Simple Summary
IFNγ is a multifunctional cytokine produced not only by activated lymphocytes but also in response to cancer immunotherapies; it has both antitumor and tumor-promoting effects. In ovarian cancer cells, the tumor-promoting effects of IFNγ are mediated by increased expression of the proto-oncogene Bcl3, the immune checkpoint PD-L1 and the proinflammatory cytokine IL-8. Recent studies have shown that IFNγ induces the expression of Bcl3, which then promotes the expression of PD-L1 and IL-8 in ovarian cancer cells, resulting in their increased proliferation and migration. In this review, we summarize the recent findings on the IFNγ tumor-promoting functions and on the mechanisms by which IFNγ induces Bcl3, PD-L1 and IL-8 expression in cancer cells, with a special focus on ovarian cancer. We also highlight the importance of a better understanding of these mechanisms to optimize the development of combinatorial approaches in cancer immunotherapies.
Abstract
IFNγ, a pleiotropic cytokine produced not only by activated lymphocytes but also in response to cancer immunotherapies, has both antitumor and tumor-promoting functions. In ovarian cancer (OC) cells, the tumor-promoting functions of IFNγ are mediated by IFNγ-induced expression of Bcl3, PD-L1 and IL-8/CXCL8, which have long been known to have critical cellular functions as a proto-oncogene, an immune checkpoint ligand and a chemoattractant, respectively. However, overwhelming evidence has demonstrated that these three genes have tumor-promoting roles far beyond their originally identified functions. These tumor-promoting mechanisms include increased cancer cell proliferation, invasion, angiogenesis, metastasis, resistance to chemotherapy and immune escape. Recent studies have shown that IFNγ-induced Bcl3, PD-L1 and IL-8 expression is regulated by the same JAK1/STAT1 signaling pathway: IFNγ induces the expression of Bcl3, which then promotes the expression of PD-L1 and IL-8 in OC cells, resulting in their increased proliferation and migration. In this review, we summarize the recent findings on how IFNγ affects the tumor microenvironment and promotes tumor progression, with a special focus on ovarian cancer and on Bcl3, PD-L1 and IL-8/CXCL8 signaling. We also discuss promising novel combinatorial strategies in clinical trials targeting Bcl3, PD-L1 and IL-8 to increase the effectiveness of cancer immunotherapies.
1. Introduction
Ovarian cancer (OC) is the most aggressive gynecologic cancer, with poor survival and high mortality rates. One of the key features of OC is its heterogeneity, partly explaining, together with the lack of specific symptoms and biomarkers for early detection, the lack of successful treatments and poor outcomes [1,2,3,4,5,6,7,8]. The current treatment strategy includes tumor reduction surgery followed by platinum and taxane-based chemotherapy. However, since most patients relapse and develop chemoresistance, new therapeutic strategies are urgently needed. Immunotherapies have shown great promise in treating a variety of cancers, but in OC, the results have been disappointing. Further studies are needed to better understand the nature of immune signaling in OC and develop more effective therapeutic approaches to increase the effectiveness of cancer immunotherapies.
IFNγ is a pleiotropic cytokine that plays a major role in the immune response and cancer immunity [9,10,11,12,13]. In the tumor microenvironment (TME), IFNγ is produced predominantly by tumor-infiltrating lymphocytes (TILs), particularly by activated CD8 cytotoxic T cells. In addition, IFNγ expression is induced in response to immune checkpoint blockade (ICB) or radiation therapy used in cancer treatment [14,15,16,17,18]. However, since most OC tumors do not have high levels of TILs, other immune cells likely contribute to IFNγ expression in OC TME. In this regard, macrophages were reported to produce IFNγ [19], and since in OC, tumor-associated macrophages (TAMs) constitute over 50% of cells in the peritoneal tumor implants and the ascites [20], they likely represent another source of IFNγ. Furthermore, a study by Nowak et al. showed that peripheral blood mononuclear cells (PBMCs) isolated from OC patients produce appreciable levels of IFNγ [21], suggesting that mononuclear cells may also contribute to IFNγ expression in the TME. In addition to OC cells, IFNγ likely regulates other cells in the TME, including TILs, TAMs, dendritic cells (DCs), myeloid-derived suppressor cells (MDSCs), as well as cancer-associated fibroblasts (CAFs), thus having a central regulatory function in the TME (Figure 1).
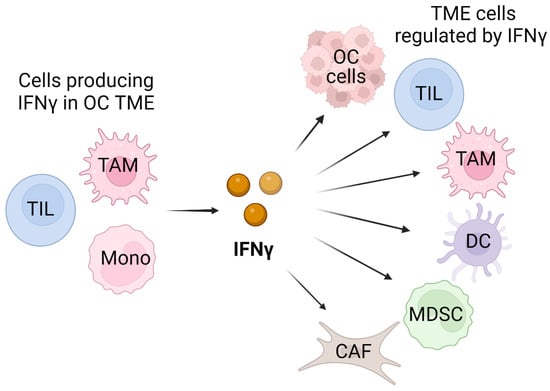
Figure 1.
Schematic illustration of cellular sources and targets of IFNγ in OC TME. In addition to tumor-infiltrating lymphocytes (TILs), IFNγ can be produced by tumor-associated macrophages (TAMs) and monocytic cells (Monos) present in TME. The cellular targets of the produced IFNγ include OC cells and also TILs, TAMs, dendritic cells (DCs), myeloid-derived suppressor cells (MDSCs), as well as cancer-associated fibroblasts (CAFs). Created with BioRender.com with granted permission and license.
Early studies revealed numerous antitumor effects of IFNγ, including enhanced MHC expression and antigen presentation on cancer cells, increased T-cell cytotoxic activity, and increased cancer cell cycle arrest and apoptosis [9,10,11,12,13,22,23,24,25,26,27,28,29]. Because of these antitumor properties, IFNγ has been used in cancer treatment [9,10,11,12,13]. However, most clinical trials using IFNγ have produced mixed or disappointing results [30,31,32,33,34]. Indeed, recent studies have shown that IFNγ also has important tumor-promoting functions that are less well understood and include the ability of IFNγ to induce the expression of immune checkpoints and T-cell exhaustion and increase cancer cell proliferation and migration [35,36,37,38,39,40,41,42,43,44]. In ovarian cancer, IFNγ induces the expression of the cancer antigen CA-125 (MUC-16) [45], human leukocyte antigen-E (HLA-E) [46], the immune checkpoint ligand PD-L1 [36,38,47,48,49], the proto-oncogene Bcl3 [47,50], and the pro-angiogenic and pro-inflammatory chemokine IL-8 [50,51], resulting in increased proliferation and migration of OC cells, immune escape, and tumor growth (Table 1).

Table 1.
Antitumor and tumorigenic functions of IFNγ in ovarian cancer cells.
Understanding the tumor-promoting mechanisms and cellular and molecular targets of IFNγ in ovarian cancer is crucial for minimizing its tumor-promoting functions in PD-1/PD-L1-blocking cancer immunotherapies that induce IFNγ expression and in other therapies associated with IFNγ release. Since Bcl3, PD-L1 and IL-8 promote tumorigenesis, combinatorial strategies targeting the IFNγ-induced Bcl3, PD-L1 and IL-8 expression in ovarian cancer may enhance the antitumor effects of cancer immunotherapies.
In this review, we summarize the current knowledge on the mechanisms by which IFNγ induces the expression of Bcl3, PD-L1 and IL-8/CXCL8 in cancer cells, with a special focus on ovarian cancer, and highlight the importance of a better understanding of these mechanisms to optimize the development of combinatorial approaches in cancer immunotherapies.
2. IFNγ Signaling in Ovarian Cancer
The biological effects of IFNγ are mediated by its binding to cell surface receptors IFNGR1 and IFNGR2, resulting in oligomerization of the receptor and phosphorylation and activation of the tyrosine Janus protein kinases JAK1 and JAK2 [9,52,53]. Activated JAK1/JAK2 then phosphorylate the transcription factor STAT1 at Tyr-701, resulting in its dimerization and nuclear translocation [52,53]. In addition to STAT1 phosphorylation at Tyr-701 by JAK1/2, STAT1 can be phosphorylated at Ser-727, which is required for its maximum transcriptional activity; however, the regulation of STAT1 phosphorylation on Ser-727 is cell specific [9,52]. In some cells, IFNγ signaling may also activate IκB kinase (IKK) to promote the expression of NFκB-dependent genes [54,55,56]. Furthermore, several studies have shown that IFNγ induces promoter histone acetylation by recruiting the histone acetyltransferases (HATs) p300 and CBP, resulting in increased promoter accessibility and transcription of interferon stimulated genes (ISGs) [57,58,59,60].
In ovarian cancer cells, IFNγ induces Bcl3, PD-L1 and IL-8 expression by mechanisms involving increased promoter acetylation, JAK1-STAT1 signaling, p65 NFκB signaling and Bcl3, resulting in increased OC cell proliferation and migration [47,48,49,50,51]; these mechanisms are schematically illustrated in Figure 2. In addition, a recent study has shown that IFNγ upregulates the expression of suppressor of cytokine signaling 1 (SOCS1) in OC cells, thus suppressing STAT3 and STAT5 and decreasing OC cell proliferation [61].
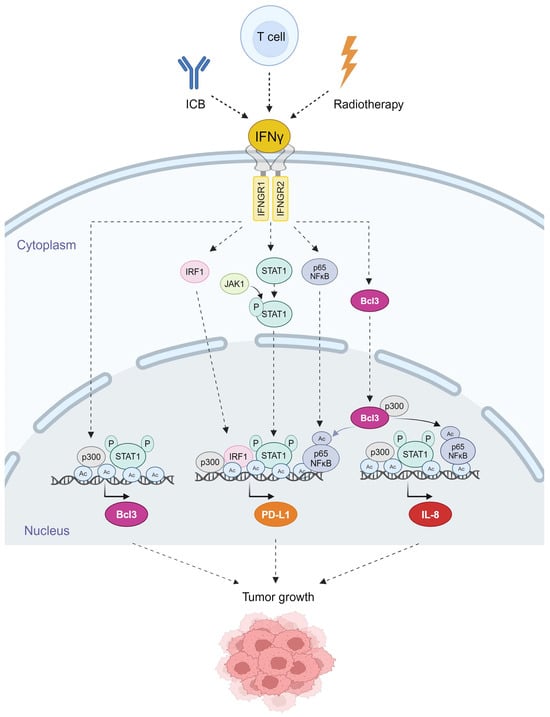
Figure 2.
Schematic illustration of the mechanisms regulating IFNγ-induced Bcl3, PD-L1 and IL-8 expression in OC cells. IFNγ binds to its receptors IFNGR1 and IFNGR2, resulting in the activation of JAK1 kinase, and increased expression of the transcription regulators IRF1, STAT1, p65 NFκB and Bcl3 in OC cells [47,49,50]. JAK1 phosphorylates STAT1 at Tyr-701, which is required for the nuclear translocation of STAT1. STAT1 is then phosphorylated at Ser-727, which promotes its recruitment to the Bcl3, PD-L1 and IL-8 promoters. In addition, IFNγ induces p300-mediated acetylation of p65 NFκB at K314/315, resulting in increased p65 transcriptional activity and recruitment to PD-L1 and IL-8 promoters [47,49]. IFNγ also induces acetylation of histones (Ac) at the Bcl3, PD-L1 and IL-8 promoters in OC cells, thus facilitating transcription factor recruitment and transcription [47,48,49,50,51]. Created with BioRender.com with granted permission and license.
