Double PitNETs: A Case Report and Literature Review
Simple Summary
Abstract
1. Introduction
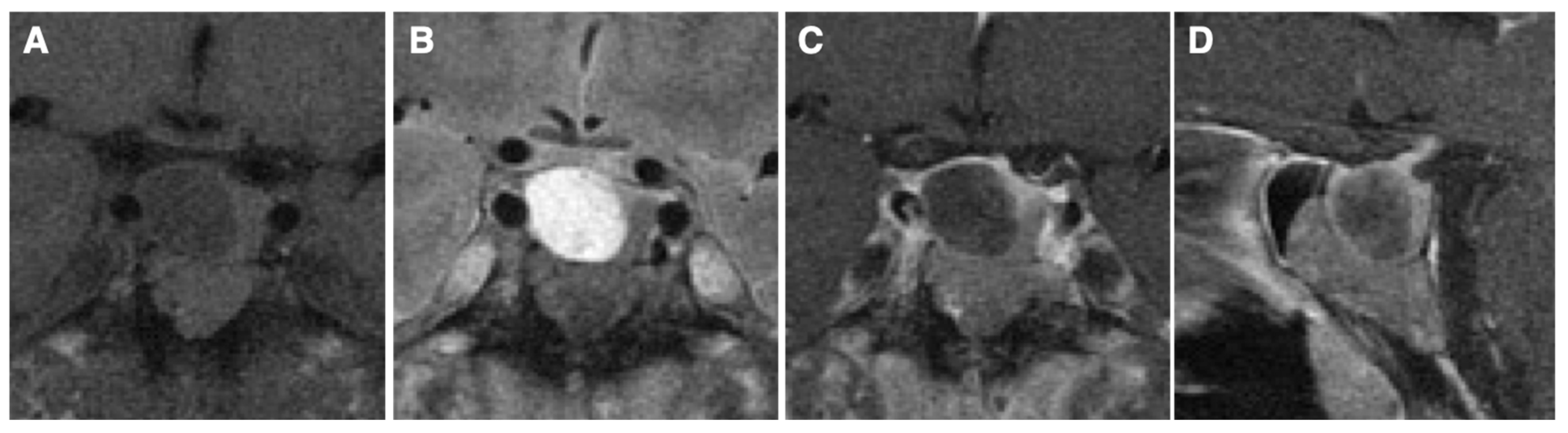
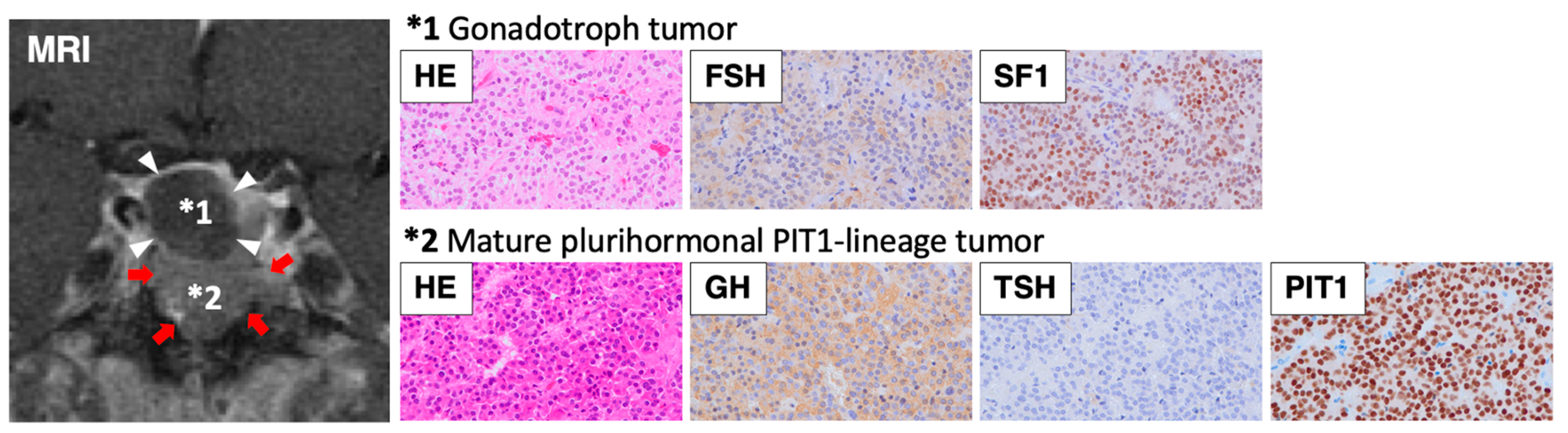
2. Case Presentation
3. Literature Review
4. Statistical Analysis
5. Epidemiology
6. Pathogenesis
7. Clinical Phenotype
8. Pathological Findings
9. Radiological Findings
10. Treatment
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| FSH | follicle-stimulating hormone |
| FT3 | free triiodothyronine |
| FT4 | free thyroxine |
| GH | growth hormone |
| IGF-1 | insulin-like growth factor 1 |
| IHC | immunohistochemistry |
| LH | luteinizing hormone |
| MRI | magnetic resonance imaging |
| PIT1 | pituitary transcription factor 1 |
| PitNET | pituitary neuroendocrine tumor |
| PRL | prolactin |
| SF1 | steroidogenic factor 1 |
| TPIT | T-Box family member TRX19 |
| TSH | thyroid-stimulating hormone |
| TSHoma | thyroid-stimulating hormone producing tumor |
References
- Melmed, S.; Kaiser, U.B.; Lopes, M.B.; Bertherat, J.; Syro, L.V.; Raverot, G.; Reincke, M.; Johannsson, G.; Beckers, A.; Fleseriu, M.; et al. Clinical Biology of the Pituitary Adenoma. Endocr. Rev. 2022, 43, 1003–1037. [Google Scholar] [CrossRef] [PubMed]
- Tritos, N.A.; Miller, K.K. Diagnosis and Management of Pituitary Adenomas: A Review. JAMA 2023, 329, 1386–1398. [Google Scholar] [CrossRef]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, H.; Inoshita, N. New WHO classification of pituitary adenomas (4th edition): Assessment of pituitary transcription factors and the prognostic histological factors. Brain Tumor Pathol. 2018, 35, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Budan, R.M.; Georgescu, C.E. Multiple Pituitary Adenomas: A Systematic Review. Front. Endocrinol. 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgos, G.; Thodou, E. Double adenomas of the pituitary: An imaging, pathological, and clinical diagnostic challenge. Hormones 2019, 18, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Mete, O.; Asa, S.L. Structure, Function, and Morphology in the Classification of Pituitary Neuroendocrine Tumors: The Importance of Routine Analysis of Pituitary Transcription Factors. Endocr. Pathol. 2020, 31, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Wilson, C.B. Simultaneously occurring prolactinomas. Case report. J. Neurosurg. 1981, 55, 124–126. [Google Scholar] [CrossRef]
- Woosley, R.E. Multiple secreting microadenomas as a possible cause of selective transsphenoidal adenomectomy failure. Case report. J. Neurosurg. 1983, 58, 267–269. [Google Scholar] [CrossRef]
- Blevins, L.S., Jr.; Hall, G.S.; Madoff, D.H.; Laws, E.R., Jr.; Wand, G.S. Case report: Acromegaly and Cushing’s disease in a patient with synchronous pituitary adenomas. Am. J. Med. Sci. 1992, 304, 294–297. [Google Scholar] [CrossRef]
