Patterns of Treatment Delay in Patients with Symptomatic Metastatic Epidural Spinal Cord Compression
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Parameters
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Predictive Factors of Postoperative Ambulatory Status
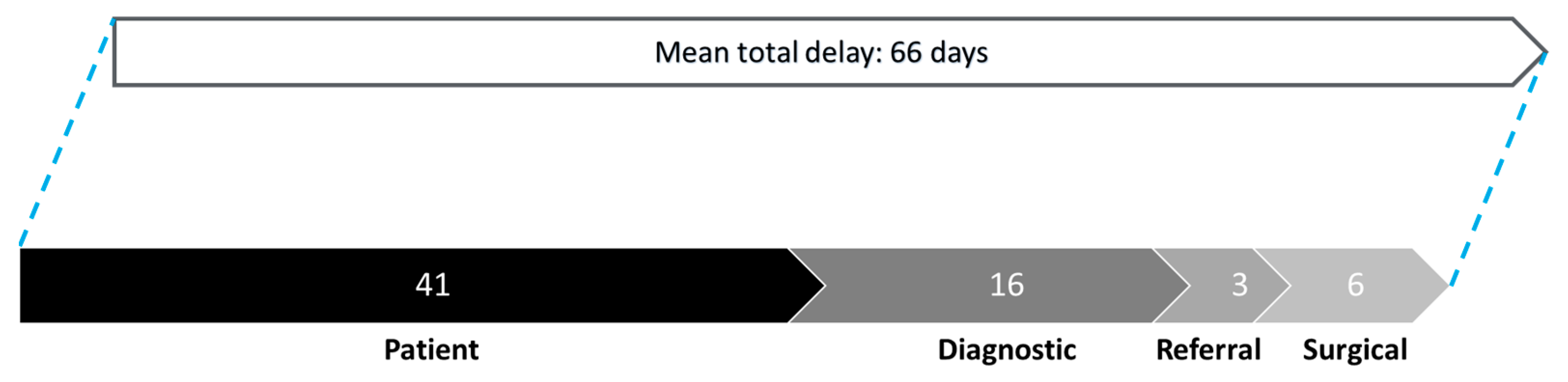
3.3. Patterns of Delay
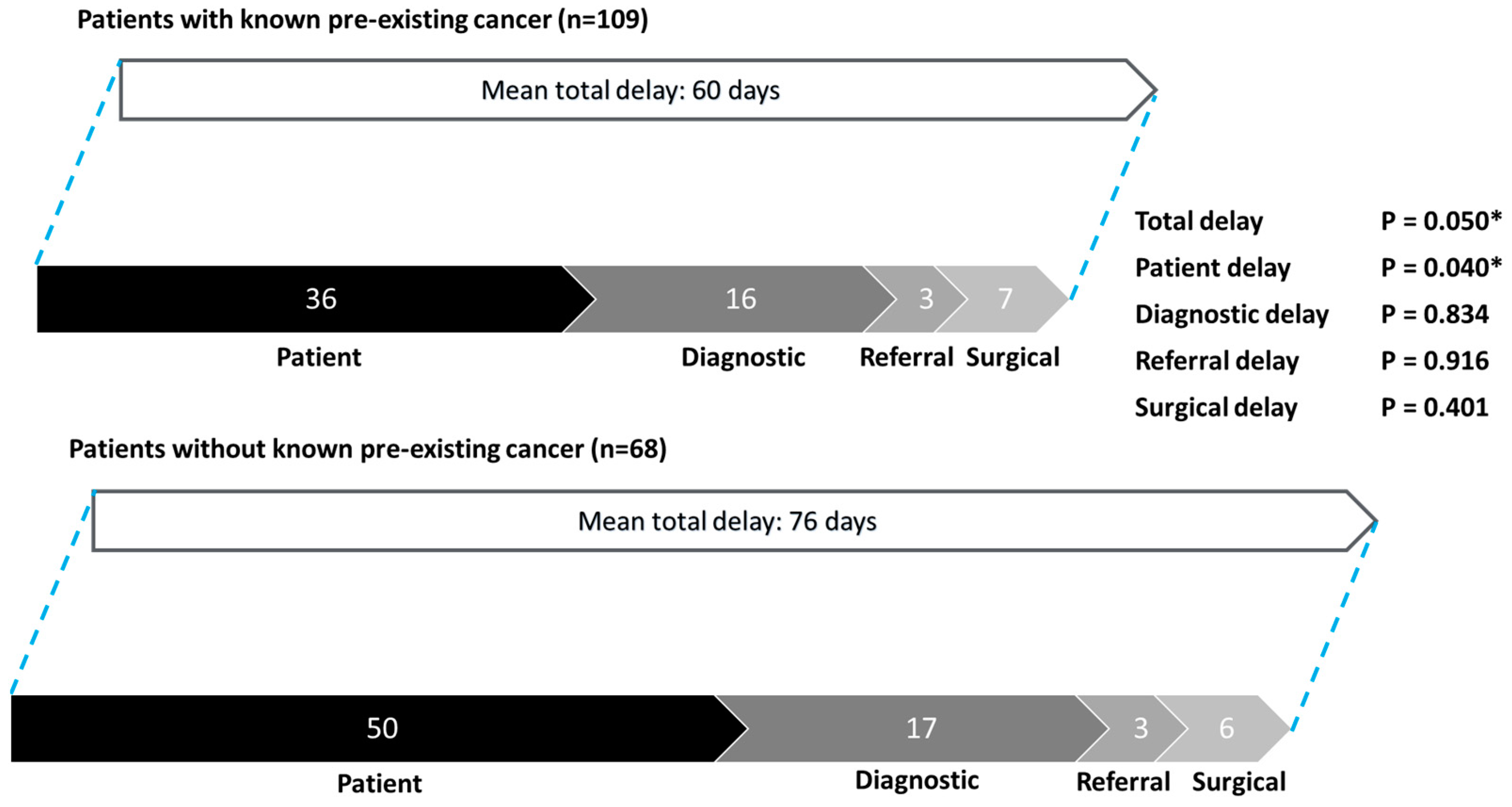
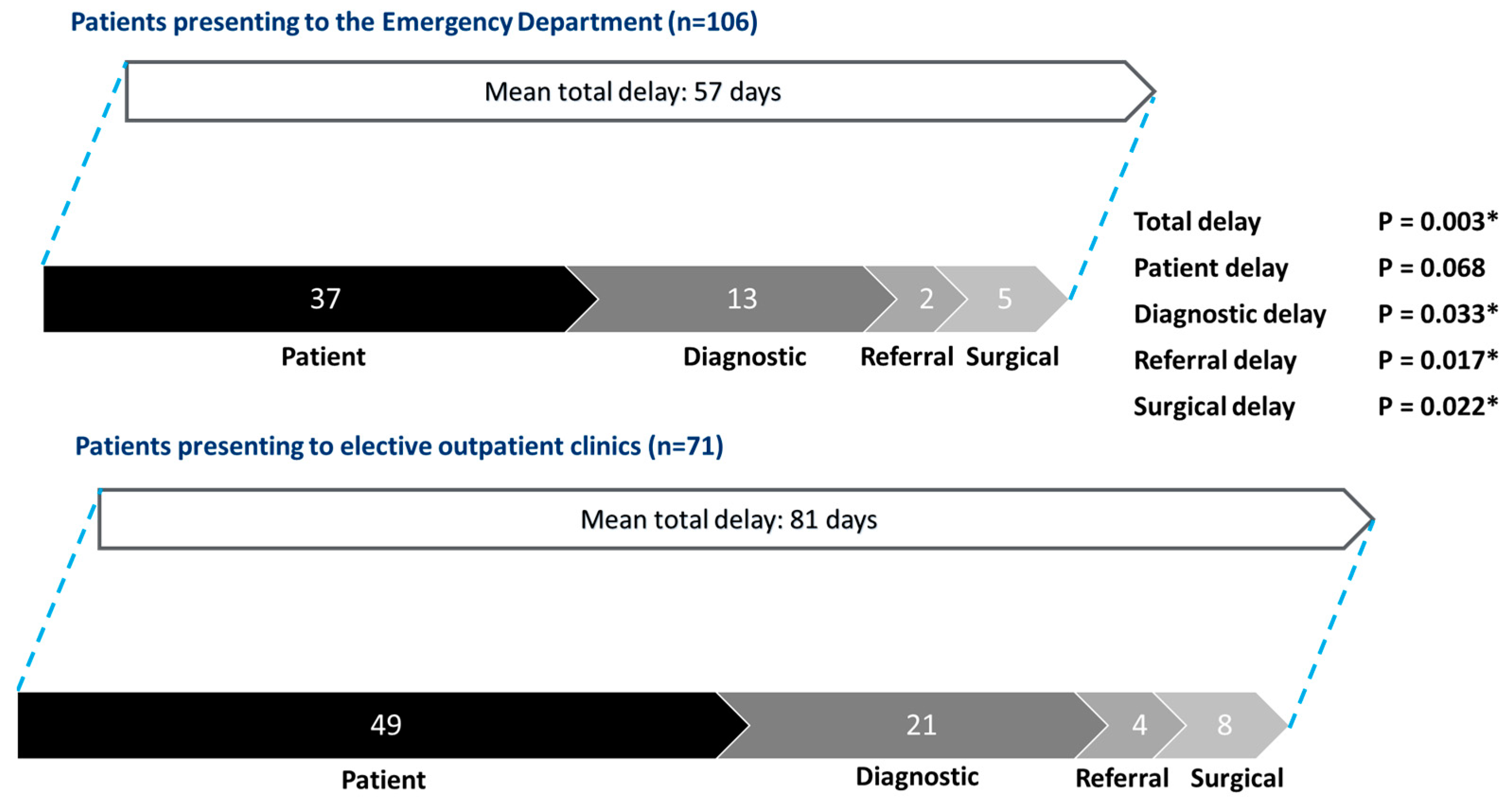
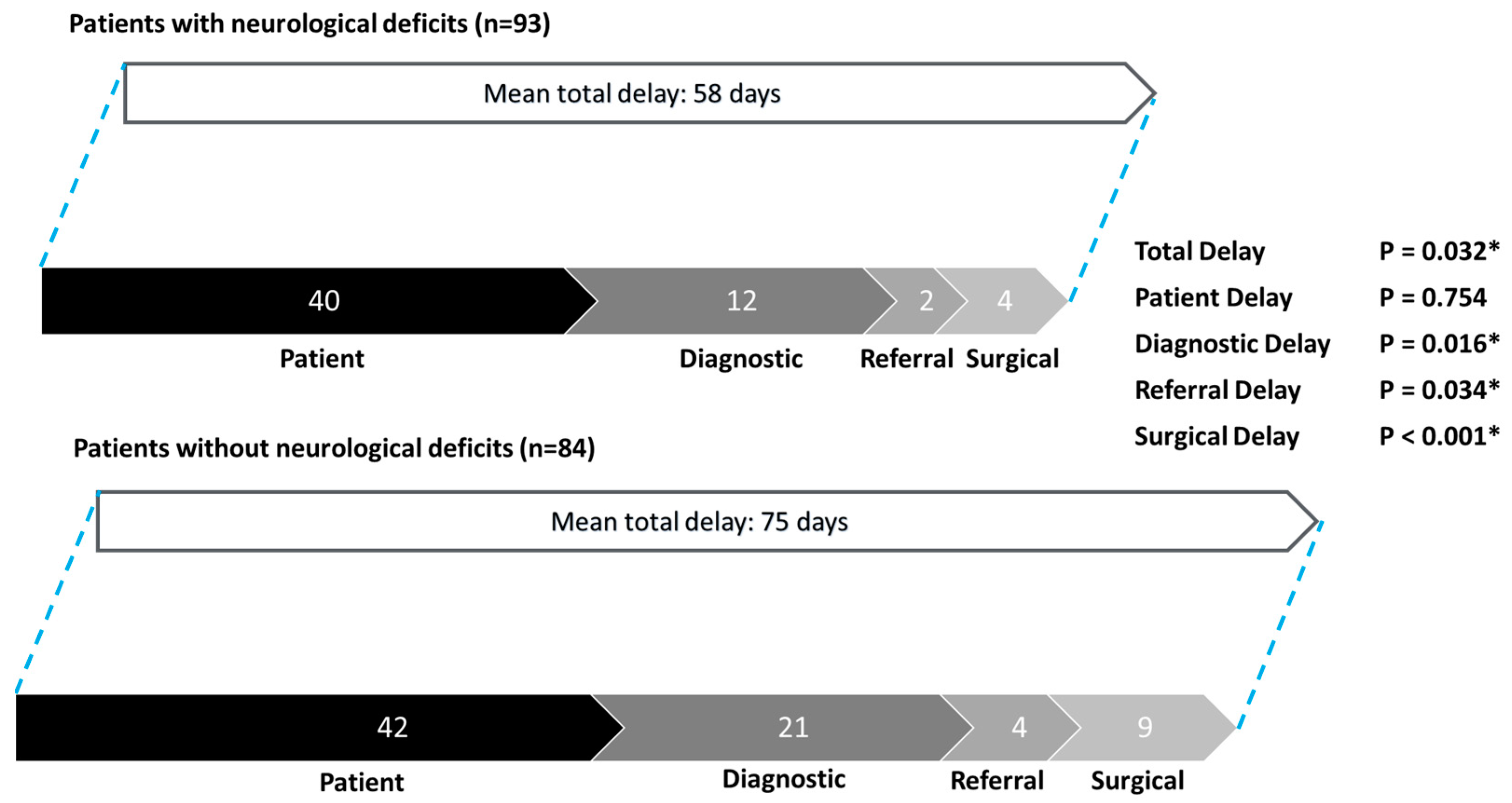
3.4. Subgroup Analysis
4. Discussion
4.1. Importance of Early Surgical Treatment of MESCC
4.2. Reducing Patient Delay Through Patient Education and Collaboration with Primary Care Providers
4.3. Optimizing Multidisciplinary Care of MESCC Patients to Reduce Delays
4.4. Utilizing Artificial Intelligence and Machine Learning Diagnostic Tools to Reduce Diagnostic Delay
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| ANOVA | Analysis of variance |
| CI | Confidence interval |
| COVID-19 | Coronavirus disease |
| CT | Computed tomography |
| CT-TAP | Computed tomography scan of the thorax, abdomen, and pelvis |
| ECOG | Eastern Cooperative Oncology Group |
| MRI | Magnetic resonance imaging |
| MESCC | Metastatic epidural spinal cord compression |
| NICE | National Institute of Health and Care Excellence (of the United Kingdom) |
| OR | Odds ratio |
| SINS | Spinal instability neoplastic score |
| SORG | Skeletal Oncology Research Group |
References
- Fornasier, V.L.; Horne, J.G. Metastases to the vertebral column. Cancer 1975, 36, 590–594. [Google Scholar] [CrossRef]
- Wong, D.A.; Fornasier, V.L.; MacNab, I. Spinal metastases: The obvious, the occult, and the impostors. Spine 1990, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Goncalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar]
