Impact of Systemic and Radiation Therapy on Survival of Primary Central Nervous System Lymphoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Treatment Characteristics
3.3. Associations with Patient Outcomes
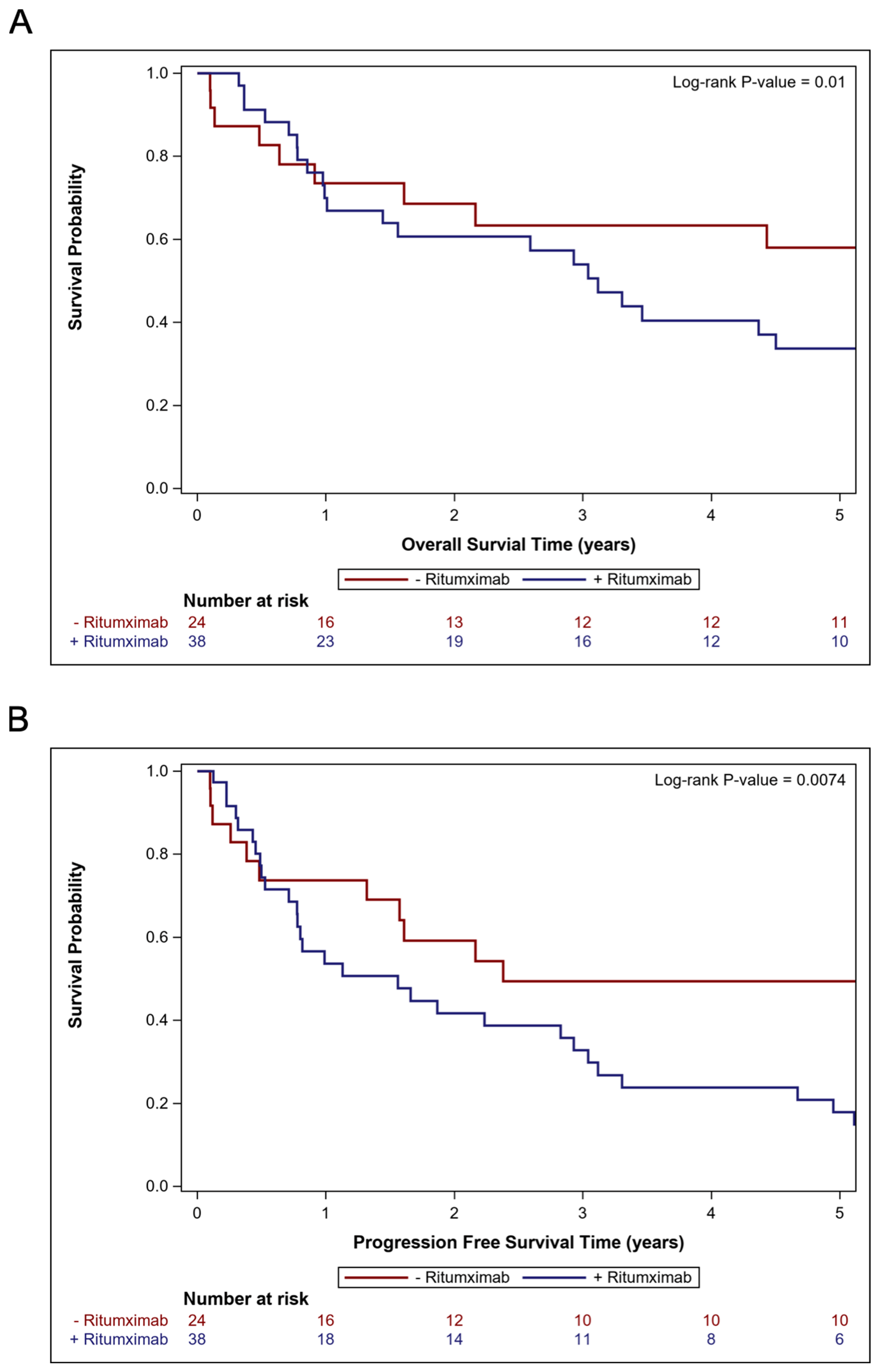
3.4. Comparisons of Cohorts Treated with and Without Rituximab
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendez, J.S.; Ostrom, Q.T.; Gittleman, H.; Kruchko, C.; Deangelis, L.M.; Barnholtz-Sloan, J.S.; Grommes, C. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro. Oncol. 2018, 20, 687–694. [Google Scholar] [CrossRef]
- Bataille, B.; Delwail, V.; Menet, E.; Vandermarcq, P.; Ingrand, P.; Wager, M.; Guy, G.; Lapierre, F. Primary intracerebral malignant lymphoma: Report of 248 cases. J. Neurosurg. 2000, 92, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Abrey, L.E.; Ben-Porat, L.; Panageas, K.S.; Yahalom, J.; Berkey, B.; Curran, W.; Schultz, C.; Leibel, S.; Nelson, D.; Mehta, M.; et al. Primary central nervous system lymphoma: The memorial sloan-kettering cancer center prognostic model. J. Clin. Oncol. 2006, 24, 5711–5715. [Google Scholar] [CrossRef] [PubMed]
- Jahr, G.; Broi, M.D.; Holte, H.; Beiske, K.; Meling, T.R. Evaluation of Memorial Sloan-Kettering Cancer Center and International Extranodal Lymphoma Study Group prognostic scoring systems to predict Overall Survival in intracranial Primary CNS lymphoma. Brain Behav. 2018, 8, e00928. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Blay, J.Y.; Reni, M.; Pasini, F.; Spina, M.; Ambrosetti, A.; Calderoni, A.; Rossi, A.; Vavassori, V.; Conconi, A.; et al. Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group experience. J. Clin. Oncol. 2003, 21, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Burt Nabors, L.; Portnow, J.; Baehring, J.; Brem, S.; Butowski, N.; Campian, J.L.; Cannon, D.M.; Chao, S.; Chheda, M.G.; Clark, S.W.; et al. NCCN Guidelines Version 2.2021 Central Nervous System Cancers Continue NCCN Guidelines Panel Disclosures. 2021. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1425 (accessed on 7 February 2025).
- Thiel, E.; Korfel, A.; Martus, P.; Kanz, L.; Griesinger, F.; Rauch, M.; Röth, A.; Hertenstein, B.; von Toll, T.; Hundsberger, T.; et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010, 11, 1036–1047. [Google Scholar] [CrossRef]
- Poortmans, P.M.P.; Kluin-Nelemans, H.C.; Haaxma-Reiche, H.; Van’t Veer, M.; Hansen, M.; Soubeyran, P.; Taphoorn, M.; Thomas, J.; Van Den Bent, M.; Fickers, M.; et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J. Clin. Oncol. 2003, 21, 4483–4488. [Google Scholar] [CrossRef]
