Alternative Splicing as a Modulator of the Interferon-Gamma Pathway
Simple Summary
Abstract
1. Introduction
2. Interferon-Gamma: Structure and Function
2.1. Cellular Production and Genetic Regulation
2.2. Immune Response Modulation
2.3. Antitumor Immunity
2.4. Signaling Mechanisms
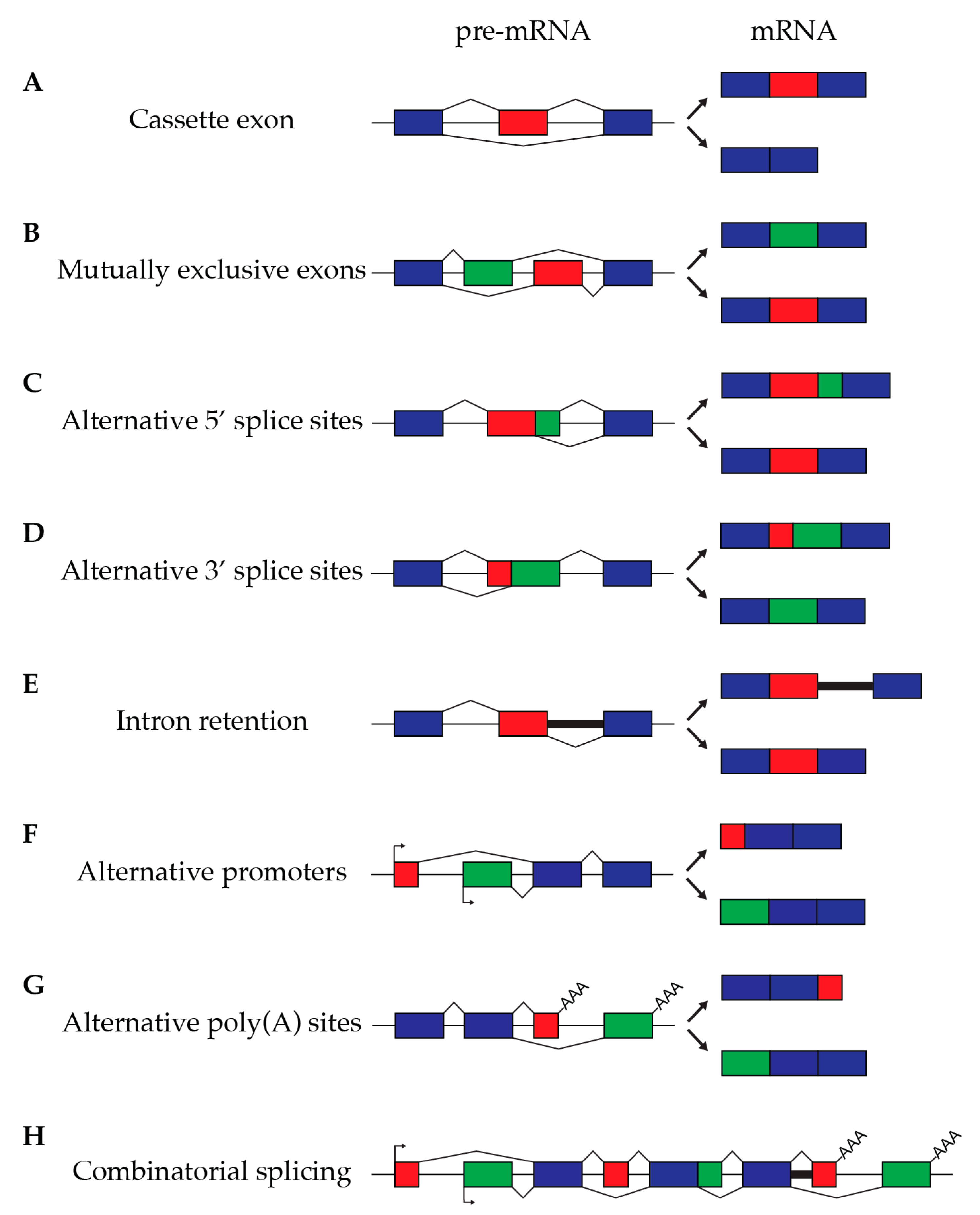
3. Alternative Splicing
4. Upstream Regulators of IFN-γ and Their Associated Co-Factors
4.1. Tumor Neopeptides
4.2. IRF8
4.3. TAp63
4.4. FOXP3
4.5. SRSF1
4.6. RBM39
5. Downstream Effectors of IFN-γ Activity
5.1. IFNGR1
5.2. STAT1
5.3. IRF1
5.4. OAS1
5.5. CD20
5.6. TrpRS
6. Therapeutic Perspectives
- (a)
- Compounds that influence alternative splicing, including Spliceostatin, Sudemycins, and FD-895, which exert direct effects on the spliceosome [202].
- (b)
- Isoginkgetin, a splicing inhibitor that functions by obstructing the recruitment of U4/U5/U6 small nuclear ribonucleoproteins (snRNPs) [203].
- (c)
- Small molecules that modulate splicing factors by targeting their regulatory kinases [204].
- (d)
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Ealick, S.; Cook, W.; Vijay-Kumar, S.; Carson, M.; Nagabhushan, T.; Trotta, P.; Bugg, C.E. Three-dimensional structure of recombinant human interferon-gamma. Science 1991, 252, 698–702. [Google Scholar] [CrossRef]
- De Veer, M.J.; Holko, M.; Frevel, M.; Walker, E.; Der, S.; Paranjape, J.M.; Silverman, R.H.; Williams, B.R. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 2001, 69, 912–920. [Google Scholar] [CrossRef]
- Munder, M.; Mallo, M.; Eichmann, K.; Modolell, M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. J. Exp. Med. 1998, 187, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Fricke, I.; Mitchell, D.; Mittelstädt, J.; Lehan, N.; Heine, H.; Goldmann, T.; Böhle, A.; Brandau, S. Mycobacteria induce IFN-gamma production in human dendritic cells via triggering of TLR2. J. Immunol. 2006, 176, 5173–5182. [Google Scholar] [CrossRef] [PubMed]
- Sercan, O.; Hämmerling, G.J.; Arnold, B.; Schüler, T. Innate immune cells contribute to the IFN-gamma-dependent regulation of antigen-specific CD8+ T cell homeostasis. J. Immunol. 2006, 176, 735–739. [Google Scholar] [CrossRef]
- Baldridge, M.T.; King, K.Y.; Boles, N.C.; Weksberg, D.C.; Goodell, M.A. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature 2010, 465, 793–797. [Google Scholar] [CrossRef]
- Duque, G.; Huang, D.C.; Dion, N.; Macoritto, M.; Rivas, D.; Li, W.; Yang, X.F.; Li, J.; Lian, J.; Marino, F.T.; et al. Interferon-γ plays a role in bone formation in vivo and rescues osteoporosis in ovariectomized mice. J. Bone Miner. Res. 2011, 26, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Street, S.E.; Cretney, E.; Smyth, M.J. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 2001, 97, 192–197. [Google Scholar] [CrossRef]
- Street, S.E.A.; Trapani, J.A.; MacGregor, D.; Smyth, M.J. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J. Exp. Med. 2002, 196, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Testi, M.G.; Pasetto, M.; Picchio, M.C.; Innamorati, G.; Mazzocco, M.; Ugel, S.; Cingarlini, S.; Bronte, V.; Zanovello, P.; et al. IFN-gamma-mediated upmodulation of MHC class I expression activates tumor-specific immune response in a mouse model of prostate cancer. Vaccine 2010, 28, 3548–3557. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kohli, K.; Black, R.G.; Yao, L.; Spadinger, S.M.; He, Q.; Pillarisetty, V.G.; Cranmer, L.D.; Van Tine, B.A.; Yee, C.; et al. Systemic Interferon-γ Increases MHC Class I Expression and T-cell Infiltration in Cold Tumors: Results of a Phase 0 Clinical Trial. Cancer Immunol. Res. 2019, 7, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- A Young, H.; Hardy, K.J. Role of interferon-gamma in immune cell regulation. J. Leukoc. Biol. 1995, 58, 373–381. [Google Scholar] [CrossRef]
- Vigneron, N. Human Tumor Antigens and Cancer Immunotherapy. BioMed Res. Int. 2015, 2015, 948501. [Google Scholar] [CrossRef]
- Chin, Y.E.; Kitagawa, M.; Su, W.C.; You, Z.H.; Iwamoto, Y.; Fu, X.Y. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science 1996, 272, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Harvat, B.L.; Seth, P.; Jetten, A.M. The role of p27Kip1 in gamma interferon-mediated growth arrest of mammary epithelial cells and related defects in mammary carcinoma cells. Oncogene 1997, 14, 2111–2122. [Google Scholar] [CrossRef]
- Chawla-Sarkar, M.; Lindner, D.J.; Liu, Y.-F.; Williams, B.R.; Sen, G.C.; Silverman, R.H.; Borden, E.C. Apoptosis and interferons: Role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 2003, 8, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.J.; Basagoudanavar, S.H.; Nogusa, S.; Irrinki, K.; Mallilankaraman, K.; Slifker, M.J.; Beg, A.A.; Madesh, M.; Balachandran, S. NF-kappaB protects cells from gamma interferon-induced RIP1-dependent necroptosis. Mol. Cell Biol. 2011, 31, 2934–2946. [Google Scholar] [CrossRef] [PubMed]
- Relation, T.; Yi, T.; Guess, A.J.; La Perle, K.; Otsuru, S.; Hasgur, S.; Dominici, M.; Breuer, C.; Horwitz, E.M. Intratumoral Delivery of Interferonγ-Secreting Mesenchymal Stromal Cells Repolarizes Tumor-Associated Macrophages and Suppresses Neuroblastoma Proliferation In Vivo. Stem Cells 2018, 36, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Overacre-Delgoffe, A.E.; Chikina, M.; Dadey, R.E.; Yano, H.; Brunazzi, E.A.; Shayan, G.; Horne, W.; Moskovitz, J.M.; Kolls, J.K.; Sander, C.; et al. Interferon-γ Drives Treg Fragility to Promote Anti-tumor Immunity. Cell 2017, 169, 1130–1141.e11. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.R.; Merlino, G. The two faces of interferon-γ in cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef]
- Beatty, G.L.; Paterson, Y. IFN-gamma can promote tumor evasion of the immune system in vivo by down-regulating cellular levels of an endogenous tumor antigen. J. Immunol. 2000, 165, 5502–5508. [Google Scholar] [CrossRef] [PubMed]