- Kontogeorgos, G.; Scheithauer, B.W.; Horvath, E.; Kovacs, K.; Lloyd, R.V.; Smyth, H.S.; Rologis, D. Double adenomas of the pituitary: A clinicopathological study of 11 tumors. Neurosurgery 1992, 31, 840–849; discussion 849. [Google Scholar] [CrossRef] [PubMed]
- Wynne, A.G.; Scheithauer, B.W.; Young, W.F., Jr.; Kovacs, K.; Ebersold, M.J.; Horvath, E. Coexisting corticotroph and lactotroph adenomas: Case report with reference to the relationship of corticotropin and prolactin excess. Neurosurgery 1992, 30, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Shintani, Y.; Yoshimoto, K.; Horie, H.; Sano, T.; Kanesaki, Y.; Hosoi, E.; Yokogoshi, Y.; Bando, H.; Iwahana, H.; Kannuki, S.; et al. Two different pituitary adenomas in a patient with multiple endocrine neoplasia type 1 associated with growth hormone-releasing hormone-producing pancreatic tumor: Clinical and genetic features. Endocr. J. 1995, 42, 331–340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kannuki, S.; Matsumoto, K.; Sano, T.; Shintani, Y.; Bando, H.; Saito, S. Double pituitary adenoma--two case reports. Neurol. Med. Chir. 1996, 36, 818–821. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sano, T.; Horiguchi, H.; Xu, B.; Li, C.; Hino, A.; Sakaki, M.; Kannuki, S.; Yamada, S. Double pituitary adenomas: Six surgical cases. Pituitary 1999, 1, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Meij, B.P.; Lopes, M.B.; Vance, M.L.; Thorner, M.O.; Laws, E.R., Jr. Double pituitary lesions in three patients with Cushing’s disease. Pituitary 2000, 3, 159–168. [Google Scholar] [CrossRef]
- Ratliff, J.K.; Oldfield, E.H. Multiple pituitary adenomas in Cushing’s disease. J. Neurosurg. 2000, 93, 753–761. [Google Scholar] [CrossRef]
- Syro, L.V.; Horvath, E.; Kovacs, K. Double adenoma of the pituitary: A somatotroph adenoma colliding with a gonadotroph adenoma. J. Endocrinol. Investig. 2000, 23, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Tosaka, M.; Kohga, H.; Kobayashi, S.; Zama, A.; Tamura, M.; Murakami, M.; Sasaki, T. Double pituitary adenomas detected on preoperative magnetic resonance images. Case illustration. J. Neurosurg. 2000, 92, 361. [Google Scholar] [CrossRef] [PubMed]
- McKelvie, P.A.; McNeill, P. Double pituitary adenomas: A series of three patients. Pathology 2002, 34, 57–60. [Google Scholar] [CrossRef]
- Kim, K.; Yamada, S.; Usui, M.; Sano, T. Preoperative identification of clearly separated double pituitary adenomas. Clin. Endocrinol. 2004, 61, 26–30. [Google Scholar] [CrossRef]
- Shimizu, C.; Koike, T.; Sawamura, Y. Double pituitary adenomas with distinct histological features and immunophenotypes. J. Neurol. Neurosurg. Psychiatry 2004, 75, 140. [Google Scholar] [PubMed]