- DeAngelis, L.M.; Seiferheld, W.; Clifford Schold, S.; Fisher, B.; Schultz, C.J. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group study 93-10. J. Clin. Oncol. 2002, 20, 4643–4648. [Google Scholar] [CrossRef]
- Pulczynski, E.J.; Kuittinen, O.; Erlanson, M.; Hagberg, H.; Fosså, A.; Eriksson, M.; Nordstrøm, M.; Østenstad, B.; Fluge, Ø.; Leppä, S.; et al. Successful change of treatment strategy in elderly patients with primary central nervous system lymphoma by de-escalating induction and introducing temozolomide maintenance: Results from a phase II study by the Nordic Lymphoma Group. Haematologica 2015, 100, 534–540. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Cwynarski, K.; Pulczynski, E.; Fox, C.P.; Schorb, E.; La Rosée, P.; Binder, M.; Fabbri, A.; Torri, V.; Minacapelli, E.; et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: Results of the second randomisation of the International Extranodal L. Lancet Haematol. 2017, 4, e510–e523. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Cwynarski, K.; Pulczynski, E.; Ponzoni, M.; Deckert, M.; Politi, L.S.; Torri, V.; Fox, C.P.; Rosée, P.L.; Schorb, E.; et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: Results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016, 3, e217–e227. [Google Scholar] [CrossRef] [PubMed]
- Illerhaus, G.; Ferreri, A.J.M.; Binder, M.; Borchmann, P.; Hasenkamp, J.; Stilgenbauer, S.; Roeth, A.; Weber, T.; Egerer, G.; Ernst, T.; et al. Effects on Survival of Non-Myeloablative Chemoimmunotherapy Compared to High-Dose Chemotherapy Followed By Autologous Stem Cell Transplantation (HDC-ASCT) As Consolidation Therapy in Patients with Primary CNS Lymphoma-Results of an International Randomi. Blood 2022, 140, LBA-3. [Google Scholar] [CrossRef]
- Franklin, J.M.; Patorno, E.; Desai, R.J.; Glynn, R.J.; Martin, D.; Quinto, K.; Pawar, A.; Bessette, L.G.; Lee, H.; Garry, E.M.; et al. Emulating Randomized Clinical Trials with Nonrandomized Real-World Evidence Studies: First Results from the RCT DUPLICATE Initiative. Circulation 2021, 143, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.E.C.; Issa, S.; Bakunina, K.; Minnema, M.C.; Seute, T.; Durian, M.; Cull, G.; Schouten, H.C.; Stevens, W.B.C.; Zijlstra, J.M.; et al. Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): A randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2019, 20, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Chang, S.M.; Van Den Bent, M.J.; Vogelbaum, M.A.; Macdonald, D.R.; Lee, E.Q. Response assessment in neuro-oncology clinical trials. J. Clin. Oncol. 2017, 35, 2439–2449. [Google Scholar] [CrossRef]