- Le Poole, I.C.; Riker, A.I.; Quevedo, M.E.; Stennett, L.S.; Wang, E.; Marincola, F.M.; Kast, W.M.; Robinson, J.K.; Nickoloff, B.J. Interferon-gamma reduces melanosomal antigen expression and recognition of melanoma cells by cytotoxic T cells. Am. J. Pathol. 2002, 160, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Finbloom, D.S.; Hoover, D.L.; Wahl, L.M. The characteristics of binding of human recombinant interferon-gamma to its receptor on human monocytes and human monocyte-like cell lines. J. Immunol. 1985, 135, 300–305. [Google Scholar] [CrossRef]
- Windsor, W.T.; Walter, L.J.; Syto, R.; Fossetta, J.; Cook, W.J.; Nagabhushan, T.L.; Walter, M.R. Purification and crystallization of a complex between human interferon gamma receptor (extracellular domain) and human interferon gamma. Proteins 1996, 26, 108–114. [Google Scholar] [CrossRef]
- Bach, E.A.; Aguet, M.; Schreiber, R.D. The IFN gamma receptor: A paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 1997, 15, 563–591. [Google Scholar] [CrossRef] [PubMed]
- Ramana, C.V.; Gil, M.P.; Schreiber, R.D.; Stark, G.R. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 2002, 23, 96–101. [Google Scholar] [CrossRef]
- Johnson, H.M.; Noon-Song, E.N.; Dabelic, R.; Ahmed, C.M. IFN signaling: How a non-canonical model led to the development of IFN mimetics. Front. Immunol. 2013, 4, 53708. [Google Scholar] [CrossRef]
- Tau, G.; Rothman, P. Biologic functions of the IFN-gamma receptors. Allergy 1999, 54, 1233–1251. [Google Scholar] [CrossRef]
- Lighvani, A.A.; Frucht, D.M.; Jankovic, D.; Yamane, H.; Aliberti, J.; Hissong, B.D.; Nguyen, B.V.; Gadina, M.; Sher, A.; Paul, W.E.; et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc. Natl. Acad. Sci. USA 2001, 98, 15137–15142. [Google Scholar] [CrossRef] [PubMed]
- Djuretic, I.M.; Levanon, D.; Negreanu, V.; Groner, Y.; Rao, A.; Ansel, K.M. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat. Immunol. 2007, 8, 145–153. [Google Scholar] [CrossRef]
- Afkarian, M.; Sedy, J.R.; Yang, J.; Jacobson, N.G.; Cereb, N.; Yang, S.Y.; Murphy, T.L.; Murphy, K.M. T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat. Immunol. 2002, 3, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, E.F.; Sibley, W.A. Circulating Virus, Interferon and Antibody After Vaccination with the 17-D Strain of Yellow-Fever Virus. N. Engl. J. Med. 1965, 273, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- The, C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 1998, 282, 2012–2018. [Google Scholar]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef]
- International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, J.; Lin, W.; Li, S.; Li, H.; Zhou, J.; Ni, P.; Dong, W.; Hu, S.; Zeng, C.; et al. The Genomes of Oryza sativa: A history of duplications. PLoS Biol. 2005, 3, e38. [Google Scholar] [CrossRef] [PubMed]
- Berget, S.M.; Moore, C.; Sharp, P.A. Spliced segments at the 5’ terminus of adenovirus 2 late mRNA. Proc. Natl. Acad. Sci. USA 1977, 74, 3171–3175. [Google Scholar] [CrossRef] [PubMed]
- Graveley, B.R. Alternative splicing: Increasing diversity in the proteomic world. Trends Genet. 2001, 17, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Mouse Genome Sequencing Consortium; Waterston, R.H.; Lindblad-Toh, K.; Birney, E.; Rogers, J.; Abril, J.F.; Agarwal, P.; Agarwala, R.; Ainscough, R.; Alexandersson, M.; et al. Initial sequencing and comparative analysis of the mouse genome. Nature 2002, 420, 520–562. [Google Scholar] [PubMed]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Ramani, A.K.; Calarco, J.A.; Pan, Q.; Mavandadi, S.; Wang, Y.; Nelson, A.C.; Lee, L.J.; Morris, Q.; Blencowe, B.J.; Zhen, M.; et al. Genome-wide analysis of alternative splicing in Caenorhabditis elegans. Genome Res. 2010, 21, 342–348. [Google Scholar] [CrossRef]
- Gibilisco, L.; Zhou, Q.; Mahajan, S.; Bachtrog, D. Alternative Splicing within and between Drosophila Species, Sexes, Tissues, and Developmental Stages. PLoS Genet. 2016, 12, e1006464. [Google Scholar] [CrossRef] [PubMed]
- Marasco, L.E.; Kornblihtt, A.R. The physiology of alternative splicing. Nat. Rev. Mol. Cell Biol. 2022, 24, 242–254. [Google Scholar] [CrossRef]
- Kim, E.; Magen, A.; Ast, G. Different levels of alternative splicing among eukaryotes. Nucleic Acids Res. 2007, 35, 125–131. [Google Scholar] [CrossRef]
- Scarpato, M.; Federico, A.; Ciccodicola, A.; Costa, V. Novel transcription factor variants through RNA-sequencing: The importance of being “alternative”. Int. J. Mol. Sci. 2015, 16, 1755–1771. [Google Scholar] [CrossRef]
- Bessa, C.; Matos, P.; Jordan, P.; Gonçalves, V. Alternative Splicing: Expanding the Landscape of Cancer Biomarkers and Therapeutics. Int. J. Mol. Sci. 2020, 21, 9032. [Google Scholar] [CrossRef]
- Wright, C.J.; Smith, C.W.J.; Jiggins, C.D. Alternative splicing as a source of phenotypic diversity. Nat. Rev. Genet. 2022, 23, 697–710. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Du, F.; Wang, J.; Bao, J.; Mi, J.; Sun, X. Primary unilateral and epilepsy adrenal tuberculosis misdiagnosed as adrenal tumor: Report of two cases. Asian J. Surg. 2021, 44, 1461–1463. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.G.; Wilusz, J.E. Non-AUG translation: A new start for protein synthesis in eukaryotes. Genes. Dev. 2017, 31, 1717–1731. [Google Scholar] [CrossRef] [PubMed]
- Grützmann, K.; Szafranski, K.; Pohl, M.; Voigt, K.; Petzold, A.; Schuster, S. Fungal alternative splicing is associated with multicellular complexity and virulence: A genome-wide multi-species study. DNA Res. 2014, 21, 27–39. [Google Scholar] [CrossRef]
- Kalyna, M.; Simpson, C.G.; Syed, N.H.; Lewandowska, D.; Marquez, Y.; Kusenda, B.; Marshall, J.; Fuller, J.; Cardle, L.; McNicol, J.; et al. Alternative splicing and nonsense-mediated decay modulate expression of important regulatory genes in Arabidopsis. Nucleic Acids Res. 2012, 40, 2454–2469. [Google Scholar] [CrossRef]
- Gonzalez-Hilarion, S.; Paulet, D.; Lee, K.-T.; Hon, C.-C.; Lechat, P.; Mogensen, E.; Moyrand, F.; Proux, C.; Barboux, R.; Bussotti, G.; et al. Intron retention-dependent gene regulation in Cryptococcus neoformans. Sci. Rep. 2016, 6, 32252. [Google Scholar] [CrossRef]
