. These discordant results with respect to the prognostic significance of KIF14 in NSCLC could reflect differences in methods for quantifying its expression as well as clinical, molecular, and/or demographic differences in the patient populations examined. As such, additional studies using standardized protocols for measuring KIF14 expression in large independent cohorts are required to resolve them. Recently, KIF14 was identified as a putative target of miR-195-3p and miR-144-3p in LUAD models, with circ-0002727 functioning as a competing endogenous RNA that relieves miR-144-3p-mediated suppression of KIF14 expression [31,32]. Akinduro et al. also reported that KIF14 is a YAP target gene whose expression was reduced by the treatment of lung cancer cells with the YAP-TEAD inhibitor, verteporfin [33].
While most studies indicate that KIF14 behaves as an oncogene, it has been described to have both oncogenic and tumour suppressive effects in lung cancer [23,27,30,34]. Consistent with an oncogenic role, KIF14 is located within a minimal common region of DNA amplification on chromosome 1q that occurs in ~21% of lung cancers and is positively associated with tumour progression and metastasis in LUSC [26,35,36]. Like the gene expression studies described above, functional studies in lung cancer models have also shown conflicting results. One study reported that KIF14 depletion decreased cell proliferation and colony formation in H1299 cells [27], while another reported that knockdown enhanced migration and overexpression reduced migration and invasion in the C1-5 cell line [30]. This highlights the need to investigate KIF14 in additional models representing the major subtypes of NSCLC to resolve its therapeutic significance, which may be subtype-specific.
4.2. Kinesin-4 Family: KIF4A
KIF4A is a plus-end-directed mitotic kinesin involved in chromosome condensation, segregation during anaphase, and cytokinesis. It has also been linked to cellular response to DNA damage because of its interaction with BRCA2 [19,37]. Since it contains a DNA binding domain, KIF4A is considered a chromokinesin. KIF4A influences chromosome congression on the metaphase plate by generating polar ejection forces that counteract those from minus-end-directed dyneins at kinetochores [38]. Consistent with these functions, KIF4A depletion causes mitotic defects, including chromosome misalignment and missegregation, abnormal anaphase spindle dynamics, and aneuploidy [39]. KIF4A protein expression was detected by IHC in both NSCLC and SCLC tumours [40], and KIF4A transcript levels were identified as overexpressed in LUAD compared to non-malignant tissues [28,41]. Multiple studies found that high KIF4A expression at both the protein and RNA levels was associated with poor prognosis and tumour stage in LUAD [28,40,41,42,43,44,45]. KIF4A was also identified as a component of mitotic spindle- and microtubule-related gene expression signatures associated with poor survival of LUAD patients [46,47].
KIF4A knockdown reduced proliferation, viability, migration, and invasive properties of NSCLC cells in vitro, and their expression of epithelial-to-mesenchymal transition (EMT) markers [40,42,43,48]. Suppression of KIF4A led to reductions in sphere-forming capability, self-renewal capacity, and cell survival after radiation, while overexpression increased cancer stem cell (CSC) marker expression and cell motility in the A549 model [42], suggesting KIF4A could play a role in maintaining a stem-like phenotype in lung cancer cells. KIF4A has also been implicated in lung cancer response to chemotherapy. Two independent groups reported that KIF4A was overexpressed in cisplatin-resistant LUAD cells and promoted chemotherapy resistance [49,50]. Pan et al. identified that KIF4A interacted directly with lung resistance-related protein (LRP), a drug efflux pump, in A549 cells and proposed a putative mechanism whereby KIF4A contributes to cisplatin resistance by transporting LRP through the cytoplasm to the cell membrane [50]. A different study identified that KIF4A was upregulated upon treatment with cisplatin and that its depletion induced cell cycle arrest and enhanced cisplatin-induced cytotoxicity in H1299 cells, possibly by impairing BRCA2 and Rad51 responses to DNA damage [51]. Moreover, Zhang and colleagues found that KIF4A knockdown sensitized A549 to the cytotoxic chemotherapy, doxorubicin [48]. These findings indicate a potential role for KIF4A in mediating multidrug resistance through non-mitotic, intracellular transport functions or regulation of the DNA damage response. Collectively, they indicate KIF4A’s therapeutic potential in lung cancer and rationalize the development of KIF4A-targeted inhibitors that could be dually effective by antagonizing its transport and mitotic functions. To this end, Yan et al. recently mined gene expression and drug response data available in the Connectivity Map and identified WZ-3146 as a putative KIF4A inhibitor, reporting that it downregulated KIF4A expression and exhibited anti-cancer effects in glioma models [52]. However, no evidence was provided to show that WZ-3146 inhibited the enzymatic activity of KIF4A, indicating the need for experimental evidence to confirm this supposition.
4.3. Kinesin-5 Family: KIF11 (Eg5)
KIF11 encodes Eg5, or KSP, and is involved in bipolar spindle assembly, making it essential for accurate chromosome segregation [53]. It forms homotetramers consisting of two antiparallel dimers with motor domains at each end that crosslink spindle microtubules emanating from distinct centrosomes. With this configuration, its plus-end-directed motor activity enables Eg5 to generate forces that push antiparallel microtubules apart, driving centrosome separation and establishing a mitotic spindle with bipolar orientation [53]. Consistent with this function, Eg5 localizes to the centrosome and spindle poles during prophase, moving to the spindle microtubules during metaphase. Eg5 inhibition and overactivation have been tied to failed centrosome separation and premature centrosome separation, respectively, two phenotypes associated with centrosome amplification [53]. Inhibition of Eg5 causes the formation of monopolar or “monastral” spindles that activate the spindle assembly checkpoint and arrest cells in mitosis, leading to mitotic catastrophe and subsequent cell death in cancer models [53,54]. Extensive research has confirmed that Eg5 is associated with and enables cancer progression in a variety of malignancies, including colorectal cancer, glioblastoma, and NSCLC, among others, emphasizing its potential clinical utility as a prognostic marker and therapeutic target [53].
Several studies have identified KIF11/Eg5 as overexpressed at the RNA and protein levels in both NSCLC and SCLC compared to normal tissues, with high expression correlating with greater pathological stage, lymph node metastasis, and poor progression-free and overall survival rates [41,55,56,57,58,59,60,61,62,63]. High expression of Eg5 quantified by IHC was also found to predict brain metastasis following primary tumour resection in LUAD patients and to predict response to platinum chemotherapy combined with anti-mitotic agents in a retrospective NSCLC study [64,65]. Eg5 was also upregulated in EGFR-mutant H1975 and MDA-L-011 NSCLC lines resistant to EGFR tyrosine kinase inhibitors (TKIs) [66]. In SCLC, RNA expression of KIF11 was correlated with the proliferation marker Ki-67, an established prognostic indicator of tumour aggression [60]. At the molecular level, a study by Ling and colleagues found that the tobacco smoke carcinogen, benzo(a)pyrene, upregulated Eg5 expression in lung cancer cells [56], while hsa-miR-16-5p was reported to suppress it [67].
Genetic and pharmacologic inhibition of Eg5 arrested SCLC cells in the G2/M phase of the cell cycle and induced potent cytotoxicity in vitro, establishing Eg5 as a dependency in several SCLC cell lines [60,68]. Combined inhibition of Eg5 and BCL2L1 had an even stronger therapeutic effect in SCLC models [60]. Similarly in NSCLC cells, Eg5 depletion induced G2/M arrest, enhanced apoptosis, and reduced proliferation, migration, and invasion, while its overexpression promoted migration and angiogenesis [58,59]. Kato et al. also demonstrated that siRNA targeting KIF11 suppressed the growth of highly invasive H460SM NSCLC tumour xenografts in mice [69], and Good et al. reported that Eg5 inhibition elicited potent anti-tumour efficacy in a patient-derived xenograft model of lung cancer [70]. Furthermore, inhibition of Eg5 demonstrated efficacy in EGFR-TKI-resistant and cisplatin-resistant lung cancer cells, and combining Eg5 and BCL2L1 inhibitors effectively killed EGFR-mutant LUAD cells refractory to EGFR TKIs. These findings suggest that targeting Eg5 could be an effective approach for overcoming resistance to standard of care chemo- and targeted therapies in lung cancer [66,71,72].
The therapeutic potential of Eg5 is supported by its prominent upregulation in malignant compared to non-malignant cells. This provides a therapeutic window that has been validated in preclinical models, as cancer cells have shown greater sensitivity to Eg5 inhibition than normal cells [73]. Additional studies have demonstrated that Eg5 depletion exhibits greater cytotoxicity in tetraploid cancer cells compared to diploid counterparts, consistent with the idea that Eg5 can be exploited as a target in cancers with aneuploidy and/or CIN [74]. The robust anti-cancer effects of Eg5 suppression seen across diverse preclinical cancer models spurred the discovery and development of numerous Eg5 inhibitors, including but not limited to monastrol, Ispinesib, SB-743921, and Arry-520 (Filanesib). A number of these drugs have been evaluated in clinical trials [53]. Encouragingly, Eg5 inhibitors have been well tolerated and have not induced neurotoxicities commonly seen with microtubule inhibitors, but their therapeutic efficacy has been poor [75]. However, Filanesib was found to be effective in multiple myeloma when combined with the proteasome inhibitor, bortezomib, and the anti-inflammatory agent, dexamethasone [76]. Ongoing research is being conducted to develop additional Eg5 inhibitors and to understand how their clinical efficacy can be improved by discovering response predictive biomarkers, synergistic therapeutic combinations, and mechanisms of drug resistance. A recent study used a chemoinformatics approach to identify compounds capable of inhibiting Eg5, which were functionally screened in a panel of lung cancer cell lines. This revealed (-)-Cochlactone A, Phelligridin C, Sterenin E, and Cyathusal A as Eg5 inhibitors with cytotoxic activity, nominating these compounds as scaffolds for developing additional Eg5-targeted drugs with enhanced anti-tumour efficacy [59,60].
4.4. Kinesin-6 Family: KIF20A (MKLP2), KIF20B (MPP1), and KIF23 (MKLP1)
KIF20A encodes mitotic kinesin-like protein 2 (MKLP2), a plus-end-directed kinesin essential for cytokinesis that has also been implicated in regulating Golgi apparatus positioning and transport via interactions with the small GTPase, Rab6 [20]. Accordingly, its inhibition using antibodies blocked cytokinesis and produced binucleated cells [77]. MKLP2 accumulates in the nucleus during prophase and is found in the cytoplasm during metaphase [78]. Later in mitosis, it complexes with the chromosomal passenger complex to facilitate cleavage furrow formation [78]. Phosphorylation by PLK1 is known to regulate MKLP2’s motor activity and ability to mediate cytokinesis [19,37]. With respect to its transcriptional regulation, KIF20A has been identified as a target of Hedgehog (Hh) signalling [79,80] and of FOXM1 in response to radiation [81].
KIF20A/MKLP2 is overexpressed in many cancers, including lung cancer, raising interest in its potential as a therapeutic target [78]. Overexpression of KIF20A RNA has been reported in several studies that analyzed NSCLC and SCLC tumours and is associated with poor patient prognosis; KIF20A was also identified as a component of prognostic gene expression signatures associated with drug resistance and microtubules [28,41,46,47,82,83,84,85,86,87,88,89]. Zhao et al. demonstrated ubiquitous MKLP2 expression in LUAD tissues but absent or weak staining in matched non-malignant tissues by IHC. They also reported that high expression was associated with poor prognosis, suggesting KIF20A’s prognostic utility is consistent regardless of whether RNA or protein is assessed [41,46,82,83,84,85,86,87,88].
Multiple groups have investigated the therapeutic potential of MKLP2 in lung cancer models using RNAi-based methods. MKLP2 depletion was found to reduce cell proliferation and colony-forming capacity, enhance apoptosis, and induce G1 arrest in H1975 and A549 LUAD cells in vitro, and its knockdown inhibited the growth of A549 tumour xenografts in mice [87,88]. Similarly, Sun et al. reported that MKLP2 knockdown in A549 and PC9 cells inhibited proliferation and colony formation, induced G2/M phase arrest, and enhanced apoptosis [90]. An independent group also knocked down MKLP2 in A549 and PC9 cells and observed reduced colony formation, increased reactive oxygen species production, and enhanced sensitivity to the chemotherapeutic agent, gemcitabine [91]. Lastly, KIF20A/MKLP2 expression was upregulated after treatment with radiation, and its knockdown impaired the ability of A549 cells to survive radiotherapy [81]. These latter studies implicate MKLP2 in regulating multimodal therapeutic resistance, possibly through a FOXM1-driven signalling axis, but further research is needed to confirm this hypothesis.
Its tumour-dominant expression patterns and oncogenic functions make MKLP2 an attractive target for anti-cancer therapies. As such, numerous strategies for inhibiting MKLP2 have been identified, including natural compounds and small molecule inhibitors [78,92]. Three small molecule inhibitors have been reported: Paprotrain, Compound 9a, and BKS0349. Paprotrain is a non-competitive inhibitor of ATP and microtubules that disrupts the mobility of the chromosomal passenger complex and cytokinesis, resulting in binucleated cells without affecting other kinesin proteins [93]. Characterization of paprotrain’s effects in another study indicated that it disrupted kinetochore-microtubule attachments, resulting in chromosome alignment errors and CIN [94], providing evidence that MKLP2 inhibition could be leveraged to potentiate instability in cancer cells. Compound 9a is an analogue of paprotrain that exhibited enhanced inhibition of MKLP2 ATPase and microtubule-stimulating activity and demonstrated promising cytotoxicity in human cancer cell lines [92,95]. Most recently, BKS0349, another derivative of paprotrain, was described as an antagonist of MKLP2, but it has not been evaluated in cancer models [96]. Interestingly, berberine, a traditional Chinese medicine, has been proposed to inhibit LUAD tumour models in vivo by suppressing KIF20A and CCNE2 expression [97]. However, whether berberine physically interacts with MKLP2 to inhibit its activity is unknown, and experimental evidence is required to support this idea. Each of these MKLP2 compounds requires rigorous studies to determine their effects on non-malignant cells and toxicities in mice to better understand their therapeutic potential.
An early finding that KIF20A expression was largely restricted to malignant tissues, particularly pancreatic cancer, with minimal expression in most adult normal tissues spurred Imai et al. to hypothesize that peptides derived from MKLP2 could be tumour-associated antigens (TAAs) and targets for cancer immunotherapy [98]. Subsequent work led to the discovery that 3 different HLA-A2-restricted MKLP2-derived peptides could activate human cytotoxic CD8+ T cells and that such T cells could effectively kill HLA-A2+ MKLP2-expressing cancer cells in vitro [98]. Several follow-up studies provided additional evidence to support the development of vaccines to stimulate anti-tumour immunity against MKLP2-derived TAAs expressed in cancer cells, which have emerged as another mode of targeting MKLP2 [92]. As recently reviewed by Moon, multiple MKLP2 vaccines have been evaluated in phase I clinical trials, including in advanced NSCLC and SCLC patients, and have demonstrated tolerability and encouraging abilities to antagonize several tumour types [92]. Although this therapeutic strategy is independent of MKLP2’s role in cell division and genome maintenance, it is a promising approach for treating lung cancers with high MKLP2 expression. Ongoing trials will determine its clinical utility.
KIF20B, also known as MPP1, is a plus-end-directed kinesin that localizes to the spindle midbody to regulate abscission, and its knockdown leads to cytokinesis failure and multinucleation of dividing cells [99]. KIF20B was identified as overexpressed at the mRNA level in the TCGA’s cohort of LUAD tumours compared to normal tissue controls, and higher expression was correlated with advanced stage and worse survival [100]. Knockdown of MPP1 reduced migration and survival and increased apoptosis in A549 cells, which was associated with elevated p53 and Bax expression and downregulation of BCL2 [100]. Although no clinical-grade inhibitors are available, a screen for MPP1 inhibitors revealed that depsidones, which are natural compounds derived from lichen, inhibited MPP1 [101]. The marine natural product, adociasulfate-2 (AS-2), was also identified as a non-specific kinesin antagonist capable of inhibiting multiple kinesins, including MPP1 [102]. These chemical probes may prove useful for informing the future development of potent and selective MPP1 inhibitors.
KIF23, also known as mitotic kinesin-like protein 1 (MKLP1), is part of the centralspindlin complex, which bundles microtubules to regulate spindle midbody formation and cytokinesis [19,37]. Loss of MKLP1 function causes cytokinesis failure and the production of binucleated and multinucleated cells [19,37]. Numerous studies have demonstrated that KIF23 mRNA and/or protein is overexpressed in NSCLC and SCLC tumours, which are associated with poor prognosis of patients, specifically greater tumour stage, and worse overall and recurrence-free survival [103,104,105,106,107,108,109,110]. One study demonstrated the absence of MKLP1 staining in non-malignant lung tissues [104]. The activity of MKLP1 is regulated through phosphorylation by multiple mitotic kinases, including AURKB, CDK1, and PLK1 [19,37]. More recently, YAP was shown to regulate KIF23 at the transcriptional level, as YAP inhibition with verteporfin or siRNA reduced KIF23/MKLP1 RNA and protein expression [33,111].
At the phenotypic level, Iltzsche et al. discovered that MKLP1 was required for the development of lung tumours in genetically engineered mouse models of NSCLC driven by the loss of p53 and KRAS activation [112]. They also showed that MKLP1 depletion with shRNA inhibited proliferation and induced apoptosis in 4 lung cancer cell lines and impaired lung tumour growth in vivo, which was associated with abnormalities including giant multinucleated cells [112]. An independent group showed that MKLP1 depletion also reduced cell proliferation in H2122 LUAD cells [104,112]. Although no specific MKLP1 inhibitors have been reported, the natural marine product AS-2 was found to inhibit MKLP1 [102], indicating its amenability to pharmacologic inhibition. Based on the evidence in preclinical models of lung and other cancers, MKLP1 represents an attractive therapeutic target warranting the development of specific inhibitors. Interestingly, kinesin-6 family members have a unique 8–10 kDa sequence in their motor domain that may provide an opportunity to develop kinesin-selective inhibitors [19].