- Liu, Y.; Nickleach, D.C.; Zhang, C.; Switchenko, J.M.; Kowalski, J. Carrying out streamlined routine data analyses with reports for observational studies: Introduction to a series of generic SAS ®macros. F1000Research 2019, 7, 1955. [Google Scholar] [CrossRef]
- Chen, C.; Sun, P.; Cui, J.; Yan, S.; Chen, H.; Xia, Y.; Bi, X.; Liu, P.; Wang, Y.; Yang, H.; et al. High-dose Methotrexate plus temozolomide with or without rituximab in patients with untreated primary central nervous system lymphoma: A retrospective study from China. Cancer Med. 2019, 8, 1359–1367. [Google Scholar] [CrossRef]
- Swinnen, L.J.; O’Neill, A.; Imus, P.H.; Gujar, S.; Schiff, D.; Kleinberg, L.R.; Advani, R.H.; Dunbar, E.M.; Moore, D.; Grossman, S.A. Phase II study of rituximab given in conjunction with standard chemotherapy in primary central nervous system lymphoma (PCNSL): A trial of the ECOG-ACRIN cancer research group (E1F05). Oncotarget 2018, 9, 766–773. [Google Scholar] [CrossRef]
- Mishima, K.; Nishikawa, R.; Narita, Y.; Mizusawa, J.; Sumi, M.; Koga, T.; Sasaki, N.; Kinoshita, M.; Nagane, M.; Arakawa, Y.; et al. Randomized phase III study of high-dose methotrexate and whole-brain radiotherapy with/without temozolomide for newly diagnosed primary CNS lymphoma: JCOG1114C. Neuro. Oncol. 2022, 25, 687–698. [Google Scholar] [CrossRef]
- Calimeri, T.; Steffanoni, S.; Gagliardi, F.; Chiara, A.; Ferreri, A.J.M. How we treat primary central nervous system lymphoma. ESMO Open 2021, 6, 100213. [Google Scholar] [CrossRef]
- Hoang-Xuan, K.; Bessell, E.; Bromberg, J.; Hottinger, A.F.; Preusser, M.; Rudà, R.; Schlegel, U.; Siegal, T.; Soussain, C.; Abacioglu, U.; et al. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: Guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015, 16, e322–e332. [Google Scholar] [CrossRef] [PubMed]
- Grommes, C.; DeAngelis, L.M. Primary CNS lymphoma. J. Clin. Oncol. 2017, 35, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.P.; Phillips, E.H.; Smith, J.; Linton, K.; Gallop-Evans, E.; Hemmaway, C.; Auer, D.P.; Fuller, C.; Davies, A.J.; McKay, P.; et al. Guidelines for the diagnosis and management of primary central nervous system diffuse large B-cell lymphoma. Br. J. Haematol. 2019, 184, 348–363. [Google Scholar] [CrossRef]
- Janopaul-Naylor, J.R.; Shen, Y.; Qian, D.C.; Buchwald, Z.S. The abscopal effect: A review of pre-clinical and clinical advances. Int. J. Mol. Sci. 2021, 22, 11061. [Google Scholar] [CrossRef] [PubMed]
- LOC-R01 Study of Lenalidomide and Ibrutinib in Association With Rituximab-Methotrexate Procarbazine Vincristin (R-MPV). Available online: https://clinicaltrials.gov/study/NCT04446962 (accessed on 10 November 2023).