- Shakola, F.; Suri, P.; Ruggiu, M. Splicing Regulation of Pro-Inflammatory Cytokines and Chemokines: At the Interface of the Neuroendocrine and Immune Systems. Biomolecules 2015, 5, 2073–2100. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, J.; Gu, C.; Yang, Y. Alternative splicing and cancer: A systematic review. Signal Transduct. Target. Ther. 2021, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, J.F.; Kornblihtt, A.R. Alternative splicing: Multiple control mechanisms and involvement in human disease. Trends Genet. 2002, 18, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Cartegni, L.; Chew, S.L.; Krainer, A.R. Listening to silence and understanding nonsense: Exonic mutations that affect splicing. Nat. Rev. Genet. 2002, 3, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Faustino, N.A.; Cooper, T.A. Pre-mRNA splicing and human disease. Genes Dev. 2003, 17, 419–437. [Google Scholar] [CrossRef]
- Matlin, A.J.; Clark, F.; Smith, C.W.J. Understanding alternative splicing: Towards a cellular code. Nat. Rev. Mol. Cell Biol. 2005, 6, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, O.; Convertini, P.; Zhang, Z.; Wen, Y.; Shen, M.; Falaleeva, M.; Stamm, S. Function of alternative splicing. Gene 2013, 514, 1–30. [Google Scholar] [CrossRef]
- Li, D.; McIntosh, C.S.; Mastaglia, F.L.; Wilton, S.D.; Aung-Htut, M.T. Neurodegenerative diseases: A hotbed for splicing defects and the potential therapies. Transl. Neurodegener. 2021, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Lu, L.; Cai, S.; Chen, J.; Lin, W.; Han, F. Alternative Splicing: A New Cause and Potential Therapeutic Target in Autoimmune Disease. Front. Immunol. 2021, 12, 713540. [Google Scholar] [CrossRef]
- Katsikis, P.D.; Ishii, K.J.; Schliehe, C. Challenges in developing personalized neoantigen cancer vaccines. Nat. Rev. Immunol. 2024, 24, 213–227. [Google Scholar] [CrossRef]
- Xu, R.; Du, S.; Zhu, J.; Meng, F.; Liu, B. Neoantigen-targeted TCR-T cell therapy for solid tumors: How far from clinical application. Cancer Lett. 2022, 546, 215840. [Google Scholar] [CrossRef]
- Mariuzza, R.A.; Wu, D.; Pierce, B.G. Structural basis for T cell recognition of cancer neoantigens and implications for predicting neoepitope immunogenicity. Front. Immunol. 2023, 14, 1303304. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Boesch, M.; Baty, F.; Rothschild, S.I.; Tamm, M.; Joerger, M.; Früh, M.; Brutsche, M.H. Tumour neoantigen mimicry by microbial species in cancer immunotherapy. Br. J. Cancer 2021, 125, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiong, B.; Shuai, X.; Li, J.; Wang, C.; Guo, J.; Cheng, Z.; Liu, S. Downregulation of HNRNPA1 induced neoantigen generation via regulating alternative splicing. Mol. Med. 2024, 30, 85. [Google Scholar] [CrossRef]
- McClorey, G.; Fletcher, S.; Wilton, S. Splicing intervention for Duchenne muscular dystrophy. Curr. Opin. Pharmacol. 2005, 5, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Spitali, P.; Aartsma-Rus, A. Splice modulating therapies for human disease. Cell 2012, 148, 1085–1088. [Google Scholar] [CrossRef]
- van Roon-Mom, W.M.C.; Aartsma-Rus, A. Overview on applications of antisense-mediated exon skipping. Methods Mol. Biol. 2012, 867, 79–96. [Google Scholar] [PubMed]
- El Marabti, E.; Abdel-Wahab, O. Therapeutic Modulation of RNA Splicing in Malignant and Non-Malignant Disease. Trends Mol. Med. 2021, 27, 643–659. [Google Scholar] [CrossRef]
- Santos, J.I.; Gonçalves, M.; Matos, L.; Moreira, L.; Carvalho, S.; Prata, M.J.; Coutinho, M.F.; Alves, S. Splicing Modulation as a Promising Therapeutic Strategy for Lysosomal Storage Disorders: The Mucopolysaccharidoses Example. Life 2022, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Mercatante, D.R.; Sazani, P.; Kole, R. Modification of alternative splicing by antisense oligonucleotides as a potential chemotherapy for cancer and other diseases. Curr. Cancer Drug Targets 2001, 1, 211–230. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; Arechavala-Gomeza, V.; Khoo, B. Splicing modulation therapy in the treatment of genetic diseases. Appl. Clin. Genet. 2014, 7, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Havens, M.A.; Hastings, M.L. Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Res. 2016, 44, 6549–6563. [Google Scholar] [CrossRef] [PubMed]
- Sciarrillo, R.; Wojtuszkiewicz, A.; El Hassouni, B.; Funel, N.; Gandellini, P.; Lagerweij, T.; Buonamici, S.; Blijlevens, M.; van der Laan, E.A.Z.; Zaffaroni, N.; et al. Splicing modulation as novel therapeutic strategy against diffuse malignant peritoneal mesothelioma. EBioMedicine 2019, 39, 215–225. [Google Scholar] [CrossRef]
- Schneider-Poetsch, T.; Chhipi-Shrestha, J.K.; Yoshida, M. Splicing modulators: On the way from nature to clinic. J. Antibiot. 2021, 74, 603–616. [Google Scholar] [CrossRef]
- Huang, P.; Wen, F.; Tuerhong, N.; Yang, Y.; Li, Q. Neoantigens in cancer immunotherapy: Focusing on alternative splicing. Front. Immunol. 2024, 15, 1437774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mao, M.; Lv, Y.; Yang, Y.; He, W.; Song, Y.; Wang, Y.; Yang, Y.; Al Abo, M.; Freedman, J.A.; et al. A widespread length-dependent splicing dysregulation in cancer. Sci. Adv. 2022, 8, eabn9232. [Google Scholar] [CrossRef]
- Skotheim, R.I.; Nees, M. Alternative splicing in cancer: Noise, functional, or systematic? Int. J. Biochem. Cell Biol. 2007, 39, 1432–1449. [Google Scholar] [CrossRef]
- Biamonti, G.; Catillo, M.; Pignataro, D.; Montecucco, A.; Ghigna, C. The alternative splicing side of cancer. Semin. Cell Dev. Biol. 2014, 32, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Cazzola, M.; Boultwood, J.; Malcovati, L.; Vyas, P.; Bowen, D.; Pellagatti, A.; Wainscoat, J.S.; Hellstrom-Lindberg, E.; Gambacorti-Passerini, C.; et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N. Engl. J. Med. 2011, 365, 1384–1395. [Google Scholar] [CrossRef]
- Yoshida, K.; Sanada, M.; Shiraishi, Y.; Nowak, D.; Nagata, Y.; Yamamoto, R.; Sato, Y.; Sato-Otsubo, A.; Kon, A.; Nagasaki, M.; et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 2011, 478, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lawrence, M.S.; Wan, Y.; Stojanov, P.; Sougnez, C.; Stevenson, K.; Werner, L.; Sivachenko, A.; DeLuca, D.S.; Zhang, L.; et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N. Engl. J. Med. 2011, 365, 2497–2506. [Google Scholar] [CrossRef]