- Jastania, R.A.; Alsaad, K.O.; Al-Shraim, M.; Kovacs, K.; Asa, S.L. Double adenomas of the pituitary: Transcription factors Pit-1, T-pit, and SF-1 identify cytogenesis and differentiation. Endocr. Pathol. 2005, 16, 187–194. [Google Scholar] [CrossRef]
- Oyama, K.; Yamada, S.; Hukuhara, N.; Hiramatsu, R.; Taguchi, M.; Yazawa, M.; Matsuda, A.; Ohmura, E.; Imai, Y. FSH-producing macroadenoma associated in a patient with Cushing’s disease. Neuro Endocrinol. Lett. 2006, 27, 733–736. [Google Scholar] [PubMed]
- Andrioli, M.; Pecori Giraldi, F.; Losa, M.; Terreni, M.; Invitti, C.; Cavagnini, F. Cushing’s disease due to double pituitary ACTH-secreting adenomas: The first case report. Endocr. J. 2010, 57, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Coiré, C.I.; Smyth, H.S.; Rosso, D.; Horvath, E.; Kovacs, K. A double pituitary adenoma presenting as a prolactin-secreting tumor with partial response to medical therapy. Case report. Endocr. Pathol. 2010, 21, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Magri, F.; Villa, C.; Locatelli, D.; Scagnelli, P.; Lagonigro, M.S.; Morbini, P.; Castellano, M.; Gabellieri, E.; Rotondi, M.; Solcia, E.; et al. Prevalence of double pituitary adenomas in a surgical series: Clinical, histological and genetic features. J. Endocrinol. Investig. 2010, 33, 325–331. [Google Scholar] [CrossRef]
- Mohammed, S.; Cusimano, M.D.; Scheithauer, B.W.; Rotondo, F.; Horvath, E.; Kovacs, K. O-methylguanine-DNA methyltransferase immunoexpression in a double pituitary adenoma: Case report. Neurosurgery 2010, 66, E421–E422. [Google Scholar] [CrossRef] [PubMed]
- Koutourousiou, M.; Kontogeorgos, G.; Wesseling, P.; Grotenhuis, A.J.; Seretis, A. Collision sellar lesions: Experience with eight cases and review of the literature. Pituitary 2010, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, F.; Khatun, N.; Scheithauer, B.W.; Horvath, E.; Marotta, T.R.; Cusimano, M.; Kovacs, K. Unusual double pituitary adenoma: A case report. Pathol. Int. 2011, 61, 42–46. [Google Scholar] [CrossRef]
- Zieliński, G.; Maksymowicz, M.; Podgórski, J.; Olszewski, W.T. Double, synchronous pituitary adenomas causing acromegaly and Cushing’s disease. A case report and review of literature. Endocr. Pathol. 2013, 24, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Iacovazzo, D.; Bianchi, A.; Lugli, F.; Milardi, D.; Giampietro, A.; Lucci-Cordisco, E.; Doglietto, F.; Lauriola, L.; De Marinis, L. Double pituitary adenomas. Endocrine 2013, 43, 452–457. [Google Scholar] [CrossRef]