- LTA Pilot Study of Glucarpidase in Patients With Central Nervous System Lymphoma. Available online: https://clinicaltrials.gov/study/NCT03684980 (accessed on 10 November 2023).
- Pembrolizumab, Ibrutinib and Rituximab in PCNSL. Available online: https://clinicaltrials.gov/study/NCT04421560 (accessed on 10 November 2023).
- Study on Pembrolizumab for Recurrent Primary Central Nervous System Lymphoma (PCNSL). Available online: https://clinicaltrials.gov/study/NCT02779101 (accessed on 10 November 2023).
- A Study of Nivolumab in Relapsed/Refractory Primary Central Nervous System Lymphoma (PCNSL) and Relapsed/Refractory Primary Testicular Lymphoma (PTL). Available online: https://clinicaltrials.gov/study/NCT02857426 (accessed on 10 November 2023).
- Nivolumab and Ibrutinib in Treating Patients with Relapsed or Refractory Central Nervous System Lymphoma. Available online: https://clinicaltrials.gov/study/NCT03770416 (accessed on 10 November 2023).
- Kim, S.K.; Park, J.E.; Kim, K.H.; Cho, J.M.; Moon, J.; Yoon, W.-S.; Kim, S.H.; Kim, Y., II; Kim, Y.Z.; Kim, H.S.; et al. A National Consensus Survey for Current Practice in Brain Tumor Management III: Brain Metastasis and Primary Central Nervous System Lymphoma. Brain Tumor Res. Treat. 2020, 8, 20. [Google Scholar] [CrossRef]
- Khan, R.B.; Shi, W.; Thaler, H.T.; DeAngelis, L.M.; Abrey, L.E. Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma? J. Neurooncol. 2002, 58, 175–178. [Google Scholar] [CrossRef]
- Otani, R.; Yamada, R.; Kushihara, Y.; Inazuka, M.; Shinoura, N. Continuous intrathecal injection therapy of methotrexate is a therapeutic option in primary CNS lymphoma. J. Clin. Neurosci. 2019, 69, 26–30. [Google Scholar] [CrossRef]
- Qian, L.; Zhou, C.; Shen, J.; Cen, J.; Yin, W. Treatment of newly diagnosed B-cell origin primary CNS lymphoma with systemic R-IDARAM chemotherapy and intrathecal immunochemotherapy. Oncotarget 2016, 7, 25783–25790. [Google Scholar] [CrossRef][Green Version]