- Furney, S.J.; Pedersen, M.; Gentien, D.; Dumont, A.G.; Rapinat, A.; Desjardins, L.; Turajlic, S.; Piperno-Neumann, S.; de la Grange, P.; Roman-Roman, S.; et al. SF3B1 mutations are associated with alternative splicing in uveal melanoma. Cancer Discov. 2013, 3, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Roberson, E.D.O.; Anbunathan, H.; Onken, M.D.; Worley, L.A.; Bowcock, A.M. Recurrent mutations at codon 625 of the splicing factor SF3B1 in uveal melanoma. Nat. Genet. 2013, 45, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Maßhöfer, L.; Temming, P.; Rahmann, S.; Metz, C.; Bornfeld, N.; van de Nes, J.; Klein-Hitpass, L.; Hinnebusch, A.G.; Horsthemke, B.; et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat. Genet. 2013, 45, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Krauthammer, M.; Halaban, R. Rare SF3B1 R625 mutations in cutaneous melanoma. Melanoma Res. 2014, 24, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gong, Q.; Wang, Y.; Li, M.; Wang, L.; Ding, H.; Li, P. The biological function and clinical significance of SF3B1 mutations in cancer. Biomark. Res. 2020, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Benbarche, S.; Abdel-Wahab, O. Splicing factor mutations in hematologic malignancies. Blood 2021, 138, 599–612. [Google Scholar] [CrossRef]
- Cusan, M.; Shen, H.; Zhang, B.; Liao, A.; Yang, L.; Jin, M.; Fernandez, M.; Iyer, P.; Wu, Y.; Hart, K.; et al. SF3B1 mutation and ATM deletion codrive leukemogenesis via centromeric R-loop dysregulation. J. Clin. Investig. 2023, 133, e163325. [Google Scholar] [CrossRef]
- Yao, T.; Zhang, Z.; Li, Q.; Huang, R.; Hong, Y.; Li, C.; Zhang, F.; Huang, Y.; Fang, Y.; Cao, Q.; et al. Long-Read Sequencing Reveals Alternative Splicing-Driven, Shared Immunogenic Neoepitopes Regardless of SF3B1 Status in Uveal Melanoma. Cancer Immunol. Res. 2023, 11, 1671–1687. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Bies, J.; Tamura, T.; Ozato, K.; Wolff, L. The interferon regulatory factor ICSBP/IRF-8 in combination with PU.1 up-regulates expression of tumor suppressor p15(Ink4b) in murine myeloid cells. Blood 2004, 103, 4142–4149. [Google Scholar] [CrossRef][Green Version]
- Zhu, C.; Saberwal, G.; Lu, Y.; Platanias, L.C.; Eklund, E.A. The interferon consensus sequence-binding protein activates transcription of the gene encoding neurofibromin 1. J. Biol. Chem. 2004, 279, 50874–50885. [Google Scholar] [CrossRef]
- Xia, X.; Wang, W.; Yin, K.; Wang, S. Interferon regulatory factor 8 governs myeloid cell development. Cytokine Growth Factor Rev. 2020, 55, 48–57. [Google Scholar] [CrossRef]
- Li, W.; Nagineni, C.N.; Ge, H.; Efiok, B.; Chepelinsky, A.B.; Egwuagu, C.E. Interferon consensus sequence-binding protein is constitutively expressed and differentially regulated in the ocular lens. J. Biol. Chem. 1999, 274, 9686–9691. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.-S.; Wei, X.; Liu, Y.; Zhang, Y.; Chen, K.; Gao, L.; Zhou, H.; Zhu, X.-H.; Liu, P.P.; Lau, W.B.; et al. IRF8 suppresses pathological cardiac remodelling by inhibiting calcineurin signalling. Nat. Commun. 2014, 5, 3303. [Google Scholar] [CrossRef] [PubMed]
- Kesper, C.; Viestenz, A.; Wiese-Rischke, C.; Scheller, M.; Hammer, T. Impact of the transcription factor IRF8 on limbal epithelial progenitor cells in a mouse model. Exp. Eye Res. 2022, 218, 108985. [Google Scholar] [CrossRef] [PubMed]
- Tailor, P.; Tamura, T.; Morse, H.C.; Ozato, K. The BXH2 mutation in IRF8 differentially impairs dendritic cell subset development in the mouse. Blood 2008, 111, 1942–1945. [Google Scholar] [CrossRef] [PubMed]
- E Grajales-Reyes, G.; Iwata, A.; Albring, J.; Wu, X.; Tussiwand, R.; Kc, W.; Kretzer, N.M.; Briseño, C.G.; Durai, V.; Bagadia, P.; et al. Batf3 maintains autoactivation of Irf8 for commitment of a CD8α(+) conventional DC clonogenic progenitor. Nat. Immunol. 2015, 16, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Sichien, D.; Scott, C.L.; Martens, L.; Vanderkerken, M.; Van Gassen, S.; Plantinga, M.; Joeris, T.; De Prijck, S.; Vanhoutte, L.; Vanheerswynghels, M.; et al. IRF8 Transcription Factor Controls Survival and Function of Terminally Differentiated Conventional and Plasmacytoid Dendritic Cells, Respectively. Immunity 2016, 45, 626–640. [Google Scholar] [CrossRef]
- Stirewalt, D.L.; Alonzo, T.A.; Pogosova-Agadjanyan, E.L.; Gerbing, R.B.; Franklin, J.; Lee, H.; Raimondi, S.C.; Hirsch, B.A.; Gamis, A.S.; Meshinchi, S. Novel IRF8 Splice Variants Are Validated As a New Prognostic Biomarker for Adverse Outcome in an Independent Population of Pediatric Patients with AML. Blood 2012, 120, 2550. [Google Scholar] [CrossRef]
- Pogosova-Agadjanyan, E.L.; Kopecky, K.J.; Ostronoff, F.; Appelbaum, F.R.; Godwin, J.; Lee, H.; List, A.F.; May, J.J.; Oehler, V.G.; Petersdorf, S.; et al. The prognostic significance of IRF8 transcripts in adult patients with acute myeloid leukemia. PLoS ONE 2013, 8, e70812. [Google Scholar] [CrossRef]
- Zhuang, H.; Li, F.; Xu, Y.; Pei, R.; Chen, D.; Liu, X.; Li, S.; Ye, P.; Yuan, J.; Lian, J.; et al. Loss of IRF8 inhibits the growth of acute myeloid leukemia cells. Ann. Hematol. 2023, 102, 1063–1072. [Google Scholar] [CrossRef]
- Mehta, S.Y.; Morten, B.C.; Antony, J.; Henderson, L.; Lasham, A.; Campbell, H.; Cunliffe, H.; Horsfield, J.A.; Reddel, R.R.; Avery-Kiejda, K.A.; et al. Regulation of the interferon-gamma (IFN-γ) pathway by p63 and Δ133p53 isoform in different breast cancer subtypes. Oncotarget 2018, 9, 29146–29161. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, H.; Wiesmüller, L.; Chen, M. Canonical and non-canonical functions of p53 isoforms: Potentiating the complexity of tumor development and therapy resistance. Cell Death Dis. 2024, 15, 412. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Murakami, R. The adaptability of regulatory T cells and Foxp3. Int. Immunol. 2021, 33, 803–807. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Mailer, R.K.W. Alternative Splicing of FOXP3-Virtue and Vice. Front. Immunol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Smith, E.L.; Finney, H.M.; Nesbitt, A.M.; Ramsdell, F.; Robinson, M.K. Splice variants of human FOXP3 are functional inhibitors of human CD4+ T-cell activation. Immunology 2006, 119, 203–211. [Google Scholar] [CrossRef]