While early studies suggested that increased IFNγ levels correlate with improved clinical outcomes in patients with ovarian cancer [62], more recent studies have shown that high IFNγ expression in OC tissues promotes cancer progression and correlates with poor survival [36,46,63]. This discrepancy might reflect the high heterogeneity of OC tissues, different analytical methods used and the low stability of endogenous IFNγ in human serum and tissues.
3. Bcl3 in Ovarian Cancer
3.1. Bcl3 Signaling
The proto-oncogene and transcriptional regulator Bcl3 is a member of the IκB family of NFκB inhibitors, but unlike other IκB proteins, Bcl3 is localized predominantly in the nucleus. Since Bcl3 contains a transactivation domain, it can be recruited to NFκB-dependent promoters, resulting in transcriptional activation or repression, depending on the subunit composition of the NFκB complexes [64,65,66,67,68,69,70]. By binding to other NFκB proteins, Bcl3 regulates transcription of many NFκB-dependent genes, including genes involved in the regulation of the cell cycle, survival, proliferation, migration, chemotherapy resistance and cancer stemness, as well as pro- and anti-inflammatory genes [71,72,73,74,75,76,77,78,79,80,81]. In addition, Bcl3 can directly bind other transcriptional regulators, including histone acetyltransferases (HATs) and histone deacetylases (HDACs), thus regulating other signaling pathways [71,72,73].
Although Bcl3 was first identified in patients with chronic lymphocytic leukemia [64], its increased expression has been demonstrated in many types of hematological malignancies [82,83,84,85,86,87,88], as well as in solid tumors, including ovarian cancer, hepatocellular and nasopharyngeal carcinoma and colorectal, cervical, breast and prostate cancers [47,71,78,79,89,90,91,92,93].
3.2. Bcl3 Expression and Function in OC
Bcl3 expression is regulated by microRNA miR-125b, which is downregulated in OC tissues [94,95]. Overexpression of miR-125b decreases the Bcl3 levels in OC cells, resulting in decreased cell proliferation and cell cycle arrest and decreased tumorigenesis when injected into nude mice [94]. Bcl3 gene expression is increased in ovarian clear-cell adenocarcinoma, ovarian endometrioid adenocarcinoma, ovarian mucinous adenocarcinoma, ovarian serous adenocarcinoma, and ovarian serous surface papillary carcinoma [47]. Interestingly, compared to other OC types, ovarian serous surface papillary carcinoma has the highest levels of Bcl3 [47]; since tumors are often highly heterogeneous, it is plausible that a subset of OC cells might express considerably higher Bcl3 levels.
In vitro studies demonstrated that Bcl3 suppression in OC cells induces their apoptosis and inhibits cell migration [47]. In addition, Dai et al. [96] showed that Bcl3 promotes survival, migration and invasion of OC cells by inducing the expression of the copper-carrying plasma protein ceruloplasmin. Intriguingly, we found that Bcl3 expression in OC cells is further enhanced by IFNγ, thus identifying Bcl3 as a new member of the ISGs [47,48,49,50]. IFNγ-induced Bcl3 facilitates the expression of IL-8 and PD-L1, linking the function of Bcl3 in OC to angiogenesis and immune escape [47,48,49,50,51]. Thus, the newly emerging functions of Bcl3 in ovarian cancer include the induction of OC cell proliferation, survival, migration and invasion and also angiogenesis and immune escape (Figure 3).
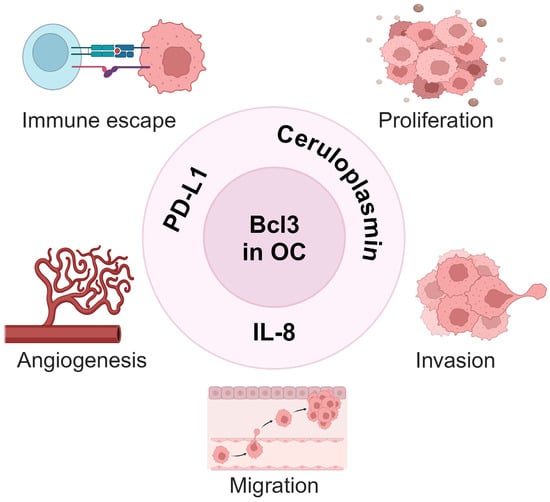
Figure 3.
Bcl3 tumor-promoting functions in ovarian cancer. By inducing the expression of ceruloplasmin [96] and IL-8 [50,51], Bcl3 promotes proliferation, invasion and angiogenesis in OC cells. By inducing the expression of PD-L1 [47,49], Bcl3 also promotes immune escape in ovarian cancer. Created with BioRender.com.
3.3. Mechanisms Regulating IFNγ-Induced Bcl3 Expression in OC
IFNγ-induced Bcl3 expression is facilitated by increased acetylation of the Bcl3 promoter and is dependent on both p65 NFκB and JAK1/STAT1 signaling [47,50] (Figure 2). IFNγ stimulation of OC cells increases acetylation of histones at the Bcl3 promoter, with a simultaneous promoter recruitment of Ser-727 pSTAT1, which is required for maximum transcriptional activity of STAT1 [50]. Although IFNγ-induced Bcl3 expression in OC cells is also dependent on p65 NFκB, p65 NFκB is not directly recruited to the Bcl3 promoter in OC cells [50]. However, p65 NFκB can mediate IFNγ-induced Bcl3 expression through binding to a downstream enhancer or through a cooperative interaction with STAT1. The latter scenario seems to be supported by several previous studies that reported crosstalk between NFκB and STAT1 in the regulation of IFNγ-induced inflammatory genes [9,97,98,99]. Future studies are needed to analyze in detail the mechanisms by which IFNγ induces the Bcl3 expression.
4. PD-L1 in Ovarian Cancer
4.1. PD-L1 Signaling
PD-L1 (also known as B7-H1 and CD274) was first identified as a transmembrane protein expressed on the surface of leukocytes, as well as non-lymphoid cells, including vascular endothelial cells in peripheral tissues [100,101,102,103]. By binding to PD-1 expressed on the surface of T cells, PD-L1 inhibits effector T-cell functions and immune responses. While the PD-1/PD-L1 pathway is central to maintaining immune tolerance and preventing autoimmunity, PD-L1 can also be expressed on tumor cells, thus attenuating tumor-specific immunity and promoting tumor growth [104,105,106,107,108,109]. PD-L1 expression in cancer cells is upregulated by IFNγ and other pro-inflammatory cytokines released by activated lymphocytes and other immune cells in the TME [36,38,39,40,41,104,105,106,107,108,109]. In addition, PD-L1 expression in cancer cells can be induced by IFNγ produced in response to chemotherapy, radiation or immune checkpoint blockade used in cancer treatment [10,110,111].
4.2. PD-L1 Expression and Function in OC
Immunotherapies blocking the PD-1/PD-L1 interaction have demonstrated great promise in treating a variety of cancers, but most patients fail to respond or develop resistance [41,104,105,112]. One of the factors determining the effectiveness of PD-1/PD-L1-blocking therapies is the surface expression of PD-L1. However, PD-L1 expression in tumors is highly heterogeneous, and varies among specific tumor types, cases, tissues and sampling times [36,104,112]. One plausible explanation for the high variability in PD-L1 in cancer tissues may be its dependence on the expression of IFNγ, which rapidly upregulates PD-L1 on most tumor cells [36,104,107,108,112]. In addition, the most widely used PD-L1 assay, immunohistochemistry, often measures mainly the surface levels of PD-L1 but not its intracellular levels; thus, additional mechanisms regulated by intracellular PD-L1 might be responsible for the limited responsiveness to PD-L1 targeted immunotherapies and the development of resistance.
Although PD-L1 was originally identified as a cell surface membrane protein [100,101,102,103], more recent studies have demonstrated its presence also in the cytoplasm and in the nucleus [113,114,115,116,117,118,119,120,121]. While the specific functions of intracellular PD-L1 are just emerging, several studies have shown that cytoplasmic PD-L1 acts as an RNA binding protein and regulates mRNA stability, while nuclear PDL1 can associate with DNA and regulate transcription [122,123,124,125]. In ovarian cancer, cell-intrinsic PD-L1 regulates cell proliferation, stemness gene expression, mTOR signaling, DNA damage response, autophagy and development of chemotherapy resistance [115,116,117,126,127,128,129].
High PD-L1 expression in OC tissues has been associated with poor outcomes; however, in contrast to other solid cancers, such as non-small-cell lung cancer (NSCLC), where PD-L1 expression has been used as a clinical indicator to select patients for anti-PD-1/PD-L1 therapy, in ovarian cancer, surface PD-L1 expression is not a good biomarker, and PD-1/PD-L1 blockade therapy does not significantly prolong progression-free survival [130,131,132,133,134,135,136,137,138,139]. Multiple causes are likely responsible for the limited effectiveness of PD-L1 as a clinical biomarker and therapeutic target in PD-L1 blocking therapies in OC; they may include the highly heterogeneous nature of OC tumors, the high inducibility and dependence of PD-L1 expression on IFNγ signaling and the incompletely understood functions of intracellular PD-L1, which is inaccessible to the currently used PD-1/PD-L1-blocking therapies. A better understanding of the mechanisms that regulate PD-L1 expression in OC cells and its intracellular functions should lead to the development of more effective PD-L1-targeting strategies for OC patients.
4.3. Mechanisms Regulating IFNγ-Induced PD-L1 Expression in OC
Although IFNγ is the main inducer of PD-L1 in most cells, the specific mechanisms and signaling pathways involved appear to be cell specific. The human PD-L1/CD274 promoter contains binding sites for the transcription factors NFκB, STAT1, IRF1 and hypoxia-inducible transcription factor (HIF) [47,140,141]. Consistent with the promoter multiple p65 NFκB binding sites, PD-L1 expression in OC cells treated with the chemotherapeutic drugs gemcitabine and paclitaxel is dependent on p65 NFκB signaling [110]. IFNγ induces the expression of p65 NFκB, its Bc3-facilitated acetylation and recruitment to PD-L1 promoter in OC cells [47,49] (Figure 2).
In addition, IFNγ-induced PD-L1 expression in OC cells is regulated by the canonical pathway, which involves the JAK1/STAT1 signaling [49]. IFNγ induces the expression of the transcription factor IRF1, which is then recruited to PD-L1 promoter and is required for maximum PD-L1 expression in OC cells [49]. Thus, IFNγ-induced PD-L1 expression in OC cells is dependent on simultaneous IFNγ-induced and p300-dependent PD-L1 promoter acetylation and promoter recruitment of the transcription factors IRF1, Ser-727-phosphorylated STAT1 and K314/315-acetylated p65 NFκB [44] (Figure 2).