- Morris, P.G.; Correa, D.D.; Yahalom, J.; Raizer, J.J.; Schiff, D.; Grant, B.; Grimm, S.; Lai, R.K.; Reiner, A.S.; Panageas, K.; et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: Final results and long-term outcome. J. Clin. Oncol. 2013, 31, 3971–3979. [Google Scholar] [CrossRef]
- Weigel, R.; Senn, P.; Weis, J.; Krauss, J.K. Severe complications after intrathecal methotrexate (MTX) for treatment of primary central nervous system lymphoma (PCNSL). Clin. Neurol. Neurosurg. 2004, 106, 82–87. [Google Scholar] [CrossRef]
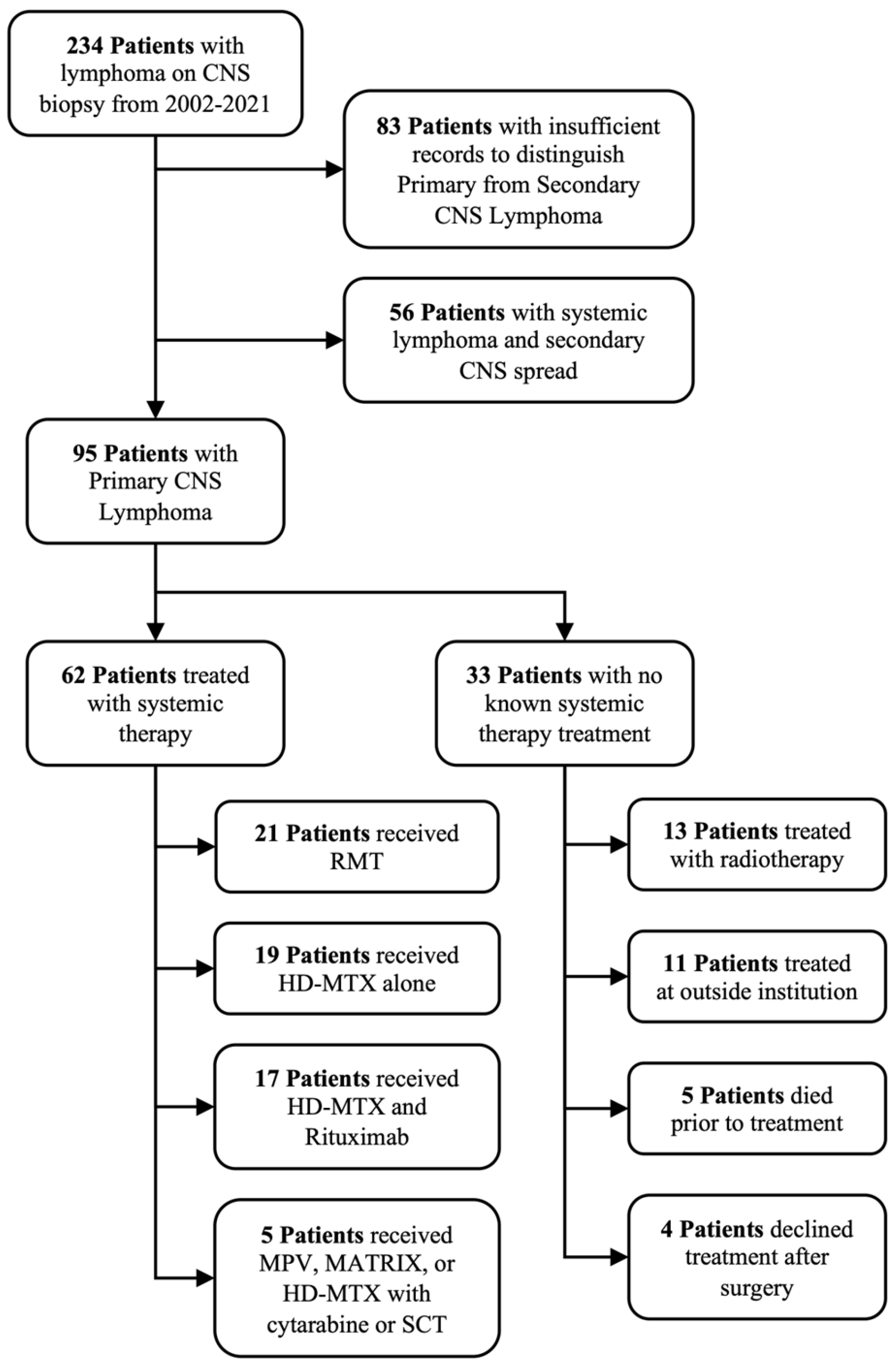

| Variable | Level | Overall Cohort (n = 95) | Systemic Therapy Cohort (n = 62) | WBRT Cohort (n = 13) |
|---|---|---|---|---|
| Age | ≥65 | 36 (37.9%) | 25 (40.3%) | 3 (23.1%) |
| <65 | 59 (62.1%) | 37 (59.7%) | 10 (76.9%) | |
| KPS | ≥70 | 61 (64.2%) | 44 (71.0%) | 4 (30.8%) |
| <70 | 34 (35.8%) | 18 (29.0%) | 9 (69.2%) | |
| HIV | Positive | 10 (10.5%) | 1 (1.6%) | 5 (38.5%) |
| Negative | 85 (89.5%) | 61 (98.4%) | 8 (61.5%) | |
| Solid Organ Transplant | Yes | 4 (4.2%) | 1 (1.6%) | 3 (23.1%) |
| No | 91 (95.8%) | 61 (98.4%) | 10 (76.9%) | |
| Initial Number of Lesions | 2+ | 36 (51.4%) | 26 (51.0%) | 3 (50.0%) |
| 1 | 34 (48.6%) | 25 (49.0%) | 3 (50.0%) | |
| Missing | 25 | 1 | 7 | |
| Initial Size of Lesions | ≥14 cc | 32 (45.7%) | 23 (45.1%) | 2 (33.3%) |
| <14 cc | 38 (54.3%) | 28 (54.9%) | 4 (66.7%) | |
| Missing | 25 | 11 | 7 | |
| Histology | DLBCL | 87 (91.6%) | 56 (90.3%) | 13 (100.0) |
| Marginal Zone | 5 (5.3%) | 3 (4.8%) | 0 (0.0%) | |
| T-Cell | 3 (3.2%) | 3 (4.8%) | 0 (0.0%) | |
| CSF Cytology | Positive | 18 (30.0%) | 18 (39.1%) | 0 (0.0%) |
| Negative | 42 (70.0%) | 28 (60.9%) | 5 (100.0%) | |
| Missing | 35 | 16 | 8 | |
| Cycles of HD-MTX | ≥6 | 33 (34.7%) | 35 (56.5%) | 0 (0.0%) |
| 0–5 | 62 (65.3%) | 27 (43.5%) | 13 (100.0%) | |
| Upfront Rituximab | Yes | 38 (61.3%) | 38 (61.3%) | 0 (0.0%) |
| No | 24 (38.7%) | 24 (38.7%) | 13 (100.0%) | |
| Missing | 33 | 0 | 0 | |
| Upfront Temozolomide | Yes | 21 (33.9%) | 41 (66.1%) | 0 (0.0%) |
| No | 41 (66.1%) | 21 (33.9%) | 13 (100.0%) | |