4.5. Kinesin-7 Family: KIF10 (CENPE)
Centromere-associated protein-E (CENPE), encoded by KIF10, is a plus-end-directed kinesin critical for faithful chromosome segregation during mitosis because of its roles in attaching kinetochores to the spindle and chromosome alignment, as well as SAC regulation [113]. CENPE forms homotetramers that localize to the kinetochores from nuclear envelope breakdown to anaphase [114]. Accordingly, CENPE loss of function increases chromosome misalignment, mitotic arrest, aberrant spindle positioning, CIN, and mitotic catastrophe in cancer, fibroblast, and liver regeneration models [113,115]. Given its involvement in suppressing CIN, CENPE has emerged as a promising therapeutic target in several malignancies [113].
Numerous independent analyses of gene expression databases, clinical tumour samples, and in vitro cell lines have revealed that KIF10/CENPE is overexpressed in both LUAD and LUSC forms of NSCLC [82,116,117,118,119,120]. Upregulation of KIF10 RNA is associated with worse patient prognosis in LUAD and LUSC, although the association may be weaker in LUSC [116,117]. While all tumour grades have been shown to express elevated levels of KIF10 compared to non-malignant tissues, multiple reports indicate that KIF10 RNA expression is significantly higher in stage II, III, and IV compared to stage I tumours and associated with worse patient outcomes [118,119]. Transcriptomic analyses of clinical NSCLC samples identified KIF10 as a hub gene implicated in various cellular processes, including cell division, cell cycle, DNA replication, angiogenesis, and cell migration [121]. Another correlative study discovered that somatic mutations in the KIF10 gene were associated with distant recurrence in patients, leading the authors to speculate that genetic disruption of KIF10 drives lung cancer progression by enhancing CIN [122]. KIF10 mutations were also identified in tumour tissues of patients with sporadic lung cancers, as well as in the constitutional DNA of multiple members of a family with suspected familial lung cancer, nominating germline KIF10 mutations as putative lung cancer risk factors [123].
While most studies of CENPE in lung cancer have been correlative, some have studied its loss of function using genetic or pharmacological means, revealing that CENPE inhibition reduces proliferation and colony-forming capacity and arrests cells in G2/M in multiple lung cancer cell lines (A549, PC9, H460, H522) [117,118,120]. The study by Shan et al. additionally discovered a putative regulatory relationship between CENPE and the FOXM1 transcription factor, indicating that FOXM1 binds to the KIF10 gene promoter to control its expression [118]. Since FOXM1 is a downstream target of several oncogenic pathways, including PI3K/Akt, Ras/ERK, and JNK/p38 MAPK, it is possible that CENPE is induced to support mitotic fidelity in rapidly proliferating cells [118,124].
Several inhibitors of CENPE exist and were described in a recent review [113]. Of these, GSK923295 has been the most studied in preclinical models, and a first-in-human phase I clinical trial revealed it had an acceptable toxicity profile and resulted in stable disease in one-third of previously treated patients with solid tumours [125,126]. GSK923295 is a potent and specific allosteric inhibitor of CENPE that has been shown to inhibit cell proliferation in vitro and tumour growth in vivo in lung cancer and several other malignancies [113,127,128]. However, like many anti-mitotic drugs, CENPE inhibitors have had limited clinical efficacy as monotherapies, which may be attributable to “mitotic slippage” of cancer cells that experience prolonged mitotic arrest in response to such drugs. Mitotic slippage is characterized by cells exiting mitosis before sufficient apoptotic signals accumulate to drive cell death, allowing them to continue dividing [129]. This finding has prompted the exploration of combinations to bolster the therapeutic effects of CENPE inhibitors like GSK923295.
In lung cancer models specifically, CENPE inhibition has shown promising synergistic effects when combined with Navitoclax, a BH3-mimetic that inhibits anti-apoptotic proteins, as well as MEK/ERK inhibitors [120,130,131]. Both combinations yielded enhanced anti-tumour effects compared with monotherapy and abrogated the post-mitotic survival of cells that underwent mitotic slippage in A549, H460, H522, and Lewis lung carcinoma cells [117,120]. Interestingly, CENPE inhibition with GSK923295 was recently described to synergize with PD-L1-targeted immunotherapy in NSCLC tumour models, which was attributed to CENPE inhibitor-mediated upregulation of PD-L1 [117]. Moreover, CENPE was identified as part of a 3-gene prognostic signature whose high expression correlated with worse survival and greater T cell infiltration in EGFR-mutant LUAD patients [132]. This finding warrants validation studies and additional investigations to determine whether CENPE expression has value for predicting immunotherapy response. In line with mitotic kinesins being strategic targets for amplifying CIN to lethal levels, Tucker et al. recently demonstrated that CENPE inhibition enhanced CIN and synergized with microtubule-disrupting agents in breast cancer [133]. Additionally, a study by Yoshizawa et al. found that the anti-proliferative effects of GSK923295 were greater in tetraploid compared to diploid HAP1 leukemia cells, suggesting that CENPE inhibitor cytotoxicity could be restricted to aneuploid tumours or cancers with CIN [134]. However, combinatorial therapeutic approaches will likely be required to achieve maximal therapeutic benefit with CENPE inhibitors.
4.6. Kinesin-8 Family: KIF18A, KIF18B
KIF18A functions as both a plus-end-directed motor protein and a microtubule depolymerase [135]. It localizes to the plus end of kinetochore microtubules and regulates chromosome alignment during mitosis by influencing kinetochore-microtubule dynamics in prometaphase through metaphase [19,135]. KIF18A loss of function causes a failure of chromosome alignment, hyperstable microtubules, and a loss of spindle tension that activates the SAC [19]. While KIF18A expression is low in non-malignant tissues, elevated KIF18A expression has been detected at both RNA and protein levels in NSCLC tissues, consistent with reports for several other human cancers [136,137,138,139,140]. High KIF18A RNA and protein expression in NSCLC has been associated with higher tumour stage, tumour differentiation, lymph node metastasis, and tumour mutation burden [136,138,139,140]. Concordantly, multiple studies have identified KIF18A as a prognostic marker in LUAD, as elevated KIF18A expression was correlated with reduced overall survival, disease-free survival, and recurrence-free survival in LUAD but not LUSC patients [136,137,139]. KIF18A was also reported as a putative serum biomarker that could predict patients with asbestosis who have a high risk of developing lung cancer [141].
Depletion of KIF18A has been shown to induce excessive CIN and death of genomically unstable cancer cells [142]. Independent functional studies have shown that KIF18A depletion impairs proliferation, migration, and invasion; increases apoptosis; induces cell cycle arrest in the A549 and H1975 LUAD models; and also inhibits tumour growth and metastasis in in vivo tumour models [136,137,140]. A flurry of recent studies has implicated KIF18A as a tractable therapeutic vulnerability specifically in cancers with aneuploidy, CIN, weakened APC/C activity, and SAC persistence [142,143,144,145,146]. Supporting KIF18A’s therapeutic promise in genomically unstable cancers like triple-negative breast cancer and high-grade serous ovarian cancers, multiple KIF18A inhibitors have been developed and were recently reviewed by Chen and colleagues [147]. Based on encouraging preclinical studies demonstrating selective cytotoxicity in models with aneuploidy and CIN compared to diploid and genomically stable counterparts, several KIF18A inhibitors have progressed to evaluation in clinical trials [147]. Notably, one inhibitor, GSC000190, appears to exhibit anti-cancer efficacy in tumours resistant to cisplatin and olaparib, highlighting the potential utility of targeting KIF18A in the context of therapy resistance, which is often associated with CIN [148]. Given these exciting discoveries, the oncology field ardently awaits the results of ongoing clinical trials.
KIF18A’s kinesin-8 family member, KIF18B, is also an N-type plus-end-directed microtubule depolymerase that localizes to the plus-ends of microtubules to regulate astral microtubule length and chromosome alignment [149]. It complexes with MCAK (KIF2C) to control microtubule depolymerization [149]. KIF18B has been identified as a differentially expressed, upregulated gene in both LUAD and LUSC tumours in the TCGA database based on transcriptomic analyses [28,41,44,150]. Additionally, it was identified as upregulated in LUAD tumours compared to normal tissues using IHC, Western blotting, and qPCR approaches [150,151,152]. High KIF18B RNA levels were reported to correlate with worse overall survival, but only in LUAD patients [28,41,44,150,151]. Additional correlative analyses in LUAD models revealed that cell lines with higher basal proliferation rates had higher expression of KIF18B compared to those with lower proliferation rates [44]. In the same study, gene set enrichment analyses of TCGA LUAD tumours with high versus low KIF18B mRNA expression suggested KIF18B-high tumours were enriched for gene expression signatures indicative of mTORC1 signalling, G2/M checkpoint, MYC, E2F, and mitotic spindle regulation pathways, consistent with KIF18B’s roles in mitosis and its correlation with a proliferative capacity [44]. Functionally, the knockdown of KIF18B reduced proliferation, migration, and invasion of A549 cells, which was associated with reductions in the expression of Rac1-GTP, phospho-AKT, and phospho-mTOR [151], suggesting it may also be therapeutically relevant in lung cancer.
4.7. Kinesin-10 Family: KIF22 (KID)
KIF22, or kinesin-like DNA-binding protein (KID), is a monomeric chromokinesin capable of binding to microtubules through both its motor and tail domains and to DNA via its tail domain [19]. It localizes to spindle microtubules and chromosomes to regulate chromosome congression and alignment by generating polar ejection forces. As such, KID loss of function leads to mitotic defects, including chromosome misalignment, shortened spindles, and anaphase delay [19]. KID also regulates chromosome compaction during anaphase, and its depletion increases the prevalence of lagging chromosomes and multinucleated cells [153]. KIF22 was identified as a significantly upregulated gene in LUAD tumours at the RNA level but was not associated with patient prognosis [28].
Pike et al. explored the functional relevance of KID in lung cancer. In A549 cells, KID depletion reduced EGF-dependent proliferation, which was associated with a reduction in ERK phosphorylation, suggesting KID’s involvement in regulating EGFR signalling [154]. EGF was also shown to stimulate the binding of KID to the coxsackie and adenovirus receptor (CAR), implicating CAR in regulating EGFR signalling through its interaction with KID. Moreover, KID was identified to influence cytoskeletal dynamics in an EGF-dependent manner by stabilizing microtubules, which contributed to the retention of EGFR at the plasma membrane [154]. Therefore, although further studies are required to understand the generalizability of these findings, KID inhibition could potentially suppress lung cancer growth by disrupting its contributions to maintaining genome stability and EGFR signalling.
4.8. Kinesin-12 Family: KIF15
KIF15 is a homotetrameric kinesin involved in spindle elongation and maintenance of bipolarity [19,155]. It was reported to regulate the length of K-fibres and to form a complex with TPX2 that crosslinks and slides microtubules apart to separate centrosomes during spindle assembly [155]. Interestingly, KIF15 was identified as non-essential for bipolar spindle assembly when Eg5 is active but essential upon loss of Eg5 activity [155], indicating that KIF15 compensates for Eg5 when necessary. KIF15 RNA and protein expression were found to be higher in LUAD and LUSC tumour tissues compared to normal tissues based on mining gene expression databases, IHC, and immunoblotting [28,155,156,157,158]. High RNA and protein expression of KIF15 correlated with worse overall survival of LUAD but not LUSC patients [28,41,156,157], and KIF15 was identified as a component of metabolism-, microtubule-, and mitotic spindle-associated gene expression signatures that correlated with poor survival in LUAD [46,47,159]. High KIF15 protein levels were also found to correlate with advanced tumour stage [157].
Multiple studies have indicated KIF15’s functional relevance in lung cancer. Two independent groups found that KIF15 depletion inhibited proliferation, migration, and invasion of lung cancer cells and that these anti-cancer effects were only seen in LUAD (A549, H1299) and large-cell carcinoma (H460) models with no effect in LUSC (H226) cells [156,157]. KIF15 knockdown in NSCLC cell lines also increased apoptosis and induced G1 arrest, which was associated with reduced Cyclin D1 and increased p27 expression [156,157]. Furthermore, the growth of H460 lung tumour xenografts expressing KIF15-targeted shRNA was significantly impaired in mice, and stunted tumour growth was associated with the downregulation of components of the Raf-Mek-ERK signalling pathway, including phospho-ERK, ATF2, phospho-MEK, and phospho-c-Raf [156]. Taken together, these studies suggest KIF15 has therapeutic potential in lung cancer, particularly LUAD, due to its positive regulation of oncogenic signalling pathways. Given its functions in mitosis, KIF15 inhibition is likely to influence genomic stability; however, the effects of its inhibition on CIN and mitotic phenotypes remain to be determined in lung cancer cells. The ability of KIF15 to compensate for loss of Eg5 function led Rath and Kozielski to hypothesize that KIF15 can confer resistance to Eg5 inhibitors, which was later demonstrated in multiple studies [160,161]. Accordingly, KIF15 inhibitors have demonstrated synergy with Eg5 inhibition [162,163], rationalizing further development of KIF15-targeted agents and studies to characterize this therapeutic combination. While multiple KIF15-targeting tool compounds have been reported (e.g., GW108X, Munesib-1, Fift-IN, dihydropyrazole and dihydropyrrole derivatives), none have progressed beyond preclinical stages of development [163,164,165].
4.9. Kinesin-13 Family: KIF2A, KIF2B, KIF2C, and KIF24
KIF2A regulates microtubule dynamics to influence spindle assembly and chromosome alignment through its microtubule depolymerizing activity [19]. It localizes to spindle microtubules and spindle poles during mitosis. Cells lacking KIF2A form monopolar spindles, indicating its importance for bipolar spindle assembly [19]. KIF2A is overexpressed in NSCLC cell lines compared to non-malignant lines [166,167] and protein expression is upregulated in NSCLC tumours relative to non-malignant tissues [168,169]. KIF2A expression is also clinically relevant, being associated with multiple prognostic factors in lung cancer patients. For instance, high KIF2A expression was correlated with tumour stage and lymph node metastasis in a cohort of LUAD tumours [168]. A larger study of NSCLC patients found that high KIF2A protein expression was associated with increased pathological tumour grade and size, the presence of lymph node metastasis, and worse disease-free and overall survival [169].
Functional studies in lung cancer models have implicated KIF2A in regulating multiple oncogenic phenotypes. KIF2A knockdown decreased cell viability, increased apoptosis, decreased invasive properties, and reduced expression of EMT markers in A549, H1975, and H1299 LUAD cells [166,170]. Concordantly, KIF2A overexpression promoted cell proliferation, migration, and invasion in NSCLC cells in vitro [167,169]. KIF2A knockdown was also reported to reduce stem-like features of LUAD cell lines, including sphere-forming ability and the proportion of CD133+ cells [166,167]. KIF2A was also shown to influence lung cancer response to chemotherapy, as knockdown sensitized A549, H1975, and H1299 cells to cisplatin and paclitaxel [166,169], while overexpression conferred resistance to cisplatin [167]. Moreover, KIF2A influences the Notch, Wnt, PI3K-Akt, and VEGF signalling pathways [166]; its knockdown was found to reduce the expression of PI3K, phospho-AKT, and VEGF [167], and its overexpression increased Wnt1, GSK-3, and β-catenin levels [170]. Expression of KIF2A in NSCLC cells can be regulated by multiple non-coding RNA axes, including circ_0010235-mediated regulation of miR-338-3p and circ_SATB-mediated regulation of miR-760, as well as miR-451a, as each of these microRNAs targets KIF2A to reduce its expression [171,172,173]. Recruitment of KIF2A to spindle microtubules and its depolymerase activity is further regulated in a cell-cycle-specific manner by multiple kinases, including PLK1, AURKA, and AURKB [19].
KIF2C, also known as mitotic centromere-associated kinesin (MCAK), also depolymerizes microtubules to regulate kinetochore-microtubule attachments as well as chromosome congression and alignment [19]. Depleting MCAK in non-malignant cells induces chromosome missegregation and CIN, demonstrating its role in maintaining genomic integrity [19]. KIF2C has also been implicated in DNA repair, cellular senescence, and immune modulation, although the mechanisms underlying these putative functions are not well understood [174]. Multiple independent studies of NSCLC have demonstrated that lung tumours express higher levels of KIF2C mRNA than normal lung tissues, and that high expression is associated with a worse prognosis of NSCLC patients [28,41,175,176,177]. Specifically, high KIF2C expression correlated with greater tumour stage, lymph node metastasis, disease recurrence, and worse overall survival [174,176,177,178]. Moreover, KIF2C was a component gene of multiple mRNA signatures associated with poor prognosis in lung cancer patients [23,179,180].
In cultured H1299 and A549 LUAD and H226 LUSC models, MCAK silencing inhibited cell proliferation, promoted apoptosis, and impaired migration, invasion, and EMT [175,176,177]. In vivo, knockdown of MCAK reduced the growth of LA-4 tumour xenografts [177], and an independent group found that the reduction in lung tumour size associated with MCAK knockdown was associated with increased E-cadherin and suppressed vimentin expression [177], implicating MCAK in EMT. MCAK has also been shown to promote resistance to microtubule-disrupting and DNA-damaging chemotherapies in lung and other cancer models [174]. At the cellular level, KIF2C overexpression resulted in increased expression of β-catenin, phosphorylated GSK-3β, and phosphorylated AKT, suggesting MCAK influences Wnt/β-catenin signalling [175]. Its own expression can be regulated by miR-186-3p, which is often downregulated in lung cancer cells. Transfection with miR-186-3p phenocopied MCAK knockdown in LUAD cells, slowing proliferation, increasing apoptosis, and reducing expression of several Wnt/β-catenin pathway components [175]. Given that it supports multiple malignant phenotypes and chromosome segregation, MCAK is an attractive therapeutic target that could be inhibited to potentiate CIN to lethal levels. In support of this idea, MCAK inhibition has been shown to induce aneuploidy and reduce the viability of genomically unstable cancers like triple-negative breast cancer [181], warranting additional research to develop MCAK-targeted drugs.