5. IL-8 in Ovarian Cancer
5.1. IL-8 Signaling
Interleukin-8 (IL-8, CXCL8), which was originally discovered as a neutrophil chemoattractant and inducer of leukocyte-mediated inflammation, plays a crucial role in cancer progression through its induction of tumor cell proliferation, migration, invasion, angiogenesis and metastasis [142,143,144,145]. Furthermore, by binding to its receptors CXCR1/CXCR2, tumor-derived IL-8 recruits neutrophils (PMNs) and myeloid-derived suppressor cells (MDSCs), which play critical roles in tumorigenesis [146,147,148,149,150,151,152,153]. In contrast to the pro-inflammatory function of PMNs, the majority of recruited MDSCs are PMN-MDSCs with immunosuppressive activity [146,147,148,149,150,151,152,153]. In addition, the recruited PMNs and PMN-MDSCs release proteases that are associated with neutrophil extracellular traps and promote cancer progression [154]. IL-8 levels are increased in patients with advanced solid cancers, reflect the tumor burden and correlate with poor prognosis [155].
5.2. IL-8 Expression and Function in OC
IL-8 levels are increased in OC ascites, serum and tumor tissues, and increased IL-8 expression correlates with poor prognosis and survival in OC patients [156,157,158,159,160,161,162,163,164,165,166,167]. In addition, IL-8 polymorphisms have been associated with a greater risk of ovarian cancer [168]. IL-8 expression in OC cells is induced under stress conditions, such as hypoxia [157], acidic pH [169], the chemotherapeutic drug paclitaxel (Taxol) [170,171], the neurotransmitter hormone norepinephrine [172], proteasome inhibition [173,174], inhibition of histone deacetylation [175,176], surgical peritoneal stress [177] and fluid shear stress [178]. In addition, recent studies have shown that the IL-8 expression in OC cells is induced by IFNγ, adding IL-8 to the list of IFNγ-regulated genes [51].
Studies using human OC cells and tissues have shown that increased IL-8 expression enhances OC cell survival, proliferation, migration, and metastasis [50,51,179,180,181,182,183,184,185]. In addition, IL-8 increases OC growth by promoting epithelial-to-mesenchymal transition (EMT) [186,187,188], angiogenesis [189], cancer cell stemness [190] and immune evasion mediated by tumor-associated neutrophil infiltration [191] (Figure 4).
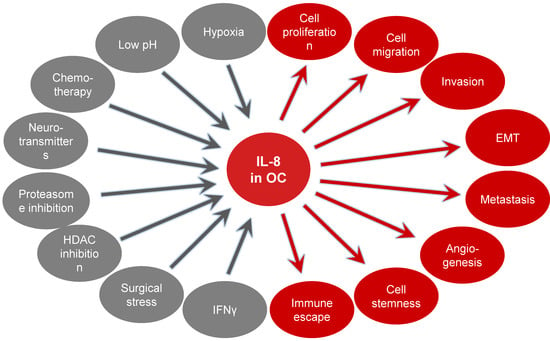
Figure 4.
Mechanisms inducing IL-8 expression in ovarian cancer and the tumor promoting effects of IL-8 in OC cells.
5.3. Mechanisms Regulating IFNγ-Induced IL-8 Expression in OC
Since mice do not have a homolog of the IL-8/CXCL8 gene, which is present in other species, including humans [192], compared to other cytokines, our understanding of the mechanisms that regulate IL-8 expression in cancer cells has been lagging. The IL-8 transcription is regulated predominantly by the transcription factor NFκB; in addition, in some cells, the transcription factors AP-1 and C/EBP are required for maximum IL-8 expression [193,194,195,196,197,198,199]. The human IL-8 promoter also contains a binding site for the transcription factor HIF-1α, which is adjacent to the NFκB site [199], consistent with hypoxia-induced IL-8 expression in OC cells [157].
In OC cells, IL-8 expression is regulated predominantly by p65 NFκB homodimers phosphorylated at serine 536 by IKK [173,174,175]. In addition, the IL-8 expression in OC cells is facilitated by increased acetylation of the IL-8 promoter and recruitment of IKK [175,176]. Inhibition of IKK suppresses IL-8 expression in vitro and increases the effectiveness of proteasome and HDAC inhibitors in vivo [175,176]. In this context, several studies have correlated the use of nonsteroidal anti-inflammatory drugs targeting the IKK enzymes with a reduced risk of ovarian cancer [200,201].
In IFNγ-stimulated OC cells, IL-8 expression is dependent on both JAK1/STAT1 and p65/Bcl3 signaling and is facilitated by concomitant promoter acetylation by p300 and recruitment of Ser-727 pSTAT1 and K314/315 ac-p65 NFκB (Figure 2). Neutralization of IFNγ-induced IL-8 using an anti-IL-8 blocking antibody reduces IFNγ-induced migration of OC cells and their invasion ability in 3D spheroids [51], indicating that IFNγ-induced IL-8 expression contributes to the pro-tumorigenic effects of IFNγ in ovarian cancer cells.
6. Bcl3, PD-L1 and IL-8 Co-Expression in Ovarian Cancer
6.1. Genomic Studies in OC
Considering that the expression of Bcl3, PD-L1 and IL-8 in ovarian cancer cells is induced by IFNγ and dependent on JAK1/STAT1 signaling [47,48,49,50,51], we examined the gene co-expression profiles of Bcl3, PD-L1/CD274 and IL-8/CXCL8 in OC tissues using The Cancer Genome Atlas (TCGA) database and the UCSC Xena Browser (https://xena.ucsc.edu (accessed on 1 April 2024) [202]. Heatmap analysis of 379 OC samples from the GDC-TCGA database revealed a positive correlation between Bcl3, PD-L1/CD274 and IL-8/CXCL8 mRNA expression (Figure 5A). In addition, Pearson’s and Spearman’s correlation tests revealed positive correlations between Bcl3 and PD-L1/CD274, between Bcl3 and IL-8/CXCL8 and between IL-8/CXCL8 and PD-L1/CD274 expression in the GDC-TCGA database (Figure 5B). The positive correlation between IL-8 and PD-L1 in OC was also demonstrated by Wang et al., who showed that co-culture of ascites-derived ovarian cancer cells with CD8+ T cells resulted in increased gene and protein levels of IL-8 and PD-L1 in OC cells [203], and by Wu et al., who demonstrated IL-8 and PD-L1 co-expression in ovarian cancer organoids [204].
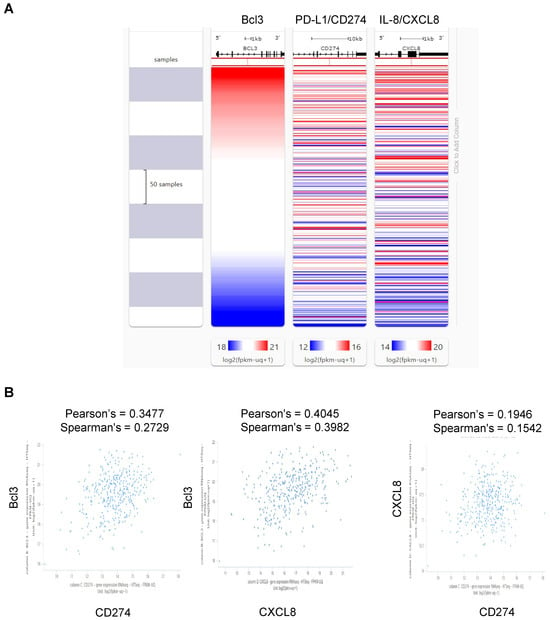
Figure 5.
Bcl3, PD-L1 and IL-8 co-expression in ovarian cancer tissues. (A) Heatmap of Bcl3, PD-L1/CD274 and IL-8/CXCL8 mRNA co-expression in 379 OC samples in the GDC-TCGA database using the UCSC Xena platform. (B) Scatter plots showing associations between Bcl3 and PD-L1/CD274, between Bcl3 and IL-8/CXCL8 and between IL-8/CXCL8 and PD-L1/CD274 gene expression in GDC-TCGA ovarian cancer samples (n = 379) using the UCSC Xena browser.
We have also examined the TCGA datasets using the cBioPortal for Cancer Genomics (https://www.cbioportal.org (accessed on 14 July 2024) [205] to evaluate whether there is any difference in overall survival (OS) in OC patients with altered expression of Bcl3, PD-L1 and IL-8. Analysis of 1949 samples revealed a statistical difference (p < 0.05) in OS in OC patients with altered Bcl3 expression but not PD-L1 or IL-8 expression; this is consistent with a model in which OS is driven mainly by the expression of Bcl3. However, more studies are needed to confirm these genome-wide studies also on the protein level.
6.2. Mechanisms Regulating Bcl3, PD-L1 and IL-8 Co-Expression in OC
The expression of Bcl3, PD-L1 and IL-8 in OC cells is induced by IFNγ; the underlying mechanisms involve the IFNγ-induced canonical JAK1/STAT1 pathway, as well as p65 NFκB signaling [47,48,49,50,51]. In addition, IFNγ enhances promoter acetylation, resulting in increased promoter recruitment and transcription of Bcl3, PD-L1 and IL-8. Interestingly, the proto-oncogene and transcriptional regulator Bcl3 is required for the maximum expression of both PD-L1 and IL-8 in IFNγ-stimulated OC cells [47,48,49,50,51] (Figure 2). These findings indicate that IFNγ first induces the expression of Bcl3, which then promotes the transcription of PD-L1 and IL-8 by facilitating promoter acetylation and p65 NFκB recruitment to PD-L1 and IL-8 promoters [47,48,49,50,51] (Figure 2). Since both PD-L1 and IL-8 induce proliferation, migration and invasion in OC cells, their IFNγ-induced expression likely contributes to the tumor-promoting effects of IFNγ in ovarian cancer (Figure 6).
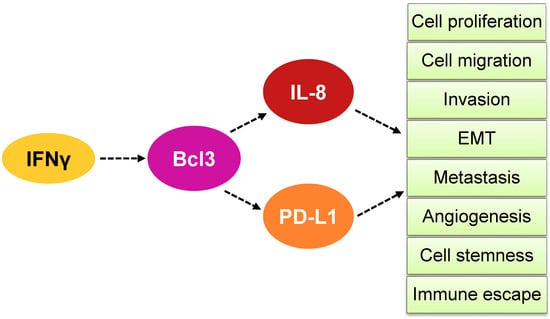
Figure 6.
Model of how IFNγ induces Bcl3-dependent IL-8 and PD-L1 expression in OC cells. IFNγ induces first the expression of Bcl3 in OC cells, resulting in increased transcription of IL-8 and PD-L1. The increased expression of IL-8 and PD-L1 enhances OC cell proliferation, migration, invasion, epithelial-to-mesenchymal transition (EMT), metastasis, angiogenesis, cell stemness and immune escape.
Interestingly, several reports have indicated that IL-8 can directly upregulate PD-L1 expression in gastric, colorectal and esophageal cancer by mechanisms that include NFκB, p38 MAP kinase and mTOR signaling [206,207,208,209]. It will be important to determine whether IL-8 can also induce PD-L1 expression in OC cells and to identify the responsible mechanisms.
Furthermore, recent studies in other cancers have shown that increased systemic and tumor-associated IL-8 levels correlate with reduced clinical benefits of ICB therapies used in advanced melanoma, NSCLC, urothelial carcinoma, renal cell carcinoma, and glioma [210,211,212,213]. Since ICB therapies also enhance IFNγ expression [15,16,17,106], one of the underlying mechanisms may involve the IFNγ-induced IL-8 expression, resulting in increased cancer cell growth and resistance to ICB. These data suggest that targeting IL-8 signaling may increase the effectiveness of PD-L1-blocking therapies in ovarian cancer. To this end, future studies should determine whether immunotherapies targeting the PD-1/PD-L1 axis in OC patients increase their serum levels of IL-8.