- Zhdanov, D.D.; Gladilina, Y.A.; Blinova, V.G.; Abramova, A.A.; Shishparenok, A.N.; Eliseeva, D.D. Induction of FoxP3 Pre-mRNA Alternative Splicing to Enhance the Suppressive Activity of Regulatory T Cells from Amyotrophic Lateral Sclerosis Patients. Biomedicines 2024, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhang, Y.; Saleh, Q.W.; Zhang, J.; Zhu, Z.; Tepel, M. Metabolic regulation of forkhead box P3 alternative splicing isoforms and their impact on health and disease. Front. Immunol. 2023, 14, 1278560. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Zhao, X.; Guo, J.; Jin, Q.; Wang, T.; Xu, W.; Li, L.; Zhang, J.; Zhang, W.; Hong, S.; et al. The splicing isoform Foxp3Δ2 differentially regulates tTreg and pTreg homeostasis. Cell Rep. 2023, 42, 112877. [Google Scholar] [CrossRef] [PubMed]
- Mailer, R.K. IPEX as a Consequence of Alternatively Spliced FOXP3. Front. Pediatr. 2020, 8, 594375. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, K.N.; Domeier, P.P.; Ziegler, S.F. A Splice of Life: The Discovery, Function, and Clinical Implications of FOXP3 Isoforms in Autoimmune Disease. Int. Immunol. 2024, 37, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Huang, C.; Zhou, B.; Ziegler, S.F. Isoform-specific inhibition of RORα-mediated transcriptional activation by human FOXP3. J. Immunol. 2008, 180, 4785–4792. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, K.; Yoshida, H.; Wakabayashi, Y.; Chinen, T.; Saeki, K.; Nakaya, M.; Takaesu, G.; Hori, S.; Yoshimura, A.; Kobayashi, T. Foxp3 Inhibits RORγt-mediated IL-17A mRNA Transcription through Direct Interaction with RORγt. J. Biol. Chem. 2008, 283, 17003–17008. [Google Scholar] [CrossRef] [PubMed]
- Kröger, B.; Spohn, M.; Mengel, M.; Sperhake, J.-P.; Ondruschka, B.; Mailer, R.K. Expression of full-length FOXP3 exceeds other isoforms in thymus and stimulated CD4+ T cells. J. Clin. Immunol. 2024, 44, 114. [Google Scholar] [CrossRef]
- Mailer, R.K.W.; Falk, K.; Rötzschke, O. Absence of leucine zipper in the natural FOXP3Δ2Δ7 isoform does not affect dimerization but abrogates suppressive capacity. PLoS ONE 2009, 4, e6104. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Goodall, J.C.; Jarvis, L.B.; Gaston, J.H. Characterisation of Foxp3 splice variants in human CD4+ and CD8+ T cells--identification of Foxp3Δ7 in human regulatory T cells. Mol. Immunol. 2010, 48, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Blinova, V.G.; Novachly, N.S.; Gippius, S.N.; Hilal, A.; Gladilina, Y.A.; Eliseeva, D.D.; Zhdanov, D.D. Phenotypical and functional characteristics of human regulatory T cells during ex vivo maturation from CD4+ T lymphocytes. Appl. Sci. 2021, 11, 5776. [Google Scholar] [CrossRef]
- Sato, Y.; Liu, J.; Lee, E.; Perriman, R.; Roncarolo, M.G.; Bacchetta, R. Co-Expression of FOXP3FL and FOXP3Δ2 Isoforms Is Required for Optimal Treg-Like Cell Phenotypes and Suppressive Function. Front. Immunol. 2021, 12, 752394. [Google Scholar] [CrossRef] [PubMed]
- Joly, A.-L.; Liu, S.; Dahlberg, C.I.; Mailer, R.K.; Westerberg, L.S.; Andersson, J. Foxp3 lacking exons 2 and 7 is unable to confer suppressive ability to regulatory T cells in vivo. J. Autoimmun. 2015, 63, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Saleh, Q.W.; Mohammadnejad, A.; Tepel, M. FOXP3 splice variant expression in males and females in healthy populations and in kidney transplant recipients. Sci. Rep. 2024, 14, 12112. [Google Scholar] [CrossRef]
- Lu, L.; Barbi, J.; Pan, F. The regulation of immune tolerance by FOXP3. Nat. Rev. Immunol. 2017, 17, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Magg, T.; Wiebking, V.; Conca, R.; Krebs, S.; Arens, S.; Schmid, I.; Klein, C.; Albert, M.H.; Hauck, F. IPEX due to an exon 7 skipping FOXP3 mutation with autoimmune diabetes mellitus cured by selective TReg cell engraftment. Clin. Immunol. 2018, 191, 52–58. [Google Scholar] [CrossRef]
- Du, J.; Wang, Q.; Yang, S.; Chen, S.; Fu, Y.; Spath, S.; Domeier, P.; Hagin, D.; Anover-Sombke, S.; Haouili, M.; et al. FOXP3 exon 2 controls Treg stability and autoimmunity. Sci. Immunol. 2022, 7, eabo5407. [Google Scholar] [CrossRef] [PubMed]
- Bin Li, B.; Samanta, A.; Song, X.; Iacono, K.T.; Brennan, P.; Chatila, T.A.; Roncador, G.; Banham, A.H.; Riley, J.L.; Wang, Q.; et al. FOXP3 is a homo-oligomer and a component of a supramolecular regulatory complex disabled in the human XLAAD/IPEX autoimmune disease. Int. Immunol. 2007, 19, 825–835. [Google Scholar] [CrossRef]
- Song, X.; Li, B.; Xiao, Y.; Chen, C.; Wang, Q.; Liu, Y.; Berezov, A.; Xu, C.; Gao, Y.; Li, Z.; et al. Structural and biological features of foxp3 dimerization relevant to regulatory T cell function. Cell Rep. 2012, 1, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, T.; Li, H.; Krishfield, S.M.; Kyttaris, V.C.; Moulton, V.R. Splicing factor SRSF1 limits IFN-γ production via RhoH and ameliorates experimental nephritis. Rheumatology 2021, 60, 420–429. [Google Scholar] [CrossRef]
- Katsuyama, T.; Li, H.; Comte, D.; Tsokos, G.C.; Moulton, V.R. Splicing factor SRSF1 controls T cell hyperactivity and systemic autoimmunity. J. Clin. Investig. 2019, 129, 5411–5423. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, T.; Moulton, V.R. Splicing factor SRSF1 is indispensable for regulatory T cell homeostasis and function. Cell Rep. 2021, 36, 109339. [Google Scholar] [CrossRef]
- Le Pen, J.; Paniccia, G.; Kinast, V.; Moncada-Velez, M.; Ashbrook, A.W.; Bauer, M.; Hoffmann, H.-H.; Pinharanda, A.; Ricardo-Lax, I.; Stenzel, A.F.; et al. A genome-wide arrayed CRISPR screen identifies PLSCR1 as an intrinsic barrier to SARS-CoV-2 entry that recent virus variants have evolved to resist. PLoS Biol. 2024, 22, e3002767. [Google Scholar] [CrossRef]
- Mai, S.; Qu, X.; Li, P.; Ma, Q.; Cao, C.; Liu, X. Global regulation of alternative RNA splicing by the SR-rich protein RBM39. Biochim. Biophys. Acta 2016, 1859, 1014–1024. [Google Scholar] [CrossRef]