- Selvaggi, G.; Scagliotti, G.V. Management of bone metastases in cancer: A review. Crit. Rev. Oncol./Hematol. 2005, 56, 365–378. [Google Scholar] [CrossRef]
- Wewel, J.T.; O’Toole, J.E. Epidemiology of spinal cord and column tumors. Neurooncol Pr. 2020, 7 (Suppl. 1), i5–i9. [Google Scholar] [CrossRef] [PubMed]
- Van den Brande, R.; Cornips, E.M.J.; Peeters, M.; Ost, P.; Billiet, C.; Van de Kelft, E. Epidemiology of spinal metastases, metastatic epidural spinal cord compression and pathologic vertebral compression fractures in patients with solid tumors: A systematic review. J. Bone Oncol. 2022, 35, 100446. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Nottebaert, M.; von Hochstetter, A.R.; Exner, G.U.; Schreiber, A. Metastatic carcinoma of the spine. A study of 92 cases. Int. Orthop. 1987, 11, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.H.J.; Hallinan, J.T.P.D.; Lee, R.; Chan, Y.H.; Tan, T.H.; Ang, S.W.; Tan, L.T.I.; Tan, J.H.I.; Sin, Q.S.; Hey, D.H.W.; et al. Trends in surgical management of spinal metastases in a Singaporean tertiary referral center: A 17-year retrospective review. Front. Oncol. 2023, 13, 1297553. [Google Scholar] [CrossRef]
- van Tol, F.R.; Choi, D.; Verkooijen, H.M.; Oner, F.C.; Verlaan, J.J. Delayed presentation to a spine surgeon is the strongest predictor of poor postoperative outcome in patients surgically treated for symptomatic spinal metastases. Spine J. Off. J. N. Am. Spine Soc. 2019, 19, 1540–1547. [Google Scholar] [CrossRef]
- Brodowicz, T.; Hadji, P.; Niepel, D.; Diel, I. Early identification and intervention matters: A comprehensive review of current evidence and recommendations for the monitoring of bone health in patients with cancer. Cancer Treat. Rev. 2017, 61, 23–34. [Google Scholar] [CrossRef] [PubMed]