7. Targeting IFNγ-Induced Bcl3, PD-L1 and IL-8 Expression
7.1. Targeting Bcl3 in OC
Bcl3 expression in OC cells promotes the expression of PD-L1 and IL-8, resulting in increased OC cell proliferation, migration, invasion and immune escape (Figure 6). Since Bcl3 gene expression is significantly increased in ovarian clear-cell adenocarcinoma, endometrioid adenocarcinoma, ovarian mucinous adenocarcinoma, ovarian serous adenocarcinoma and ovarian serous surface papillary carcinoma [47], Bcl3 might serve as a potential novel biomarker in OC. In this context, Bcl3 protein expression analyzed by immunohistochemistry is currently being tested as a potential biomarker to predict the response to alkylating chemotherapy in glioma patients (NCT03011671) [76,214]. Future studies should determine whether Bcl3 protein levels are increased in OC tissues and whether they correlate with PD-L1 and IL-8 expression.
In contrast to NFκB knockouts, Bcl3 knockout mice are viable, indicating that suppression of Bcl3 may represent a possible novel anticancer strategy [73,215]. Indeed, a recent study demonstrated that Bcl3-/- mice exhibit increased sensitivity to cisplatin chemotherapy in colorectal cancer, thus offering a rationale for targeting Bcl3 as an adjuvant to conventional therapies [216]. In this regard, Soukupova et al. recently developed small-molecule Bcl3 inhibitors with promising antimetastatic activity and low toxicity in triple-negative breast cancer [81]. Future studies are warranted to determine whether Bcl3 may serve as a potential biomarker in ovarian cancer and whether Bcl3 inhibition might be used as an antimetastatic strategy in ovarian cancer and other solid tumors.
7.2. Targeting PD-L1 in OC
In contrast to other solid tumors, such as NSCLC or melanoma, in ovarian cancer, surface PD-L1 expression has not been proven as a reliable biomarker to select patients for anti-PD-1/PD-L1 therapy and targeting the PD-1/PD-L1 signaling has produced disappointing results [106]. The underlying mechanisms likely include the highly heterogeneous nature of OC tumors, the high dependence of PD-L1 expression on IFNγ signaling, and the incompletely understood functions of intracellular PD-L1, which is inaccessible to currently used PD-1/PD-L1-blocking therapies. Since stability and intracellular localization and functions of PD-L1 are regulated by PD-L1 post-translational modifications that include acetylation and glycosylation, targeting these post-translational modifications may improve the effectiveness of PD-L1-targeting immunotherapies [123,217,218].
Interestingly, studies in melanoma, NSCLC, metastatic urothelial carcinoma and metastatic renal cell carcinoma have shown that resistance to PD-1/PD-L1-blocking immunotherapies correlates with increased serum levels of IL-8 [210,211,212,219,220], suggesting that IL-8 might serve as a biomarker predictive of resistance to PD-1/PD-L1-blocking therapies. Since IL-8 has multiple pro-tumorigenic effects, these studies have also indicated that suppressing IL-8 signaling might improve the outcomes of PD-L1-blocking therapies [210,211,212,219,220]. Indeed, numerous studies have evaluated the combination potential of PD-1/PD-L1-blocking immunotherapies with inhibiting IL-8 signaling, either by using small-molecule inhibitors that interfere with the IL-8 receptors CXCR1/CXCR2 or by using anti-IL-8 neutralizing antibodies [143,221,222,223].
7.3. Targeting IL-8 in OC
IL-8 signaling greatly influences the TME and promotes cancer progression by increasing cancer cell proliferation, angiogenesis and metastasis. In ovarian cancer, increased IL-8 expression induces proliferation and migration of OC cells, and increased IL-8 levels correlate with poor prognosis in OC patients [50,51,156,157,158,159,160,161,162,163,164,165,166,167,179,180,181,182,183,184,185]. In pre-clinical studies, blocking IL-8 signaling with the CXCR1/2 small-molecule inhibitors SX-682 or reparixin resulted in increased antitumor immunity and significantly slower tumor growth in gastric cancer [207], pancreatic cancer [224,225], squamous cell carcinoma [226], NSCLC [227], glioma [213] and hepatocellular carcinoma [228]. In addition, simultaneous inhibition of CXCR1/2 signaling by SX-682 and inhibition of TGFβ and PD-L1 signaling synergized to reduce mesenchymal tumor features in murine models of breast and lung cancer [229]. Blocking IL-8 expression with IL-8-neutralizing antibodies also suppressed the invasion of IFNγ-stimulated ovarian cancer cells grown in 3D spheroids [51]. Furthermore, blocking IL-8 signaling using an anti-IL-8 antibody resulted in decreased tumor growth and improved survival in a glioma mouse model treated with a PD-1-blocking antibody compared to PD-1 antibody alone [214].
HuMax-IL8 (BMS-986253) is a fully human monoclonal antibody that neutralizes IL-8 [230]. A phase I clinical study evaluated the safety and tolerability of HuMax-IL8, as well as changes in IL-8 serum levels in patients with incurable metastatic or unresectable solid tumors [230]. Although no objective tumor responses were observed, the antibody was safe and well-tolerated and associated with decreased serum IL-8 levels [230]. The acceptable safety profile of HuMax-IL8 (BMS-986253) suggested its potential in combination immunotherapies [230].
7.4. Clinical Studies Based on Simultaneous Inhibition of IL-8 and PD-1/PD-L1 Signaling
Several clinical trials have evaluated the use of combination immunotherapy based on simultaneous inhibition of PD-1/PD-L1 signaling and IL-8 signaling by using either the IL-8-neutralizing antibody HuMax-IL8 (BMS-986253) or blocking the IL-8 CXCR1/2 receptor. Ongoing clinical trials are evaluating the combination of HuMax-IL8 (BMS-986253) and PD-1/PD-L1-blocking immunotherapies in melanoma and advanced cancers (NCT03400332; NCT04572451), hepatocellular carcinoma (NCT04050462), prostate cancer (NCT03689699), pancreatic cancer (NCT02451982), head and neck squamous-cell carcinoma (NCT04848116), and colon carcinoma (NCT03026140) (Table 2).

Table 2.
Active clinical trials based on simultaneous inhibition of IL-8 and PD-1/PD-L1 signaling.
In addition, there are ongoing clinical trials assessing the efficacy of PD-1/PD-L1-blocking antibodies and the small-molecule and orally bioavailable CXCR1/2 inhibitor SX-682 in treating melanoma (NCT03161431), metastatic colorectal cancer (NCT04599140; NCT06149481), pancreatic adenocarcinoma (NCT05604560; NCT04477343) and NSCLC (NCT05570825) (Table 2).
There have been no clinical trials evaluating the efficacy of combined inhibition of IL-8 and PD-1/PD-L1 signaling in ovarian cancer. However, considering that IL-8 expression is increased in OC tissues and that the neutralizing IL-8 antibody HuMax-IL8 (BMS-986253) and the CXCR1/2 inhibitor SX-682 are well tolerated, future clinical studies should assess whether the combination of IL-8 and PD-1/PD-L1 blockade might increase the effectiveness of PD-1/PD-L1-targeting immunotherapies in ovarian cancer patients.
8. Conclusions and Perspectives
Bcl3, PD-L1 and IL-8 have long been known to have important cellular functions as a proto-oncogene, an immune checkpoint ligand and a neutrophil chemoattractant, respectively. However, overwhelming evidence shows that these three genes have tumor-promoting functions far beyond their originally identified functions in many types of cancer, including ovarian cancer. These tumor-promoting mechanisms include increased cancer cell proliferation, migration, invasion, angiogenesis, EMT, metastasis, cancer cell stemness, resistance to chemotherapy and immune escape. The expression of Bcl3, PD-L1 and IL-8 is induced by IFNγ, which is produced not only by activated T cells but also in response to PD-1/PD-L1-blocking cancer immunotherapies. Perhaps not surprisingly, recent studies have shown that the IFNγ-induced Bcl3, PD-L1 and IL-8 expression is regulated by the same JAK1/STAT1 signaling pathway: IFNγ first induces the expression of Bcl3, which then promotes the expression of PD-L1 and IL-8 in ovarian cancer cells (Figure 6).
Since increased systemic and tumor-associated IL-8 levels correlate with reduced clinical benefit of PD-1/PD-L1-blocking therapies in solid cancers, including melanoma, NSCLC and glioma [210,211,212,213], serum IL-8 levels have been used as a biomarker for responsiveness to PD-1/PD-L1-targeting immunotherapies in those cancers. Based on recent in vitro studies in OC cells, future clinical studies are warranted to determine whether serum IL-8 levels may serve as a prognostic biomarker for PD-1/PD-L1-targeting therapies in ovarian cancer patients. In addition, considering the tumor-promoting functions of IL-8 and the low toxicity of the IL-8-neutralizing human antibody HuMax-IL8 (BMS-986253), blocking IL-8 signaling with IL-8-neutralizing antibody may increase the effectiveness of PD-1/PD-L1 immunotherapies in ovarian cancer patients.
As the expression of Bcl3, PD-L1 and IL-8 is induced by IFNγ, the expression of which greatly varies in cancer tissues, it will be important to analyze the expression levels of Bcl3, PD-L1, IL-8 and IFNγ in tumor tissues at the single-cell level. Future investigations should also determine whether immunotherapies targeting PD-1/PD-L1 signaling and other therapies associated with induced IFNγ release increase IL-8 serum levels and Bcl3 expression in tumor tissues.
Further studies are needed to better understand the nature of immune signaling in the ovarian cancer TME. There are many outstanding questions to be addressed: What are the specific cells, in addition to TILs, responsible for IFNγ production, and what are the cellular and molecular targets of IFNγ in OC TME? What are the specific mechanisms that regulate the stability and the transcriptional activity of Bcl3 and its ability to promote PD-L1 and IL-8 expression in OC? What are the specific functions of nuclear and cytoplasmic PD-L1 and what are the mechanisms that regulate the intracellular localization of PD-L1 and its stability? A more thorough understanding of these mechanisms will lead to the development of more reliable biomarkers and PD-1/PD-L1-targeting cancer immunotherapies. In addition, future clinical trials are warranted to assess whether the combination of IL-8 and PD-1/PD-L1 blockade might increase the effectiveness of PD-1/PD-L1-targeting immunotherapies in ovarian cancer patients.
Author Contributions
Conceptualization: I.V.; Acquisition of data: S.U.R., F.Z.S., A.V. and I.V.; Funding acquisition: I.V.; Writing, review and editing: S.U.R., F.Z.S., A.V. and I.V. All authors have read and agreed to the published version of the manuscript.
Funding
The work in I. Vancurova’s laboratory is funded by the NIH grant R16GM149263.
