Role of Spatially Fractionated Radiotherapy (LATTICE) Treatment in Inoperable Bulky Soft-Tissue Sarcomas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
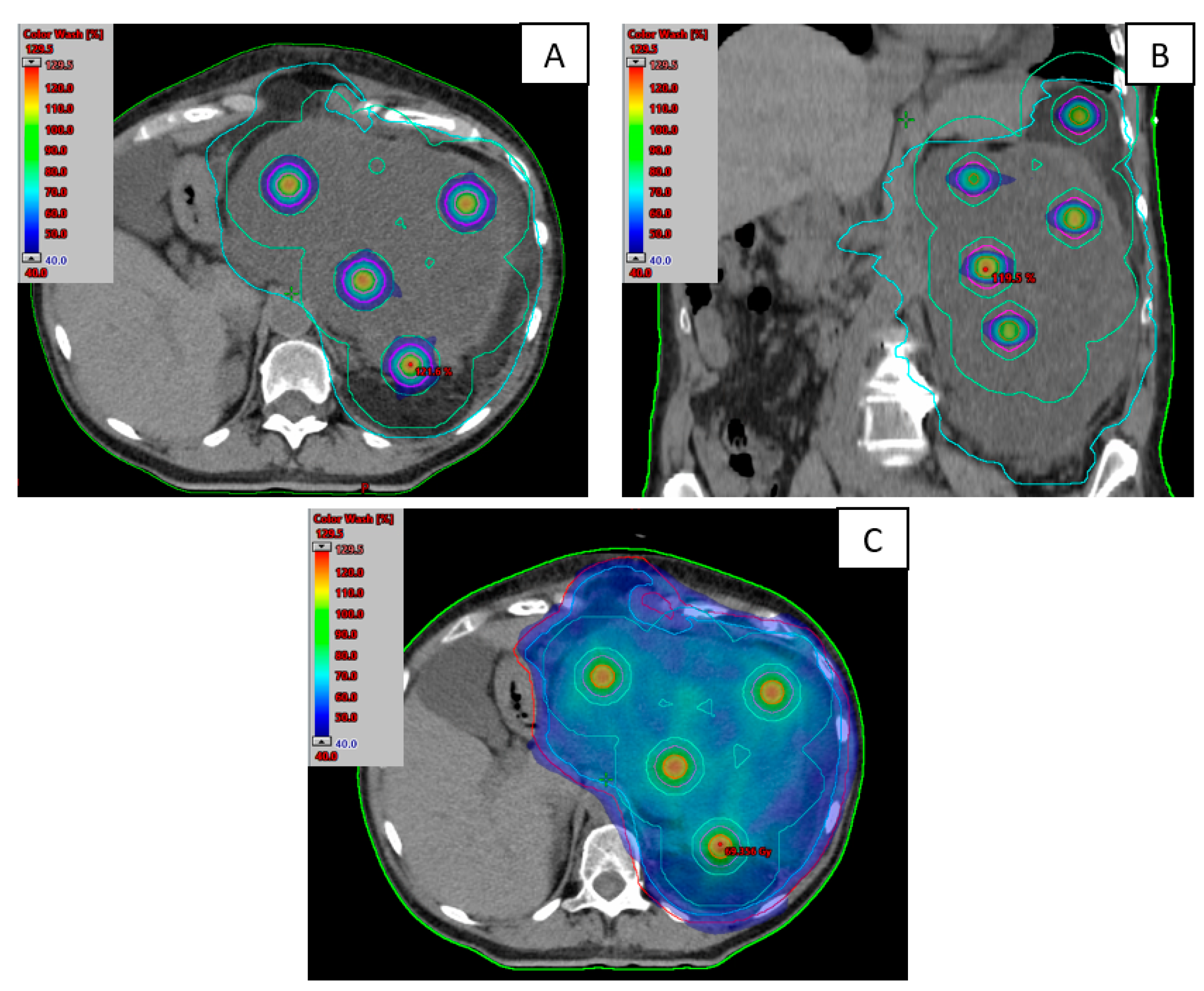
2.1. Treatment Planification
2.2. Clinical Evaluation
3. Results
3.1. Patients’ Characteristics
3.2. Treatment Characteristics
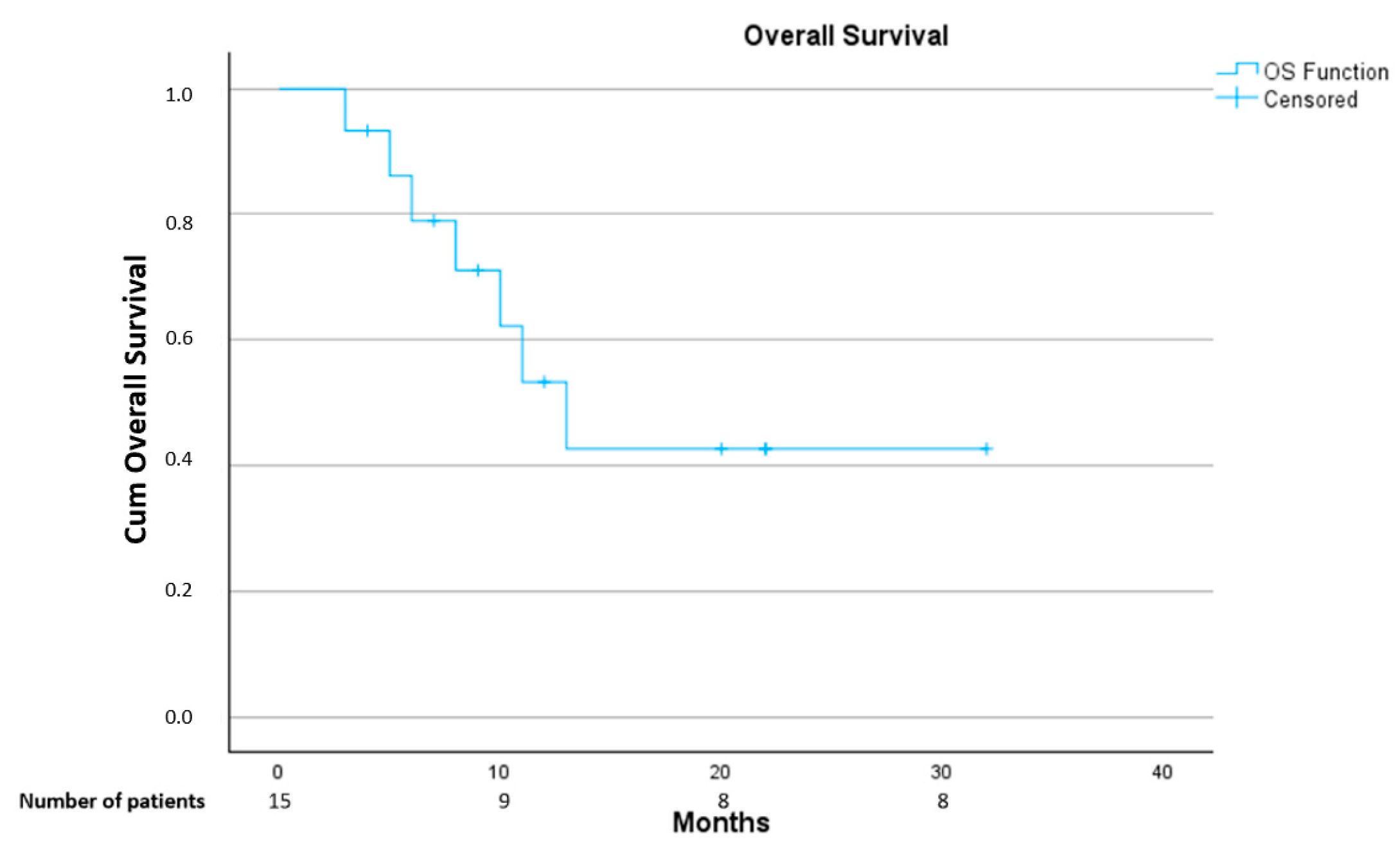
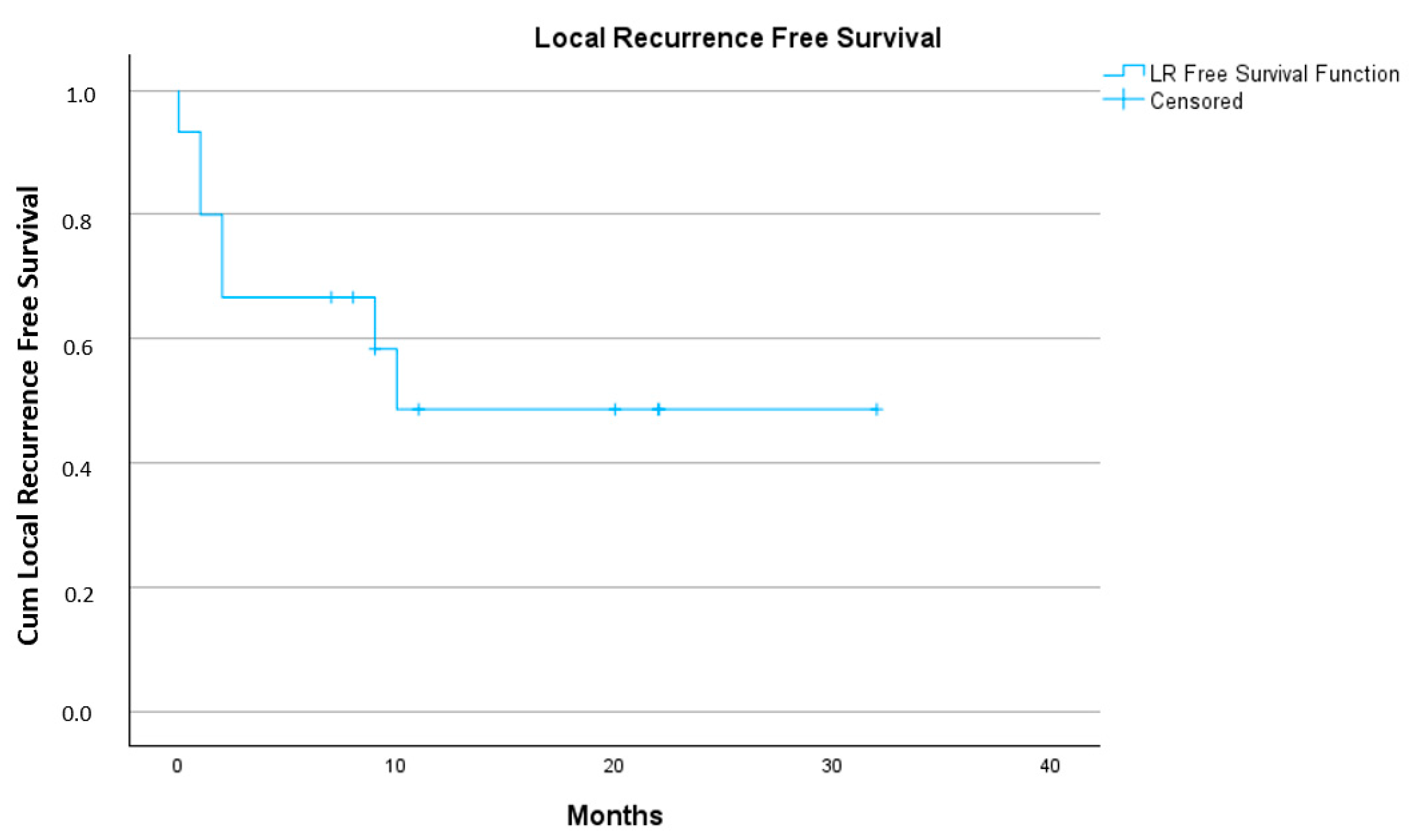
3.3. Treatment Outcome
3.4. Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lahat, G.; Lazar, A.; Lev, D. Sarcoma Epidemiology and Etiology: Potential Environmental and Genetic Factors. Surg. Clin. N. Am. 2008, 88, 451–481. [Google Scholar] [CrossRef]
- Armstrong, S.A.; Bishop, A.J.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Dry, S.; Eulo, V.; Ganjoo, K.N.; Gonzalez, R.J.; Holder, A.; et al. NCCN Guidelines Version 2.2023 Soft Tissue Sarcoma; National Comprehensive Cancer Network: Philadelphia, PA, USA, 2023. [Google Scholar]
- Kepka, L.; DeLaney, T.F.; Suit, H.D.; Goldberg, S.I. Results of radiation therapy for unresected soft-tissue sarcomas. Int. J. Radiat. Oncol. 2005, 63, 852–859. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Allibhai, Z.; Taremi, M.; Bezjak, A.; Brade, A.; Hope, A.J.; Sun, A.; Cho, B.C.J. The Impact of Tumor Size on Outcomes After Stereotactic Body Radiation Therapy for Medically Inoperable Early-Stage Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. 2013, 87, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.J.; Ahmed, M.M.; Amendola, B.; Belyakov, O.; Bentzen, S.M.; Butterworth, K.T.; Chang, S.; Coleman, C.N.; Djonov, V.; Formenti, S.C.; et al. Understanding High-Dose, Ultra-High Dose Rate, and Spatially Fractionated Radiation Therapy. Int. J. Radiat. Oncol. 2020, 107, 766–778. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Fujita, M.; Regine, W.F.; Megooni, A.S.; Ibbott, G.S.; Ahmed, M.M. High-dose spatially-fractionated radiation (GRID): A new paradigm in the management of advanced cancers. Int. J. Radiat. Oncol. 