- Mendola, M.; Dolci, A.; Piscopello, L.; Tomei, G.; Bauer, D.; Corbetta, S.; Ambrosi, B. Rare case of Cushing’s disease due to double ACTH-producing adenomas, one located in the pituitary gland and one into the stalk. Hormones 2014, 13, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Takei, M.; Ohkubo, Y.; Kakizawa, Y.; Matoba, H.; Kumagai, M.; Takeda, T.; Suzuki, S.; Komatsu, M. A somatotropin-producing pituitary adenoma with an isolated adrenocorticotropin-producing pituitary adenoma in a female patient with acromegaly, subclinical Cushing’s disease and a left adrenal tumor. Endocr. J. 2014, 61, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.U.; Montgomery, B.K.; Raghavan, P.; Sharma, S.; Nieman, L.K.; Patronas, N.; Oldfield, E.H.; Chittiboina, P. Different imaging characteristics of concurrent pituitary adenomas in a patient with Cushing’s disease. J. Clin. Neurosci. 2015, 22, 891–894. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eytan, S.; Kim, K.Y.; Bleich, D.; Raghuwanshi, M.; Eloy, J.A.; Liu, J.K. Isolated double pituitary adenomas: A silent corticotroph adenoma and a microprolactinoma. J. Clin. Neurosci. 2015, 22, 1676–1678. [Google Scholar] [CrossRef]
- Pu, J.; Wang, Z.; Zhou, H.; Zhong, A.; Jin, K.; Ruan, L.; Yang, G. Isolated double adrenocorticotropic hormone-secreting pituitary adenomas: A case report and review of the literature. Oncol. Lett. 2016, 12, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Borges, M.T.; Lillehei, K.O.; Kleinschmidt-DeMasters, B.K. Double separate versus contiguous pituitary adenomas: MRI features and endocrinological follow up. Pituitary 2016, 19, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Mete, O.; Alshaikh, O.M.; Cintosun, A.; Ezzat, S.; Asa, S.L. Synchronous Multiple Pituitary Neuroendocrine Tumors of Different Cell Lineages. Endocr. Pathol. 2018, 29, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, N.; Doi, R.; Kuramoto, T.; Sakata, K.; Tahara, S.; Sugita, Y.; Morioka, M. Double pituitary adenomas associated with persistent trigeminal artery: A rare case report and the review of literature. Neurosurg. Rev. 2018, 41, 341–345. [Google Scholar] [CrossRef]
- Collazo-Gutiérrez, N.; de Jesús, O.; Villamil-Jarauta, M.; Alvarado, M.; González, L.; Ramírez, M.; Carlo-Chevere, V.J. Double Pituitary Adenomas with Synchronous Somatotroph and Corticotroph Clinical Presentation of Acromegaly and Cushing’s Disease. World Neurosurg. 2019, 132, 161–164. [Google Scholar] [CrossRef]
- Zieliński, G.; Sajjad, E.A.; Maksymowicz, M.; Pękul, M.; Koziarski, A. Double pituitary adenomas in a large surgical series. Pituitary 2019, 22, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Saindane, A.M.; Neill, S.G.; Oyesiku, N.M.; Ioachimescu, A.G. The Intriguing Case of a Double Pituitary Adenoma. World Neurosurg. 