Data Availability Statement
All data are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dutta, S.; Wang, F.Q.; Phalen, A.; Fishman, D.A. Biomarkers for ovarian cancer detection and therapy. Cancer Biol. Ther. 2010, 9, 668–677. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kossai, M.; Leary, A.; Scoazec, J.Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2018, 85, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Elias, K.M.; Guo, J.; Bast, R.C., Jr. Early Detection of Ovarian Cancer. Hematol. Oncol. Clin. North. Am. 2018, 32, 903–914. [Google Scholar] [CrossRef] [PubMed]
- James, N.E.; Woodman, M.; DiSilvestro, P.A.; Ribeiro, J.R. The Perfect Combination: Enhancing Patient Response to PD-1-Based Therapies in Epithelial Ovarian Cancer. Cancers 2020, 12, 2150. [Google Scholar] [CrossRef] [PubMed]
- Morand, S.; Devanaboyina, M.; Staats, H.; Stanbery, L.; Nemunaitis, J. Ovarian Cancer Immunotherapy and Personalized Medicine. Int. J. Mol. Sci. 2021, 22, 6532. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Karakasis, K.; Rottapel, R.; Oza, A.M. Advances in ovarian cancer, from biology to treatment. Nat. Cancer 2021, 2, 6–8. [Google Scholar] [CrossRef]
- Tang, L.; Li, J.; Bao, M.; Xiang, J.; Chen, Y.; Wang, Y. Genetic association between HER2 and ESR2 polymorphisms and ovarian cancer: A meta-analysis. Onco Targets Ther. 2018, 11, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Garlisi, B.; Lauks, S.; Aitken, C.; Ogilvie, L.M.; Lockington, C.; Petrik, D.; Eichhorn, J.S.; Petrik, J. The Complex Tumor Microenvironment in Ovarian Cancer: Therapeutic Challenges and Opportunities. Curr. Oncol. 2024, 31, 3826–3844. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Goncalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-γ at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Ni, L.; Lu, J. Interferon-γ in cancer immunotherapy. Cancer Med. 2018, 7, 4509–4516. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.R. The Interferon-Gamma Paradox in Cancer. J. Interferon Cytokine Res. 2019, 39, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Wang, X.; Yang, X.; Fu, Y.; Zheng, Y.; Gong, H.; He, Z. The role of interferons in ovarian cancer progression: Hinderer or promoter? Front. Immunol. 2022, 13, 1087620. [Google Scholar] [CrossRef] [PubMed]
- Lugade, A.A.; Sorensen, E.W.; Gerber, S.A.; Moran, J.P.; Frelinger, J.G.; Lord, E.M. Radiation-induced IFN-γ production within the tumor microenvironment influences antitumor immunity. J. Immunol. 2008, 180, 3132–3139. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef] [PubMed]
- Liakou, C.I.; Kamat, A.; Tang, D.N.; Chen, H.; Sun, J.; Troncoso, P.; Logothetis, C.; Sharma, P. CTLA-4 blockade increases IFNγ-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc. Natl. Acad. Sci. USA 2008, 105, 14987–14992. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Liu, C.; Xu, C.; Lou, Y.; Chen, J.; Yang, Y.; Yagita, H.; Overwijk, W.W.; Lizee, G.; Radvanyi, L.; et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012, 72, 5209–5218. [Google Scholar] [CrossRef] [PubMed]
- Goedegebuure, R.S.A.; de Klerk, L.K.; Bass, A.J.; Derks, S.; Thijssen, V. Combining Radiotherapy With Anti-angiogenic Therapy and Immunotherapy; A Therapeutic Triad for Cancer? Front. Immunol. 2018, 9, 3107. [Google Scholar] [CrossRef] [PubMed]
- Darwich, L.; Coma, G.; Peña, R.; Bellido, R.; Blanco, E.J.; Este, J.A.; Borras, F.E.; Clotet, B.; Ruiz, L.; Rosell, A.; et al. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology 2009, 126, 386–393. [Google Scholar] [CrossRef]
- Gupta, V.; Yull, F.; Khabele, D. Bipolar Tumor-Associated Macrophages in Ovarian Cancer as Targets for Therapy. Cancers 2018, 10, 366. [Google Scholar] [CrossRef]
- Nowak, M.; Klink, M.; Glowacka, E.; Sulowska, Z.; Kulig, A.; Szpakowski, M.; Szyllo, K.; Tchorzewski, H. Production of cytokines during interaction of peripheral blood mononuclear cells with autologous ovarian cancer cells or benign ovarian tumour cells. Scand. J. Immunol. 2010, 71, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Mobus, V.J.; Asphal, W.; Knapstein, P.G.; Kreienberg, R. Effects of interferon-γ on the proliferation and modulation of cell-surface structures of human ovarian carcinoma cell lines. J. Cancer Res. Clin. Oncol. 1993, 120, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.S.; Kudelka, A.P.; Kavanagh, J.J.; Verschraegen, C.; Edwards, C.L.; Nash, M.; Levy, L.; Atkinson, E.N.; Zhang, H.Z.; Melichar, B.; et al. Clinical and biological effects of intraperitoneal injections of recombinant interferon-γ and recombinant interleukin 2 with or without tumor-infiltrating lymphocytes in patients with ovarian or peritoneal carcinoma. Clin. Cancer Res. 2000, 6, 2268–2278. [Google Scholar] [PubMed]
- Ogawa, M.; Yu, W.G.; Umehara, K.; Iwasaki, M.; Wijesuriya, R.; Tsujimura, T.; Kubo, T.; Fujiwara, H.; Hamaoka, T. Multiple roles of interferon-γ in the mediation of interleukin 12-induced tumor regression. Cancer Res. 1998, 58, 2426–2432. [Google Scholar] [PubMed]
- Kim, E.J.; Lee, J.M.; Namkoong, S.E.; Um, S.J.; Park, J.S. Interferon regulatory factor-1 mediates interferon-γ-induced apoptosis in ovarian carcinoma cells. J. Cell Biochem. 2002, 85, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Wall, L.; Burke, F.; Barton, C.; Smyth, J.; Balkwill, F. IFN-γ induces apoptosis in ovarian cancer cells in vivo and in vitro. Clin. Cancer Res. 2003, 9, 2487–2496. [Google Scholar] [PubMed]
- Marth, C.; Cronauer, M.V.; Doppler, W.; Ofner, D.; Ullrich, A.; Daxenbichler, G. Effects of interferons on the expression of the proto-oncogene HER-2 in human ovarian carcinoma cells. Int. J. Cancer 1992, 50, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Peccatori, F.; Paganin, C.; Bini, S.; Brandely, M.; Mangioni, C.; Mantovani, A.; Allavena, P. Anti-tumor and immunomodulatory activity of intraperitoneal IFN-γ in ovarian carcinoma patients with minimal residual tumor after chemotherapy. Int. J. Cancer 1992, 51, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Fady, C.; Gardner, A.; Gera, J.F.; Lichtenstein, A. Interferon-γ-induced increased sensitivity of HER2/neu-overexpressing tumor cells to lymphokine-activated killer cell lysis: Importance of ICAM-1 in binding and post-binding events. Cancer Immunol. Immunother. 1993, 37, 329–336. [Google Scholar] [CrossRef]
- Windbichler, G.H.; Hausmaninger, H.; Stummvoll, W.; Graf, A.H.; Kainz, C.; Lahodny, J.; Denison, U.; Muller-Holzner, E.; Marth, C. Interferon-γ in the first-line therapy of ovarian cancer: A randomized phase III trial. Br. J. Cancer 2000, 82, 1138–1144. [Google Scholar] [CrossRef]
- Marth, C.; Windbichler, G.H.; Hausmaninger, H.; Petru, E.; Estermann, K.; Pelzer, A.; Mueller-Holzner, E. Interferon-γ in combination with carboplatin and paclitaxel as a safe and effective first-line treatment option for advanced ovarian cancer: Results of a phase I/II study. Int. J. Gynecol. Cancer 2006, 16, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Alberts, D.S.; Marth, C.; Alvarez, R.D.; Johnson, G.; Bidzinski, M.; Kardatzke, D.R.; Bradford, W.Z.; Loutit, J.; Kirn, D.H.; Clouser, M.C.; et al. Randomized phase 3 trial of interferon gamma-1b plus standard carboplatin/paclitaxel versus carboplatin/paclitaxel alone for first-line treatment of advanced ovarian and primary peritoneal carcinomas: Results from a prospectively designed analysis of progression-free survival. Gynecol. Oncol. 2008, 109, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.H.; Pugh, M.; Kirkwood, J.M.; Karp, D.; Larson, M.; Borden, E. Eastern cooperative group trial of interferon gamma in metastatic melanoma: An innovative study design. Clin. Cancer Res. 1996, 2, 29–36. [Google Scholar] [PubMed]
- Gleave, M.E.; Elhilali, M.; Fradet, Y.; Davis, I.; Venner, P.; Saad, F.; Klotz, L.H.; Moore, M.J.; Paton, V.; Bajamonde, A. Interferon gamma-1b compared with placebo in metastatic renal-cell carcinoma. Canadian Urologic Oncology Group. N. Engl. J. Med. 1998, 338, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- He, Y.F.; Wang, X.H.; Zhang, G.M.; Chen, H.T.; Zhang, H.; Feng, Z.H. Sustained low-level expression of interferon-γ promotes tumor development: Potential insights in tumor prevention and tumor immunotherapy. Cancer Immunol. Immunother. 2005, 54, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Abiko, K.; Matsumura, N.; Hamanishi, J.; Horikawa, N.; Murakami, R.; Yamaguchi, K.; Yoshioka, Y.; Baba, T.; Konishi, I.; Mandai, M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 2015, 112, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, R.; Martin, A.; Bommarito, D.; Wang, K.; Hansen, S.H.; Freeman, G.J.; Ritz, J. Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. Oncoimmunology 2015, 4, e1008824. [Google Scholar] [CrossRef]
- Mandai, M.; Hamanishi, J.; Abiko, K.; Matsumura, N.; Baba, T.; Konishi, I. Dual Faces of IFNγ in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro-and Antitumor Immunity. Clin. Cancer Res. 2016, 22, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Benci, J.L.; Xu, B.; Qiu, Y.; Wu, T.J.; Dada, H.; Twyman-Saint Victor, C.; Cucolo, L.; Lee, D.S.M.; Pauken, K.E.; Huang, A.C.; et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell 2016, 167, 1540–1554.e12. [Google Scholar] [CrossRef]