| Missing | 33 | 0 | 0 | |
| Intrathecal Chemotherapy | Yes | 12 (19.4%) | 49 (79.0%) | 0 (0.0%) |
| No | 50 (80.6%) | 13 (21.0%) | 13 (100.0%) | |
| Missing | 33 | 0 | 0 | |
| Type of Surgery | Biopsy | 70 (73.7%) | 49 (79.0%) | 12 (92.3%) |
| GTR | 13 (13.7%) | 6 (9.7%) | 0 (0.0%) | |
| STR | 12 (12.6%) | 7 (11.3%) | 1 (7.7%) | |
| Radiotherapy Use | None | 54 (65.9%) | 47 (75.8%) | 0 (0.0%) |
| First-Line | 16 (19.5%) | 3 (4.8%) | 13 (100.0%) | |
| Salvage | 12 (14.6%) | 12 (19.4%) | 0 (0.0%) | |
| Missing | 13 | 0 | 0 |
| Overall Survival | Progression-Free Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UVA | MVA | UVA | MVA | |||||||
| Variable | Level | n | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value |
| Age | ≥65 | 25 | 2.11 (1.12–3.95) | 0.020 | 1.89 (0.98–3.62) | 0.057 | 1.68 (0.96–2.96) | 0.070 | 1.51 (0.85–2.68) | 0.160 |
| <65 | 37 | - | - | - | - | - | - | - | - | |
| KPS | ≥70 | 18 | - | - | - | - | ||||
| <70 | 44 | 1.39 (0.69–2.78) | 0.356 | 1.26 (0.66–2.39) | 0.488 | |||||
| HIV | Positive | 1 | 1.09 (0.15–7.99) | 0.935 | 0.88 (0.12–6.46) | 0.903 | ||||
| Negative | 61 | - | - | - | - | |||||
| Solid Organ Transplant | Yes | 1 | 10.98 (1.28–94.08) | 0.029 | 11.34 (1.32–97.09) | 0.027 | ||||
| No | 61 | - | - | - | - | |||||
| Initial Number of Lesions | 2+ | 26 | 1.36 (0.71–2.59) | 0.349 | 1.26 (0.70–2.26) | 0.442 | ||||
| 1 | 25 | - | - | - | - | |||||
| Initial Size of Lesions | ≥14 cc | 23 | 1.30 (0.69–2.44) | 0.418 | 1.09 (0.60–1.99) | 0.766 | ||||
| <14 cc | 28 | - | - | - | - | |||||
| CSF Cytology | Positive | 18 | - | - | - | - | ||||
| Negative | 28 | 1.19 (0.59–2.41) | 0.624 | 1.07 (0.56–2.04) | 0.847 | |||||
| Cycles of HD-MTX | ≥6 | 35 | 0.40 (0.21–0.76) | 0.005 | 0.40 (0.21–0.78) | 0.007 | 0.44 (0.24–0.79) | 0.006 | 0.44 (0.24–0.80) | 0.007 |
| 0–5 | 27 | - | - | - | - | - | - | - | - | |
| Upfront Rituximab | Yes | 38 | 2.46 (1.22–4.97) | 0.012 | 2.82 (1.37–5.83) | 0.005 | 2.31 (1.24–4.33) | 0.009 | 2.37 (1.26–4.48) | 0.008 |
| No | 24 | - | - | - | - | - | - | - | - | |