2019, 126, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Kinoshita, Y.; Tominaga, A.; Amatya, V.J.; Takeshima, Y.; Yamasaki, F. Metachronous Double Pituitary Adenoma with Altered Transcriptional Factor Profile: A Case Report and Literature Review. NMC Case Rep. J. 2021, 8, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Hagel, C.; Schüller, U.; Flitsch, J.; Knappe, U.J.; Kellner, U.; Bergmann, M.; Buslei, R.; Buchfelder, M.; Rüdiger, T.; Herms, J.; et al. Double adenomas of the pituitary reveal distinct lineage markers, copy number alterations, and epigenetic profiles. Pituitary 2021, 24, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Damiani, L.; Riccioni, L.; Nuzzi, D.; Celico, M.; Panzacchi, R.; Ragazzini, C.; Tosatto, L.; Nasi, M.T.; Balestrieri, A. Two Cases of Double Pituitary Adenomas in a Surgical Series Over 16 Years in a Single Centre. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1518–1523. [Google Scholar] [CrossRef]
- Pecorari, I.L.; Mahali, L.P.; Funari, A.; Fecher, R.; Suda, N.; Agarwal, V. Silent Corticotroph and Somatotroph Double Pituitary Adenoma: A Case Report and Review of Literature. J. Neurol. Surg. Rep. 2022, 83, e33–e38. [Google Scholar] [CrossRef]
- Demirci, H.; Kahraman, D.; Kuzucu, P.; Şenol, Ö.; Uğur, K.; Ergün, M.A.; Keskil, S.; Akdemir Özışık, P. Growth hormone-releasing pituitary microadenoma overshaded by a macroadenoma: A case of double pituitary adenomas and review of the literature. Br. J. Neurosurg. 2022, 38, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.A.; Tavakoli, S.; Shah, I.; Laing, B.R.; Coss, D.; Ioachimescu, A.G.; Findling, J.; Zwagerman, N.T. Consecutive resections of double pituitary adenoma for resolution of Cushing disease: Illustrative case. J. Neurosurg. Case Lessons 2023, 6, CASE23485. [Google Scholar] [CrossRef]
- Nakazato, I.; Oyama, K.; Ishikawa, H.; Tabei, Y.; Inomoto, C.; Osamura, Y.; Teramoto, A.; Matsuno, A. Double pituitary neuroendocrine tumors in a patient with normal growth hormone level acromegaly: A case report and review of the literature. Surg. Neurol. Int. 2023, 14, 425. [Google Scholar] [CrossRef]
- Ullah, M.T.; Lopes, M.B.S.; Jane, J.A., Jr.; Hong, G.K.; Love, K.M. Co-occurrence of Functional Gonadotroph Adenoma and Lactotroph Adenoma: A Case Report and Literature Review. AACE Clin. Case Rep. 2023, 9, 5–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, X.; Pu, J.; Liu, J.; Ye, Z.; Zhu, H.; Lu, L.; Pan, H.; Deng, K.; Yao, Y. Double pituitary adenomas: Report of two cases and systematic review of the literature. Front. Endocrinol. 2024, 15, 1373869. [Google Scholar] [CrossRef]
- Trofimiuk-Müldner, M.; Domagała, B.; Maksymowicz, M.; Pękul, M.; Zieliński, G.; Hubalewska-Dydejczyk, A. Asynchronous, double, growth hormone-secreting pituitary neuroendocrine tumor of a variable proliferative potential. Pol. Arch. Intern. Med. 2024, 134, 16716. [Google Scholar]