- van Tol, F.R.; Massier, J.R.A.; Frederix, G.W.J.; Öner, F.C.; Verkooijen, H.M.; Verlaan, J.J. Costs Associated With Timely and Delayed Surgical Treatment of Spinal Metastases. Glob. Spine J. 2022, 12, 1661–1666. [Google Scholar] [CrossRef]
- van Tol, F.R.; Versteeg, A.L.; Verkooijen, H.M.; Öner, F.C.; Verlaan, J.J. Time to Surgical Treatment for Metastatic Spinal Disease: Identification of Delay Intervals. Glob. Spine J. 2023, 13, 316–323. [Google Scholar] [CrossRef]
- de Groot, T.M.; Ramsey, D.; Groot, O.Q.; Fourman, M.; Karhade, A.V.; Twining, P.K.; Berner, E.A.; Fenn, B.P.; Collins, A.K.; Raskin, K.; et al. Does the SORG Machine-learning Algorithm for Extremity Metastases Generalize to a Contemporary Cohort of Patients? Temporal Validation From 2016 to 2020. Clin. Orthop. Relat. Res. 2023, 481, 2419–2430. [Google Scholar] [CrossRef]
- Katagiri, H.; Okada, R.; Takagi, T.; Takahashi, M.; Murata, H.; Harada, H.; Nishimura, T.; Asakura, H.; Ogawa, H. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014, 3, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Spinal Metastases and Metastatic Spinal Cord Compression; NICE: London, UK, 2023; NICE Guideline [NG234]. [Google Scholar]
- Choi, D.; Crockard, A.; Bunger, C.; Harms, J.; Kawahara, N.; Mazel, C.; Melcher, R.; Tomita, K. Review of metastatic spine tumour classification and indications for surgery: The consensus statement of the Global Spine Tumour Study Group. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2010, 19, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.H.J.; Hallinan, J.T.P.D.; Ang, S.W.; Tan, T.H.; Tan, H.I.J.; Tan, L.T.I.; Sin, Q.S.; Lee, R.; Hey, H.W.D.; Chan, Y.H.; et al. Outcomes and Complications of Surgery for Symptomatic Spinal Metastases; a Comparison Between Patients Aged ≥70 and <70. Glob. Spine J. 2023, 21925682231209624. [Google Scholar] [CrossRef] [PubMed]