1999, 45, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Marks, H. Clinical experience with irradiation through a grid. Radiology 1952, 58, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Perez, N.C.; Zheng, Y.; Li, X.; Jiang, L.; Amendola, B.E.; Xu, B.; Mayr, N.A.; Lu, J.J.; Hatoum, G.F.; et al. The Technical and Clinical Implementation of LATTICE Radiation Therapy (LRT). Radiat. Res. 2020, 194, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Billena, C.; Khan, A.J. A Current Review of Spatial Fractionation: Back to the Future? Int. J. Radiat. Oncol. 2019, 104, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Duriseti, S.; Kavanaugh, J.A.; Szymanski, J.; Huang, Y.; Basarabescu, F.; Chaudhuri, A.; Henke, L.; Samson, P.; Lin, A.; Robinson, C.; et al. LITE SABR M1: A phase I trial of Lattice stereotactic body radiotherapy for large tumors. Radiother. Oncol. 2022, 167, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Iori, F.; Cappelli, A.; D’Angelo, E.; Cozzi, S.; Ghersi, S.F.; De Felice, F.; Ciammella, P.; Bruni, A.; Iotti, C. Lattice Radiation Therapy in clinical practice: A systematic review. Clin. Transl. Radiat. Oncol. 2023, 39, 100569. [Google Scholar] [CrossRef] [PubMed]
- Borzov, E.; Bar-Deroma, R.; Lutsyk, M. Physical aspects of a spatially fractionated radiotherapy technique for large soft tissue sarcomas. Phys. Imaging Radiat. Oncol. 2022, 22, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Grams, M.P.; Owen, D.; Park, S.S.; Petersen, I.A.; Haddock, M.G.; Jeans, E.B.; Finley, R.R.; Ma, D.J. VMAT Grid Therapy: A Widely Applicable Planning Approach. Pract. Radiat. Oncol. 2021, 11, e339–e347. [Google Scholar] [CrossRef] [PubMed]
- Iori, F.; Botti, A.; Ciammella, P.; Cozzi, S.; Orlandi, M.; Iori, M.; Iotti, C. How a very large sarcomatoid lung cancer was efficiently managed with lattice radiation therapy: A case report. Ann. Palliat. Med. 2022, 11, 3555–3561. [Google Scholar] [CrossRef]