| Upfront Temozolomide | Yes | 21 | 1.68 (0.87–3.24) | 0.120 | 1.77 (0.96–3.27) | 0.069 | ||||
| No | 41 | - | - | - | - | |||||
| Intrathecal Chemotherapy | Yes | 13 | 0.59 (0.27–1.27) | 0.174 | 0.68 (0.35–1.33) | 0.262 | ||||
| No | 49 | - | - | - | - | |||||
| Type of Surgery | Biopsy | 49 | 1.78 (0.72–4.40) | 0.213 | 2.15 (0.89–5.19) | 0.09 | ||||
| GTR | 6 | 1.85 (0.55–6.28) | 0.323 | 2.62 (0.81–8.44) | 0.107 | |||||
| STR | 7 | - | - | - | - | |||||
| Consolidation Radiotherapy | No | 59 | - | - | - | - | ||||
| Yes | 3 | 0.24 (0.03–1.74) | 0.156 | 0.37 (0.09–1.59) | 0.182 | |||||
| Variable | Level | RMT Regimen (n = 21) | HD-MTX and Rituximab (n = 17) | HD-MTX Alone (n = 19) | p-Value |
|---|---|---|---|---|---|
| Age | ≥65 | 11 (52.4%) | 10 (58.8%) | 12 (63.2%) | 0.785 |
| <65 | 10 (47.6%) | 7 (41.2%) | 7 (36.8%) | ||
| KPS | ≥70 | 13 (61.9%) | 12 (70.6%) | 15 (78.9%) | 0.500 |
| <70 | 8 (38.1%) | 5 (29.4%) | 4 (21.1%) | ||
| HIV | Positive | 0 (0.0%) | 1 (5.9%) | 0 (0.0%) | 0.302 |
| Negative | 21 (100.0%) | 16 (94.1%) | 19 (100.0%) | ||
| Solid Organ Transplant | Yes | 0 (0.0%) | 1 (5.9%) | 0 (0.0%) | 0.302 |
| No | 21 (100.0%) | 16 (94.1%) | 19 (100.0%) | ||
| Initial Number of Lesions | 2+ | 12 (60.0%) | 6 (37.5%) | 8 (61.5%) | 0.314 |
| 1 | 8 (40.0%) | 10 (62.5%) | 5 (38.5%) | ||
| Missing | 1 | 1 | 6 | ||
| Initial Size of Lesions | ≥14 cc | 9 (45.0%) | 8 (50.0%) | 5 (38.5%) | 0.824 |
| <14 cc | 11 (55.0%) | 8 (50.0%) | 8 (61.5%) | ||
| Missing | 1 | 1 | 6 | ||
| Histology | DLBCL | 20 (95.2%) | 16 (94.1%) | 16 (84.2%) | 0.383 |
| Marginal Zone | 1 (4.8%) | 1 (5.9%) | 1 (5.3%) | ||
| T-Cell | 0 (0.0%) | 0 (0.0%) | 2 (10.5%) | ||
| CSF Cytology | Positive | 8 (42.1%) | 2 (18.2%) | 6 (46.2%) | 0.310 |
| Negative | 11 (57.9%) | 9 (81.8%) | 7 (53.8%) | ||
| Missing | 2 | 6 | 6 | ||
| Cycles of HD-MTX | ≥6 | 15 (71.4%) | 7 (41.2%) | 11 (57.9%) | 0.171 |
| 0–5 | 6 (28.6%) | 10 (58.8%) | 8 (42.1%) | ||
| Upfront Rituximab | Yes | 21 (100.0%) | 17 (100.0%) | 0 (0.0%) | <0.001 |