- Amelot, A.; Terrier, L.M.; Le Nail, L.R.; Buffenoir, K.; Cook, A.R.; François, P.; Marie-Hardy, L.; Mathon, B. Spine Metastasis: Patients With Poor Performance Status (ECOG) Could benefit From Palliative Surgical Care! A Prospective Cohort Study. Spine 2023, 48, 476–483. [Google Scholar] [CrossRef]
- Dea, N.; Versteeg, A.L.; Sahgal, A.; Verlaan, J.J.; Charest-Morin, R.; Rhines, L.D.; Sciubba, D.M.; Schuster, J.M.; Weber, M.H.; Lazary, A.; et al. Metastatic Spine Disease: Should Patients with Short Life Expectancy Be Denied Surgical Care? An International Retrospective Cohort Study. Neurosurgery 2020, 87, 303–311. [Google Scholar] [CrossRef]
- Hsieh, C.-J.; Wu, C.-Y.; Lin, Y.-H.; Huang, Y.-C.; Yang, W.-C.; Chen, T.W.-W.; Ma, W.-L.; Lin, W.-H.; Hsu, F.-M.; Xiao, F.; et al. Delay of Surgery for Spinal Metastasis due to the COVID-19 Outbreak Affected Patient Outcomes. Neurospine 2023, 20, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Levack, P.; Graham, J.; Collie, D.; Grant, R.; Kidd, J.; Kunkler, I.; Gibson, A.; Hurman, D.; McMillan, N.; Rampling, R.; et al. Don’t wait for a sensory level--listen to the symptoms: A prospective audit of the delays in diagnosis of malignant cord compression. Clin. Oncol. (R. Coll. Radiol. (Great Br.)) 2002, 14, 472–480. [Google Scholar] [CrossRef]
- Husband, D.J. Malignant spinal cord compression: Prospective study of delays in referral and treatment. BMJ (Clin. Res. Ed.) 1998, 317, 18–21. [Google Scholar] [CrossRef]