The other two members of the kinesin-13 family, KIF2B and KIF24, which are also microtubule depolymerases, have been less studied in the context of lung cancer. Laucius et al. found that KIF2B influences CIN by reducing chromosome segregation defects in a genetically engineered mouse model of Kras-driven lung cancer [182]. This finding is consistent with the established role of KIF2B in maintaining genomic integrity by regulating and correcting kinetochore-microtubule attachments [19,183]. KIF24’s involvement in mediating microtubule dynamics has been shown to regulate cilia formation [184]. Mutations in KIF24 are associated with NSCLC metastasis [185], but functional studies are required to confirm whether this can be explained by its regulation of cilia assembly.
4.10. Kinesin-14A Family: KIFC1
KIFC1, or HSET, the lone kinesin-14A member, is a minus-end-directed homodimeric kinesin whose functions include spindle formation and pole focusing, as well as vesicle, organelle, and double-stranded DNA transport [19,186]. KIFC1 is highly expressed in many cancers at both the RNA and protein levels, including breast, ovarian, bladder, prostate, and kidney tumours, among others [186,187,188]. In lung cancer, both RNA and protein expression of KIFC1 is elevated in LUAD, LUSC, and SCLC tumours compared to healthy lung tissues, which may be attributable to loss of regulatory DNA methylation (i.e., hypomethylation) at the KIFC1 genomic locus [41,189,190,191]. Elevated KIFC1 expression in NSCLC is correlated with advanced TNM stage, lymph node metastasis, smoking history, and poor patient prognosis, specifically worse progression-free and overall survival [189,190,191,192]. Moreover, overexpression of KIFC1 was found to be enriched in tumours with TP53 mutations [193]. KIFC1 was identified as a component gene in an expression signature predictive of NSCLC metastasis to the brain [194], and its expression was elevated in the serum of lung cancer patients compared to healthy individuals [190], indicating its potential utility as a prognostic biomarker.
Functionally, KIFC1 depletion is associated with various anti-cancer effects, including suppressed proliferation, colony formation, cell growth, invasion, and migration, while its overexpression enhanced malignant phenotypes in H1299, PC9, A549, and SPC-A1 lung cancer cells [189,191,192]. Notably, it has been described as non-essential in non-malignant cells, providing a therapeutic window for targeting KIFC1 [195,196]. Silencing of KIFC1 has also been shown to impair EMT, evident by expression changes in E-cadherin, N-cadherin, and Vimentin in LUAD cells, by regulating the TGF-β/SMAD signalling pathway [192,197]. Gene set enrichment analyses comparing TCGA lung tumours with high and low KIFC1 mRNA expression suggested KIFC1 involvement in the cell cycle, DNA replication, and TP53 signalling pathways [193].
Using a CRISPR/Cas9 screen, we recently discovered KIFC1 as a vulnerability specifically in LUAD cells with centrosome amplification (CA), a malignancy-associated phenotype known to drive CIN [189,191,192]. The presence of extra centrosomes during mitosis leads to multipolar mitotic spindles that are prone to causing major chromosome segregation errors, lethal aneuploidies, and mitotic catastrophes if left unmanaged [22]. Our assessment of KIFC1 dependency across 6 LUAD models confirmed that KIFC1 depletion only reduced the viability of cell lines with high basal levels of CA and KIFC1 expression (H1299, H1975, H2030), nominating these features as putative biomarkers for predicting sensitivity to KIFC1 inhibition [189,191,192]. Mechanistically, loss of KIFC1 function in vulnerable models was associated with an inability to effectively group extra centrosomes into mitotic spindles with a bipolar orientation, attributable to KIFC1’s role in centrosome clustering and pole focusing [189,191,192].
Due to its promotion of several oncogenic phenotypes and the dependence of cancer cells on KIFC1 to avoid the deleterious effects of CA-associated multipolar mitotic spindles, KIFC1 is a promising target in cancers in which CA is prevalent [22,191]. Several compounds with the ability to inhibit KIFC1 have been described and were recently summarized and computationally evaluated in a comprehensive review [188]. Briefly, these compounds aim to antagonize the centrosome clustering function of KIFC1 to induce severe chromosome segregation errors that lead to intolerable CIN and mitotic catastrophe. However, it is important to note that they may also exert therapeutic effects by blocking KIFC1-mediated intracellular transport of macromolecules and organelles. Despite their utility as tool compounds, none of these agents have progressed to clinical studies due to a lack of potency, selectivity for KIFC1, poor pharmacological profiles, or a combination of these limitations. Thus, continued efforts are required to develop effective strategies for targeting KIFC1, requiring advances in medicinal chemistry and improved knowledge of KIFC1 structure to exploit its therapeutic potential [188].
4.11. Kinesin-14B Family: KIFC3
KIFC3 is a tetrameric kinesin that contributes to multiple mitotic processes, including centrosome cohesion, spindle assembly, and cytokinesis, as well as the transport of intracellular cargo [20,198,199]. It was recently reported to localize to the cytoplasm and nucleus and was identified as overexpressed and positively correlated with tumour size, stage, lymph node metastasis, and worse overall survival and poor prognosis in NSCLC patients [200]. Functionally, KIFC3 knockdown reduced proliferation, colony formation, migration, invasion, and expression of phospho-AKT, Cyclin D1, CDK4, CDK6, RhoA, and RhoC in A549 and H1299 cells, while its overexpression enhanced these phenotypes [200]. Overexpression of KIFC3 also enhanced subcutaneous tumour growth and metastasis to the lungs in xenograft models of A549 and H1299 [200,201]. As such, KIFC3 appears to promote multiple phenotypes associated with lung cancer growth and progression.
5. Non-Mitotic Kinesins Implicated in Lung Cancer
A number of non-mitotic kinesins have been described as prognostically or functionally relevant in lung cancer, with putative roles in regulating malignant phenotypes and oncogenic signalling pathways. Although the anti-cancer effects of targeting these kinesins are likely driven by cargo transport and other cellular functions rather than by influencing CIN, here we briefly describe evidence of their significance in lung cancer.
The functions of KIF26B (kinesin-11 family member) include regulation of cell adhesion, migration, and polarity [202,203]. A study in NSCLC found that KIF26B expression was higher in tumours compared to normal tissues and was associated with poor overall survival of patients [204]. KIF26B silencing in H1299 and H520 cell lines slowed proliferation, impaired invasion, induced cell cycle arrest in vitro, and suppressed the growth of H520 tumour xenografts in mice [204]. Knockdown also enhanced the sensitivity of lung cancer cells to cisplatin chemotherapy [204]. Furthermore, KIF26B silencing led to the downregulation of vimentin and N-cadherin, upregulation of E-cadherin, and diminished Wnt/β-catenin pathway activity, implicating KIF26B as a positive regulator of EMT and Wnt/β-catenin signalling in lung cancer cells [204]. These findings are consistent with an oncogenic role for KIF26B in NSCLC, but this remains to be corroborated in additional models.
KIF7 is a member of the kinesin-4 family and a microtubule-associated protein involved in Hedgehog signalling and cilia formation through its regulation of microtubule dynamics [205,206]. Hu et al. found that the HPV-associated oncoprotein, E6, reduced the expression of KIF7, which was associated with the downregulation of the tumour suppressor LKB1 in H1299 cells [207]. In murine respiratory epithelial cells, KIF7 was shown to negatively regulate microtubule stability, S phase entry, mitotic exit, and cell proliferation [208]. KIF7 loss of function was associated with an increase in Hedgehog effector proteins, GLI1 and GLI2 [208]. Unlike other kinesins, these findings are consistent with KIF7 behaving as a tumour suppressor in lung cancer. Its regulation of the cell cycle, particularly mitotic exit, suggests it may influence genomic integrity during mitosis, but functional experiments are required to support this idea.
KIF21B is another kinesin-4 family member that regulates cytoskeletal transport, neuronal morphology, and microtubule dynamics by inhibiting the growth of microtubules through its tail domain [209,210]. Sun et al. investigated the clinical and functional relevance of KIF21B in NSCLC [211]. IHC staining for KIF21B revealed it was localized primarily to the cytoplasm and was highly overexpressed in tumours compared to normal lung tissues. Moreover, high KIF21B expression was positively correlated with TNM stage and worse prognosis. In functional experiments, KIF21B silencing selectively reduced the proliferation, colony formation, migration, and invasion capacity of two LUAD cell lines (H1299 and A549) compared to non-malignant lung cells (BEAS-2B). Knockdown also increased apoptosis in lung cancer models, which was associated with reduced expression of BCL2, reduced phosphorylation of Akt, and decreased Cyclin D1 expression specifically in malignant models [211]. Finally, they found that KIF21B-targeted shRNA significantly impaired the growth of H1299 lung tumour xenografts in nude mice [211]. The finding that KIF21B promotes multiple oncogenic phenotypes suggests that KIF21B may be a promising therapeutic target that warrants further exploration in NSCLC.
The kinesin-1 family includes the neuron-specific KIF5A and ubiquitously expressed KIF5B, both of which are involved in the axonal transport of various cargoes including organelles, neurofilaments, proteins, and RNA [212]. KIF5A was identified as a putative serum biomarker that could predict lung cancer risk in patients with asbestosis and has also been associated with resistance to docetaxel in LUAD models, consistent with its role in mediating drug resistance in other cancer types [141,213]. KIF5B is a common translocation partner of the oncogenes ALK, RET, and MET in NSCLC tumours [214,215,216,217]. These oncogenic rearrangements drive constitutive activation of the receptor tyrosine kinases involved because the 5′ end of the fusions contains a portion of KIF5B’s coiled-coil domain, which enables dimerization that mimics activation normally induced by ligand binding to the wild-type receptor. As a result, fusions stimulate receptor activation and downstream tumour-promoting functions [218].
Members of the kinesin-3 family, KIF13A and KIF13B, are also involved in genetic rearrangements, with KIF13A-RET, KIF13A-ALK, and KIF13B-NRG1 being identified in LUAD or not otherwise specified lung cancer patients [219,220,221]. KIF13A regulates recycling endosomes and cargo transport, while KIF13B is involved in endocytosis [222,223]. KIF13B has also been shown to contribute to angiogenesis by transporting VEGFR2 along microtubules to the surface of endothelial cells to stimulate angiogenic signalling [224]. A peptide designed to block the interaction between VEGFR2 and KIF13B inhibited VEGF-stimulated endothelial cell migration and the growth of human H460 lung tumour xenografts in mice [224], offering a putative anti-angiogenic strategy.
The single nucleotide polymorphism rs1555195 in the gene encoding another kinesin-3 family member, KIF16B, was associated with reduced expression of KIF16B mRNA in lung tissues and cells in the blood, as well as poor survival of NSCLC patients [225]. KIF16B is involved in endosomal transport, as well as recycling and degradation of receptors like EGFR [226]. KIF16B expression was also implicated in the growth of pre-metastatic tumours in human models of the lung-to-brain metastasis, and low mRNA expression of KIF16B was associated with poor disease-free survival in LUAD patients [227], indicating its prognostic significance in lung cancer. Similarly, KIF1C, a kinesin-3 family member that regulates the transport of mRNA and Golgi-derived vesicles to the endoplasmic reticulum [228,229], was reported as a component of a 3-gene mRNA expression signature that could predict brain metastasis in NSCLC patients, which was validated at the protein level using IHC [194].
The kinesin-2 family member, KIF3A, plays an important role in the formation and function of cilia by regulating intraflagellar transport [54,230]. KIF3A forms a heterodimer with KIF3B that functions as a plus-end-directed motor for the anterograde transport of organelles [231]. KIF3A was also found to be required for Hedgehog signalling downstream of Patch1 [232]. In lung cancer, KIF3A was identified as a fusion partner of ALK in a LUAD patient [220]. Yang et al. proposed KIF3A as a tumour suppressor in NSCLC since its knockdown in H520 cells enhanced proliferation, invasion, and migration and inhibited apoptosis [233]. Protein expression of KIF3A was significantly lower in NSCLC compared to adjacent normal tissue and lower in LUAD than in LUSC tumours; low expression was correlated with higher TNM stage in LUAD and lymph node metastasis in LUSC, revealing KIF3A as a putative prognostic factor in NSCLC [233]. Another group confirmed KIF3A’s tumour suppressive function in the human LUSC model SW900 and additionally discovered that knockdown led to hyperactivation of Wnt/β-catenin signalling, which was associated with increased proliferation, spheroid formation, migration, and expression of stemness markers in vitro as well as enhanced growth of the human LUSC tumour model SW900 in mice [234]. Kim et al. further showed that KIF3A formed a complex with β-arrestin to inhibit Wnt/β-catenin signalling, and IHC revealed that low KIF3A expression was correlated with higher β-catenin expression and poor prognosis of NSCLC patients [234]. In contrast, KIF3A was required for the development of SCLC tumours in genetically engineered mouse models driven by conditional deletion of Trp53 and Rb1 in the airways [235], suggesting its effects on lung tumorigenesis are lineage-specific.
Another kinesin-2 member, KIF3C, which regulates microtubule dynamics, axon growth, and regeneration, was recently described as a putative oncogene in NSCLC [236,237]. Liu et al. found that KIF3C RNA and protein expression was significantly higher in NSCLC compared to non-malignant tissues and positively correlated with TNM stage, worse overall survival, shorter progression-free survival, and shorter post-progression survival of NSCLC patients [28,236]. In functional experiments, KIF3C knockdown suppressed proliferation, migration, and invasion of A549 cells, while overexpression enhanced these phenotypes and lung metastasis in vivo in the H226 model [236]. Furthermore, both miR-150-5p and miR-186-3p were identified as negative regulators of KIF3C expression [236]. These functions are consistent with an oncogenic role for KIF3C in lung cancer, which requires validation in additional models.
6. Outlook and Perspective
Mitotic kinesins regulate several processes critical for faithful chromosome segregation, bestowing them with integral roles in maintaining genomic integrity. As a result, their loss of function potentiates CIN and induces severe aneuploidies, highlighting the potential to harness kinesin inhibition to induce synthetic lethality in genomically unstable cancers like lung cancer. Notably, the therapeutic potential of some kinesins can also be attributed to their regulation of intracellular transport, oncogenic signalling pathways, and other hallmarks of cancer that drive tumour growth and progression.
Most studies investigating kinesin inhibition in lung cancer have used a limited number of common cell line or xenograft models, limiting our understanding of the generalizability of phenotypes induced and its effectiveness in heterogeneous patient-derived xenografts that more accurately model lung tumours in patients. Nevertheless, ample preclinical evidence exists to support the pursuit of several kinesins as targets in lung cancer. To date, the kinesin targets that have progressed the furthest along the translational therapeutic pathway are Eg5 and CENPE. Inhibitors of these mitotic kinesins were found to have acceptable toxicity profiles in clinical trials, with neutropenia and fatigue being the most common adverse events observed in patients [75,125]. Little neurotoxicity has been seen, which is a common, severe, and sometimes long-lasting side effect of anti-mitotic microtubule-binding agents like taxanes [75,125,238]. Unfortunately, however, besides the combinatorial activity of the Eg5 inhibitor, filanesib, with bortezomab and dexamethasone in multiple myeloma [75,239], kinesin inhibitors have yielded low or no objective response rates in advanced cancer patients. This indicates that the promising anti-tumour activity observed in preclinical models has not translated to patients in the clinic. Below, we discuss several challenges that must be addressed to drive progress in the development of effective kinesin-targeted therapies to unleash their potential to benefit lung and other cancer patients.
6.1. Potency and Selectivity of Kinesin Inhibitors
Established kinesin-targeted inhibitors and drugs demonstrate the feasibility of inhibiting kinesins pharmacologically. While clinical-grade inhibitors of Eg5, CENPE, and KIF18A have been reported to selectively and potently inhibit their targets [128,135,240,241], the specificity and/or potency of other kinesin inhibitors has been poor, suboptimal, or not robustly characterized. For instance, numerous KIFC1 inhibitors have been evaluated preclinically, but none have progressed to clinical studies [188]. The first KIFC1 inhibitor described, AZ82, induced non-specific cytotoxicity in yeast and exhibited poor bioavailability in rodents [188,242]. Our own work characterizing the sensitivity of LUAD models to KIFC1 loss of function revealed that AZ82 exhibited the strongest cytotoxic effects in a model with low KIFC1 expression that was insensitive to genetically mediated KIFC1 depletion [189]. Furthermore, micromolar concentrations of AZ82 and other KIFC1 inhibitors are required to achieve cytotoxic effects in cancer cells [188,189,243,244], raising the likelihood of undesirable off-target effects. These findings illustrate the need for novel inhibitors with improved selectivity and potency to harness the therapeutic potential of promising targets like KIFC1.
One obstacle limiting the development of KIFC1 inhibitors is the lack of structural knowledge needed to inform drug-binding sites and guide structure–function-driven design of compounds with improved pharmacokinetic and pharmacodynamic profiles [188]. To address this challenge, Sharma et al. recently performed an in silico drug docking analysis of available KIFC1 inhibitors to map putative drug binding sites in the KIFC1 protein, yielding structural insights that could be used to engineer novel KIFC1 inhibitors with superior pharmacologic activity [188]. Applying this conceptual framework to other kinesins that can be targeted with non-specific inhibitors (e.g., KIF2C, KIF20A, and KIF15) could also be useful. Alternatively, available compounds with documented abilities to inhibit kinesin functions could be used as scaffolds to engineer more potent and selective derivatives, and high-throughput screens could be used to discover completely new compounds. Given the success of KRAS G12C inhibitors that bind within the switch-II pocket to regulate KRAS’ affinity for GTP [245], compounds targeting allosteric sites that disrupt kinesin associations with ATP or microtubules may demonstrate enhanced kinesin-specific selectivity. Such an approach could mitigate the challenge of high homology between motor domains of different kinesins and other ATPases. We are optimistic that continued drug development efforts will guide the synthesis of small molecule inhibitors with enhanced potencies and improved kinesin specificity, ultimately resulting in clinically useful compounds.
