Evaluation of the Quality of Results of Lung Cancer Surgery in France Using the PMSI National Database
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Database and Inclusion
2.2. Patient Characteristics
2.3. Region and Hospital Characteristics
2.4. Outcome (Quality Indicator)
2.5. Statistical Analyses
2.6. Ethics, Patient and Public Involvement
3. Results
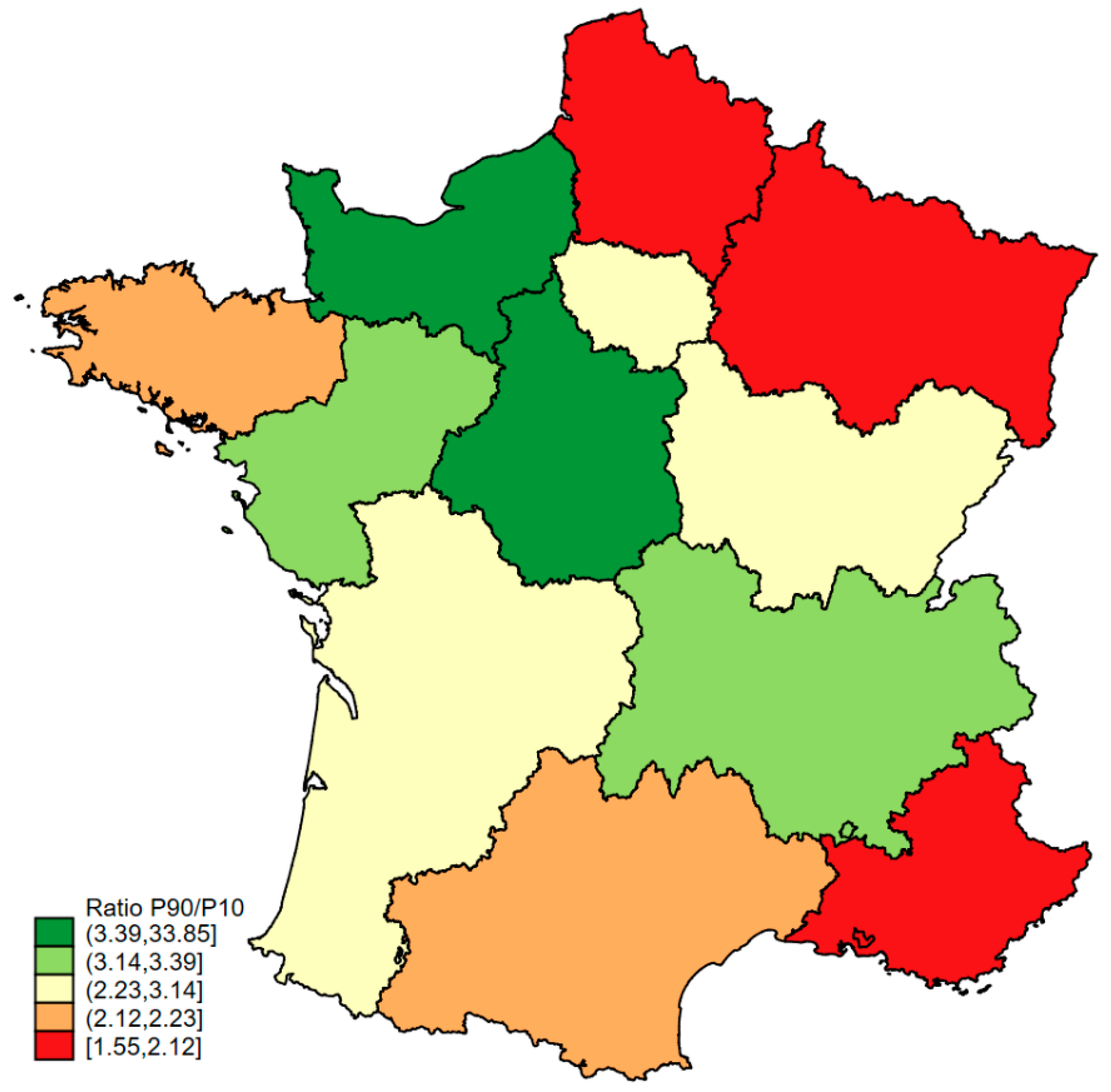
3.1. Regions and Characteristics of Hospitals
3.2. Standardised Rate of Severe Complications (Clavien–Dindo > 2) in the Current Situation: Estimation and Modelling
3.3. Estimate of the Standardised Rate of Severe Complications (Clavien–Dindo > 2) After Simulation of a Reorganisation of Hospitals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Ministère de l’Enseignement Supérieur et de la Recherche; Ministère de la Santé et des Sports. Plan Cancer 2009–2013. Available online: http://www.e-cancer.fr/Plan-cancer/Les-Plans-cancer-de-2003-a-2013/Le-Plancancer-2009-2013 (accessed on 1 March 2016).