- Guzik, G. Analysis of factors delaying the surgical treatment of patients with neurological deficits in the course of spinal metastatic disease. BMC Palliat. Care 2018, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Q.H.; Lee, K.H.; Low, L.L. A systematic review of health status, health seeking behaviour and healthcare utilisation of low socioeconomic status populations in urban Singapore. Int. J. Equity Health 2018, 17, 39. [Google Scholar] [CrossRef]
- Green, T.; Atkin, K.; Macleod, U. Cancer detection in primary care: Insights from general practitioners. Br. J. Cancer 2015, 112, S41–S49. [Google Scholar] [CrossRef]
- Silbermann, M.; Pitsillides, B.; Al-Alfi, N.; Omran, S.; Al-Jabri, K.; Elshamy, K.; Ghrayeb, I.; Livneh, J.; Daher, M.; Charalambous, H.; et al. Multidisciplinary care team for cancer patients and its implementation in several Middle Eastern countries. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24 (Suppl. 7), vii41–vii47. [Google Scholar] [CrossRef] [PubMed]
- Berardi, R.; Morgese, F.; Rinaldi, S.; Torniai, M.; Mentrasti, G.; Scortichini, L.; Giampieri, R. Benefits and Limitations of a Multidisciplinary Approach in Cancer Patient Management. Cancer Manag. Res. 2020, 12, 9363–9374. [Google Scholar] [CrossRef] [PubMed]
- Hallinan, J.T.P.D.; Ge, S.; Zhu, L.; Zhang, W.; Lim, Y.T.; Thian, Y.L.; Jagmohan, P.; Kuah, T.; Lim, D.S.W.; Low, X.Z.; et al. Diagnostic Accuracy of CT for Metastatic Epidural Spinal Cord Compression. Cancers 2022, 14, 4231. [Google Scholar] [CrossRef] [PubMed]