6.2. Therapeutic Index
The ability of kinesin inhibitors to exert their cytotoxic effects preferentially in cancer cells will greatly influence their clinical utility. Ideal targets are kinesins whose expression is largely restricted to cancer cells and/or whose function is dispensable in non-malignant cells, as has been described for KIF20A, KIFC1, and KIF18A [98,142,145,195,196]. Observations that Eg5 and CENPE inhibitors have been well-tolerated in patients suggest that inhibiting these kinesins in normal cells is relatively innocuous [75,125]; although, on-treatment tumour biopsies would be required to confirm effective suppression of target activity. The therapeutic indices for targeting other kinesins are not as well established because their inhibition has not been robustly tested in non-malignant cells, and/or the lack of specific inhibitors has precluded their evaluation in animal models where off-tumour toxicities would be evident. As such, studies to better define the dependence of healthy, non-transformed cells on individual kinesins are imperative. At least in theory, targeting kinesins with the intent of exacerbating CIN in cancer cells with pre-existing instability widens the therapeutic window for kinesin-targeted therapy. Preclinical work demonstrating greater efficacy of CENPE, Eg5, and KIF18A inhibitors in aneuploid, tetraploid, and CIN-positive cancer models supports this concept [74,134,142,145]. This highlights the need for similar studies to determine whether other kinesins are vulnerabilities specific to cancer cells with CIN to better understand their therapeutic potential.
6.3. Effects of CIN in Non-Malignant Cells
It cannot be overlooked that treatments designed to potentiate CIN in cancer cells may induce low to moderate levels of instability in diploid non-malignant cells, causing cell death or rendering them susceptible to neoplastic transformation [10,12]. To address this potential adverse effect, thorough investigations of the effects of kinesin inhibition on the karyotypes of normal cells are needed. Although CENPE deletion in a model of liver regeneration in adult mice induced chromosome misalignment and CIN, livers recovered with normal hepatocyte differentiation and function [115], consistent with the idea that non-malignant cells can protect themselves from CIN and its potentially lethal consequences. Inducing genetic alterations in non-malignant cells as a byproduct of kinesin inhibition could also initiate cellular transformation, leading to secondary tumour formation [10,12]. While this unwanted effect is not trivial, it is a risk associated with many standard-of-care treatments that induce DNA damage, including chemo- and radiotherapy, especially in younger patients with longer life expectancies [246]. The development of alternative modes of delivering anti-cancer drugs and their cytotoxic effects preferentially to tumour tissues is an active area of research in oncology, exemplified by studies investigating the use of prodrugs, nanoparticles, antibody-drug conjugates, and other tumour-directed drug delivery strategies [247,248,249,250]. If effective, such approaches could be applied to kinesin-targeted therapy to increase its therapeutic index and minimize potential adverse effects caused by inducing CIN in normal cells.
6.4. Enhancing Efficacy with Combination Strategies
The results of trials evaluating Eg5 and CENPE inhibitors in cancer patients indicate that they are ineffective as monotherapies. Several theories to explain the failure of Eg5 and CENPE inhibitors to date have been proposed, including mitosis-specific expression of the targets combined with low mitotic indices in solid tumours, mitotic slippage to evade cell death, suboptimal pharmacodynamic and pharmacokinetic profiles, resistance conferred by mutations in the targeted kinesins or CIN-induced, and compensation by functionally redundant kinesins such as KIF15 in the case of Eg5 inhibition [113,251,252]. The most recent kinesin inhibitors to enter clinical trials are those targeting KIF18A, sovilnesib and VLS-1488. The field eagerly awaits their results, which will determine whether they also suffer from limited efficacy and tumour resistance. As noted above, Eg5 inhibition was effective when combined with bortezomab and dexamethasone in relapsed or refractory multiple myeloma patients [76]. This combination achieved an overall response rate of 39% with a median progression-free survival of 8.5 months. Therefore, combining kinesin-targeted therapy with other drugs may be useful for augmenting anti-tumour efficacy, and preclinical studies to identify rational combinations are warranted.
Several combinatorial approaches have been described. To overcome KIF15-mediated resistance to Eg5 inhibitors, combining Eg5 and KIF15 inhibitors is a logical solution. Implementing this strategy requires research to develop KIF15 inhibitors, which is currently underway [162,163]. Recently, Tucker and colleagues discovered that CENPE inhibitors could overcome resistance to microtubule-binding agents like taxanes by exacerbating CIN to induce breast cancer cell death, nominating this combination as a synergistic therapeutic approach [133]. As we have described, inhibition of many mitotic kinesins interferes with kinetochore attachments to spindle microtubules, producing unbound chromosomes that activate the SAC and induce mitotic arrest. Prolonged arrest normally induces cell death, but cancer cells can escape this via mitotic slippage, also known as checkpoint adaptation [239,253]. Thus, another rational strategy to enhance the efficacy of kinesin inhibitors is to combine them with drugs that inhibit anti-apoptotic proteins or mitotic exit [254,255,256]. Proof of concept for this approach has been shown in lung cancer models, where the Eg5 inhibitor SB743921 synergized with the BCL2L1 inhibitor WEHI-539 in two SCLC cell lines [60]. Similarly, combining filanesib with inhibition of the pro-survival factor, MCL-1, enhanced mitotic cell death in multiple myeloma cells, rationalizing studies to further explore this combination [257]. To complement these hypothesis-driven strategies, unbiased approaches could also be used to discover effective therapeutic combinations. These include high-throughput drug screens to identify compounds that synergize with kinesin inhibitors, as well as genetic screens to discover synthetic lethal interactions that could be targeted to sensitize cancer cells to kinesin-targeted therapy.
6.5. Biomarkers to Inform Patient Selection
As for any targeted therapy, the success of kinesin inhibitors will depend on the ability of clinicians to identify patients likely to benefit from the drugs. Therefore, studies to identify response-predictive biomarkers should also be prioritized when developing kinesin-targeted therapies. First and foremost, the expression of the target should be confirmed, requiring the establishment of robust IHC assays for measuring kinesins in tumour tissues. Second, identifying patients whose tumours exhibit CIN is essential given the therapeutic premise for kinesin-targeted therapy. Strictly speaking, classifying tumours as CIN-positive requires the detection of dynamic changes in the genome, which is not feasible with tumour biopsies that capture its state at one moment in time [8]. The expanding clinical use of liquid biopsies, which could be used to characterize DNA in circulating tumour cells, may be helpful in this regard. However, quantifying copy number alterations and aneuploidies as phenotypic indicators of CIN in diagnostic specimens is currently the most tractable approach. For this purpose, multiplexed fluorescence in situ hybridization (M-FISH) techniques can be used to detect structural and numerical chromosomal abnormalities or centromere copy numbers in tumour cells from fixed specimens [258]. Gene expression signatures of CIN, such as CIN70 [259], and next-generation sequencing assays, particularly single-cell profiling strategies that can quantify the molecular heterogeneity produced by CIN, have also been used to gauge instability [258,260]. Additional proxies for CIN include observations of abnormal chromosome segregation in mitotic tumour cells, changes in the nuclear area, and the presence of micronuclei [12]. Thus, several strategies for measuring surrogates of CIN in clinical tumour tissues exist and could be integrated into the clinic given their compatibility with different sample inputs (i.e., tissue, RNA, DNA).
Encouragingly, some CIN-related biomarkers associated with response to kinesin inhibitors have already been identified. For example, tetraploidy was associated with enhanced efficacy of Eg5 inhibitors in cancer models [74], and KIF18A inhibitors were more effective in models with whole genome duplication (WGD), aneuploidy, TP53 mutations, and CIN [142,143,144,145]. Notably, genomic analyses of TCGA tumours indicate that a high proportion of LUAD (59%) and LUSC (64%) exhibit WGD and signatures of CIN, implicating their susceptibility to KIF18A- and Eg5-targeted drugs [13,14,15]. Additionally, our own work and the work of others have nominated centrosome amplification, a malignancy-associated phenotype with a well-established role in promoting CIN, as a putative biomarker of sensitivity to KIFC1 inhibition [189,196]. Lastly, the mitotic index of tumours may be relevant for predicting response to kinesin-targeted therapy [251,261]. As discussed above, tumours in patients often have a lower proportion of dividing cells than in preclinical cancer models. This “proliferation rate paradox” has been implicated in resistance to several anti-mitotic drugs, including those targeting mitotic kinases and kinesins, which simply may not be as effective in slow-growing cancers [251,252,261]. This suggests that biomarkers indicative of mitotic rates, such as enumeration of mitotic cells or Ki67 staining in tumour tissues, may be needed to assign kinesin-targeted therapy [262].
7. Conclusions
Several members of the kinesin superfamily have emerged as promising targets in lung and other tumours, providing an opportunity to develop a new class of targeted therapy for lung cancer patients. Preclinical evidence supports the concept that mitotic kinesins can be inhibited to potentiate CIN beyond a tolerable threshold in genomically unstable tumours, tipping the delicate balance of CIN and survival towards lethality (Figure 2). However, although targeting kinesins to exploit CIN is an attractive strategy, further work is needed to realize its therapeutic potential. Recently initiated clinical trials evaluating KIF18A inhibitors have reinvigorated enthusiasm for targeting kinesins in the oncology field. Insights gleaned from their results, combined with knowledge gained from unsuccessful Eg5 and CENPE inhibitors, can be leveraged to inform improved strategies for designing effective kinesin-targeted therapies. Such strategies are likely to involve novel inhibitors and alternative modes of administering them, as well as patient selection based on CIN biomarkers to enhance their therapeutic index and anti-tumour efficacy.
Author Contributions
Conceptualization, C.Z. and K.L.T.; writing—original draft preparation, C.Z. and K.L.T.; writing—review and editing, C.Z., B.Z.W. and K.L.T.; visualization, C.Z.; supervision, K.L.T.; funding acquisition, K.L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds from Unity Health Toronto, the Canada Research Chairs Program (Grant #233198), and the Canadian Institutes for Health Research (CIHR, Grant #439607). C.Z. is supported by scholarship funds from CIHR.
Acknowledgments
All figures were generated using BioRender (https://www.biorender.com/).
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, S.; Lam, S.; Lockwood, W.W. New Insights into the Biology and Development of Lung Cancer in Never Smokers-Implications for Early Detection and Treatment. J. Transl. Med. 2023, 21, 585. [Google Scholar] [CrossRef]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-Small-Cell Lung Cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef]
- Politi, K.; Herbst, R.S. Lung Cancer in the Era of Precision Medicine. Clin. Cancer Res. 2015, 21, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non–small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Pikor, L.; Thu, K.; Vucic, E.; Lam, W. The Detection and Implication of Genome Instability in Cancer. Cancer Metastasis Rev. 2013, 32, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Sansregret, L.; Vanhaesebroeck, B.; Swanton, C. Determinants and Clinical Implications of Chromosomal Instability in Cancer. Nat. Rev. Clin. Oncol. 2018, 15, 139–150. [Google Scholar] [CrossRef]
- Cunningham, C.E.; MacAuley, M.J.; Yadav, G.; Vizeacoumar, F.S.; Freywald, A.; Vizeacoumar, F.J. Targeting the CINful Genome: Strategies to Overcome Tumor Heterogeneity. Prog. Biophys. Mol. Biol. 2019, 147, 77–91. [Google Scholar] [CrossRef]
- Hosea, R.; Hillary, S.; Naqvi, S.; Wu, S.; Kasim, V. The Two Sides of Chromosomal Instability: Drivers and Brakes in Cancer. Signal Transduct. Target. Ther. 2024, 9, 75. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.L.; Jeusset, L.M.-P.; Lepage, C.C.; McManus, K.J. Evolving Therapeutic Strategies to Exploit Chromosome Instability in Cancer. Cancers 2017, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Drews, R.M.; Hernando, B.; Tarabichi, M.; Haase, K.; Lesluyes, T.; Smith, P.S.; Morrill Gavarró, L.; Couturier, D.-L.; Liu, L.; Schneider, M.; et al. A Pan-Cancer Compendium of Chromosomal Instability. Nature 2022, 606, 976–983. [Google Scholar] [CrossRef]
- Zack, T.I.; Schumacher, S.E.; Carter, S.L.; Cherniack, A.D.; Saksena, G.; Tabak, B.; Lawrence, M.S.; Zhsng, C.-Z.; Wala, J.; Mermel, C.H.; et al. Pan-Cancer Patterns of Somatic Copy Number Alteration. Nat. Genet. 2013, 45, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.D.; Abbasi, A.; Islam, S.M.A.; Bowes, A.L.; Khandekar, A.; Haase, K.; Hames-Fathi, S.; Ajayi, D.; Verfaillie, A.; Dhami, P.; et al. Signatures of Copy Number Alterations in Human Cancer. Nature 2022, 606, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Andor, N.; Maley, C.C.; Ji, H.P. Genomic Instability in Cancer: Teetering on the Limit of Tolerance. Cancer Res. 2017, 77, 2179–2185. [Google Scholar] [CrossRef]
- Thu, K.L.; Soria-Bretones, I.; Mak, T.W.; Cescon, D.W. Targeting the Cell Cycle in Breast Cancer: Towards the next Phase. Cell Cycle 2018, 17, 1871–1885. [Google Scholar] [CrossRef] [PubMed]
- Slade, D. PARP and PARG Inhibitors in Cancer Treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef]
- Rath, O.; Kozielski, F. Kinesins and Cancer. Nat. Rev. Cancer 2012, 12, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Noda, Y.; Tanaka, Y.; Niwa, S. Kinesin Superfamily Motor Proteins and Intracellular Transport. Nat. Rev. Mol. Cell Biol. 2009, 10, 682–696. [Google Scholar] [CrossRef]
- Lucanus, A.J.; Yip, G.W. Kinesin Superfamily: Roles in Breast Cancer, Patient Prognosis and Therapeutics. Oncogene 2018, 37, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Sabat-Pośpiech, D.; Fabian-Kolpanowicz, K.; Prior, I.A.; Coulson, J.M.; Fielding, A.B. Targeting Centrosome Amplification, an Achilles’ Heel of Cancer. Biochem. Soc. Trans. 2019, 47, 1209–1222. [Google Scholar] [CrossRef]