- Mojic, M.; Takeda, K.; Hayakawa, Y. The Dark Side of IFN-γ: Its Role in Promoting Cancer Immunoevasion. Int. J. Mol. Sci. 2017, 19, 89. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Aqbi, H.F.; Wallace, M.; Sappal, S.; Payne, K.K.; Manjili, M.H. IFN-γ orchestrates tumor elimination, tumor dormancy, tumor escape, and progression. J. Leukoc. Biol. 2018, 103, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Li, Z.L.; Qiu, S.F. IFN-γ Induces Gastric Cancer Cell Proliferation and Metastasis Through Upregulation of Integrin beta3-Mediated NFκB Signaling. Transl. Oncol. 2018, 11, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Benci, J.L.; Johnson, L.R.; Choa, R.; Xu, Y.; Qiu, J.; Zhou, Z.; Xu, B.; Ye, D.; Nathanson, K.L.; June, C.H.; et al. Opposing Functions of Interferon Coordinate Adaptive and Innate Immune Responses to Cancer Immune Checkpoint Blockade. Cell 2019, 178, 933–948.e14. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Fuith, L.C.; Bock, G.; Daxenbichler, G.; Dapunt, O. Modulation of ovarian carcinoma tumor marker CA-125 by gamma-interferon. Cancer Res. 1989, 49, 6538–6542. [Google Scholar] [PubMed]
- Zheng, H.; Guan, X.; Meng, X.; Tong, Y.; Wang, Y.; Xie, S.; Guo, L.; Lu, R. IFN-γ in ovarian tumor microenvironment upregulates HLA-E expression and predicts a poor prognosis. J. Ovarian Res. 2023, 16, 229. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Uddin, M.M.; Padmanabhan, S.; Zhu, Y.; Bu, P.; Vancura, A.; Vancurova, I. The proto-oncogene Bcl3 induces immune checkpoint PD-L1 expression, mediating proliferation of ovarian cancer cells. J. Biol. Chem. 2018, 293, 15483–15496. [Google Scholar] [CrossRef]
- Gaire, B.; Uddin, M.M.; Zou, Y.; Vancurova, I. Analysis of IFNγ-Induced Migration of Ovarian Cancer Cells. Methods Mol. Biol. 2020, 2108, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Gaire, B.; Zou, Y.; Uddin, M.M.; Vancurova, I. IFNγ-induced PD-L1 expression in ovarian cancer cells is regulated by JAK1, STAT1 and IRF1 signaling. Cell. Signal. 2022, 97, 110400. [Google Scholar] [CrossRef]
- Gaire, B.; Padmanabhan, S.; Zou, Y.; Uddin, M.M.; Reddy, S.U.; Vancurova, I. IFNγ induces Bcl3 expression by JAK1/STAT1/p65 signaling, resulting in increased IL-8 expression in ovarian cancer cells. FEBS Open Bio 2023, 13, 1495–1506. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Gaire, B.; Zou, Y.; Uddin, M.M.; DeLeon, D.; Vancurova, I. IFNγ induces JAK1/STAT1/p65 NFκB-dependent interleukin-8 expression in ovarian cancer cells, resulting in their increased migration. Int. J. Biochem. Cell Biol. 2021, 141, 106093. [Google Scholar] [CrossRef]
- Stark, G.R.; Darnell, J.E., Jr. The JAK-STAT pathway at twenty. Immunity 2012, 36, 503–514. [Google Scholar] [CrossRef]
- Gough, D.J.; Levy, D.E.; Johnstone, R.W.; Clarke, C.J. IFNγ signaling—Does it mean JAK–STAT? Cytokine Growth Factor Rev. 2008, 19, 383–394. [Google Scholar] [CrossRef]
- Sizemore, N.; Agarwal, A.; Das, K.; Lerner, N.; Sulak, M.; Rani, S.; Ransohoff, R.; Shultz, D.; Stark, G.R. Inhibitor of κB kinase is required to activate a subset of IFNγ-stimulated genes. Proc. Natl. Acad. Sci. USA 2004, 101, 7994–7998. [Google Scholar] [CrossRef] [PubMed]
- Shultz, D.B.; Rani, M.R.; Fuller, J.D.; Ransohoff, R.M.; Stark, G.R. Roles of IKK-β, IRF1, and p65 in the activation of chemokine genes by IFNγ. J. Interferon Cytokine Res. 2009, 29, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.J.; Basagoudanavar, S.H.; Nogusa, S.; Irrinki, K.; Mallilankaraman, K.; Slifker, M.J.; Beg, A.A.; Madesh, M.; Balachandran, S. NFκB protects cells from gamma interferon-induced RIP1-dependent necroptosis. Mol. Cell Biol. 2011, 31, 2934–2946. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.C.; Beresford, G.W.; Mooney, M.R.; Boss, J.M. Kinetics of a gamma interferon response: Expression and assembly of CIITA promoter IV and inhibition by methylation. Mol. Cell Biol. 2002, 22, 4781–4791. [Google Scholar] [CrossRef]
- Hiroi, M.; Ohmori, Y. The transcriptional coactivator CREB-binding protein cooperates with STAT1 and NFκB for synergistic transcriptional activation of the CXC ligand 9/monokine induced by interferon-gamma gene. J. Biol. Chem. 2003, 278, 651–660. [Google Scholar] [CrossRef]
- Garrett, S.; Dietzmann-Maurer, K.; Song, L.; Sullivan, K.E. Polarization of primary human monocytes by IFN-γ induces chromatin changes and recruits RNA Pol II to the TNF-alpha promoter. J. Immunol. 2008, 180, 5257–5266. [Google Scholar] [CrossRef]
- Qiao, Y.; Giannopoulou, E.G.; Chan, C.H.; Park, S.H.; Gong, S.; Chen, J.; Hu, X.; Elemento, O.; Ivashkiv, L.B. Synergistic activation of inflammatory cytokine genes by IFN-γ-induced chromatin remodeling and toll-like receptor signaling. Immunity 2013, 39, 454–469. [Google Scholar] [CrossRef]
- Gao, A.H.; Hu, Y.R.; Zhu, W.P. IFN-γ inhibits ovarian cancer progression via SOCS1/JAK/STAT signaling pathway. Clin. Transl. Oncol. 2022, 24, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Fiegl, H.; Zeimet, A.G.; Muller-Holzner, E.; Deibl, M.; Doppler, W.; Daxenbichler, G. Interferon-γ expression is an independent prognostic factor in ovarian cancer. Am. J. Obs. Obstet. Gynecol. 2004, 191, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Cheng, W.F.; Chang, M.C.; Lin, H.W.; Huang, C.T.; Chien, C.L.; Chen, C.A. Interferon-γ in ascites could be a predictive biomarker of outcome in ovarian carcinoma. Gynecol. Oncol. 2013, 131, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Takimoto, G.; McKeithan, T.W. The candidate proto-oncogene bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell 1990, 60, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Kerr, L.D.; Duckett, C.S.; Wamsley, P.; Zhang, Q.; Chiao, P.; Nabel, G.; McKeithan, T.W.; Baeuerle, P.A.; Verma, I.M. The proto-oncogene bcl-3 encodes an IκB protein. Genes. Dev. 1992, 6, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Wulczyn, F.G.; Naumann, M.; Scheidereit, C. Candidate proto-oncogene bcl-3 encodes a subunit-specific inhibitor of transcription factor NFκB. Nature 1992, 358, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Bours, V.; Franzoso, G.; Azarenko, V.; Park, S.; Kanno, T.; Brown, K.; Siebenlist, U. The oncoprotein Bcl-3 directly transactivates through κB motifs via association with DNA-binding p50B homodimers. Cell 1993, 72, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Nolan, G.P.; Fujita, T.; Bhatia, K.; Huppi, C.; Liou, H.C.; Scott, M.L.; Baltimore, D. The bcl-3 proto-oncogene encodes a nuclear IκB-like molecule that preferentially interacts with NFκB p50 and p52 in a phosphorylation-dependent manner. Mol. Cell Biol. 1993, 13, 3557–3566. [Google Scholar] [CrossRef]
- Zhang, Q.; Didonato, J.A.; Karin, M.; McKeithan, T.W. BCL3 encodes a nuclear protein which can alter the subcellular location of NFκB proteins. Mol. Cell Biol. 1994, 14, 3915–3926. [Google Scholar] [CrossRef]
- Na, S.Y.; Choi, J.E.; Kim, H.J.; Jhun, B.H.; Lee, Y.C.; Lee, J.W. Bcl3, an IκB protein, stimulates AP-1 transactivation and cellular proliferation. J. Biol. Chem. 1999, 274, 28491–28496. [Google Scholar] [CrossRef]
- Maldonado, V.; Melendez-Zajgla, J. Role of Bcl-3 in solid tumors. Mol. Cancer 2011, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zeng, L.; Yang, Y.; Guo, C.; Wang, H. Bcl-3: A Double-Edged Sword in Immune Cells and Inflammation. Front. Immunol. 2022, 13, 847699. [Google Scholar] [CrossRef] [PubMed]
- Seaton, G.; Smith, H.; Brancale, A.; Westwell, A.D.; Clarkson, R. Multifaceted roles for BCL3 in cancer: A proto-oncogene comes of age. Mol. Cancer 2024, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- Legge, D.N.; Shephard, A.P.; Collard, T.J.; Greenhough, A.; Chambers, A.C.; Clarkson, R.W.; Paraskeva, C.; Williams, A.C. BCL-3 promotes a cancer stem cell phenotype by enhancing beta-catenin signalling in colorectal tumour cells. Dis. Model. Mech. 2019, 12, dmm037697. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lee, D.S.; Yan, Y.T.; Shen, C.N.; Hwang, S.M.; Lee, S.T.; Hsieh, P.C. Bcl3 Bridges LIF-STAT3 to Oct4 Signaling in the Maintenance of Naive Pluripotency. Stem Cells 2015, 33, 3468–3480. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Bernal, G.M.; Cahill, K.E.; Pytel, P.; Fitzpatrick, C.A.; Mashek, H.; Weichselbaum, R.R.; Yamini, B. BCL3 expression promotes resistance to alkylating chemotherapy in gliomas. Sci. Transl. Med. 2018, 10, eaar2238. [Google Scholar] [CrossRef] [PubMed]
- Riemann, M.; Endres, R.; Liptay, S.; Pfeffer, K.; Schmid, R.M. The IκB protein Bcl-3 negatively regulates transcription of the IL-10 gene in macrophages. J. Immunol. 2005, 175, 3560–3568. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, A.; Piggott, L.; Croston, D.; Jiang, W.G.; Clarkson, R. Suppression of the NFκB Cofactor Bcl3 Inhibits Mammary Epithelial Cell Apoptosis And, in Breast Tumours, Correlates with Poor Prognosis. Breast Cancer Res. 2008, 10, O4. [Google Scholar] [CrossRef][Green Version]
- Wakefield, A.; Soukupova, J.; Montagne, A.; Ranger, J.; French, R.; Muller, W.J.; Clarkson, R.W. Bcl3 selectively promotes metastasis of ERBB2-driven mammary tumors. Cancer Res. 2013, 73, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Turnham, D.J.; Yang, W.W.; Davies, J.; Varnava, A.; Ridley, A.J.; Conlan, R.S.; Clarkson, R.W.E. Bcl-3 promotes multi-modal tumour cell migration via NFκB1 mediated regulation of Cdc42. Carcinogenesis 2020, 41, 1432–1443. [Google Scholar] [CrossRef]
- Soukupova, J.; Bordoni, C.; Turnham, D.J.; Yang, W.W.; Seaton, G.; Gruca, A.; French, R.; Lee, K.Y.; Varnava, A.; Piggott, L.; et al. The Discovery of a Novel Antimetastatic Bcl3 Inhibitor. Mol. Cancer Ther. 2021, 20, 775–786. [Google Scholar] [CrossRef] [PubMed]
- McKeithan, T.W.; Takimoto, G.S.; Ohno, H.; Bjorling, V.S.; Morgan, R.; Hecht, B.K.; Dube, I.; Sandberg, A.A.; Rowley, J.D. BCL3 rearrangements and t(14;19) in chronic lymphocytic leukemia and other B-cell malignancies: A molecular and cytogenetic study. Genes Chromosomes Cancer 1997, 20, 64–72. [Google Scholar] [CrossRef]