| No | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Upfront Temozolomide | Yes | 21 (100.0%) | 0 (0.0%) | 0 (0.0%) | <0.001 |
| No | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Intrathecal Chemotherapy | Yes | 1 (4.8%) | 4 (23.5%) | 3 (15.8%) | 0.245 |
| No | 20 (95.2%) | 13 (76.5%) | 16 (84.2%) | ||
| Type of Surgery | Biopsy | 16 (76.2%) | 13 (76.5%) | 15 (78.9%) | 0.888 |
| GTR | 3 (14.3%) | 2 (11.8%) | 1 (5.3%) | ||
| STR | 2 (9.5%) | 2 (11.8%) | 3 (15.8%) | ||
| Radiotherapy Use | None | 15 (71.4%) | 13 (76.5%) | 15 (78.9%) | 0.418 |
| First Line | 0 (0.0%) | 1 (5.9%) | 2 (10.5%) | ||
| Salvage | 6 (28.6%) | 3 (17.6%) | 2 (10.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janopaul-Naylor, J.R.; Patel, J.S.; Rupji, M.; Hoang, K.B.; McCall, N.S.; Qian, D.C.; Shoaf, M.L.; Kothari, S.; Olson, J.J.; Shu, H.-K.G.; et al. Impact of Systemic and Radiation Therapy on Survival of Primary Central Nervous System Lymphoma. Cancers 2025, 17, 618. https://doi.org/10.3390/cancers17040618
Janopaul-Naylor JR, Patel JS, Rupji M, Hoang KB, McCall NS, Qian DC, Shoaf ML, Kothari S, Olson JJ, Shu H-KG, et al. Impact of Systemic and Radiation Therapy on Survival of Primary Central Nervous System Lymphoma. Cancers. 2025; 17(4):618. https://doi.org/10.3390/cancers17040618
Chicago/Turabian StyleJanopaul-Naylor, James Robert, Jimmy S. Patel, Manali Rupji, Kimberly Bojanowski Hoang, Neal Sean McCall, David C. Qian, Madison Lee Shoaf, Shawn Kothari, Jeffrey J. Olson, Hui-Kuo G. Shu, and et al. 2025. "Impact of Systemic and Radiation Therapy on Survival of Primary Central Nervous System Lymphoma" Cancers 17, no. 4: 618. https://doi.org/10.3390/cancers17040618
APA StyleJanopaul-Naylor, J. R., Patel, J. S., Rupji, M., Hoang, K. B., McCall, N. S., Qian, D. C., Shoaf, M. L., Kothari, S., Olson, J. J., Shu, H.-K. G., Voloschin, A., Zhong, J., Neill, S. G., & Eaton, B. (2025). Impact of Systemic and Radiation Therapy on Survival of Primary Central Nervous System Lymphoma. Cancers, 17(4), 618. https://doi.org/10.3390/cancers17040618