- Pantelia, E.; Kontogeorgos, G.; Piaditis, G.; Rologis, D. Triple pituitary adenoma in Cushing’s disease: Case report. Acta Neurochir. 1998, 140, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, S.; Curtò, L.; Lania, A.; Saccomanno, K.; Salpietro, F.M.; Trimarchi, F. Unusual MRI finding of multiple adenomas in the pituitary gland: A case report and review of the literature. Magn. Reson. Imaging 1999, 17, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Burrow, G.N.; Wortzman, G.; Rewcastle, N.B.; Holgate, R.C.; Kovacs, K. Microadenomas of the pituitary and abnormal sellar tomograms in an unselected autopsy series. N. Engl. J. Med. 1981, 304, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgos, G.; Kovacs, K.; Horvath, E.; Scheithauer, B.W. Multiple adenomas of the human pituitary. A retrospective autopsy study with clinical implications. J. Neurosurg. 1991, 74, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Buurman, H.; Saeger, W. Subclinical adenomas in postmortem pituitaries: Classification and correlations to clinical data. Eur. J. Endocrinol. 2006, 154, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Schöning, J.V.; Flitsch, J.; Lüdecke, D.K.; Fahlbusch, R.; Buchfelder, M.; Buslei, R.; Knappe, U.J.; Bergmann, M.; Schulz-Schaeffer, W.J.; Herms, J.; et al. Multiple tumorous lesions of the pituitary gland. Hormones 2022, 21, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Srirangam Nadhamuni, V.; Korbonits, M. Novel Insights into Pituitary Tumorigenesis: Genetic and Epigenetic Mechanisms. Endocr. Rev. 2020, 41, 821–846. [Google Scholar] [CrossRef]
- Landis, C.A.; Masters, S.B.; Spada, A.; Pace, A.M.; Bourne, H.R.; Vallar, L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 1989, 340, 692–696. [Google Scholar] [CrossRef]
- Reincke, M.; Sbiera, S.; Hayakawa, A.; Theodoropoulou, M.; Osswald, A.; Beuschlein, F.; Meitinger, T.; Mizuno-Yamasaki, E.; Kawaguchi, K.; Saeki, Y.; et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat. Genet. 2015, 47, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Delemer, B. MEN1 and pituitary adenomas. Ann. Endocrinol. 2012, 73, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Ogando-Rivas, E.; Alalade, A.F.; Boatey, J.; Schwartz, T.H. Double pituitary adenomas are most commonly associated with GH- and ACTH-secreting tumors: Systematic review of the literature. Pituitary 2017, 20, 702–708. [Google Scholar] [CrossRef]
- Tahara, S.; Kurotani, R.; Ishii, Y.; Sanno, N.; Teramoto, A.; Osamura, R.Y. A case of Cushing’s disease caused by pituitary adenoma producing adrenocorticotropic hormone and growth hormone concomitantly: Aberrant expression of transcription factors NeuroD1 and Pit-1 as a proposed mechanism. Mod. Pathol. 2002, 15, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, K.M.; Greenman, Y.; Ram, Z.; Hershkovitz, D.; Aizenstein, O.; Ariel, O.; Asa, S.L. Plurihormonal Pituitary Tumor of Pit-1 and SF-1 Lineages, with Synchronous Collision Corticotroph Tumor: A Possible Stem Cell Phenomenon. Endocr. Pathol. 2019, 30, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Mete, O.; Riddle, N.D.; Perry, A. Multilineage Pituitary Neuroendocrine Tumors (PitNETs) Expressing PIT1 and SF1. Endocr. Pathol. 2023, 34, 273–278. [Google Scholar] [CrossRef]