- Li, T.-F.; Rothhaar, P.; Lang, A.; Gruenvogel, O.; Colasanti, O.; Ugarte, S.M.O.; Traut, J.; Piras, A.; Acosta-Rivero, N.; Magalhaes, V.G.; et al. RBM39 shapes innate immunity through transcriptional and splicing control of key factors of the interferon response. bioRxiv 2023. [Google Scholar] [CrossRef]
- Campagne, S.; Jutzi, D.; Malard, F.; Matoga, M.; Romane, K.; Feldmuller, M.; Colombo, M.; Ruepp, M.-D.; Allain, F.H.-T. Molecular basis of RNA-binding and autoregulation by the cancer-associated splicing factor RBM39. Nat. Commun. 2023, 14, 5366. [Google Scholar] [CrossRef]
- Xu, Y.; Nijhuis, A.; Keun, H.C. RNA-binding motif protein 39 (RBM39): An emerging cancer target. Br. J. Pharmacol. 2022, 179, 2795–2812. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, X.; Zhang, X.; Zhao, D.; Dou, Z.; Xie, X.; Li, H.; Yang, H.; Li, Q.; Zhang, H.; et al. RNA-binding protein 39: A promising therapeutic target for cancer. Cell Death Discov. 2021, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Spear, S.; Ma, Y.; Lorentzen, M.P.; Gruet, M.; McKinney, F.; Xu, Y.; Wickremesinghe, C.; Shepherd, M.R.; McNeish, I.; et al. Pharmacological depletion of RNA splicing factor RBM39 by indisulam synergizes with PARP inhibitors in high-grade serous ovarian carcinoma. Cell Rep. 2023, 42, 113307. [Google Scholar] [CrossRef]
- Eilers, A.; Georgellis, D.; Klose, B.; Schindler, C.; Ziemiecki, A.; Harpur, A.G.; Wilks, A.F.; Decker, T. Differentiation-regulated serine phosphorylation of STAT1 promotes GAF activation in macrophages. Mol. Cell. Biol. 1995, 15, 3579–3586. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, M.H.; van Dissel, J.T.; Holland, S.M. Human host genetic factors in nontuberculous mycobacterial infection: Lessons from single gene disorders affecting innate and adaptive immunity and lessons from molecular defects in interferon-gamma-dependent signaling. Microbes Infect. 2006, 8, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Horvath, C.M.; Wen, Z.; E Darnell, J. A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes. Dev. 1995, 9, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Blaszczyk, K.; Nowicka, H.; Kostyrko, K.; Antonczyk, A.; Wesoly, J.; Bluyssen, H.A. The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses. Cytokine Growth Factor Rev. 2016, 29, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Rosain, J.; Neehus, A.-L.; Manry, J.; Yang, R.; Le Pen, J.; Daher, W.; Liu, Z.; Chan, Y.-H.; Tahuil, N.; Türel, Ö.; et al. Human IRF1 governs macrophagic IFN-γ immunity to mycobacteria. Cell 2023, 186, 621–645.e33. [Google Scholar] [CrossRef]
- Zhang, P.; Ying, W.; Wu, B.; Liu, R.; Wang, H.; Wang, X.; Lu, Y. Complete IFN-γR1 Deficiency in a Boy Due to UPD(6)mat with IFNGR1 Novel Splicing Variant. J. Clin. Immunol. 2021, 41, 834–836. [Google Scholar] [CrossRef]
- Müller, M.; Laxton, C.; Briscoe, J.; Schindler, C.; Improta, T.; Darnell, J.; Stark, G.; Kerr, I. Complementation of a mutant cell line: Central role of the 91 kDa polypeptide of ISGF3 in the interferon-alpha and -gamma signal transduction pathways. EMBO J. 1993, 12, 4221–4228. [Google Scholar] [CrossRef] [PubMed]
- Shuai, K.; Stark, G.R.; Kerr, I.M.; Darnell, J.E. A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science 1993, 261, 1744–1746. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, N.; Lymar, E.S.; Yang, E.; Malik, S.; Zhang, J.J.; Roeder, R.G.; Darnell, J.E. Distinct transcriptional activation functions of STAT1alpha and STAT1beta on DNA and chromatin templates. J. Biol. Chem. 2003, 278, 43067–43073. [Google Scholar] [CrossRef] [PubMed]
- Semper, C.; Leitner, N.R.; Lassnig, C.; Parrini, M.; Mahlakõiv, T.; Rammerstorfer, M.; Lorenz, K.; Rigler, D.; Müller, S.; Kolbe, T.; et al. STAT1β is not dominant negative and is capable of contributing to gamma interferon-dependent innate immunity. Mol. Cell. Biol. 2014, 34, 2235–2248. [Google Scholar] [CrossRef]
- Schindler, C.; Fu, X.Y.; Improta, T.; Aebersold, R.; E Darnell, J. Proteins of transcription factor ISGF-3: One gene encodes the 91-and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc. Natl. Acad. Sci. USA 1992, 89, 7836–7839. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Y.; Kessler, D.S.; A Veals, S.; E Levy, D.; E Darnell, J. ISGF3, the transcriptional activator induced by interferon alpha, consists of multiple interacting polypeptide chains. Proc. Natl. Acad. Sci. USA 1990, 87, 8555–8559. [Google Scholar] [CrossRef]
- Kessler, D.S.; Veals, S.A.; Fu, X.Y.; Levy, D.E. Interferon-alpha regulates nuclear translocation and DNA-binding affinity of ISGF3, a multimeric transcriptional activator. Genes. Dev. 1990, 4, 1753–1765. [Google Scholar] [CrossRef]
- Ramana, C.V.; Chatterjee-Kishore, M.; Nguyen, H.; Stark, G.R. Complex roles of Stat1 in regulating gene expression. Oncogene 2000, 19, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Darnell, J.E., Jr. STATs: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Parrini, M.; Meissl, K.; Ola, M.J.; Lederer, T.; Puga, A.; Wienerroither, S.; Kovarik, P.; Decker, T.; Müller, M.; Strobl, B. The C-Terminal Transactivation Domain of STAT1 Has a Gene-Specific Role in Transactivation and Cofactor Recruitment. Front. Immunol. 2018, 9, 2879. [Google Scholar] [CrossRef] [PubMed]
- Bluyssen, H. STAT2-directed pathogen responses. Oncotarget 2015, 6, 28525–28526. [Google Scholar] [CrossRef] [PubMed]
- Göder, A.; Ginter, T.; Heinzel, T.; Stroh, S.; Fahrer, J.; Henke, A.; Krämer, O.H. STAT1 N-terminal domain discriminatively controls type I and type II IFN signaling. Cytokine 2021, 144, 155552. [Google Scholar] [CrossRef] [PubMed]
- Staab, J.; Nast, R.; Meyer, T. Do the two transcription factors form a positive feedback amplifier circuit in their common fight against pathogens? Immunol. Cell Biol. 2018, 96, 1023. [Google Scholar] [CrossRef]
- Bernard, A.; Hibos, C.; Richard, C.; Viltard, E.; Chevrier, S.; Lemoine, S.; Melin, J.; Humblin, E.; Mary, R.; Accogli, T.; et al. The Tumor Microenvironment Impairs Th1 IFNγ Secretion through Alternative Splicing Modifications of Irf1 Pre-mRNA. Cancer Immunol. Res. 2021, 9, 324–336. [Google Scholar] [CrossRef]