- Jiang, L.; Li, X.; Zhang, J.; Li, W.; Dong, F.; Chen, C.; Lin, Q.; Zhang, C.; Zheng, F.; Yan, W.; et al. Combined High-Dose LATTICE Radiation Therapy and Immune Checkpoint Blockade for Advanced Bulky Tumors: The Concept and a Case Report. Front. Oncol. 2021, 10, 548132. [Google Scholar] [CrossRef]
- Mayr, N.A.; Mohiuddin, M.; Snider, J.W.; Zhang, H.; Griffin, R.J.; Amendola, B.E.; Hippe, D.S.; Perez, N.C.; Wu, X.; Lo, S.S.; et al. Practice Patterns of Spatially Fractionated Radiation Therapy: A Clinical Practice Survey. Adv. Radiat. Oncol. 2024, 9, 101308. [Google Scholar] [CrossRef] [PubMed]
- Ferini, G.; Parisi, S.; Lillo, S.; Viola, A.; Minutoli, F.; Critelli, P.; Valenti, V.; Illari, S.I.; Brogna, A.; Umana, G.E.; et al. Impressive Results after “Metabolism-Guided” Lattice Irradiation in Patients Submitted to Palliative Radiation Therapy: Preliminary Results of LATTICE_01 Multicenter Study. Cancers 2022, 14, 3909. [Google Scholar] [CrossRef] [PubMed]
- Spałek, M.J. Lattice radiotherapy: Hype or hope? Ann. Palliat. Med. 2022, 11, 3378–3381. [Google Scholar] [CrossRef]
- Mayr, N.A.; Snider, J.W.; Regine, W.F.; Mohiuddin, M.; Hippe, D.S.; Peñagarícano, J.; Mohiuddin, M.; Kudrimoti, M.R.; Zhang, H.; Limoli, C.L.; et al. An International Consensus on the Design of Prospective Clinical–Translational Trials in Spatially Fractionated Radiation Therapy. Adv. Radiat. Oncol. 2022, 7, 100866. [Google Scholar] [CrossRef] [PubMed]
- Duriseti, S.; Kavanaugh, J.; Goddu, S.; Price, A.; Knutson, N.; Reynoso, F.; Michalski, J.; Mutic, S.; Robinson, C.; Spraker, M.B. Spatially fractionated stereotactic body radiation therapy (Lattice) for large tumors. Adv. Radiat. Oncol. 2021, 6, 100639. [Google Scholar] [CrossRef]