- Institut National Du Cancer L’organisation de l’offre de soins—Professionnels de Santé. Available online: https://www.e-cancer.fr/Professionnels-de-sante/L-organisation-de-l-offre-de-soins/ (accessed on 1 October 2024).
- Kozower, B.D.; O’Brien, S.M.; Kosinski, A.S.; Magee, M.J.; Dokholyan, R.; Jacobs, J.P.; Shahian, D.M.; Wright, C.D.; Fernandez, F.G. The Society of Thoracic Sdiscussion 726gram Performance for Lobectomy for Lung Cancer. Ann. Thorac. Surg. 2016, 101, 1379–1386, discussion 1386–1387. [Google Scholar] [CrossRef] [PubMed]
- Ten Berge, M.G.; Beck, N.; Steup, W.H.; Verhagen, A.F.T.M.; van Brakel, T.J.; Schreurs, W.H.; Wouters, M.W.J.M. Textbook outcome as a composite outcome measure in non-small-cell lung cancer surgery. Eur. J. Cardio-Thorac. Surg. 2021, 59, 92–99. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision. 2016. Available online: http://apps.who.int/classifications/icd10/browse/2016/en (accessed on 1 March 2016).
- Iezzoni, L.I. Assessing quality using administrative data. Ann. Intern. Med. 1997, 127, 666–674. [Google Scholar] [CrossRef]
- Bernard, A.; Cottenet, J.; Pagès, P.-B.; Quantin, C. Mortality and failure-to-rescue major complication trends after lung cancer surgery between 2005 and 2020: A nationwide population-based study. BMJ Open 2023, 13, e075463. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Cottenet, J.; Quantin, C. Is the Validity of Logistic Regression Models Developed with a National Hospital Database Inferior to Models Developed from Clinical Databases to Analyze Surgical Lung Cancers? Cancers 2024, 16, 734. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Seely, A.J.E.; Ivanovic, J.; Threader, J.; Al-Hussaini, A.; Al-Shehab, D.; Ramsay, T.; Gilbert, S.; Maziak, D.E.; Shamji, F.M.; Sundaresan, R.S. Systematic classification of morbidity and mortality after thoracic surgery. Ann. Thorac. Surg. 2010, 90, 936–942, Discussion 942. [Google Scholar] [CrossRef] [PubMed]
- Salati, M.; Refai, M.; Pompili, C.; Xiumè, F.; Sabbatini, A.; Brunelli, A. Major morbidity after lung resection: A comparison between the European Society of Thoracic Surgeons Database system and the Thoracic Morbidity and Mortality system. J. Thorac. Dis. 2013, 5, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W. Clinical Prediction Models: A Practical Approach to Development, Validating and Updating; Springer: New York, NY, USA, 2009. [Google Scholar]
- Normand, S.-L.T.; Shahian, D.M. Statistical and Clinical Aspects of Hospital Outcomes Profiling. Stat. Sci. 2007, 22, 206–226. [Google Scholar] [CrossRef]
- David, E.A.; Cooke, D.T.; Chen, Y.; Perry, A.; Canter, R.J.; Cress, R. Surgery in high-volume hospitals not commission on cancer accreditation leads to increased cancer-specific survival for early-stage lung cancer. Am. J. Surg. 2015, 210, 643–647. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Birkmeyer, J.D.; Siewers, A.E.; Finlayson, E.V.A.; Stukel, T.A.; Lucas, F.L.; Batista, I.; Welch, H.G.; Wennberg, D.E. Hospital volume and surgical mortality in the United States. N. Engl. J. Med. 2002, 346, 1128–1137. [Google Scholar] [CrossRef]