- Hallinan, J.T.P.D.; Zhu, L.; Zhang, W.; Lim, D.S.W.; Baskar, S.; Low, X.Z.; Yeong, K.Y.; Teo, E.C.; Kumarakulasinghe, N.B.; Yap, Q.V.; et al. Deep Learning Model for Classifying Metastatic Epidural Spinal Cord Compression on MRI. Front. Oncol. 2022, 12, 849447. [Google Scholar] [CrossRef] [PubMed]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“how-to” guide and critical reflection. Insights Into Imaging 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Z.; Ni, D.; Chou, Y.H.; Qin, J.; Tiu, C.M.; Chang, Y.C.; Huang, C.S.; Shen, D.; Chen, C.M. Computer-Aided Diagnosis with Deep Learning Architecture: Applications to Breast Lesions in US Images and Pulmonary Nodules in CT Scans. Sci. Rep. 2016, 6, 24454. [Google Scholar] [CrossRef]
- Eadie, L.H.; Taylor, P.; Gibson, A.P. A systematic review of computer-assisted diagnosis in diagnostic cancer imaging. Eur. J. Radiol. 2012, 81, e70–e76. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Hanaoka, S.; Nomura, Y.; Sato, I.; Nemoto, M.; Miki, S.; Maeda, E.; Yoshikawa, T.; Hayashi, N.; Abe, O. Deep neural network-based computer-assisted detection of cerebral aneurysms in MR angiography. J. Magn. Reson. Imaging JMRI 2018, 47, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Miki, S.; Hayashi, N.; Masutani, Y.; Nomura, Y.; Yoshikawa, T.; Hanaoka, S.; Nemoto, M.; Ohtomo, K. Computer-Assisted Detection of Cerebral Aneurysms in MR Angiography in a Routine Image-Reading Environment: Effects on Diagnosis by Radiologists. Am. J. Neuroradiol. 2016, 37, 1038. [Google Scholar] [CrossRef]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef]
- Najjar, R. Redefining Radiology: A Review of Artificial Intelligence Integration in Medical Imaging. Diagnostics 2023, 13, 2760. [Google Scholar] [CrossRef]

| Variable | n (%) |
|---|---|
| Age, years (mean (standard deviation)) | 62.6 (10.1) |
| Sex | |
| Male | 85 (48.0%) |
| Female | 92 (52.0%) |
| ECOG Score | |
| 0–2 | 166 (93.8%) |