- Yan, W.; Khan, M.K.; Wu, X.; Simone, C.B.; Fan, J.; Gressen, E.; Zhang, X.; Limoli, C.L.; Bahig, H.; Tubin, S.; et al. Spatially fractionated radiation therapy: History, present and the future. Clin. Transl. Radiat. Oncol. 2020, 20, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Petersen, I.A.; Grams, M.P.; Finley, R.R.; Haddock, M.G.; Owen, D. Spatially Fractionated Radiation Therapy in Sarcomas: A Large Single-Institution Experience. Adv. Radiat. Oncol. 2024, 9, 101401. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Memon, M.; Nobah, A.; Elsebaie, M.; AL Suhaibani, A.; Pant, R.; Shaheen, M.; Alyamani, M.; Al Dayal, F. Locally advanced high-grade extremity soft tissue sarcoma: Response with novel approach to neoadjuvant chemoradiation using induction spatially fractionated GRID radiotherapy (SFGRT). J. Clin. Oncol. 2014, 32, 10575. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Miller, T.; Ronjon, P.; Malik, U. Spatially Fractionated Grid Radiation (SFGRT): A Novel Approach in the Management of Recurrent and Unresectable Soft Tissue Sarcoma. Int. J. Radiat. Oncol. 2009, 75, S526. [Google Scholar] [CrossRef]
- Snider, J.W.; Molitoris, J.; Shyu, S.; Diwanji, T.; Rice, S.; Kowalski, E.; Decesaris, C.; Remick, J.S.; Yi, B.; Zhang, B.; et al. Spatially Fractionated Radiotherapy (GRID) Prior to Standard Neoadjuvant Conventionally Fractionated Radiotherapy for Bulky, High-Risk Soft Tissue and Osteosarcomas: Feasibility, Safety, and Promising Pathologic Response Rates. Radiat. Res. 2020, 194, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, G.F.; Temple, H.T.; Garcia, S.A.; Zheng, Y.; Kfoury, F.; Kinley, J.; Wu, X. Neoadjuvant Radiation Therapy with Interdigitated High-Dose LRT for Voluminous High-Grade Soft-Tissue Sarcoma. Cancer Manag. Res. 2023, 15, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Mohiuddin, M.M.; Jackson, G.L. Dramatic response from neoadjuvant, spatially fractionated GRID radiotherapy (SFGRT) for large, high-grade extremity sarcoma. J. Radiat. Oncol. 2013, 2, 103–106. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Collini, P.; Messina, A.; Morosi, C.; Barisella, M.; Bertulli, R.; Piovesan, C.; Dileo, P.; Torri, V.; Gronchi, A.; et al. High-Grade Soft-Tissue Sarcomas: Tumor Response Assessment—Pilot Study to Assess the Correlation between Radiologic and Pathologic Response by Using RECIST and Choi Criteria. Radiology 2009, 251, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Roets, E.; van der Graaf, W.; van Riet, B.H.G.; Haas, R.L.; Younger, E.; Sparano, F.; Wilson, R.; van der Mierden, S.; Steeghs, N.; Efficace, F.; et al. Patient-reported outcomes in randomized clinical trials of systemic therapy for advanced soft tissue sarcomas in adults: A systematic review. Crit. Rev. Oncol./Hematol. 2024, 197, 104345. [Google Scholar] [CrossRef] [PubMed]
| Characteristics (n = 15) | |
|---|---|
| Median age | 60 (range 29–81) |
| Patient gender | Male: 9 (60%) Female: 6 (40%) |
| Number of lesions treated with LRT | 1 lesion: 14 (93%) 2 lesions: 1 (7%) |
| Tumor type | Primary: 11 (73%) Metastasis: 4 (27%) |
| Median GTV/ITV volume (cm3) | 1058 (range 142–6103) |
| Treatment site | Thorax: 3 (20%) Abdomen: 7 (47%) Pelvis: 5 (33%) |
| Histology | Chondrosarcoma: 2 (13%) Dedifferentiated liposarcoma: 4 (27%) |
| Endometrial stromal sarcoma: 1 (7%) Leiomyosarcoma: 2 (13%) Malignant peripheral nerves heath tumor: 1 (7%) Synovial sarcoma: 1 (7%) Undifferentiated pleomorphic sarcoma: 1 (7%) Undifferentiated spindle cell sarcoma: 3 (20%) | |
| Performance status (PS) | 0–1: 9 (60%) 2: 6 (40%) |
| Treatment Parameters | Median (Range) |
|---|---|
| Number of spheres | 9 (4–30) |
| LRT dose (Gy) | 20 |
| EBRT dose (Gy) | 45 (20–54) |
| Number of EBRT fractions | 25 (5–27) |
| Number of days between LRT and EBRT | 1 (1–14) |
| Clinical Results and Toxicity | |
|---|---|
| Number of patients with symptoms before treatment | 11 (73%) |
| Clinical response | No: 0 Yes: 11 (100%) Worsening: 0 |
| Number of patients operated | 5 (33%) |
| RECIST1.1 criteria | Stable disease: 10 (67%) Progression: 5 (33%) |