- Ling, B.; Liao, X.; Huang, Y.; Liang, L.; Jiang, Y.; Pang, Y.; Qi, G. Identification of Prognostic Markers of Lung Cancer through Bioinformatics Analysis and in Vitro Experiments. Int. J. Oncol. 2020, 56, 193–205. [Google Scholar] [CrossRef]
- Feng, Z.; Li, B.; Chen, Q.; Zhang, H.; Guo, Z.; Qin, J. Identification and Validation of a GPX4-Related Immune Prognostic Signature for Lung Adenocarcinoma. J. Oncol. 2022, 2022, 9054983. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Xu, H.; Liu, S.; Yang, B.; Li, L.; Zhao, H.; Jiang, C. Integrated Bioinformatics Analysis Identifies a New Stemness Index-Related Survival Model for Prognostic Prediction in Lung Adenocarcinoma. Front. Genet. 2022, 13, 860268. [Google Scholar] [CrossRef] [PubMed]
- Corson, T.W.; Huang, A.; Tsao, M.-S.; Gallie, B.L. KIF14 Is a Candidate Oncogene in the 1q Minimal Region of Genomic Gain in Multiple Cancers. Oncogene 2005, 24, 4741–4753. [Google Scholar] [CrossRef] [PubMed]
- Corson, T.W.; Zhu, C.Q.; Lau, S.K.; Shepherd, F.A.; Tsao, M.-S.; Gallie, B.L. KIF14 Messenger RNA Expression Is Independently Prognostic for Outcome in Lung Cancer. Clin. Cancer Res. 2007, 13, 3229–3234. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, G.; Wang, X.; Liao, X.; Huang, R.; Huang, C.; Huang, P.; Zhang, J.; Wang, P. Genome-wide Investigation of the Clinical Significance and Prospective Molecular Mechanisms of Kinesin Family Member Genes in Patients with Lung Adenocarcinoma. Oncol. Rep. 2019, 42, 1017–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, F.; Tang, P.; Zhang, L.; Gan, Q.; Li, Y. Noncoding RNAs-Mediated Overexpression of KIF14 Is Associated with Tumor Immune Infiltration and Unfavorable Prognosis in Lung Adenocarcinoma. Aging 2022, 14, 8013–8031. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.-F.; Hong, T.-M.; Hsu, Y.-C.; Chen, H.-Y.; Chang, Y.-L.; Wu, C.-T.; Chang, G.-C.; Jou, Y.-S.; Pan, S.-H.; Yang, P.-C. The Motor Protein KIF14 Inhibits Tumor Growth and Cancer Metastasis in Lung Adenocarcinoma. PLoS ONE 2013, 8, e61664. [Google Scholar] [CrossRef]
- Li, Y.; Hong, X.; Zhai, J.; Liu, Y.; Li, R.; Wang, X.; Zhang, Y.; Lv, Q. Novel Circular RNA Circ-0002727 Regulates miR-144-3p/KIF14 Pathway to Promote Lung Adenocarcinoma Progression. Front. Cell Dev. Biol. 2023, 11, 1249174. [Google Scholar] [CrossRef]
- Lao, Y.; Li, T.; Xie, X.; Chen, K.; Li, M.; Huang, L. MiR-195-3p Is a Novel Prognostic Biomarker Associated with Immune Infiltrates of Lung Adenocarcinoma. Int. J. Gen. Med. 2022, 15, 191–203. [Google Scholar] [CrossRef]
- Akinduro, O.O.; Suarez-Meade, P.; Roberts, M.; Tzeng, S.Y.; Sarabia-Estrada, R.; Schiapparelli, P.; Norton, E.S.; Gokaslan, Z.L.; Anastasiadis, P.Z.; Guerrero-Cázares, H.; et al. Verteporfin-Loaded Microparticles for Radiosensitization of Preclinical Lung and Breast Metastatic Spine Cancer. J. Neurosurg. Spine 2023, 38, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Dehan, E.; Ben-Dor, A.; Liao, W.; Lipson, D.; Frimer, H.; Rienstein, S.; Simansky, D.; Krupsky, M.; Yaron, P.; Friedman, E.; et al. Chromosomal Aberrations and Gene Expression Profiles in Non-Small Cell Lung Cancer. Lung Cancer 2007, 56, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Baudis, M.; Cleary, M.L. Progenetix.net: An Online Repository for Molecular Cytogenetic Aberration Data. Bioinformatics 2001, 17, 1228–1229. [Google Scholar] [CrossRef]
- Ma, J.; Gao, M.; Lu, Y.; Feng, X.; Zhang, J.; Lin, D.; Xiao, T.; Hu, Z.; Yuan, J.; Su, K.; et al. Gain of 1q25-32, 12q23-24.3, and 17q12-22 Facilitates Tumorigenesis and Progression of Human Squamous Cell Lung Cancer. J. Pathol. 2006, 210, 205–213. [Google Scholar] [CrossRef]
- Wu, G.; Zhou, L.; Khidr, L.; Guo, X.E.; Kim, W.; Lee, Y.M.; Krasieva, T.; Chen, P.-L. A Novel Role of the Chromokinesin Kif4A in DNA Damage Response. Cell Cycle 2008, 7, 2013–2020. [Google Scholar] [CrossRef]
- Barisic, M.; Aguiar, P.; Geley, S.; Maiato, H. Kinetochore Motors Drive Congression of Peripheral Polar Chromosomes by Overcoming Random Arm-Ejection Forces. Nat. Cell Biol. 2014, 16, 1249–1256. [Google Scholar] [CrossRef]
- Mazumdar, M.; Sundareshan, S.; Misteli, T. Human Chromokinesin KIF4A Functions in Chromosome Condensation and Segregation. J. Cell Biol. 2004, 166, 613–620. [Google Scholar] [CrossRef]
- Taniwaki, M.; Takano, A.; Ishikawa, N.; Yasui, W.; Inai, K.; Nishimura, H.; Tsuchiya, E.; Kohno, N.; Nakamura, Y.; Daigo, Y. Activation of KIF4A as a Prognostic Biomarker and Therapeutic Target for Lung Cancer. Clin. Cancer Res. 2007, 13, 6624–6631. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, G.; Singh, P.; Gurung, R.; Hakami, M.A.; Alkhorayef, N.; Alsaiari, A.A.; Alqahtani, L.S.; Hasan, M.R.; Rashid, S.; Kumar, A.; et al. Investigating the Role of Kinesin Family in Lung Adenocarcinoma via Integrated Bioinformatics Approach. Sci. Rep. 2023, 13, 9859. [Google Scholar] [CrossRef] [PubMed]
- Kahm, Y.-J.; Kim, I.-G.; Jung, U.; Lee, J.H.; Kim, R.-K. Impact of KIF4A on Cancer Stem Cells and EMT in Lung Cancer and Glioma. Cancers 2023, 15, 5523. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tang, W.; Li, H. Identification of KIF4A and Its Effect on the Progression of Lung Adenocarcinoma Based on the Bioinformatics Analysis. Biosci. Rep. 2021, 41, BSR20203973. [Google Scholar] [CrossRef] [PubMed]
- Singharajkomron, N.; Yodsurang, V.; Seephan, S.; Kungsukool, S.; Petchjorm, S.; Maneeganjanasing, N.; Promboon, W.; Dangwilailuck, W.; Pongrakhananon, V. Evaluating the Expression and Prognostic Value of Genes Encoding Microtubule-Associated Proteins in Lung Cancer. Int. J. Mol. Sci. 2022, 23, 14724. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wan, Y.; Deng, W.; Fei, F.; Wang, L.; Qi, F.; Zheng, Z. Promising Novel Biomarkers and Candidate Small-Molecule Drugs for Lung Adenocarcinoma: Evidence from Bioinformatics Analysis of High-Throughput Data. Open Med. 2022, 17, 96–112. [Google Scholar] [CrossRef]
- Zhang, L.; He, M.; Zhu, W.; Lv, X.; Zhao, Y.; Yan, Y.; Li, X.; Jiang, L.; Zhao, L.; Fan, Y.; et al. Identification of a Panel of Mitotic Spindle-Related Genes as a Signature Predicting Survival in Lung Adenocarcinoma. J. Cell. Physiol. 2020, 235, 4361–4375. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, C.; Wei, J.; Li, W.; Qin, Y.; Liu, G. Identification and Verification of Microtubule Associated Genes in Lung Adenocarcinoma. Sci. Rep. 2023, 13, 16134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lu, W.; Samadi, N. KIF4A Knockdown Inhibits Tumor Progression and Promotes Chemo-Sensitivity via Induction of P21 in Lung Cancer Cells. Chem. Biol. Drug Des. 2023, 101, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Fu, C.; Lin, Y.; Dong, Z.; Pan, L.; Chen, W.; Zhao, J. Overexpression of Chromosome Kinesin Protein KIF4A Enhance Cisplatin Resistance in A549/DDP Cells. Int. J. Clin. Exp. Pathol. 2016, 9, 3331–3339. [Google Scholar]
- Pan, L.-N.; Zhang, Y.; Zhu, C.-J.; Dong, Z.-X. Kinesin KIF4A Is Associated with Chemotherapeutic Drug Resistance by Regulating Intracellular Trafficking of Lung Resistance-Related Protein. J. Zhejiang Univ. Sci. B 2017, 18, 1046–1054. [Google Scholar] [CrossRef]
- Wan, Q.; Shen, Y.; Zhao, H.; Wang, B.; Zhao, L.; Zhang, Y.; Bu, X.; Wan, M.; Shen, C. Impaired DNA Double-Strand Breaks Repair by Kinesin Family Member 4A Inhibition Renders Human H1299 Non-Small-Cell Lung Cancer Cells Sensitive to Cisplatin. J. Cell. Physiol. 2019, 234, 10360–10371. [Google Scholar] [CrossRef]
- Yan, T.; Jiang, Q.; Ni, G.; Ma, H.; Meng, Y.; Kang, G.; Xu, M.; Peng, F.; Li, H.; Chen, X.; et al. WZ-3146 Acts as a Novel Small Molecule Inhibitor of KIF4A to Inhibit Glioma Progression by Inducing Apoptosis. Cancer Cell Int. 2024, 24, 221. [Google Scholar] [CrossRef]
- Gao, W.; Lu, J.; Yang, Z.; Li, E.; Cao, Y.; Xie, L. Mitotic Functions and Characters of KIF11 in Cancers. Biomolecules 2024, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Tanaka, Y. Kinesin Superfamily Proteins (KIFs): Various Functions and Their Relevance for Important Phenomena in Life and Diseases. Exp. Cell Res. 2015, 334, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, X.; Lyu, Z. Analysis of Two-Gene Signatures and Related Drugs in Small-Cell Lung Cancer by Bioinformatics. Open Med. 2023, 18, 20230806. [Google Scholar] [CrossRef]
- Ling, J.; Wang, Y.; Ma, L.; Zheng, Y.; Tang, H.; Meng, L.; Zhang, L. KIF11, a plus End-Directed Kinesin, as a Key Gene in Benzo(a)pyrene-Induced Non-Small Cell Lung Cancer. Environ. Toxicol. Pharmacol. 2022, 89, 103775. [Google Scholar] [CrossRef]
- Schneider, M.A.; Christopoulos, P.; Muley, T.; Warth, A.; Klingmueller, U.; Thomas, M.; Herth, F.J.F.; Dienemann, H.; Mueller, N.S.; Theis, F.; et al. AURKA, DLGAP5, TPX2, KIF11 and CKAP5: Five Specific Mitosis-Associated Genes Correlate with Poor Prognosis for Non-Small Cell Lung Cancer Patients. Int. J. Oncol. 2017, 50, 365–372. [Google Scholar] [CrossRef]
- Li, Z.; Yu, B.; Qi, F.; Li, F. KIF11 Serves as an Independent Prognostic Factor and Therapeutic Target for Patients with Lung Adenocarcinoma. Front. Oncol. 2021, 11, 670218. [Google Scholar] [CrossRef]
- Maiti, P.; Sharma, P.; Nand, M.; Bhatt, I.D.; Ramakrishnan, M.A.; Mathpal, S.; Joshi, T.; Pant, R.; Mahmud, S.; Simal-Gandara, J.; et al. Integrated Machine Learning and Chemoinformatics-Based Screening of Mycotic Compounds against Kinesin Spindle ProteinEg5 for Lung Cancer Therapy. Molecules 2022, 27, 1639. [Google Scholar] [CrossRef]
- Sakuma, Y.; Hirai, S.; Yamaguchi, M.; Idogawa, M. Small Cell Lung Carcinoma Cells Depend on KIF11 for Survival. Int. J. Mol. Sci. 2024, 25, 7230. [Google Scholar] [CrossRef]
- Balasundaram, A. In Silico Analysis Revealed the Potential circRNA-miRNA-mRNA Regulative Network of Non-Small Cell Lung Cancer (NSCLC). Comput. Biol. Med. 2023, 152, 106315. [Google Scholar] [CrossRef]
- Mengyan, X.; Kun, D.; Xinming, J.; Yutian, W.; Yongqian, S. Identification and Verification of Hub Genes Associated with the Progression of Non-Small Cell Lung Cancer by Integrated Analysis. Front. Pharmacol. 2022, 13, 997842. [Google Scholar] [CrossRef]
- Liu, J.; Tian, Y.; Yi, L.; Gao, Z.; Lou, M.; Yuan, K. High KIF11 Expression Is Associated with Poor Outcome of NSCLC. Tumori J. 2022, 108, 40–46. [Google Scholar] [CrossRef]
- Fu, F.; Zhang, Y.; Gao, Z.; Zhao, Y.; Wen, Z.; Han, H.; Li, Y.; Chen, H. Development and Validation of a Five-Gene Model to Predict Postoperative Brain Metastasis in Operable Lung Adenocarcinoma. Int. J. Cancer 2020, 147, 584–592. [Google Scholar] [CrossRef]
- Saijo, T.; Ishii, G.; Ochiai, A.; Yoh, K.; Goto, K.; Nagai, K.; Kato, H.; Nishiwaki, Y.; Saijo, N. Eg5 Expression Is Closely Correlated with the Response of Advanced Non-Small Cell Lung Cancer to Antimitotic Agents Combined with Platinum Chemotherapy. Lung Cancer 2006, 54, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.B.; Sun, H.; Robichaux, J.; Pfeifer, M.; McDermott, U.; Travers, J.; Diao, L.; Xi, Y.; Tong, P.; Shen, L.; et al. A YAP/FOXM1 Axis Mediates EMT-Associated EGFR Inhibitor Resistance and Increased Expression of Spindle Assembly Checkpoint Components. Sci. Transl. Med. 2020, 12, eaaz4589. [Google Scholar] [CrossRef]
- Dong, A.; Wang, Z.-W.; Ni, N.; Li, L.; Kong, X.-Y. Similarity and Difference of Pathogenesis among Lung Cancer Subtypes Suggested by Expression Profile Data. Pathol. Res. Pract. 2021, 220, 153365. [Google Scholar] [CrossRef]
- Teicher, B.A.; Silvers, T.; Selby, M.; Delosh, R.; Laudeman, J.; Ogle, C.; Reinhart, R.; Parchment, R.; Krushkal, J.; Sonkin, D.; et al. Small Cell Lung Carcinoma Cell Line Screen of Etoposide/carboplatin plus a Third Agent. Cancer Med. 2017, 6, 1952–1964. [Google Scholar] [CrossRef]
- Kato, T.; Lee, D.; Huang, H.; Cruz, W.; Ujiie, H.; Fujino, K.; Wada, H.; Patel, P.; Hu, H.-P.; Hirohashi, K.; et al. Personalized siRNA-Nanoparticle Systemic Therapy Using Metastatic Lymph Node Specimens Obtained with EBUS-TBNA in Lung Cancer. Mol. Cancer Res. 2018, 16, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Good, J.A.D.; Wang, F.; Rath, O.; Kaan, H.Y.K.; Talapatra, S.K.; Podgórski, D.; MacKay, S.P.; Kozielski, F. Optimized S-Trityl-L-Cysteine-Based Inhibitors of Kinesin Spindle Protein with Potent in Vivo Antitumor Activity in Lung Cancer Xenograft Models. J. Med. Chem. 2013, 56, 1878–1893. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fu, X.; Chang, M.; Wei, T.; Lin, R.; Tong, H.; Zhang, X.; Yuan, R.; Zhou, Z.; Huang, X.; et al. The Interaction of Calcium-Sensing Receptor with KIF11 Enhances Cisplatin Resistance in Lung Adenocarcinoma via BRCA1/cyclin B1 Pathway. Int. J. Biol. Sci. 2024, 20, 3892–3910. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Hirai, S.; Sumi, T.; Niki, T.; Yamaguchi, M. Dual Inhibition of KIF11 and BCL2L1 Induces Apoptosis in Lung Adenocarcinoma Cells. Biochem. Biophys. Res. Commun. 2023, 678, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Orth, J.D.; Tang, Y.; Shi, J.; Loy, C.T.; Amendt, C.; Wilm, C.; Zenke, F.T.; Mitchison, T.J. Quantitative Live Imaging of Cancer and Normal Cells Treated with Kinesin-5 Inhibitors Indicates Significant Differences in Phenotypic Responses and Cell Fate. Mol. Cancer Ther. 2008, 7, 3480–3489. [Google Scholar] [CrossRef] [PubMed]
- Rello-Varona, S.; Vitale, I.; Kepp, O.; Senovilla, L.; Jemaá, M.; Métivier, D.; Castedo, M.; Kroemer, G. Preferential Killing of Tetraploid Tumor Cells by Targeting the Mitotic Kinesin Eg5. Cell Cycle 2009, 8, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Saez, I.; Skoufias, D.A. Eg5 Targeting Agents: From New Anti-Mitotic Based Inhibitor Discovery to Cancer Therapy and Resistance. Biochem. Pharmacol. 2021, 184, 114364. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Kaufman, J.L.; Htut, M.; Agrawal, M.; Mazumder, A.; Cornell, R.F.; Zonder, J.A.; Fay, J.W.; Modiano, M.R.; Moshier, E.L.; et al. Filanesib plus Bortezomib and Dexamethasone in Relapsed/refractory t(11;14) and 1q21 Gain Multiple Myeloma. Cancer Med. 2022, 11, 358–370. [Google Scholar] [CrossRef]
- Hill, E.; Clarke, M.; Barr, F.A. The Rab6-Binding Kinesin, Rab6-KIFL, Is Required for Cytokinesis. EMBO J. 2000, 19, 5711–5719. [Google Scholar] [CrossRef]
- Jin, Z.; Peng, F.; Zhang, C.; Tao, S.; Xu, D.; Zhu, Z. Expression, Regulating Mechanism and Therapeutic Target of KIF20A in Multiple Cancer. Heliyon 2023, 9, e13195. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Tanaka, N. The Hedgehog Signaling Networks in Lung Cancer: The Mechanisms and Roles in Tumor Progression and Implications for Cancer Therapy. Biomed Res. Int. 2016, 2016, 7969286. [Google Scholar] [CrossRef]
- Shi, C.; Huang, D.; Lu, N.; Chen, D.; Zhang, M.; Yan, Y.; Deng, L.; Lu, Q.; Lu, H.; Luo, S. Aberrantly Activated Gli2-KIF20A Axis Is Crucial for Growth of Hepatocellular Carcinoma and Predicts Poor Prognosis. Oncotarget 2016, 7, 26206–26219. [Google Scholar] [CrossRef]
- Xiu, G.; Sui, X.; Wang, Y.; Zhang, Z. FOXM1 Regulates Radiosensitivity of Lung Cancer Cell Partly by Upregulating KIF20A. Eur. J. Pharmacol. 2018, 833, 79–85. [Google Scholar] [CrossRef]