- Ueshima, C.; Kataoka, T.R.; Takei, Y.; Hirata, M.; Sugimoto, A.; Hirokawa, M.; Okayama, Y.; Blumberg, R.S.; Haga, H. CEACAM1 long isoform has opposite effects on the growth of human mastocytosis and medullary thyroid carcinoma cells. Cancer Med. 2017, 6, 845–856. [Google Scholar] [CrossRef]
- Dery, K.J.; Kujawski, M.; Grunert, D.; Wu, X.; Ngyuen, T.; Cheung, C.; Yim, J.H.; E Shively, J. IRF-1 regulates alternative mRNA splicing of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) in breast epithelial cells generating an immunoreceptor tyrosine-based inhibition motif (ITIM) containing isoform. Mol. Cancer 2014, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Dery, K.J.; Silver, C.; Yang, L.; Shively, J.E. Interferon regulatory factor 1 and a variant of heterogeneous nuclear ribonucleoprotein L coordinately silence the gene for adhesion protein CEACAM1. J. Biol. Chem. 2018, 293, 9277–9291. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Kondo, T.; Ogawa, S.; Tamura, T.; Kitagawa, M.; Tanaka, N.; Lamphier, M.S.; Hirai, H.; Taniguchi, T. Accelerated exon skipping of IRF-1 mRNA in human myelodysplasia/leukemia; a possible mechanism of tumor suppressor inactivation. Oncogene 1994, 9, 3313–3320. [Google Scholar] [PubMed]
- Testa, U.; Stellacci, E.; Pelosi, E.; Sestili, P.; Venditti, M.; Orsatti, R.; Fragale, A.; Petrucci, E.; Pasquini, L.; Belardelli, F.; et al. Impaired myelopoiesis in mice devoid of interferon regulatory factor 1. Leukemia 2004, 18, 1864–1871. [Google Scholar] [CrossRef]
- Khan, T.; Ganai, B.A.; Masood, A.; Samoon, J.; Beigh, S.R.; Qazi, F. Relation between IRF-1 gene and acute myelocytic leukemia in Kashmiri population. Asian Pac. J. Cancer Prev. 2011, 12, 1035–1039. [Google Scholar]
- Liu, L.; Wang, H.; Wen, J.; Tseng, C.-E.; Zu, Y.; Chang, C.-C.; Zhou, X. Mutated genes and driver pathways involved in myelodysplastic syndromes—A transcriptome sequencing based approach. Mol. Biosyst. 2015, 11, 2158–2166. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Jo, M.; Park, J.; Zhang, W.; Lee, J.-H. Alternative splicing variants of IRF-1 lacking exons 7, 8, and 9 in cervical cancer. Biochem. Biophys. Res. Commun. 2006, 347, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Di, H.; Elbahesh, H.; Brinton, M.A. Characteristics of Human OAS1 Isoform Proteins. Viruses 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.E.; Butler-Laporte, G.; Khan, A.; Pairo-Castineira, E.; Drivas, T.G.; Peloso, G.M.; Nakanishi, T.; COVID-19 Host Genetics Initiative; Ganna, A.; Verma, A.; et al. Multi-ancestry fine mapping implicates OAS1 splicing in risk of severe COVID-19. Nat. Genet. 2022, 54, 125–127. [Google Scholar] [CrossRef]
- Banday, A.R.; Stanifer, M.L.; Florez-Vargas, O.; Onabajo, O.O.; Papenberg, B.W.; Zahoor, M.A.; Mirabello, L.; Ring, T.J.; Lee, C.-H.; Albert, P.S.; et al. Genetic regulation of OAS1 nonsense-mediated decay underlies association with COVID-19 hospitalization in patients of European and African ancestries. Nat. Genet. 2022, 54, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Frankiw, L.; Mann, M.; Li, G.; Joglekar, A.; Baltimore, D. Alternative splicing coupled with transcript degradation modulates OAS1g antiviral activity. RNA 2020, 26, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Vauchy, C.; Gamonet, C.; Ferrand, C.; Daguindau, E.; Galaine, J.; Beziaud, L.; Chauchet, A.; Dunand, C.J.H.; Deschamps, M.; Rohrlich, P.S.; et al. CD20 alternative splicing isoform generates immunogenic CD4 helper T epitopes. Int. J. Cancer 2015, 137, 116–126. [Google Scholar] [CrossRef]
- Gamonet, C.; Bole-Richard, E.; Delherme, A.; Aubin, F.; Toussirot, E.; Garnache-Ottou, F.; Godet, Y.; Ysebaert, L.; Tournilhac, O.; Dartigeas, C.; et al. New CD20 alternative splice variants: Molecular identification and differential expression within hematological B cell malignancies. Exp. Hematol. Oncol. 2015, 5, 7, Erratum in Exp. Hematol. Oncol. 2015, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, F.; Krautwurst, S.; Salentin, S.; Haupt, V.J.; Leberecht, C.; Bittrich, S.; Labudde, D.; Schroeder, M. The structural basis of the genetic code: Amino acid recognition by aminoacyl-tRNA synthetases. Sci. Rep. 2020, 10, 12647. [Google Scholar] [CrossRef]
- Wakasugi, K.; Schimmel, P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science 1999, 284, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Wakasugi, K.; Slike, B.M.; Hood, J.; Ewalt, K.L.; Cheresh, D.A.; Schimmel, P. Induction of angiogenesis by a fragment of human tyrosyl-tRNA synthetase. J. Biol. Chem. 2002, 277, 20124–20126. [Google Scholar] [CrossRef]
- Greenberg, Y.; King, M.; Kiosses, W.B.; Ewalt, K.; Yang, X.; Schimmel, P.; Reader, J.S.; Tzima, E. The novel fragment of tyrosyl tRNA synthetase, mini-TyrRS, is secreted to induce an angiogenic response in endothelial cells. FASEB J. 2007, 22, 1597–1605. [Google Scholar] [CrossRef]
- Otani, A.; Slike, B.M.; Dorrell, M.I.; Hood, J.; Kinder, K.; Ewalt, K.L.; Cheresh, D.; Schimmel, P.; Friedlander, M. A fragment of human TrpRS as a potent antagonist of ocular angiogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 178–183. [Google Scholar] [CrossRef]
- Wakasugi, K.; Slike, B.M.; Hood, J.; Otani, A.; Ewalt, K.L.; Friedlander, M.; Cheresh, D.A.; Schimmel, P. A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Tolstrup, A.B.; Bejder, A.; Fleckner, J.; Justesen, J. Transcriptional regulation of the interferon-gamma-inducible tryptophanyl-tRNA synthetase includes alternative splicing. J. Biol. Chem. 1995, 270, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.C.; Larsen, M.R.; Roepstorff, P.; Justesen, J.; Christiansen, G.; Birkelund, S. Mapping and identification of interferon gamma-regulated HeLa cell proteins separated by immobilized pH gradient two-dimensional gel electrophoresis. Electrophoresis 1999, 20, 984–993. [Google Scholar] [CrossRef]
- Liu, J.; Shue, E.; Ewalt, K.L.; Schimmel, P. A new gamma-interferon-inducible promoter and splice variants of an anti-angiogenic human tRNA synthetase. Nucleic Acids Res. 2004, 32, 719–727. [Google Scholar] [CrossRef]