- Boguszewski, C.L.; Boguszewski, M. Growth Hormone’s Links to Cancer. Endocr. Rev. 2019, 40, 558–574. [Google Scholar] [CrossRef] [PubMed]
- Šošić-Jurjević, B.; Ajdžanović, V.; Miljić, D.; Trifunović, S.; Filipović, B.; Stanković, S.; Bolevich, S.; Jakovljević, V.; Milošević, V. Pituitary Hyperplasia, Hormonal Changes and Prolactinoma Development in Males Exposed to Estrogens—An Insight from Translational Studies. Int. J. Mol. Sci. 2020, 21, 2024. [Google Scholar] [CrossRef]
- Inoshita, N.; Yoshimoto, T.; Takazawa, Y.; Fukuhara, N.; Okada, M.; Nishioka, H.; Yamada, S. Immunohistochemical and ultrastructural review of six cases previously diagnosed as null cell PitNETs. Brain Tumor Pathol. 2023, 40, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on diagnosis and management of Cushing’s disease: A guideline update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [Google Scholar] [CrossRef] [PubMed]
- Patronas, N.; Bulakbasi, N.; Stratakis, C.A.; Lafferty, A.; Oldfield, E.H.; Doppman, J.; Nieman, L.K. Spoiled gradient recalled acquisition in the steady state technique is superior to conventional postcontrast spin echo technique for magnetic resonance imaging detection of adrenocorticotropin-secreting pituitary tumors. J. Clin. Endocrinol. Metab. 2003, 88, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Koulouri, O.; Steuwe, A.; Gillett, D.; Hoole, A.C.; Powlson, A.S.; Donnelly, N.A.; Burnet, N.G.; Antoun, N.M.; Cheow, H.; Mannion, R.J.; et al. A role for 11C-methionine PET imaging in ACTH-dependent Cushing’s syndrome. Eur. J. Endocrinol. 2015, 173, M107–M120. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Langlois, F.; Lim, D.S.T.; Varlamov, E.V.; Melmed, S. Acromegaly: Pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol. 2022, 10, 804–826. [Google Scholar] [CrossRef]
- Fukuhara, N.; Nishiyama, M.; Iwasaki, Y. Update in Pathogenesis, Diagnosis, and Therapy of Prolactinoma. Cancers 2022, 14, 3604. [Google Scholar] [CrossRef] [PubMed]
- Petersenn, S.; Fleseriu, M.; Casanueva, F.F.; Giustina, A.; Biermasz, N.; Biller, B.M.K.; Bronstein, M.; Chanson, P.; Fukuoka, H.; Gadelha, M.; et al. Diagnosis and management of prolactin-secreting pituitary adenomas: A Pituitary Society international Consensus Statement. Nat. Rev. Endocrinol. 2023, 19, 722–740. [Google Scholar] [CrossRef] [PubMed]