| 3–4 | 11 (6.2%) |
| SORG classification of primary tumor | |
| Slow growth | 63 (35.6%) |
| Moderate growth | 63 (35.6%) |
| Rapid growth | 51 (28.8%) |
| Number of extra-spinal metastases: | |
| ≥3 | 62 (35.0%) |
| 1–2 | 45 (25.4%) |
| 0 | 69 (39.0%) |
| Number of vertebral body metastases: | |
| ≥3 | 110 (62.1%) |
| 2 | 36 (20.3%) |
| 1 | 30 (16.9%) |
| Incidence of visceral metastases | 99 (55.9%) |
| Oncological history | |
| Known cancer | 109 (61.6%) |
| New diagnosis | 68 (38.4%) |
| Preoperative neurological status | |
| Neurological deficits present | 93 (52.5%) |
| No neurological deficits | 84 (47.5%) |
| Nature of symptoms | |
| Symptoms suggestive of spinal metastases | 38 (21.5%) |
| Symptoms suggestive of cord compression | 139 (78.5%) |
| Nature of surgery | |
| Elective | 91 (51.4%) |
| Emergency | 86 (48.6%) |
| Survival status | |
| Survival duration, months (median (range)) | 13.0 (1.0–92.0) |
| Deceased | 119 (67.2%) |
| Alive | 49 (27.7%) |
| Lost to follow-up | 9 (5.1%) |
| Ambulatory status | |
| Independent | 92 (52.0%) |
| Dependent | 85 (48.0%) |
| Ambulant with assistance | 8 (4.5%) |
| Walking stick | 14 (7.9%) |
| Walking frame | 29 (16.4%) |
| Wheelchair-bound | 23 (13.0%) |
| Bedbound | 11 (6.2%) |
| Variable | OR (95%CI) | p-Value | AOR (95%CI) | p-Value |
|---|---|---|---|---|
| Patient presentation to ED vs. outpatient clinic | 0.46 (0.25–0.85) | 0.014 * | 0.65 (0.32–1.30) | 0.219 |
| Known history of cancer | 0.94 (0.51–1.72) | 0.839 | - | - |
| Emergency vs. elective surgery | 0.49 (0.27–0.90) | 0.021 * | 0.80 (0.39–1.62) | 0.528 |
| No preoperative neurological deficits | 5.30 (2.78–10.11) | <0.001 * | 3.27 (1.58–6.77) | 0.001 * |