| Toxicity G0 | 5 (33%) |
| Toxicity G1 | Skin: 1 (7%) Gastrointestinal: 4 (27%) Genitourinary: 2 (13%) |
| Toxicity G2 | Gastrointestinal: 2 (13%) Asthenia: 1 (7%) |
| Toxicity G3 | Gastrointestinal: esophagitis: 1 (7%) Skin: 1 (7%) |
| Pathological response of operated patients (median, range) | 45% (10–90%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majercakova, K.; Aguilar, N.T.; Isern Verdum, J.; Bargalló, H.V.; Capel, A.V.; Mancera Soto, M.; Gómez de Segura Melcón, G.; Cordero, J.V.R.; González-López, J.A.; Rosell, S.B.; et al. Role of Spatially Fractionated Radiotherapy (LATTICE) Treatment in Inoperable Bulky Soft-Tissue Sarcomas. Cancers 2025, 17, 624. https://doi.org/10.3390/cancers17040624
Majercakova K, Aguilar NT, Isern Verdum J, Bargalló HV, Capel AV, Mancera Soto M, Gómez de Segura Melcón G, Cordero JVR, González-López JA, Rosell SB, et al. Role of Spatially Fractionated Radiotherapy (LATTICE) Treatment in Inoperable Bulky Soft-Tissue Sarcomas. Cancers. 2025; 17(4):624. https://doi.org/10.3390/cancers17040624
Chicago/Turabian StyleMajercakova, Katarina, Natalia Tejedor Aguilar, Josep Isern Verdum, Helena Vivancos Bargalló, Antonio Vila Capel, Miriam Mancera Soto, Guillermo Gómez de Segura Melcón, Jady Vivian Rojas Cordero, José Antonio González-López, Silvia Bagué Rosell, and et al. 2025. "Role of Spatially Fractionated Radiotherapy (LATTICE) Treatment in Inoperable Bulky Soft-Tissue Sarcomas" Cancers 17, no. 4: 624. https://doi.org/10.3390/cancers17040624
APA StyleMajercakova, K., Aguilar, N. T., Isern Verdum, J., Bargalló, H. V., Capel, A. V., Mancera Soto, M., Gómez de Segura Melcón, G., Cordero, J. V. R., González-López, J. A., Rosell, S. B., Jover, D. H., Mitre, S. R., Ibañez, A. P., Sebio, A., & Sancho-Pardo, G. (2025). Role of Spatially Fractionated Radiotherapy (LATTICE) Treatment in Inoperable Bulky Soft-Tissue Sarcomas. Cancers, 17(4), 624. https://doi.org/10.3390/cancers17040624