- Ge, B.; Li, O.; Wilder, P.; Rizzino, A.; McKeithan, T.W. NFκB regulates BCL3 transcription in T lymphocytes through an intronic enhancer. J. Immunol. 2003, 171, 4210–4218. [Google Scholar] [CrossRef] [PubMed]
- Mathas, S.; Johrens, K.; Joos, S.; Lietz, A.; Hummel, F.; Janz, M.; Jundt, F.; Anagnostopoulos, I.; Bommert, K.; Lichter, P.; et al. Elevated NFκB p50 complex formation and Bcl-3 expression in classical Hodgkin, anaplastic large-cell, and other peripheral T-cell lymphomas. Blood 2005, 106, 4287–4293. [Google Scholar] [CrossRef] [PubMed]
- Martin-Subero, J.I.; Wlodarska, I.; Bastard, C.; Picquenot, J.M.; Hoppner, J.; Giefing, M.; Klapper, W.; Siebert, R. Chromosomal rearrangements involving the BCL3 locus are recurrent in classical Hodgkin and peripheral T-cell lymphoma. Blood 2006, 108, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Courtois, G.; Gilmore, T.D. Mutations in the NFκB signaling pathway: Implications for human disease. Oncogene 2006, 25, 6831–6843. [Google Scholar] [CrossRef]
- Brenne, A.T.; Fagerli, U.M.; Shaughnessy, J.D., Jr.; Vatsveen, T.K.; Ro, T.B.; Hella, H.; Zhan, F.; Barlogie, B.; Sundan, A.; Borset, M.; et al. High expression of BCL3 in human myeloma cells is associated with increased proliferation and inferior prognosis. Eur. J. Haematol. 2009, 82, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.P.; Vancurova, I. Bcl3 regulates pro-survival and pro-inflammatory gene expression in cutaneous T-cell lymphoma. Biochim. Biophys. Acta 2014, 1843, 2620–2630. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, P.C.; Guttridge, D.C.; Funkhouser, W.K.; Baldwin, A.S., Jr. Selective activation of NFκB subunits in human breast cancer: Potential roles for NFκB2/p52 and for Bcl-3. Oncogene 2000, 19, 1123–1131. [Google Scholar] [CrossRef]
- Thornburg, N.J.; Pathmanathan, R.; Raab-Traub, N. Activation of NF-κB p50 homodimer/Bcl-3 complexes in nasopharyngeal carcinoma. Cancer Res. 2003, 63, 8293–8301. [Google Scholar]
- Puvvada, S.D.; Funkhouser, W.K.; Greene, K.; Deal, A.; Chu, H.; Baldwin, A.S.; Tepper, J.E.; O’Neil, B.H. NF-kB and Bcl-3 activation are prognostic in metastatic colorectal cancer. Oncology 2010, 78, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, L.; Jiang, G.; Zhan, H.; Wang, N. B-cell CLL/lymphoma 3 promotes glioma cell proliferation and inhibits apoptosis through the oncogenic STAT3 pathway. Int. J. Oncol. 2016, 49, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, W.; Zhao, Q.; Hu, G.; Deng, K.; Liu, Y. BCL3 exerts an oncogenic function by regulating STAT3 in human cervical cancer. Onco Targets Ther. 2016, 9, 6619–6629. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Yao, H.; Zheng, Z.; Qiu, G.; Sun, K. MiR-125b targets BCL3 and suppresses ovarian cancer proliferation. Int. J. Cancer 2011, 128, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wang, J.; Ma, Y.; Yao, Z.; Pan, H. PPARγ inhibits ovarian cancer cells proliferation through upregulation of miR-125b. Biochem. Biophys. Res. Commun. 2015, 462, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Niu, J.; Feng, Y. Knockdown of long non-coding RNA LINC00176 suppresses ovarian cancer progression by BCL3-mediated down-regulation of ceruloplasmin. J. Cell Mol. Med. 2020, 24, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Ramana, C.V.; Gil, M.P.; Schreiber, R.D.; Stark, G.R. Stat1-dependent and -independent pathways in IFN-γ-dependent signaling. Trends Immunol. 2002, 23, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Piaszyk-Borychowska, A.; Szeles, L.; Csermely, A.; Chiang, H.C.; Wesoly, J.; Lee, C.K.; Nagy, L.; Bluyssen, H.A.R. Signal Integration of IFN-I and IFN-II With TLR4 Involves Sequential Recruitment of STAT1-Complexes and NFκB to Enhance Pro-inflammatory Transcription. Front. Immunol. 2019, 10, 1253. [Google Scholar] [CrossRef] [PubMed]
- Platanitis, E.; Decker, T. Regulatory Networks Involving STATs, IRFs, and NFκB in Inflammation. Front. Immunol. 2018, 9, 2542. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Curiel, T.J.; Wei, S.; Dong, H.; Alvarez, X.; Cheng, P.; Mottram, P.; Krzysiek, R.; Knutson, K.L.; Daniel, B.; Zimmermann, M.C.; et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003, 9, 562–567. [Google Scholar] [CrossRef]
- Brown, J.A.; Dorfman, D.M.; Ma, F.R.; Sullivan, E.L.; Munoz, O.; Wood, C.R.; Greenfield, E.A.; Freeman, G.J. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003, 170, 1257–1266. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Matsumura, N.; Abiko, K.; Baba, T.; Konishi, I. PD-1/PD-L1 blockade in cancer treatment: Perspectives and issues. Int. J. Clin. Oncol. 2016, 21, 462–473. [Google Scholar] [CrossRef]
- Abiko, K.; Hamanishi, J.; Matsumura, N.; Mandai, M. Dynamic host immunity and PD-L1/PD-1 blockade efficacy: Developments after “IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer”. Br. J. Cancer 2023, 128, 461–467. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2019, 29, 3766. [Google Scholar] [CrossRef]
- Chen, S.; Crabill, G.A.; Pritchard, T.S.; McMiller, T.L.; Wei, P.; Pardoll, D.M.; Pan, F.; Topalian, S.L. Mechanisms regulating PD-L1 expression on tumor and immune cells. J. Immunother. Cancer 2019, 7, 305. [Google Scholar] [CrossRef]
- Kornepati, A.V.R.; Vadlamudi, R.K.; Curiel, T.J. Programmed death ligand 1 signals in cancer cells. Nat. Rev. Cancer 2022, 22, 174–189. [Google Scholar] [CrossRef]
- Peng, J.; Hamanishi, J.; Matsumura, N.; Abiko, K.; Murat, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Hosoe, Y.; Murphy, S.K.; et al. Chemotherapy Induces Programmed Cell Death-Ligand 1 Overexpression via the NF-κB to Foster an Immunosuppressive Tumor Microenvironment in Ovarian Cancer. Cancer Res. 2015, 75, 5034–5045. [Google Scholar] [CrossRef]
- Grabosch, S.; Zeng, F.; Zhang, L.; Strange, M.; Brozick, J.; Edwards, R.P.; Vlad, A. PD-L1 Biology in Response to Chemotherapy in Vitro and in Vivo in Ovarian Cancer. J. Immunother. Cancer 2015, 3, P302. [Google Scholar] [CrossRef][Green Version]
- Mandai, M.; Hamanishi, J.; Abiko, K.; Matsumura, N.; Baba, T.; Konishi, I. Anti-PD-L1/PD-1 immune therapies in ovarian cancer: Basic mechanism and future clinical application. Int. J. Clin. Oncol. 2016, 21, 456–461. [Google Scholar] [CrossRef]
- Ghebeh, H.; Lehe, C.; Barhoush, E.; Al-Romaih, K.; Tulbah, A.; Al-Alwan, M.; Hendrayani, S.F.; Manogaran, P.; Alaiya, A.; Al-Tweigeri, T.; et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: Role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010, 12, R48. [Google Scholar] [CrossRef]
- Satelli, A.; Batth, I.S.; Brownlee, Z.; Rojas, C.; Meng, Q.H.; Kopetz, S.; Li, S. Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci. Rep. 2016, 6, 28910. [Google Scholar] [CrossRef]
- Gupta, H.B.; Clark, C.A.; Yuan, B.; Sareddy, G.; Pandeswara, S.; Padron, A.S.; Hurez, V.; Conejo-Garcia, J.; Vadlamudi, R.; Li, R.; et al. Tumor cell-intrinsic PD-L1 promotes tumor-initiating cell generation and functions in melanoma and ovarian cancer. Signal Transduct. Target. Ther. 2016, 1, 16030. [Google Scholar] [CrossRef]
- Clark, C.A.; Gupta, H.B.; Sareddy, G.; Pandeswara, S.; Lao, S.; Yuan, B.; Drerup, J.M.; Padron, A.; Conejo-Garcia, J.; Murthy, K.; et al. Tumor-Intrinsic PD-L1 Signals Regulate Cell Growth, Pathogenesis, and Autophagy in Ovarian Cancer and Melanoma. Cancer Res. 2016, 76, 6964–6974. [Google Scholar] [CrossRef]
- Clark, C.A.; Gupta, H.B.; Curiel, T.J. Tumor cell-intrinsic CD274/PD-L1: A novel metabolic balancing act with clinical potential. Autophagy 2017, 13, 987–988. [Google Scholar] [CrossRef]
- Qu, Q.X.; Xie, F.; Huang, Q.; Zhang, X.G. Membranous and Cytoplasmic Expression of PD-L1 in Ovarian Cancer Cells. Cell Physiol. Biochem. 2017, 43, 1893–1906. [Google Scholar] [CrossRef]
- Escors, D.; Gato-Canas, M.; Zuazo, M.; Arasanz, H.; Garcia-Granda, M.J.; Vera, R.; Kochan, G. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct. Target. Ther. 2018, 3, 26. [Google Scholar] [CrossRef]
- Hudson, K.; Cross, N.; Jordan-Mahy, N.; Leyland, R. The Extrinsic and Intrinsic Roles of PD-L1 and Its Receptor PD-1: Implications for Immunotherapy Treatment. Front. Immunol. 2020, 11, 568931. [Google Scholar] [CrossRef]
- Nihira, N.T.; Miki, Y. Regulation of Intrinsic Functions of PD-L1 by Post-Translational Modification in Tumors. Front. Oncol. 2022, 12, 825284. [Google Scholar] [CrossRef]
- Tu, X.; Qin, B.; Zhang, Y.; Zhang, C.; Kahila, M.; Nowsheen, S.; Yin, P.; Yuan, J.; Pei, H.; Li, H.; et al. PD-L1 (B7-H1) Competes with the RNA Exosome to Regulate the DNA Damage Response and Can Be Targeted to Sensitize to Radiation or Chemotherapy. Mol. Cell 2019, 74, 1215–1226 e1214. [Google Scholar] [CrossRef]
- Gao, Y.; Nihira, N.T.; Bu, X.; Chu, C.; Zhang, J.; Kolodziejczyk, A.; Fan, Y.; Chan, N.T.; Ma, L.; Liu, J.; et al. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat. Cell Biol. 2020, 22, 1064–1075. [Google Scholar] [CrossRef]
- Du, W.; Zhu, J.; Zeng, Y.; Liu, T.; Zhang, Y.; Cai, T.; Fu, Y.; Zhang, W.; Zhang, R.; Liu, Z.; et al. KPNB1-mediated nuclear translocation of PD-L1 promotes non-small cell lung cancer cell proliferation via the Gas6/MerTK signaling pathway. Cell Death Differ. 2021, 28, 1284–1300. [Google Scholar] [CrossRef]
- Hou, J.; Zhao, R.; Xia, W.; Chang, C.W.; You, Y.; Hsu, J.M.; Nie, L.; Chen, Y.; Wang, Y.C.; Liu, C.; et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020, 22, 1264–1275. [Google Scholar] [CrossRef]