- Mushtaq, A.; Singh, P.; Tabassum, G.; Mohammad, T.; Hassan, M.I.; Syed, M.A.; Dohare, R. Unravelling Hub Genes as Potential Therapeutic Targets in Lung Cancer Using Integrated Transcriptomic Meta-Analysis and in Silico Approach. J. Biomol. Struct. Dyn. 2023, 41, 9089–9102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Mu, H.-J.; Shi, H.B.; Bi, H.X.; Jiang, Y.F.; Liu, G.H.; Zheng, H.Y.; Liu, B. Identification of Therapeutic Targets and Mechanisms of Tumorigenesis in Non-Small Cell Lung Cancer Using Multiple-Microarray Analysis. Medicine 2020, 99, e22815. [Google Scholar] [CrossRef]
- Cheng, Y.; Hou, K.; Wang, Y.; Chen, Y.; Zheng, X.; Qi, J.; Yang, B.; Tang, S.; Han, X.; Shi, D.; et al. Identification of Prognostic Signature and Gliclazide as Candidate Drugs in Lung Adenocarcinoma. Front. Oncol. 2021, 11, 665276. [Google Scholar] [CrossRef]
- Dai, B.; Ren, L.-Q.; Han, X.-Y.; Liu, D.-J. Bioinformatics Analysis Reveals 6 Key Biomarkers Associated with Non-Small-Cell Lung Cancer. J. Int. Med. Res. 2020, 48, 300060519887637. [Google Scholar] [CrossRef]
- Xing, B.; Shi, L.; Bao, Z.; Liang, Y.; Liu, B.; Liu, R. Molecular Clustering Based on Gene Set Expression and Its Relationship with Prognosis in Patients with Lung Adenocarcinoma. J. Thorac. Dis. 2022, 14, 1638–1650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, L.-L.; Li, X.; Ni, J.; Chen, P.; Ma, R.; Wu, J.; Feng, J. Overexpression of KIF20A Confers Malignant Phenotype of Lung Adenocarcinoma by Promoting Cell Proliferation and Inhibiting Apoptosis. Cancer Med. 2018, 7, 4678–4689. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, W.; Xu, X.R.; Chen, S. Drug Resistance Related Genes in Lung Adenocarcinoma Predict Patient Prognosis and Influence the Tumor Microenvironment. Sci. Rep. 2023, 13, 9682. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Liu, X.; Wu, J.; Zhang, D.; Tian, J.; Wang, T.; Liu, S.; Meng, Z.; Wang, K.; Duan, X.; et al. Identification of Candidate Biomarkers Correlated with the Pathogenesis and Prognosis of Non-Small Cell Lung Cancer via Integrated Bioinformatics Analysis. Front. Genet. 2018, 9, 469. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, H.; Zhang, C.; Wang, L. An Evaluation of KIF20A as a Prognostic Factor and Therapeutic Target for Lung Adenocarcinoma Using Integrated Bioinformatics Analysis. Front. Bioeng. Biotechnol. 2022, 10, 993820. [Google Scholar] [CrossRef]
- He, H.; Liang, L.; Huang, J.; Jiang, S.; Liu, Y.; Sun, X.; Li, Y.; Cong, L.; Jiang, Y. KIF20A Is Associated with Clinical Prognosis and Synergistic Effect of Gemcitabine Combined with Ferroptosis Inducer in Lung Adenocarcinoma. Front. Pharmacol. 2022, 13, 1007429. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O. Advancing Cancer Therapy: The Role of KIF20A as a Target for Inhibitor Development and Immunotherapy. Cancers 2024, 16, 2958. [Google Scholar] [CrossRef] [PubMed]
- Tcherniuk, S.; Skoufias, D.A.; Labriere, C.; Rath, O.; Gueritte, F.; Guillou, C.; Kozielski, F. Relocation of Aurora B and Survivin from Centromeres to the Central Spindle Impaired by a Kinesin-Specific MKLP-2 Inhibitor. Angew. Chem. Int. Ed. Engl. 2010, 49, 8228–8231. [Google Scholar] [CrossRef]
- Schrock, M.S.; Scarberry, L.; Stromberg, B.R.; Sears, C.; Torres, A.E.; Tallman, D.; Krupinski, L.; Chakravarti, A.; Summers, M.K. MKLP2 Functions in Early Mitosis to Ensure Proper Chromosome Congression. J. Cell Sci. 2022, 135, jcs259560. [Google Scholar] [CrossRef] [PubMed]
- Labrière, C.; Talapatra, S.K.; Thoret, S.; Bougeret, C.; Kozielski, F.; Guillou, C. New MKLP-2 Inhibitors in the Paprotrain Series: Design, Synthesis and Biological Evaluations. Bioorg. Med. Chem. 2016, 24, 721–734. [Google Scholar] [CrossRef]
- Ferrero, H.; Corachán, A.; Quiñonero, A.; Bougeret, C.; Pouletty, P.; Pellicer, A.; Domínguez, F. Inhibition of KIF20A by BKS0349 Reduces Endometriotic Lesions in a Xenograft Mouse Model. Mol. Hum. Reprod. 2019, 25, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, H.; Wu, Q.; Zhong, S. Berberine Targets KIF20A and CCNE2 to Inhibit the Progression of Nonsmall Cell Lung Cancer via the PI3K/AKT Pathway. Drug Dev. Res. 2023, 84, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Hirata, S.; Irie, A.; Senju, S.; Ikuta, Y.; Yokomine, K.; Harao, M.; Inoue, M.; Tomita, Y.; Tsunoda, T.; et al. Identification of HLA-A2-Restricted CTL Epitopes of a Novel Tumour-Associated Antigen, KIF20A, Overexpressed in Pancreatic Cancer. Br. J. Cancer 2011, 104, 300–307. [Google Scholar] [CrossRef]
- Abaza, A.; Soleilhac, J.-M.; Westendorf, J.; Piel, M.; Crevel, I.; Roux, A.; Pirollet, F. M Phase Phosphoprotein 1 Is a Human plus-End-Directed Kinesin-Related Protein Required for Cytokinesis. J. Biol. Chem. 2003, 278, 27844–27852. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, L.; Han, X. KIF20B Correlates with LUAD Progression and Is an Independent Risk Factor. Crit. Rev. Eukaryot. Gene Expr. 2024, 34, 49–59. [Google Scholar] [CrossRef]
- Talapatra, S.K.; Rath, O.; Clayton, E.; Tomasi, S.; Kozielski, F. Depsidones from Lichens as Natural Product Inhibitors of M-Phase Phosphoprotein 1, a Human Kinesin Required for Cytokinesis. J. Nat. Prod. 2016, 79, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Brier, S.; Carletti, E.; DeBonis, S.; Hewat, E.; Lemaire, D.; Kozielski, F. The Marine Natural Product Adociasulfate-2 as a Tool to Identify the MT-Binding Region of Kinesins. Biochemistry 2006, 45, 15644–15653. [Google Scholar] [CrossRef] [PubMed]
- Vikberg, A.-L.; Vooder, T.; Lokk, K.; Annilo, T.; Golovleva, I. Mutation Analysis and Copy Number Alterations of KIF23 in Non-Small-Cell Lung Cancer Exhibiting KIF23 over-Expression. Onco Targets Ther. 2017, 10, 4969–4979. [Google Scholar] [CrossRef]
- Kato, T.; Wada, H.; Patel, P.; Hu, H.-P.; Lee, D.; Ujiie, H.; Hirohashi, K.; Nakajima, T.; Sato, M.; Kaji, M.; et al. Overexpression of KIF23 Predicts Clinical Outcome in Primary Lung Cancer Patients. Lung Cancer 2016, 92, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Li, H.; Zhang, F.; Lv, T.; Liu, H.; Song, Y. Expression of KIF23 and its prognostic role in non-small cell lung cancer: Analysis based on the data-mining of Oncomine. Zhongguo Fei Ai Za Zhi 2017, 20, 822–826. [Google Scholar] [PubMed]
- Yang, X.; Feng, Q.; Jing, J.; Yan, J.; Zeng, Z.; Zheng, H.; Cheng, X. Identification of Differentially Expressed Genes Associated with Lung Adenocarcinoma via Bioinformatics Analysis. Gen. Physiol. Biophys. 2021, 40, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Välk, K.; Vooder, T.; Kolde, R.; Reintam, M.-A.; Petzold, C.; Vilo, J.; Metspalu, A. Gene Expression Profiles of Non-Small Cell Lung Cancer: Survival Prediction and New Biomarkers. Oncology 2010, 79, 283–292. [Google Scholar] [CrossRef]
- Wu, X.; Sui, Z.; Zhang, H.; Wang, Y.; Yu, Z. Integrated Analysis of lncRNA-Mediated ceRNA Network in Lung Adenocarcinoma. Front. Oncol. 2020, 10, 554759. [Google Scholar] [CrossRef]
- Guo, L.; Li, H.; Li, W.; Tang, J. Construction and Investigation of a Combined Hypoxia and Stemness Index lncRNA-Associated ceRNA Regulatory Network in Lung Adenocarcinoma. BMC Med. Genom. 2020, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yu, C. Identification of Immune Infiltration and Prognostic Biomarkers in Small Cell Lung Cancer Based on Bioinformatic Methods from 3 Studies. Comb. Chem. High Throughput Screen. 2023, 26, 507–516. [Google Scholar] [PubMed]
- Chow, S.-E.; Hsu, C.-C.; Yang, C.-T.; Meir, Y.-J.J. YAP Co-Localizes with the Mitotic Spindle and Midbody to Safeguard Mitotic Division in Lung-Cancer Cells. FEBS J. 2023, 290, 5704–5719. [Google Scholar] [CrossRef] [PubMed]
- Iltzsche, F.; Simon, K.; Stopp, S.; Pattschull, G.; Francke, S.; Wolter, P.; Hauser, S.; Murphy, D.J.; Garcia, P.; Rosenwald, A.; et al. An Important Role for Myb-MuvB and Its Target Gene KIF23 in a Mouse Model of Lung Adenocarcinoma. Oncogene 2017, 36, 110–121. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Wei, Y.-L.; She, Z.-Y. Kinesin-7 CENP-E in Tumorigenesis: Chromosome Instability, Spindle Assembly Checkpoint, and Applications. Front. Mol. Biosci. 2024, 11, 1366113. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.D.; Wood, K.W.; Cleveland, D.W. The Kinesin-like Protein CENP-E Is Kinetochore-Associated throughout Poleward Chromosome Segregation during Anaphase-A. J. Cell Sci. 1996, 109 Pt 5, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Putkey, F.R.; Cramer, T.; Morphew, M.K.; Silk, A.D.; Johnson, R.S.; McIntosh, J.R.; Cleveland, D.W. Unstable Kinetochore-Microtubule Capture and Chromosomal Instability Following Deletion of CENP-E. Dev. Cell 2002, 3, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Qu, T. Expression of CENPE and Its Prognostic Role in Non-Small Cell Lung Cancer. Open Med. 2019, 14, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Tian, C.; Liu, L.; Zeng, X.; Zhang, Y. Targeting CENP-E Augments Immunotherapy in Non-Small Cell Lung Cancer via Stabilizing PD-L1. Int. Immunopharmacol. 2024, 126, 111294. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Zhao, M.; Lu, Y.; Ning, H.; Yang, S.; Song, Y.; Chai, W.; Shi, X. CENPE Promotes Lung Adenocarcinoma Proliferation and Is Directly Regulated by FOXM1. Int. J. Oncol. 2019, 55, 257–266. [Google Scholar]
- Ma, Q.; Xu, Y.; Liao, H.; Cai, Y.; Xu, L.; Xiao, D.; Liu, C.; Pu, W.; Zhong, X.; Guo, X. Identification and Validation of Key Genes Associated with Non-Small-Cell Lung Cancer. J. Cell. Physiol. 2019, 234, 22742–22752. [Google Scholar] [CrossRef]
- Pinto, B.; Silva, J.P.N.; Silva, P.M.A.; Barbosa, D.J.; Sarmento, B.; Tavares, J.C.; Bousbaa, H. Maximizing Anticancer Response with MPS1 and CENPE Inhibition alongside Apoptosis Induction. Pharmaceutics 2023, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Y.; Deng, Y.; Dong, Y.; Jiang, J.; Chen, S.; Kang, W.; Deng, J.; Sun, H. Survival-Based Bioinformatics Analysis to Identify Hub Genes and Key Pathways in Non-Small Cell Lung Cancer. Transl. Cancer Res. 2019, 8, 1188–1198. [Google Scholar] [CrossRef]
- Valter, A.; Luhari, L.; Pisarev, H.; Truumees, B.; Planken, A.; Smolander, O.P.; Oselin, K. Genomic Alterations as Independent Prognostic Factors to Predict the Type of Lung Cancer Recurrence. Gene 2023, 885, 147690. [Google Scholar] [CrossRef] [PubMed]
- Tomoshige, K.; Matsumoto, K.; Tsuchiya, T.; Oikawa, M.; Miyazaki, T.; Yamasaki, N.; Mishima, H.; Kinoshita, A.; Kubo, T.; Fukushima, K.; et al. Germline Mutations Causing Familial Lung Cancer. J. Hum. Genet. 2015, 60, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Fan, L.Y.-N.; Lam, E.W.-F. The FOXO3-FOXM1 Axis: A Key Cancer Drug Target and a Modulator of Cancer Drug Resistance. Semin. Cancer Biol. 2018, 50, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Chung, V.; Heath, E.I.; Schelman, W.R.; Johnson, B.M.; Kirby, L.C.; Lynch, K.M.; Botbyl, J.D.; Lampkin, T.A.; Holen, K.D. First-Time-in-Human Study of GSK923295, a Novel Antimitotic Inhibitor of Centromere-Associated Protein E (CENP-E), in Patients with Refractory Cancer. Cancer Chemother. Pharmacol. 2012, 69, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; McDonald, A.; Zhou, H.-J.; Adams, N.D.; Parrish, C.A.; Duffy, K.J.; Fitch, D.M.; Tedesco, R.; Ashcraft, L.W.; Yao, B.; et al. Discovery of the First Potent and Selective Inhibitor of Centromere-Associated Protein E: GSK923295. ACS Med. Chem. Lett. 2010, 1, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Balamuth, N.J.; Wood, A.; Wang, Q.; Jagannathan, J.; Mayes, P.; Zhang, Z.; Chen, Z.; Rappaport, E.; Courtright, J.; Pawel, B.; et al. Serial Transcriptome Analysis and Cross-Species Integration Identifies Centromere-Associated Protein E as a Novel Neuroblastoma Target. Cancer Res. 2010, 70, 2749–2758. [Google Scholar] [CrossRef]
- Wood, K.W.; Lad, L.; Luo, L.; Qian, X.; Knight, S.D.; Nevins, N.; Brejc, K.; Sutton, D.; Gilmartin, A.G.; Chua, P.R.; et al. Antitumor Activity of an Allosteric Inhibitor of Centromere-Associated Protein-E. Proc. Natl. Acad. Sci. USA 2010, 107, 5839–5844. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Duijf, P.H.G.; Khanna, K.K. Mitotic Slippage: An Old Tale with a New Twist. Cell Cycle 2019, 18, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Tipton, A.R.; Ji, W.; Sturt-Gillespie, B.; Bekier, M.E., 2nd; Wang, K.; Taylor, W.R.; Liu, S.-T. Monopolar Spindle 1 (MPS1) Kinase Promotes Production of Closed MAD2 (C-MAD2) Conformer and Assembly of the Mitotic Checkpoint Complex. J. Biol. Chem. 2013, 288, 35149–35158. [Google Scholar] [CrossRef]
- Mayes, P.A.; Degenhardt, Y.Y.; Wood, A.; Toporovskya, Y.; Diskin, S.J.; Haglund, E.; Moy, C.; Wooster, R.; Maris, J.M. Mitogen-Activated Protein Kinase (MEK/ERK) Inhibition Sensitizes Cancer Cells to Centromere-Associated Protein E Inhibition. Int. J. Cancer 2013, 132, E149–E157. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Zhang, C.; Zhang, C.; Jiang, L.; Wang, H.; Liu, H. Identification of a Gene Signature Closely Related to Immunosuppressive Tumour Microenvironment Predicting Prognosis of Patients in EGFR Mutant Lung Adenocarcinoma. Front. Oncol. 2021, 11, 732841. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.B.; Carlsen, C.L.; Scribano, C.M.; Pattaswamy, S.M.; Burkard, M.E.; Weaver, B.A. CENP-E Inhibition Induces Chromosomal Instability and Synergizes with Diverse Microtubule-Targeting Agents in Breast Cancer. Cancer Res. 2024, 84, 2674–2689. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Matsura, A.; Shimada, M.; Ishida-Ishihara, S.; Sato, F.; Yamamoto, T.; Yaguchi, K.; Kawamoto, E.; Kuroda, T.; Matsuo, K.; et al. Tetraploidy-Linked Sensitization to CENP-E Inhibition in Human Cells. Mol. Oncol. 2023, 17, 1148–1166. [Google Scholar] [CrossRef] [PubMed]
- Mohd Amin, A.S.; Eastwood, S.; Pilcher, C.; Truong, J.Q.; Foitzik, R.; Boag, J.; Gorringe, K.L.; Holien, J.K. KIF18A Inhibition: The next Big Player in the Search for Cancer Therapeutics. Cancer Metastasis Rev. 2024, 44, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, M.; Zhang, Z.; Zhang, L.; Liang, X.; Sun, L.; Zhong, D. High Kinesin Family Member 18A Expression Correlates with Poor Prognosis in Primary Lung Adenocarcinoma. Thorac. Cancer 2019, 10, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Jiang, L.; Lin, H.; Li, X.; Long, X.; Zhou, Y.; Li, B.; Li, Z. Overexpression of KIF18A Promotes Cell Proliferation, Inhibits Apoptosis, and Independently Predicts Unfavorable Prognosis in Lung Adenocarcinoma: Biological and Prognostic Role of KIF18A in LUAD. IUBMB Life 2019, 71, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zeng, H.; Zheng, J.; He, Y.; Zhuang, X.; Cai, J.; Huang, H.; Huang, H.; Xu, M. Preliminary Study on the Clinical Significance of Kinesin Kif18a in Nonsmall Cell Lung Cancer: An Analysis of 100 Cases: An Analysis of 100 Cases. Medicine 2020, 99, e19011. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yang, K.; Chen, J.; Qi, L.; Zhou, X.; Wang, P. Comprehensive Pan-Cancer Analysis of KIF18A as a Marker for Prognosis and Immunity. Biomolecules 2023, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-T.; Zhong, F.-K. Kinesin Family Member 18A (KIF18A) Contributes to the Proliferation, Migration, and Invasion of Lung Adenocarcinoma Cells in Vitro and in Vivo. Dis. Markers 2019, 2019, 6383685. [Google Scholar] [CrossRef]