- Shimatsu, A.; Nakamura, A.; Takahashi, Y.; Fujio, S.; Satoh, F.; Tahara, S.; Nishioka, H.; Takano, K.; Yamashita, M.; Arima, H.; et al. Preoperative and long-term efficacy and safety of lanreotide autogel in patients with thyrotropin-secreting pituitary adenoma: A multicenter, single-arm, phase 3 study in Japan. Endocr. J. 2021, 68, 791–805. [Google Scholar] [CrossRef]
- Ho, K.K.Y.; Fleseriu, M.; Wass, J.; Katznelson, L.; Raverot, G.; Little, A.S.; Castaño, J.P.; Reincke, M.; Lopes, M.B.; Kaiser, U.B.; et al. A proposed clinical classification for pituitary neoplasms to guide therapy and prognosis. Lancet Diabetes Endocrinol. 2024, 12, 209–214. [Google Scholar] [CrossRef] [PubMed]
| Clinical Feature | Number (% of All Cases) | |
|---|---|---|
| Any feature (*) | ||
| Acromegaly | 61 | (45.5) |
| Cushing | 47 | (35.1) |
| Prolactinoma | 24 | (17.9) |
| TSHoma | 2 | (1.5) |
| Gonadotropinoma | 1 | (0.0) |
| None | 17 | (12.7) |
| Double features | ||
| Acromegaly + Cushing | 3 | (2.2) |
| Acromegaly + Prolactinoma | 6 | (4.5) |
| Cushing + Prolactinoma | 7 | (5.2) |
| First PitNET | Second PitNET | ||||||
|---|---|---|---|---|---|---|---|
| GH | ACTH | PRL | LH/FSH | TSH | Others | Total | |
| GH | 22 | 22 | 7 | 22 | 2 | 7 | 82 |
| ACTH | 22 | 10 | 27 | 11 | 0 | 6 | 76 |
| PRL | 7 | 27 | 4 | 8 | 1 | 3 | 50 |
| LH/FSH | 22 | 11 | 8 | 4 | 0 | 2 | 47 |
| TSH | 2 | 0 | 1 | 0 | 0 | 0 | 3 |
| Others | 7 | 6 | 3 | 2 | 0 | 8 | 26 |
| Total | 82 | 76 | 50 | 47 | 3 | 26 | 284 |
| IHC | Results | 1980–2009 | 2010–2024 | Total | p Value |
|---|---|---|---|---|---|
| GH | (+) (−) | 35 81 | 47 121 | 82 202 | 0.69 |
| ACTH | (+) (−) | 32 84 | 44 124 | 76 208 | 0.79 |
| PRL | (+) (−) | 27 89 | 23 145 | 50 234 | 0.04 * |
| LH/FSH | (+) (−) | 8 108 | 39 129 | 47 237 | <0.01 * |
| Pituitary MRI Findings | Number (% of All Cases) | |
|---|---|---|
| Single tumor | ||
| Macro-tumor | 26 | (31.7) |
| Micro-tumor | 13 | (15.9) |
| Double tumors | ||
| Two macro-tumors | 8 | (9.8) |
| Two micro-tumors | 12 | (14.6) |
| Macro-tumor + Micro-tumor | 17 | (20.7) |
| No tumor | ||
| None | 6 | (7.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiyama, M.; Fukuhara, N.; Nishioka, H.; Yamada, S. Double PitNETs: A Case Report and Literature Review. Cancers 2025, 17, 675. https://doi.org/10.3390/cancers17040675
Nishiyama M, Fukuhara N, Nishioka H, Yamada S. Double PitNETs: A Case Report and Literature Review. Cancers. 2025; 17(4):675. https://doi.org/10.3390/cancers17040675
Chicago/Turabian StyleNishiyama, Mitsuru, Noriaki Fukuhara, Hiroshi Nishioka, and Shozo Yamada. 2025. "Double PitNETs: A Case Report and Literature Review" Cancers 17, no. 4: 675. https://doi.org/10.3390/cancers17040675
APA StyleNishiyama, M., Fukuhara, N., Nishioka, H., & Yamada, S. (2025). Double PitNETs: A Case Report and Literature Review. Cancers, 17(4), 675. https://doi.org/10.3390/cancers17040675