- Finlayson, E.V.A.; Goodney, P.P.; Birkmeyer, J.D. Hospital volume and operative mortality in cancer surgery: A national study. Arch. Surg. 2003, 138, 721–725, discussion 726. [Google Scholar] [CrossRef] [PubMed]
- Lien, Y.-C.; Huang, M.-T.; Lin, H.-C. Association between surgeon and hospital volume and in-hospital fatalities after lung cancer resections: The experience of an Asian country. Ann. Thorac. Surg. 2007, 83, 1837–1843. [Google Scholar] [CrossRef]
- Bernard, A.; Riviere, A.; Cottenet, J.; Madeleine, L.; Quantin, C.; Pages, P.B. Comparaison de la mortalité des résections pulmonaires en France aux autres pays Européens. Rev. Mal. Respir. 2022, 39, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.R.; Voeten, D.M.; Gisbertz, S.S.; Ruurda, J.P.; Achiam, M.P.; Nilsson, M.; Markar, S.R.; Pera, M.; Rosati, R.; Piessen, G.; et al. Western European Variation in the Organization of Esophageal Cancer Surgical Care. Dis. Esophagus 2024, 37, doae033. [Google Scholar] [CrossRef]
- Sheetz, K.H.; Dimick, J.B.; Nathan, H. Centralization of High-Risk Cancer Surgery Within Existing Hospital Systems. J. Clin. Oncol. 2019, 37, 3234–3242. [Google Scholar] [CrossRef] [PubMed]
- Vonlanthen, R.; Lodge, P.; Barkun, J.S.; Farges, O.; Rogiers, X.; Soreide, K.; Kehlet, H.; Reynolds, J.V.; Käser, S.A.; Naredi, P.; et al. Toward a Consensus on Centralization in Surgery. Ann. Surg. 2018, 268, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Cottenet, J.; Mariet, A.-S.; Quantin, C.; Pagès, P.-B. Is an activity volume threshold really realistic for lung cancer resection? J. Thorac. Dis. 2018, 10, 5685–5694. [Google Scholar] [CrossRef]
- Piroth, L.; Cottenet, J.; Mariet, A.-S.; Bonniaud, P.; Blot, M.; Tubert-Bitter, P.; Quantin, C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir. Med. 2021, 9, 251–259. [Google Scholar] [CrossRef]
- Viennet, M.; Tapia, S.; Cottenet, J.; Bernard, A.; Ortega-Deballon, P.; Quantin, C. Increased risk of colon cancer after acute appendicitis: A nationwide, population-based study. eClinicalMedicine 2023, 63, 102196. [Google Scholar] [CrossRef] [PubMed]
- Dahan, M. Epithor. Rev. Mal. Respir. 2020, 37, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, K.; Qu, C.; Qi, W.; Fang, T.; Yue, W.; Tian, H. The effect of the enhanced recovery after surgery program on lung cancer surgery: A systematic review and meta-analysis. J. Thorac. Dis. 2021, 13, 3566–3586. [Google Scholar] [CrossRef] [PubMed]
| 2019 | 2020 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|
| n = 12,367 | n = 11,792 | n = 12,542 | n = 13,376 | n = 14,227 | |
| None | 6528 (52.8) | 6298 (53.4) | 6972 (55.6) | 7594 (56.8) | 7969 (56.0) |
| Clavien–Dindo ≤ 2 | 1863 (15.1) | 1764 (15.0) | 1911 (15.2) | 2039 (15.2) | 2331 (16.4) |
| Clavien–Dindo > 2 | |||||
| Clavien–Dindo IIIa | 1308 (10.6) | 1207 (10.2) | 1178 (9.4) | 1264 (9.4) | 1375 (9.7) |
| Clavien–Dindo IIIb | 638 (5.2) | 652 (5.5) | 634 (5.1) | 668 (5.0) | 748 (5.3) |
| Clavien–Dindo IVa | 337 (2.7) | 361 (3.1) | 408 (3.3) | 440 (3.3) | 497 (3.5) |