- Tooker, B.C.; Newman, L.S.; Bowler, R.P.; Karjalainen, A.; Oksa, P.; Vainio, H.; Pukkala, E.; Brandt-Rauf, P.W. Proteomic Detection of Cancer in Asbestosis Patients Using SELDI-TOF Discovered Serum Protein Biomarkers. Biomarkers 2011, 16, 181–191. [Google Scholar] [CrossRef]
- Marquis, C.; Fonseca, C.L.; Queen, K.A.; Wood, L.; Vandal, S.E.; Malaby, H.L.H.; Clayton, J.E.; Stumpff, J. Chromosomally Unstable Tumor Cells Specifically Require KIF18A for Proliferation. Nat. Commun. 2021, 12, 1213. [Google Scholar] [CrossRef]
- Cohen-Sharir, Y.; McFarland, J.M.; Abdusamad, M.; Marquis, C.; Bernhard, S.V.; Kazachkova, M.; Tang, H.; Ippolito, M.R.; Laue, K.; Zerbib, J.; et al. Aneuploidy Renders Cancer Cells Vulnerable to Mitotic Checkpoint Inhibition. Nature 2021, 590, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Quinton, R.J.; DiDomizio, A.; Vittoria, M.A.; Kotýnková, K.; Ticas, C.J.; Patel, S.; Koga, Y.; Vakhshoorzadeh, J.; Hermance, N.; Kuroda, T.S.; et al. Whole-Genome Doubling Confers Unique Genetic Vulnerabilities on Tumour Cells. Nature 2021, 590, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Payton, M.; Belmontes, B.; Hanestad, K.; Moriguchi, J.; Chen, K.; McCarter, J.D.; Chung, G.; Ninniri, M.S.; Sun, J.; Manoukian, R.; et al. Small-Molecule Inhibition of Kinesin KIF18A Reveals a Mitotic Vulnerability Enriched in Chromosomally Unstable Cancers. Nat. Cancer 2024, 5, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Gliech, C.R.; Yeow, Z.Y.; Tapias-Gomez, D.; Yang, Y.; Huang, Z.; Tijhuis, A.E.; Spierings, D.C.; Foijer, F.; Chung, G.; Tamayo, N.; et al. Weakened APC/C Activity at Mitotic Exit Drives Cancer Vulnerability to KIF18A Inhibition. EMBO J. 2024, 43, 666–694. [Google Scholar] [CrossRef]
- Chen, Q.; Le, X.; Li, Q.; Liu, S.; Chen, Z. Exploration of Inhibitors Targeting KIF18A with Ploidy-Specific Lethality. Drug Discov. Today 2024, 29, 104142. [Google Scholar] [CrossRef]
- Tu, P.; Zhang, C.; Lu, B.; Xia, Y.; Yang, F. Abstract 664: GSC000190, a Highly Potent Inhibitor of KIF18A, for Tumors with Chromosome Instability. Cancer Res. 2024, 84, 664. [Google Scholar] [CrossRef]
- Tanenbaum, M.E.; Macurek, L.; van der Vaart, B.; Galli, M.; Akhmanova, A.; Medema, R.H. A Complex of Kif18b and MCAK Promotes Microtubule Depolymerization and Is Negatively Regulated by Aurora Kinases. Curr. Biol. 2011, 21, 1356–1365. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, F.; Liu, F.; Liu, S.; Meng, L.; Gu, L.; Zhao, H.; Sang, M.; Shan, B. Identification of Potential Circular RNA Biomarkers in Lung Adenocarcinoma: A Bioinformatics Analysis and Retrospective Clinical Study. Oncol. Lett. 2022, 23, 144. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Pan, X.; Shang, Y.; Ni, D.-T.; Wu, F.-L. KIF18B as a Regulator in Microtubule Movement Accelerates Tumor Progression and Triggers Poor Outcome in Lung Adenocarcinoma. Tissue Cell 2019, 61, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Jiang, L.; Long, X.; Zhou, Y.; Deng, S.; Lin, H.; Li, X. Clinical Significance And Integrative Analysis Of Kinesin Family Member 18B In Lung Adenocarcinoma. Onco Targets Ther. 2019, 12, 9249–9264. [Google Scholar] [CrossRef] [PubMed]
- Ohsugi, M.; Adachi, K.; Horai, R.; Kakuta, S.; Sudo, K.; Kotaki, H.; Tokai-Nishizumi, N.; Sagara, H.; Iwakura, Y.; Yamamoto, T. Kid-Mediated Chromosome Compaction Ensures Proper Nuclear Envelope Formation. Cell 2008, 132, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Pike, R.; Ortiz-Zapater, E.; Lumicisi, B.; Santis, G.; Parsons, M. KIF22 Coordinates CAR and EGFR Dynamics to Promote Cancer Cell Proliferation. Sci. Signal. 2018, 11, eaaq1060. [Google Scholar] [CrossRef]
- Tanenbaum, M.E.; Macůrek, L.; Janssen, A.; Geers, E.F.; Alvarez-Fernández, M.; Medema, R.H. Kif15 Cooperates with eg5 to Promote Bipolar Spindle Assembly. Curr. Biol. 2009, 19, 1703–1711. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Xu, L.; Li, M.; Wu, J.; Zhou, Y.; Li, Y. Downregulation of KIF15 Inhibits the Tumorigenesis of Non-Small-Cell Lung Cancer via Inactivating Raf/MEK/ERK Signaling. Histol. Histopathol. 2022, 37, 269–285. [Google Scholar] [PubMed]
- Qiao, Y.; Chen, J.; Ma, C.; Liu, Y.; Li, P.; Wang, Y.; Hou, L.; Liu, Z. Increased KIF15 Expression Predicts a Poor Prognosis in Patients with Lung Adenocarcinoma. Cell. Physiol. Biochem. 2018, 51, 1–10. [Google Scholar] [CrossRef]
- Qin, S.; Long, X.; Zhao, Q.; Zhao, W. Co-Expression Network Analysis Identified Genes Associated with Cancer Stem Cell Characteristics in Lung Squamous Cell Carcinoma. Cancer Investig. 2020, 38, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; An, J.; Jia, R.; Liu, C.; Zhang, Y. Identification and Verification of Metabolism-Related Immunotherapy Features and Prognosis in Lung Adenocarcinoma. Curr. Med. Chem. 2024, 31, 1423–1441. [Google Scholar] [CrossRef]
- Ma, H.T.; Erdal, S.; Huang, S.; Poon, R.Y.C. Synergism between Inhibitors of Aurora A and KIF11 Overcomes KIF15-Dependent Drug Resistance. Mol. Oncol. 2014, 8, 1404–1418. [Google Scholar] [CrossRef]
- Sturgill, E.G.; Norris, S.R.; Guo, Y.; Ohi, R. Kinesin-5 Inhibitor Resistance Is Driven by Kinesin-12. J. Cell Biol. 2016, 213, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Milic, B.; Chakraborty, A.; Han, K.; Bassik, M.C.; Block, S.M. KIF15 Nanomechanics and Kinesin Inhibitors, with Implications for Cancer Chemotherapeutics. Proc. Natl. Acad. Sci. USA 2018, 115, E4613–E4622. [Google Scholar] [CrossRef] [PubMed]
- Solon, A.L.; Zaniewski, T.M.; O’Brien, P.; Clasby, M.; Hancock, W.O.; Ohi, R. Synergy between Inhibitors of Two Mitotic Spindle Assembly Motors Undermines an Adaptive Response. Mol. Biol. Cell 2022, 33, ar132. [Google Scholar] [CrossRef]
- Sebastian, J. Dihydropyrazole and Dihydropyrrole Structures Based Design of Kif15 Inhibitors as Novel Therapeutic Agents for Cancer. Comput. Biol. Chem. 2017, 68, 164–174. [Google Scholar] [CrossRef]
- Dumas, M.E.; Chen, G.-Y.; Kendrick, N.D.; Xu, G.; Larsen, S.D.; Jana, S.; Waterson, A.G.; Bauer, J.A.; Hancock, W.; Sulikowski, G.A.; et al. Dual Inhibition of Kif15 by Oxindole and Quinazolinedione Chemical Probes. Bioorg. Med. Chem. Lett. 2019, 29, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wen, J.; Liu, D.; Xu, X.; Fan, M.; Zhang, Z. The Molecular Mechanism of Kinesin Family Member 2A (KIF2A) Underlying Non-Small Cell Lung Cancer: The Effect of Its Knockdown on Malignant Behaviors, Stemness, Chemosensitivity, and Potential Regulated Signaling Pathways. Am. J. Transl. Res. 2022, 14, 68–85. [Google Scholar]
- Xu, L.; Zhang, X.; Wang, Z.; Zhao, X.; Zhao, L.; Hu, Y. Kinesin Family Member 2A Promotes Cancer Cell Viability, Mobility, Stemness, and Chemoresistance to Cisplatin by Activating the PI3K/AKT/VEGF Signaling Pathway in Non-Small Cell Lung Cancer. Am. J. Transl. Res. 2021, 13, 2060–2076. [Google Scholar]
- Xie, T.; Li, X.; Ye, F.; Lu, C.; Huang, H.; Wang, F.; Cao, X.; Zhong, C. High KIF2A Expression Promotes Proliferation, Migration and Predicts Poor Prognosis in Lung Adenocarcinoma. Biochem. Biophys. Res. Commun. 2018, 497, 65–72. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Z.; Yu, H. Kinesin Family Member 2A High Expression Correlates with Advanced Tumor Stages and Worse Prognosis in Non-Small Cell Lung Cancer Patients. J. Clin. Lab. Anal. 2020, 34, e23135. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wang, L.; Ge, L.; Xu, D.; Zhang, R. IGF2BP1 Facilitates Non-Small Cell Lung Cancer Progression by Regulating the KIF2A-Mediated Wnt/β-Catenin Pathway. Funct. Integr. Genom. 2023, 24, 4. [Google Scholar] [CrossRef]
- Zheng, P.; Jiang, J.; Li, L.; Wei, L.; Li, J.; Jin, L. Circ_SATB2 Knockdown Inhibits the Tumorigenesis of Non-Small Cell Lung Cancer via miR-760/KIF2A Axis. Histol. Histopathol. 2023, 38, 431–441. [Google Scholar] [PubMed]
- Uchida, A.; Seki, N.; Mizuno, K.; Yamada, Y.; Misono, S.; Sanada, H.; Kikkawa, N.; Kumamoto, T.; Suetsugu, T.; Inoue, H. Regulation of KIF2A by Antitumor miR-451a Inhibits Cancer Cell Aggressiveness Features in Lung Squamous Cell Carcinoma. Cancers 2019, 11, 258. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, C.; Lv, A.; Kou, C. Circular RNA circ_0010235 Sponges miR-338-3p to Play Oncogenic Role in Proliferation, Migration and Invasion of Non-Small-Cell Lung Cancer Cells through Modulating KIF2A. Ann. Med. 2021, 53, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Kreis, N.-N.; Moon, H.H.; Wordeman, L.; Louwen, F.; Solbach, C.; Yuan, J.; Ritter, A. KIF2C/MCAK a Prognostic Biomarker and Its Oncogenic Potential in Malignant Progression, and Prognosis of Cancer Patients: A Systematic Review and Meta-Analysis as Biomarker. Crit. Rev. Clin. Lab. Sci. 2024, 61, 404–434. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, W.; Sun, L.; Yu, H.; Wang, Y.; Feng, L.; Yang, H. KIF2C Accelerates the Development of Non-Small Cell Lung Cancer and Is Suppressed by miR-186-3p via the AKT-GSK3β-β-Catenin Pathway. Sci. Rep. 2023, 13, 7288. [Google Scholar] [CrossRef]
- Gan, H.; Lin, L.; Hu, N.; Yang, Y.; Gao, Y.; Pei, Y.; Chen, K.; Sun, B. KIF2C Exerts an Oncogenic Role in Nonsmall Cell Lung Cancer and Is Negatively Regulated by miR-325-3p. Cell Biochem. Funct. 2019, 37, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Miao, R.; Han, T.; Liu, Y.; Zhou, J.; Guo, J.; Xing, Y.; Bai, Y.; Wu, J.; Hu, D. KIF2C as a Potential Therapeutic Target: Insights from Lung Adenocarcinoma Subtype Classification and Functional Experiments. Mol. Omics 2024, 20, 417–429. [Google Scholar] [CrossRef]
- Bai, Y.; Xiong, L.; Zhu, M.; Yang, Z.; Zhao, J.; Tang, H. Co-Expression Network Analysis Identified KIF2C in Association with Progression and Prognosis in Lung Adenocarcinoma. Cancer Biomark. 2019, 24, 371–382. [Google Scholar] [CrossRef]
- Li, Z.; Qi, F.; Li, F. Establishment of a Gene Signature to Predict Prognosis for Patients with Lung Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 8479. [Google Scholar] [CrossRef]
- Song, Y.-J.; Tan, J.; Gao, X.-H.; Wang, L.-X. Integrated Analysis Reveals Key Genes with Prognostic Value in Lung Adenocarcinoma. Cancer Manag. Res. 2018, 10, 6097–6108. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Husted, S.; Pilrose, J.; Ems-McClung, S.C.; Stout, J.R.; Carpenter, R.L.; Walczak, C.E. MCAK Inhibitors Induce Aneuploidy in Triple-Negative Breast Cancer Models. Cancers 2023, 15, 3309. [Google Scholar] [CrossRef] [PubMed]
- Laucius, C.D.; Orr, B.; Compton, D.A. Chromosomal Instability Suppresses the Growth of K-Ras-Induced Lung Adenomas. Cell Cycle 2019, 18, 1702–1713. [Google Scholar] [CrossRef]
- Manning, A.L.; Ganem, N.J.; Bakhoum, S.F.; Wagenbach, M.; Wordeman, L.; Compton, D.A. The Kinesin-13 Proteins Kif2a, Kif2b, and Kif2c/MCAK Have Distinct Roles during Mitosis in Human Cells. Mol. Biol. Cell 2007, 18, 2970–2979. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tsang, W.Y.; Li, J.; Lane, W.; Dynlacht, B.D. Centriolar Kinesin Kif24 Interacts with CP110 to Remodel Microtubules and Regulate Ciliogenesis. Cell 2011, 145, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ni, H.; Yang, D.; Niu, Y.; Chen, K.; Xu, J.; Wang, F.; Tang, S.; Shi, Y.; Zhang, H.; et al. Driver and Novel Genes Correlated with Metastasis of Non-Small Cell Lung Cancer: A Comprehensive Analysis. Pathol. Res. Pract. 2021, 224, 153551. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-X.; Yang, W.-X. KIFC1: A Promising Chemotherapy Target for Cancer Treatment? Oncotarget 2016, 7, 48656–48670. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Duan, Y.; Gong, S.; Zhu, Q.; Liu, X.; Liu, Z. An Integrative Pan-Cancer Analysis of Kinesin Family Member C1 (KIFC1) in Human Tumors. Biomedicines 2022, 10, 637. [Google Scholar] [CrossRef]
- Sharma, N.; Setiawan, D.; Hamelberg, D.; Narayan, R.; Aneja, R. Computational Benchmarking of Putative KIFC1 Inhibitors. Med. Res. Rev. 2023, 43, 293–318. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.Z.; Di Ciano-Oliveira, C.; Wu, Y.F.; Khavkine Binstock, S.S.; Soria-Bretones, I.; Pham, N.-A.; Elia, A.J.; Chari, R.; Lam, W.L.; et al. Identification of KIFC1 as a Putative Vulnerability in Lung Cancers with Centrosome Amplification. Cancer Gene Ther. 2024, 31, 1559–1570. [Google Scholar] [CrossRef]
- Celik, B.; Pasin, O.; Sen, S.; Tuncer, S.B.; Kayım, Z.Y.; Erciyas, S.K.; Erdogan, O.S.; Gultaslar, B.K.; Ghafour, A.A.; Yazıcı, H.; et al. DNA Methylation of KIFC1 Gene in Determination of Histological Diagnosis, Prognosis and Metastasis of Lung Cancer. Pathol. Res. Pract. 2023, 249, 154742. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, P.; Zhou, Z.; Xing, Z.; Zhu, S.; Ma, C.; Li, Q.; Zhu, Q.; Miao, Y.; Zhang, J.; et al. The Overexpression of KIFC1 Was Associated with the Proliferation and Prognosis of Non-Small Cell Lung Cancer. J. Thorac. Dis. 2016, 8, 2911–2923. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, W.; Miao, X.; Wang, X. KIFC1 Aggravates Non-Small-Cell Lung Cancer Cell Proliferation and Metastasis via Provoking TGF-β/SMAD Signal. Cell. Mol. Biol. 2023, 69, 293–299. [Google Scholar] [PubMed]
- Li, X.; Wang, S.; Ruan, P.; Bajinka, O.; Zhang, W. High Expression of KIFC1 Is a Poor Prognostic Biomarker and Correlates with TP53 Mutation in Lung Cancer. Medicine 2024, 103, e37286. [Google Scholar] [CrossRef]
- Grinberg-Rashi, H.; Ofek, E.; Perelman, M.; Skarda, J.; Yaron, P.; Hajdúch, M.; Jacob-Hirsch, J.; Amariglio, N.; Krupsky, M.; Simansky, D.A.; et al. The Expression of Three Genes in Primary Non-Small Cell Lung Cancer Is Associated with Metastatic Spread to the Brain. Clin. Cancer Res. 2009, 15, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Godinho, S.A.; Chandhok, N.S.; Ganem, N.J.; Azioune, A.; Thery, M.; Pellman, D. Mechanisms to Suppress Multipolar Divisions in Cancer Cells with Extra Centrosomes. Genes Dev. 2008, 22, 2189–2203. [Google Scholar] [CrossRef]
- Patel, N.; Weekes, D.; Drosopoulos, K.; Gazinska, P.; Noel, E.; Rashid, M.; Mirza, H.; Quist, J.; Brasó-Maristany, F.; Mathew, S.; et al. Integrated Genomics and Functional Validation Identifies Malignant Cell Specific Dependencies in Triple Negative Breast Cancer. Nat. Commun. 2018, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Zhu, X.-M.; Pan, X.-Y.; Du, M.; Sun, Z.; Li, Z.-M. MicroRNA-527 Inhibits TGF-β/SMAD Induced Epithelial-Mesenchymal Transition via Downregulating SULF2 Expression in Non-Small-Cell Lung Cancer. Math. Biosci. Eng. 2019, 16, 4607–4621. [Google Scholar] [CrossRef] [PubMed]
- Hata, S.; Pastor Peidro, A.; Panic, M.; Liu, P.; Atorino, E.; Funaya, C.; Jäkle, U.; Pereira, G.; Schiebel, E. The Balance between KIFC3 and EG5 Tetrameric Kinesins Controls the Onset of Mitotic Spindle Assembly. Nat. Cell Biol. 2019, 21, 1138–1151. [Google Scholar] [CrossRef]