| Presence of red flag symptoms suggestive of cord compression | 0.14 (0.06–0.36) | <0.001 * | 0.26 (0.09–0.70) | 0.008 * |
| Variable | Adjusted OR (95% CI) | p-Value |
|---|---|---|
| Total delay | 1.00 (0.99–1.01) | 0.248 |
| Patient delay | 1.00 (0.99–1.01) | 0.975 |
| Diagnostic delay | 1.01 (0.99–1.02) | 0.142 |
| Referral delay | 1.11 (1.02–1.20) | 0.013 * |
| Surgical delay | 1.04 (0.99–1.08) | 0.075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Hallinan, J.T.P.D.; Tan, C.L.H.; Chua, K.G.E.; Teo, A.Q.A.; Kumar, N.; Liu, G.; Hey, H.W.D.; Thambiah, J.; Lau, L.-L.; et al. Patterns of Treatment Delay in Patients with Symptomatic Metastatic Epidural Spinal Cord Compression. Cancers 2025, 17, 595. https://doi.org/10.3390/cancers17040595
Wang S, Hallinan JTPD, Tan CLH, Chua KGE, Teo AQA, Kumar N, Liu G, Hey HWD, Thambiah J, Lau L-L, et al. Patterns of Treatment Delay in Patients with Symptomatic Metastatic Epidural Spinal Cord Compression. Cancers. 2025; 17(4):595. https://doi.org/10.3390/cancers17040595
Chicago/Turabian StyleWang, Shilin, James T. P. D. Hallinan, Cherie Lin Hui Tan, Khye Gin Eugene Chua, Alex Quok An Teo, Naresh Kumar, Gabriel Liu, Hwee Weng Dennis Hey, Joseph Thambiah, Leok-Lim Lau, and et al. 2025. "Patterns of Treatment Delay in Patients with Symptomatic Metastatic Epidural Spinal Cord Compression" Cancers 17, no. 4: 595. https://doi.org/10.3390/cancers17040595
APA StyleWang, S., Hallinan, J. T. P. D., Tan, C. L. H., Chua, K. G. E., Teo, A. Q. A., Kumar, N., Liu, G., Hey, H. W. D., Thambiah, J., Lau, L.-L., Wong, H.-K., Chan, Y.-H., & Tan, J. H. J. (2025). Patterns of Treatment Delay in Patients with Symptomatic Metastatic Epidural Spinal Cord Compression. Cancers, 17(4), 595. https://doi.org/10.3390/cancers17040595