| Clavien–Dindo IVb | 1412 (11.4) | 1240 (10.5) | 1196 (9.5) | 1151 (8.6) | 1088 (7.6) |
| Clavien–Dindo V | 281 (2.3) | 270 (2.3) | 243 (1.9) | 220 (1.6) | 219 (1.5) |
| Region | Number of Hospitals | Number of Procedures | ||||
|---|---|---|---|---|---|---|
| p10 | p25 | Median | p75 | p90 | ||
| ARA | 24 | 10 | 10 | 50 | 87 | 106 |
| BFC | 5 | 10 | 20 | 35 | 43 | 281 |
| BRE | 13 | 10 | 35 | 70 | 97 | 128 |
| COR | 2 | 16 | 16 | 20 | 23 | 23 |
| CVL | 8 | 10 | 23 | 44 | 68 | 122 |
| GE | 13 | 10 | 56 | 121 | 139 | 218 |
| HdF | 12 | 10 | 20 | 71 | 108 | 222 |
| IdF | 19 | 10 | 11 | 54 | 135 | 327 |
| NA | 17 | 10 | 27 | 41 | 96 | 138 |
| NOR | 8 | 10 | 18 | 42 | 135 | 261 |
| OCC | 17 | 10 | 27 | 53 | 87 | 238 |
| PACA | 19 | 10 | 17 | 51 | 101 | 229 |
| PdL | 14 | 10 | 10 | 43 | 56 | 113 |
| Regions | Quantiles of the Standardised Severe Complication (Clavien–Dindo > 2) Rate | |||
|---|---|---|---|---|
| <23% | 23–26% | 27–35% | >35% | |
| ARA | 7 | 6 | 7 | 4 |
| 29.17 | 25.00 | 29.17 | 16.67 | |
| BFC | 1 | 2 | 2 | 0 |
| 20.00 | 40.00 | 40.00 | 0.00 | |
| BRE | 7 | 2 | 3 | 1 |
| 53.85 | 15.38 | 23.08 | 7.69 | |
| CVL | 1 | 1 | 4 | 2 |
| 12.50 | 12.50 | 50.00 | 25.00 | |
| COR | 0 | 0 | 0 | 2 |
| 0.00 | 0.00 | 0.00 | 100.00 | |
| GE | 2 | 0 | 5 | 6 |
| 15.38 | 0.00 | 38.46 | 46.15 | |
| HdF | 5 | 1 | 4 | 2 |
| 41.67 | 8.33 | 33.33 | 16.67 | |
| IdF | 5 | 3 | 2 | 9 |
| 26.32 | 15.79 | 10.53 | 47.37 | |
| NOR | 6 | 1 | 0 | 1 |
| 75.00 | 12.50 | 0.00 | 12.50 | |
| NA | 5 | 2 | 6 | 4 |
| 29.41 | 11.76 | 35.29 | 23.53 | |
| OCC | 7 | 5 | 1 | 4 |
| 41.18 | 29.41 | 5.88 | 23.53 | |
| PdL | 8 | 2 | 2 | 2 |
| 57.14 | 14.29 | 14.29 | 14.29 | |
| PACA | 3 | 5 | 5 | 6 |
| 15.79 | 26.32 | 26.32 | 31.58 | |
| aOR | 95% CI | p-Value | |
|---|---|---|---|
| Number of annual procedures | 0.0001 | ||
| <100 | 1 | ||
| 101–250 | 0.83 | 0.77–0.89 | |
| >250 | 0.85 | 0.77–0.93 | |
| Type of hospital | 0.0001 | ||
| Non-academic hospital | 1 | ||
| Academic (teaching) hospital | 0.98 | 0.89–1.08 | |
| Non-profit private hospital | 1.35 | 1.19–1.52 | |
| Private hospital | 1.10 | 1.01–1.19 | |
| Inter-regional variance | 0.034 | 0.015–0.077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernard, A.; Cottenet, J.; Quantin, C. Evaluation of the Quality of Results of Lung Cancer Surgery in France Using the PMSI National Database. Cancers 2025, 17, 617. https://doi.org/10.3390/cancers17040617
Bernard A, Cottenet J, Quantin C. Evaluation of the Quality of Results of Lung Cancer Surgery in France Using the PMSI National Database. Cancers. 2025; 17(4):617. https://doi.org/10.3390/cancers17040617
Chicago/Turabian StyleBernard, Alain, Jonathan Cottenet, and Catherine Quantin. 2025. "Evaluation of the Quality of Results of Lung Cancer Surgery in France Using the PMSI National Database" Cancers 17, no. 4: 617. https://doi.org/10.3390/cancers17040617
APA StyleBernard, A., Cottenet, J., & Quantin, C. (2025). Evaluation of the Quality of Results of Lung Cancer Surgery in France Using the PMSI National Database. Cancers, 17(4), 617. https://doi.org/10.3390/cancers17040617