- Turpaev, K.T.; Zakhariev, V.M.; Sokolova, I.V.; Narovlyansky, A.N.; Amchenkova, A.M.; Justesen, J.; Frolova, L.Y. Alternative processing of the tryptophanyl-tRNA synthetase mRNA from interferon-treated human cells. Eur. J. Biochem. 1996, 240, 732–737. [Google Scholar] [CrossRef]
- Epely, S.; Gros, C.; Labouesse, J.; Lemaire, G. Limited proteolysis of tryptophanyl-tRNA synthetase from beef pancreas. Eur. J. Biochem. 1976, 61, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Scheinker, V.; Beresten, S.; Degtyarev, S.; Kisselev, L. The effect of tRNA and tryptophanyl adenylate on limited proteolysis of beef pancreas tryptophanyl-tRNA synthetase. Nucleic Acids Res. 1979, 7, 625–637. [Google Scholar] [CrossRef][Green Version]
- Favorova, O.O.; Zargarova, T.A.; Rukosuyev, V.S.; Beresten, S.F.; Kisselev, L.L. Molecular and cellular studies of tryptophanyl-tRNA synthetases using monoclonal antibodies. Remarkable variations in the content of tryptophanyl-tRNA synthetase in the pancreas of different mammals. Eur. J. Biochem. 1989, 184, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Lu, J. Interferon gamma in cancer immunotherapy. Cancer Med. 2018, 7, 4509–4516. [Google Scholar] [CrossRef] [PubMed]
- Anczuków, O.; Krainer, A.R. Splicing-factor alterations in cancers. RNA 2016, 22, 1285–1301. [Google Scholar] [CrossRef]
- Song, X.; Zeng, Z.; Wei, H.; Wang, Z. Alternative splicing in cancers: From aberrant regulation to new therapeutics. Semin. Cell Dev. Biol. 2018, 75, 13–22. [Google Scholar] [CrossRef]
- Rahman, M.A.; Nasrin, F.; Bhattacharjee, S.; Nandi, S. Hallmarks of Splicing Defects in Cancer: Clinical Applications in the Era of Personalized Medicine. Cancers 2020, 12, 1381. [Google Scholar] [CrossRef]
- Lai, J.Y.; Ho, J.X.; Kow, A.S.F.; Liang, G.; Tham, C.L.; Ho, Y.-C.; Lee, M.T. Interferon therapy and its association with depressive disorders—A review. Front. Immunol. 2023, 14, 1048592. [Google Scholar] [CrossRef]
- Viborg, N.; Pavlidis, M.A.; Barrio-Calvo, M.; Friis, S.; Trolle, T.; Sørensen, A.B.; Thygesen, C.B.; Kofoed, S.V.; Kleine-Kohlbrecher, D.; Hadrup, S.R.; et al. DNA based neoepitope vaccination induces tumor control in syngeneic mouse models. NPJ Vaccines 2023, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, Y.; Wong, M.S.; Martí-Gómez, C.; Ayaz, A.; Kooshkbaghi, M.; Hanson, S.M.; McCandlish, D.M.; Krainer, A.R.; Kinney, J.B. Specificity, synergy, and mechanisms of splice-modifying drugs. Nat. Commun. 2024, 15, 1880. [Google Scholar] [CrossRef]
- Herrero-Vicente, J.; Black, D.L.; Valcárcel, J. Splice-modifying drug mechanisms. Nat. Chem. Biol. 2024, 20, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Convertini, P.; Shen, M.; Potter, P.M.; Palacios, G.; Lagisetti, C.; de la Grange, P.; Horbinski, C.; Fondufe-Mittendorf, Y.N.; Webb, T.R.; Stamm, S. Sudemycin E influences alternative splicing and changes chromatin modifications. Nucleic Acids Res. 2014, 42, 4947–4961. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Matlin, A.J.; Lowell, A.M.; Moore, M.J. The biflavonoid isoginkgetin is a general inhibitor of Pre-mRNA splicing. J. Biol. Chem. 2008, 283, 33147–33154. [Google Scholar] [CrossRef]
- Bouton, L.; Ecoutin, A.; Malard, F.; Campagne, S. Small molecules modulating RNA splicing: A review of targets and future perspectives. RSC Med. Chem. 2024, 15, 1109–1126. [Google Scholar] [CrossRef]
- Han, T.; Goralski, M.; Gaskill, N.; Capota, E.; Kim, J.; Ting, T.C.; Xie, Y.; Williams, N.S.; Nijhawan, D. Anticancer sulfonamides target splicing by inducing RBM39 degradation via recruitment to DCAF15. Science 2017, 356, eaal3755. [Google Scholar] [CrossRef]
- Wang, E.; Lu, S.X.; Pastore, A.; Chen, X.; Imig, J.; Lee, S.C.-W.; Hockemeyer, K.; Ghebrechristos, Y.E.; Yoshimi, A.; Inoue, D.; et al. Targeting an RNA-Binding Protein Network in Acute Myeloid Leukemia. Cancer Cell 2019, 35, 369–384.e7. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Quarni, W.; Goralski, M.; Wan, S.; Jin, H.; Van de Velde, L.-A.; Fang, J.; Wu, Q.; Abu-Zaid, A.; Wang, T.; et al. Targeting the spliceosome through RBM39 degradation results in exceptional responses in high-risk neuroblastoma models. Sci. Adv. 2021, 7, eabj5405. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Yang, Y.; Yu, J.; Yin, H.; Chu, X.; Yang, P.; Xu, L.; Wang, X.; Hu, S.; Li, Y.; et al. Targeting RBM39 through indisulam induced mis-splicing of mRNA to exert anti-cancer effects in T-cell acute lymphoblastic leukemia. J. Exp. Clin. Cancer Res. 2024, 43, 205. [Google Scholar] [CrossRef] [PubMed]

| Factor | Function | Splicing Event (s) |
|---|---|---|
| AMZ2P1-neo; MZT2B-neo | MHC-bound tumor neopeptides | Exon skipping; alternative splice sites; alternative first exons; intron retention |
| IRF8 | Transcription factor | Exon skipping; alternative first exon |
| Tap63 | Transcription factor | Exon skipping |
| FOXP3 | Transcription factor | Exon skipping |
| SRSF1 | Splicing factor | SRSF1-regulated RhoH expression |
| RBM39 | Splicing factor | RBM39-regulated IRF3 expression; inclusion of poison exon in RBM39 |
| Factor | Function | Splicing Event (s) |
|---|---|---|
| IFNGR1 | Cell surface receptor subunit | Potential exon skipping |
| STAT1 | Transcription factor | Alternative C-terminal domains |
| IRF1 | Transcription factor | Exon skipping |
| OAS1 | Antiviral defense | Poison exon |
| CD20 | Cell surface receptor | Exon skipping |
| TrpRS | Tryptophanyl-tRNA synthetase | Truncated splice variants |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suri, P.; Badalov, A.; Ruggiu, M. Alternative Splicing as a Modulator of the Interferon-Gamma Pathway. Cancers 2025, 17, 594. https://doi.org/10.3390/cancers17040594
Suri P, Badalov A, Ruggiu M. Alternative Splicing as a Modulator of the Interferon-Gamma Pathway. Cancers. 2025; 17(4):594. https://doi.org/10.3390/cancers17040594
Chicago/Turabian StyleSuri, Parul, Ariana Badalov, and Matteo Ruggiu. 2025. "Alternative Splicing as a Modulator of the Interferon-Gamma Pathway" Cancers 17, no. 4: 594. https://doi.org/10.3390/cancers17040594
APA StyleSuri, P., Badalov, A., & Ruggiu, M. (2025). Alternative Splicing as a Modulator of the Interferon-Gamma Pathway. Cancers, 17(4), 594. https://doi.org/10.3390/cancers17040594