- Nachbar, J.; Lázaro-Diéguez, F.; Prekeris, R.; Cohen, D.; Müsch, A. KIFC3 Promotes Mitotic Progression and Integrity of the Central Spindle in Cytokinesis. Cell Cycle 2014, 13, 426–433. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Jiang, X.; Guan, J.; Wang, H.; Zhang, J.; Tong, Y.; Qiu, X.; Zhou, R. KIFC3 Promotes the Proliferation, Migration and Invasion of Non-Small Cell Lung Cancer through the PI3K/AKT Signaling Pathway. Sci. Rep. 2024, 14, 20471. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Liu, H.; Luo, A.; Zhang, Q. KIFC3 Promotes the Progression of Non-Small Cell Lung Cancer Cells through the PI3K/Akt Pathway. Thorac. Cancer 2024, 15, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, Y.; Sakaguchi, M.; Terabayashi, T.; Inenaga, T.; Inoue, S.; Kobayashi, C.; Oshima, N.; Kiyonari, H.; Nakagata, N.; Sato, Y.; et al. Kif26b, a Kinesin Family Gene, Regulates Adhesion of the Embryonic Kidney Mesenchyme. Proc. Natl. Acad. Sci. USA 2010, 107, 9240–9245. [Google Scholar] [CrossRef] [PubMed]
- Guillabert-Gourgues, A.; Jaspard-Vinassa, B.; Bats, M.-L.; Sewduth, R.N.; Franzl, N.; Peghaire, C.; Jeanningros, S.; Moreau, C.; Roux, E.; Larrieu-Lahargue, F.; et al. Kif26b Controls Endothelial Cell Polarity through the Dishevelled/Daam1-Dependent Planar Cell Polarity-Signaling Pathway. Mol. Biol. Cell 2016, 27, 941–953. [Google Scholar] [CrossRef]
- Chen, N.; Wu, Q.; Zhang, G.; Fu, J.; Geng, Q.; Zhang, Y. Deactivation of AKT/GSK-3β-Mediated Wnt/β-Catenin Pathway by Silencing of KIF26B Weakens the Malignant Behaviors of Non-Small Cell Lung Cancer. Tissue Cell 2022, 76, 101750. [Google Scholar] [CrossRef]
- Cheung, H.O.-L.; Zhang, X.; Ribeiro, A.; Mo, R.; Makino, S.; Puviindran, V.; Law, K.K.L.; Briscoe, J.; Hui, C.-C. The Kinesin Protein Kif7 Is a Critical Regulator of Gli Transcription Factors in Mammalian Hedgehog Signaling. Sci. Signal. 2009, 2, ra29. [Google Scholar] [CrossRef] [PubMed]
- Klejnot, M.; Kozielski, F. Structural Insights into Human Kif7, a Kinesin Involved in Hedgehog Signalling. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 154–159. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, M.-Z.; Gu, N.-J.; Xu, H.-T.; Li, Q.-C.; Wu, G.-P. Human Papillomavirus 16 (HPV 16) E6 but Not E7 Inhibits the Antitumor Activity of LKB1 in Lung Cancer Cells by Downregulating the Expression of KIF7. Thorac. Cancer 2020, 11, 3175–3180. [Google Scholar] [CrossRef]
- Coles, G.L.; Baglia, L.A.; Ackerman, K.G. KIF7 Controls the Proliferation of Cells of the Respiratory Airway through Distinct Microtubule Dependent Mechanisms. PLoS Genet. 2015, 11, e1005525. [Google Scholar] [CrossRef] [PubMed]
- Muhia, M.; Thies, E.; Labonté, D.; Ghiretti, A.E.; Gromova, K.V.; Xompero, F.; Lappe-Siefke, C.; Hermans-Borgmeyer, I.; Kuhl, D.; Schweizer, M.; et al. The Kinesin KIF21B Regulates Microtubule Dynamics and Is Essential for Neuronal Morphology, Synapse Function, and Learning and Memory. Cell Rep. 2016, 15, 968–977. [Google Scholar] [CrossRef] [PubMed]
- van Riel, W.E.; Rai, A.; Bianchi, S.; Katrukha, E.A.; Liu, Q.; Heck, A., Jr.; Hoogenraad, C.C.; Steinmetz, M.O.; Kapitein, L.C.; Akhmanova, A. Kinesin-4 KIF21B Is a Potent Microtubule Pausing Factor. eLife 2017, 6, e24746. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-G.; Pan, F.; Shao, J.-B.; Yan, Q.-Q.; Lu, L.; Zhang, N. Kinesin Superfamily Protein 21B Acts as an Oncogene in Non-Small Cell Lung Cancer. Cancer Cell Int. 2020, 20, 233. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Noda, Y. Intracellular Transport and Kinesin Superfamily Proteins, KIFs: Structure, Function, and Dynamics. Physiol. Rev. 2008, 88, 1089–1118. [Google Scholar] [CrossRef]
- Dong, L.; Feng, C.; Cheng, W.; Huang, A.; Ying, K. FOXP3 Targets KIF5A to Increase Lactate Production and Promote Docetaxel Resistance in Lung Adenocarcinoma. Acta Biochim. Biophys. Sin. 2024, 56, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, R.; Auger, N.; Auclin, E.; Besse, B. Clinical and Translational Implications of RET Rearrangements in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Choi, Y.L.; Togashi, Y.; Soda, M.; Hatano, S.; Inamura, K.; Takada, S.; Ueno, T.; Yamashita, Y.; Satoh, Y.; et al. KIF5B-ALK, a Novel Fusion Oncokinase Identified by an Immunohistochemistry-Based Diagnostic System for ALK-Positive Lung Cancer. Clin. Cancer Res. 2009, 15, 3143–3149. [Google Scholar] [CrossRef] [PubMed]
- Gow, C.-H.; Liu, Y.-N.; Li, H.-Y.; Hsieh, M.-S.; Chang, S.-H.; Luo, S.-C.; Tsai, T.-H.; Chen, P.-L.; Tsai, M.-F.; Shih, J.-Y. Oncogenic Function of a KIF5B-MET Fusion Variant in Non-Small Cell Lung Cancer. Neoplasia 2018, 20, 838–847. [Google Scholar] [CrossRef]
- Cho, J.H.; Ku, B.M.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Park, K.; Ahn, M.-J. KIF5B-MET Gene Rearrangement with Robust Antitumor Activity in Response to Crizotinib in Lung Adenocarcinoma. J. Thorac. Oncol. 2018, 13, e29–e31. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K. Discovery Stories of RET Fusions in Lung Cancer: A Mini-Review. Front. Physiol. 2019, 10, 216. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Liu, C.; Wang, W.; Li, M.; Lv, D.; Sun, G.; Chen, H.; Dong, X.; Miao, Z.; et al. Identification of a Novel KIF13A-RET Fusion in Lung Adenocarcinoma by next-Generation Sequencing. Lung Cancer 2018, 118, 27–29. [Google Scholar] [CrossRef]
- Mo, Z.; Cai, C.; Yao, J.; Zhao, J.; Zhang, M.; Liu, H.; Mu, X. First Case Report of a Novel KIF13A-ALK Fusion in a Lung Adenocarcinoma Patient and Response to Alectinib with a 4-Year Follow-Up. Front. Genet. 2023, 14, 1289346. [Google Scholar] [CrossRef]
- Xia, D.; Le, L.P.; Iafrate, A.J.; Lennerz, J. KIF13B-NRG1 Gene Fusion and KRAS Amplification in a Case of Natural Progression of Lung Cancer. Int. J. Surg. Pathol. 2017, 25, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Thankachan, J.M.; Setty, S.R.G. KIF13A-A Key Regulator of Recycling Endosome Dynamics. Front. Cell Dev. Biol. 2022, 10, 877532. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Wang, D.; Hirokawa, N. KIF13B Enhances the Endocytosis of LRP1 by Recruiting LRP1 to Caveolae. J. Cell Biol. 2014, 204, 395–408. [Google Scholar] [CrossRef]
- Yamada, K.H.; Kang, H.; Malik, A.B. Antiangiogenic Therapeutic Potential of Peptides Derived from the Molecular Motor KIF13B That Transports VEGFR2 to Plasmalemma in Endothelial Cells. Am. J. Pathol. 2017, 187, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tang, D.; Zhao, Y.C.; Liu, H.; Luo, S.; Stinchcombe, T.E.; Glass, C.; Su, L.; Shen, S.; Christiani, D.C.; et al. Novel Genetic Variants in KIF16B and NEDD4L in the Endosome-Related Genes Are Associated with Nonsmall Cell Lung Cancer Survival. Int. J. Cancer 2020, 147, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Hoepfner, S.; Severin, F.; Cabezas, A.; Habermann, B.; Runge, A.; Gillooly, D.; Stenmark, H.; Zerial, M. Modulation of Receptor Recycling and Degradation by the Endosomal Kinesin KIF16B. Cell 2005, 121, 437–450. [Google Scholar] [CrossRef]
- Singh, M.; Venugopal, C.; Tokar, T.; McFarlane, N.; Subapanditha, M.K.; Qazi, M.; Bakhshinyan, D.; Vora, P.; Murty, N.K.; Jurisica, I.; et al. Therapeutic Targeting of the Premetastatic Stage in Human Lung-to-Brain Metastasis. Cancer Res. 2018, 78, 5124–5134. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, R.P.; Moss, K.R.; Janusz-Kaminska, A.; Knudson, L.A.; Denes, L.T.; Saxena, T.; Boggupalli, D.P.; Li, Z.; Lin, K.; Bassell, G.J.; et al. Muscleblind-like Proteins Use Modular Domains to Localize RNAs by Riding Kinesins and Docking to Membranes. Nat. Commun. 2023, 14, 3427. [Google Scholar] [CrossRef] [PubMed]
- Dorner, C.; Ciossek, T.; Müller, S.; Møller, P.H.; Ullrich, A.; Lammers, R. Characterization of KIF1C, a New Kinesin-like Protein Involved in Vesicle Transport from the Golgi Apparatus to the Endoplasmic Reticulum. J. Biol. Chem. 1998, 273, 20267–20275. [Google Scholar] [CrossRef]
- Scholey, J.M. Kinesin-2: A Family of Heterotrimeric and Homodimeric Motors with Diverse Intracellular Transport Functions. Annu. Rev. Cell Dev. Biol. 2013, 29, 443–469. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Nakata, T.; Okada, Y.; Hirokawa, N. KIF3A/B: A Heterodimeric Kinesin Superfamily Protein That Works as a Microtubule plus End-Directed Motor for Membrane Organelle Transport. J. Cell Biol. 1995, 130, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, D.; Liu, A.; Rakeman, A.S.; Murcia, N.S.; Niswander, L.; Anderson, K.V. Hedgehog Signalling in the Mouse Requires Intraflagellar Transport Proteins. Nature 2003, 426, 83–87. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Li, R.; Zhang, M.; Wang, H.; Qu, Y. Kinesin Family Member 3A Inhibits the Carcinogenesis of Non-small Cell Lung Cancer and Prolongs Survival. Oncol. Lett. 2020, 20, 348. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Suh, Y.-A.; Oh, J.-H.; Lee, B.R.; Kim, J.; Jang, S.J. KIF3A Binds to β-Arrestin for Suppressing Wnt/β-Catenin Signalling Independently of Primary Cilia in Lung Cancer. Sci. Rep. 2016, 6, 32770. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, C.R.; Vaghjiani, V.; Szczepny, A.; Jayasekara, W.S.N.; Gonzalez-Rajal, A.; Kikuchi, K.; McCaughan, G.W.; Burgess, A.; Gough, D.J.; Watkins, D.N.; et al. Trp53 and Rb1 Regulate Autophagy and Ligand-Dependent Hedgehog Signaling. J. Clin. Investig. 2020, 130, 4006–4018. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, R.; Hao, M.; Zhao, X.; Li, C. Kinesin Family Member 3C (KIF3C) Is a Novel Non-Small Cell Lung Cancer (NSCLC) Oncogene Whose Expression Is Modulated by microRNA-150-5p (miR-150-5p) and microRNA-186-3p (miR-186-3p). Bioengineered 2021, 12, 3077–3088. [Google Scholar] [CrossRef]
- Gumy, L.F.; Chew, D.J.; Tortosa, E.; Katrukha, E.A.; Kapitein, L.C.; Tolkovsky, A.M.; Hoogenraad, C.C.; Fawcett, J.W. The Kinesin-2 Family Member KIF3C Regulates Microtubule Dynamics and Is Required for Axon Growth and Regeneration. J. Neurosci. 2013, 33, 11329–11345. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.; Passos, G.F.; Quintão, N.L.M.; Fernandes, E.S.; Maia, J.R.L.C.B.; Campos, M.M.; Calixto, J.B. Taxane-Induced Neurotoxicity: Pathophysiology and Therapeutic Perspectives. Br. J. Pharmacol. 2020, 177, 3127–3146. [Google Scholar] [CrossRef]
- Shahin, R.; Aljamal, S. Kinesin Spindle Protein Inhibitors in Cancer: From High Throughput Screening to Novel Therapeutic Strategies. Future Sci. OA 2022, 8, FSO778. [Google Scholar] [CrossRef]
- Lad, L.; Luo, L.; Carson, J.D.; Wood, K.W.; Hartman, J.J.; Copeland, R.A.; Sakowicz, R. Mechanism of Inhibition of Human KSP by Ispinesib. Biochemistry 2008, 47, 3576–3585. [Google Scholar] [CrossRef]
- Lemieux, C.; DeWolf, W.; Voegtli, W.; DeLisle, R.K.; Laird, E.; Wallace, E.; Woessner, R.; Corrette, C.; Allen, S.; Hans, J. ARRY-520, a Novel, Highly Selective KSP Inhibitor with Potent Anti-Proliferative Activity. Proc. Am. Assoc. Cancer Res. 2007, 48, 5590. [Google Scholar]
- Yukawa, M.; Yamauchi, T.; Kurisawa, N.; Ahmed, S.; Kimura, K.-I.; Toda, T. Fission Yeast Cells Overproducing HSET/KIFC1 Provides a Useful Tool for Identification and Evaluation of Human Kinesin-14 Inhibitors. Fungal Genet. Biol. 2018, 116, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Watts, C.A.; Richards, F.M.; Bender, A.; Bond, P.J.; Korb, O.; Kern, O.; Riddick, M.; Owen, P.; Myers, R.M.; Raff, J.; et al. Design, Synthesis, and Biological Evaluation of an Allosteric Inhibitor of HSET That Targets Cancer Cells with Supernumerary Centrosomes. Chem. Biol. 2013, 20, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhai, L.; Wang, Y.; Boohaker, R.J.; Lu, W.; Gupta, V.V.; Padmalayam, I.; Bostwick, R.J.; White, E.L.; Ross, L.J.; et al. Discovery of a Novel Inhibitor of Kinesin-like Protein KIFC1. Biochem. J. 2016, 473, 1027–1035. [Google Scholar] [CrossRef]
- Punekar, S.R.; Velcheti, V.; Neel, B.G.; Wong, K.-K. The Current State of the Art and Future Trends in RAS-Targeted Cancer Therapies. Nat. Rev. Clin. Oncol. 2022, 19, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Demoor-Goldschmidt, C.; de Vathaire, F. Review of Risk Factors of Secondary Cancers among Cancer Survivors. Br. J. Radiol. 2019, 92, 20180390. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody Drug Conjugate: The “Biological Missile” for Targeted Cancer Therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Mahato, R.; Tai, W.; Cheng, K. Prodrugs for Improving Tumor Targetability and Efficiency. Adv. Drug Deliv. Rev. 2011, 63, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, R.; Gorospe, K.A.; Labouta, H.I.; Azad, T.; Lee, W.L.; Thu, K.L. Alternative Strategies for Delivering Immunotherapeutics Targeting the PD-1/PD-L1 Immune Checkpoint in Cancer. Pharmaceutics 2024, 16, 1181. [Google Scholar] [CrossRef] [PubMed]
- Komlodi-Pasztor, E.; Sackett, D.L.; Fojo, A.T. Inhibitors Targeting Mitosis: Tales of How Great Drugs against a Promising Target Were Brought down by a Flawed Rationale. Clin. Cancer Res. 2012, 18, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.M.; Collins, I. Recent Findings and Future Directions for Interpolar Mitotic Kinesin Inhibitors in Cancer Therapy. Future Med. Chem. 2016, 8, 463–489. [Google Scholar] [CrossRef] [PubMed]
- Gascoigne, K.E.; Taylor, S.S. Cancer Cells Display Profound Intra- and Interline Variation Following Prolonged Exposure to Antimitotic Drugs. Cancer Cell 2008, 14, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gong, H.; Huang, K. Oncogenic Role of Kinesin Proteins and Targeting Kinesin Therapy. Cancer Sci. 2013, 104, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Doménech, E.; Malumbres, M. Mitosis-Targeting Therapies: A Troubleshooting Guide. Curr. Opin. Pharmacol. 2013, 13, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.C.; Ribeiro, D.; Pedrosa, J.; Sarmento, B.; Silva, P.M.A.; Bousbaa, H. Mitosis Inhibitors in Anticancer Therapy: When Blocking the Exit Becomes a Solution. Cancer Lett. 2019, 440–441, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Tunquist, B.J.; Woessner, R.D.; Walker, D.H. Mcl-1 Stability Determines Mitotic Cell Fate of Human Multiple Myeloma Tumor Cells Treated with the Kinesin Spindle Protein Inhibitor ARRY-520. Mol. Cancer Ther. 2010, 9, 2046–2056. [Google Scholar] [CrossRef] [PubMed]
- Lepage, C.C.; Morden, C.R.; Palmer, M.C.L.; Nachtigal, M.W.; McManus, K.J. Detecting Chromosome Instability in Cancer: Approaches to Resolve Cell-to-Cell Heterogeneity. Cancers 2019, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.L.; Eklund, A.C.; Kohane, I.S.; Harris, L.N.; Szallasi, Z. A Signature of Chromosomal Instability Inferred from Gene Expression Profiles Predicts Clinical Outcome in Multiple Human Cancers. Nat. Genet. 2006, 38, 1043–1048. [Google Scholar] [CrossRef]
- Greene, S.B.; Dago, A.E.; Leitz, L.J.; Wang, Y.; Lee, J.; Werner, S.L.; Gendreau, S.; Patel, P.; Jia, S.; Zhang, L.; et al. Chromosomal Instability Estimation Based on next Generation Sequencing and Single Cell Genome Wide Copy Number Variation Analysis. PLoS ONE 2016, 11, e0165089. [Google Scholar] [CrossRef]
- Mitchison, T.J. The Proliferation Rate Paradox in Antimitotic Chemotherapy. Mol. Biol. Cell 2012, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kriegsmann, M.; Warth, A. What Is Better/reliable, Mitosis Counting or Ki67/MIB1 Staining? Transl. Lung Cancer Res. 2016, 5, 543–546. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).