- Murray, C.; Galvan, E.; Ontiveros, C.; Deng, Y.; Bai, H.; Padron, A.S.; Hinchee-Rodriguez, K.; Garcia, M.G.; Kornepati, A.; Conejo-Garcia, J.; et al. Pharmacologic Tumor PDL1 Depletion with Cefepime or Ceftazidime Promotes DNA Damage and Sensitivity to DNA-Damaging Agents. Int. J. Mol. Sci. 2022, 23, 5129. [Google Scholar] [CrossRef]
- Kornepati, A.V.R.; Boyd, J.T.; Murray, C.E.; Saifetiarova, J.; de la Pena Avalos, B.; Rogers, C.M.; Bai, H.; Padron, A.S.; Liao, Y.; Ontiveros, C.; et al. Tumor Intrinsic PD-L1 Promotes DNA Repair in Distinct Cancers and Suppresses PARP Inhibitor-Induced Synthetic Lethality. Cancer Res. 2022, 82, 2156–2170. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, J.; Ren, X. PD-L1 regulates tumorigenesis and autophagy of ovarian cancer by activating mTORC signaling. Biosci. Rep. 2019, 39, BSR20191041. [Google Scholar] [CrossRef]
- Zuo, Y.; Zheng, W.; Liu, J.; Tang, Q.; Wang, S.S.; Yang, X.S. MiR-34a-5p/PD-L1 axis regulates cisplatin chemoresistance of ovarian cancer cells. Neoplasma 2020, 67, 93–101. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Iwasaki, M.; Okazaki, T.; Tanaka, Y.; Yamaguchi, K.; Higuchi, T.; Yagi, H.; Takakura, K.; Minato, N.; et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 3360–3365. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Abiko, K.; Matsumura, N.; Baba, T.; Yoshioka, Y.; Kosaka, K.; Konishi, I. The comprehensive assessment of local immune status of ovarian cancer by the clustering of multiple immune factors. Clin. Immunol. 2011, 141, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Abiko, K.; Mandai, M.; Hamanishi, J.; Yoshioka, Y.; Matsumura, N.; Baba, T.; Yamaguchi, K.; Murakami, R.; Yamamoto, A.; Kharma, B.; et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin. Cancer Res. 2013, 19, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Maine, C.J.; Aziz, N.H.; Chatterjee, J.; Hayford, C.; Brewig, N.; Whilding, L.; George, A.J.; Ghaem-Maghami, S. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol. Immunother. 2014, 63, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Parvathareddy, S.K.; Siraj, A.K.; Al-Badawi, I.A.; Tulbah, A.; Al-Dayel, F.; Al-Kuraya, K.S. Differential expression of PD-L1 between primary and metastatic epithelial ovarian cancer and its clinico-pathological correlation. Sci. Rep. 2021, 11, 3750. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016, 9, 5023–5039. [Google Scholar] [CrossRef]
- Zhu, J.; Wen, H.; Bi, R.; Wu, Y.; Wu, X. Prognostic value of programmed death-ligand 1 (PD-L1) expression in ovarian clear cell carcinoma. J. Gynecol. Oncol. 2017, 28, e77. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, O.; Annibali, D.; Aguzzi, C.; Tuyaerts, S.; Amant, F.; Morelli, M.B.; Santoni, G.; Amantini, C.; Maggi, F.; Nabissi, M. The Controversial Role of PD-1 and Its Ligands in Gynecological Malignancies. Front. Oncol. 2019, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zuo, F.; Wang, H.; Jing, J.; He, X. The current landscape of predictive and prognostic biomarkers for immune checkpoint blockade in ovarian cancer. Front. Immunol. 2022, 13, 1045957. [Google Scholar] [CrossRef]
- Zerdes, I.; Matikas, A.; Bergh, J.; Rassidakis, G.Z.; Foukakis, T. Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: Biology and clinical correlations. Oncogene 2018, 37, 4639–4661. [Google Scholar] [CrossRef]
- Devanaboyina, M.; Kaur, J.; Whiteley, E.; Lin, L.; Einloth, K.; Morand, S.; Stanbery, L.; Hamouda, D.; Nemunaitis, J. NFκB Signaling in Tumor Pathways Focusing on Breast and Ovarian Cancer. Oncol. Rev. 2022, 16, 10568. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Dominguez, C.; Hamilton, D.H.; Palena, C. The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor. Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.D.; Houghton, A.M. Tumor-associated neutrophils: New targets for cancer therapy. Cancer Res. 2011, 71, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef]
- Giese, M.A.; Hind, L.E.; Huttenlocher, A. Neutrophil plasticity in the tumor microenvironment. Blood 2019, 133, 2159–2167. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ko, S.Y.; Mohamed, M.S.; Kenny, H.A.; Lengyel, E.; Naora, H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J. Exp. Med. 2019, 216, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Aparicio, M.; Alfaro, C. Influence of Interleukin-8 and Neutrophil Extracellular Trap (NET) Formation in the Tumor Microenvironment: Is There a Pathogenic Role? J. Immunol. Res. 2019, 2019, 6252138. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Carranza-Rua, O.; Alfaro, C.; Onate, C.; Martin-Algarra, S.; Perez, G.; Landazuri, S.F.; Gonzalez, A.; Gross, S.; Rodriguez, I.; et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin. Cancer Res. 2014, 20, 5697–5707. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, K.; Runesson, E.; Sundfeldt, K.; Haeger, M.; Hedin, L.; Janson, P.O.; Brannstrom, M. The chemotactic cytokine interleukin-8--a cyst fluid marker for malignant epithelial ovarian cancer? Gynecol. Oncol. 1998, 71, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xie, K.; Mukaida, N.; Matsushima, K.; Fidler, I.J. Hypoxia-induced elevation in interleukin-8 expression by human ovarian carcinoma cells. Cancer Res. 1999, 59, 5822–5829. [Google Scholar] [PubMed]
- Xu, L.; Fidler, I.J. Interleukin 8: An autocrine growth factor for human ovarian cancer. Oncol. Res. 2000, 12, 97–106. [Google Scholar] [CrossRef]
- Kassim, S.K.; El-Salahy, E.M.; Fayed, S.T.; Helal, S.A.; Helal, T.; Azzam Eel, D.; Khalifa, A. Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clin. Biochem. 2004, 37, 363–369. [Google Scholar] [CrossRef]
- Lokshin, A.E.; Winans, M.; Landsittel, D.; Marrangoni, A.M.; Velikokhatnaya, L.; Modugno, F.; Nolen, B.M.; Gorelik, E. Circulating IL-8 and anti-IL-8 autoantibody in patients with ovarian cancer. Gynecol. Oncol. 2006, 102, 244–251. [Google Scholar] [CrossRef]
- Merritt, W.M.; Lin, Y.G.; Spannuth, W.A.; Fletcher, M.S.; Kamat, A.A.; Han, L.Y.; Landen, C.N.; Jennings, N.; De Geest, K.; Langley, R.R.; et al. Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J. Natl. Cancer Inst. 2008, 100, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Aune, G.; Stunes, A.K.; Lian, A.M.; Reseland, J.E.; Tingulstad, S.; Torp, S.H.; Syversen, U. Circulating interleukin-8 and plasminogen activator inhibitor-1 are increased in women with ovarian carcinoma. Results Immunol. 2012, 2, 190–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dobrzycka, B.; Mackowiak-Matejczyk, B.; Terlikowska, K.M.; Kulesza-Bronczyk, B.; Kinalski, M.; Terlikowski, S.J. Serum levels of IL-6, IL-8 and CRP as prognostic factors in epithelial ovarian cancer. Eur. Cytokine Netw. 2013, 24, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Browne, A.; Sriraksa, R.; Guney, T.; Rama, N.; Van Noorden, S.; Curry, E.; Gabra, H.; Stronach, E.; El-Bahrawy, M. Differential expression of IL-8 and IL-8 receptors in benign, borderline and malignant ovarian epithelial tumours. Cytokine 2013, 64, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Sanguinete, M.M.M.; Oliveira, P.H.; Martins-Filho, A.; Micheli, D.C.; Tavares-Murta, B.M.; Murta, E.F.C.; Nomelini, R.S. Serum IL-6 and IL-8 Correlate with Prognostic Factors in Ovarian Cancer. Immunol. Invest. 2017, 46, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Shen, W.; Zhong, Y.; Bast, R.C., Jr.; Jazaeri, A.; Sood, A.K.; Liu, J. Expression of B7-H4 and IDO1 is associated with drug resistance and poor prognosis in high-grade serous ovarian carcinomas. Hum. Pathol. 2021, 113, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Smycz-Kubanska, M.; Stepien, S.; Gola, J.M.; Kruszniewska-Rajs, C.; Wendlocha, D.; Krolewska-Daszczynska, P.; Strzelec, A.; Strzelczyk, J.; Szanecki, W.; Witek, A.; et al. Analysis of CXCL8 and its receptors CXCR1/CXCR2 at the mRNA level in neoplastic tissue, as well as in serum and peritoneal fluid in patients with ovarian cancer. Mol. Med. Rep. 2022, 26, 12812. [Google Scholar] [CrossRef] [PubMed]
- Koensgen, D.; Bruennert, D.; Ungureanu, S.; Sofroni, D.; Braicu, E.I.; Sehouli, J.; Sumnig, A.; Delogu, S.; Zygmunt, M.; Goyal, P.; et al. Polymorphism of the IL-8 gene and the risk of ovarian cancer. Cytokine 2015, 71, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Fidler, I.J. Acidic pH-induced elevation in interleukin 8 expression by human ovarian carcinoma cells. Cancer Res. 2000, 60, 4610–4616. [Google Scholar]
- Lee, L.F.; Schuerer-Maly, C.C.; Lofquist, A.K.; van Haaften-Day, C.; Ting, J.P.; White, C.M.; Martin, B.K.; Haskill, J.S. Taxol-dependent transcriptional activation of IL-8 expression in a subset of human ovarian cancer. Cancer Res. 1996, 56, 1303–1308. [Google Scholar]
- Lee, L.F.; Haskill, J.S.; Mukaida, N.; Matsushima, K.; Ting, J.P. Identification of tumor-specific paclitaxel (Taxol)-responsive regulatory elements in the interleukin-8 promoter. Mol. Cell Biol. 1997, 17, 5097–5105. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.M.; Arevalo, J.M.; Armaiz-Pena, G.N.; Lu, C.; Stone, R.L.; Moreno-Smith, M.; Nishimura, M.; Lee, J.W.; Jennings, N.B.; Bottsford-Miller, J.; et al. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J. Biol. Chem. 2010, 285, 35462–35470. [Google Scholar] [CrossRef] [PubMed]
- Singha, B.; Gatla, H.R.; Manna, S.; Chang, T.P.; Sanacora, S.; Poltoratsky, V.; Vancura, A.; Vancurova, I. Proteasome inhibition increases recruitment of IκB kinase beta (IKKβ), S536P-p65, and transcription factor EGR1 to interleukin-8 (IL-8) promoter, resulting in increased IL-8 production in ovarian cancer cells. J. Biol. Chem. 2014, 289, 2687–2700. [Google Scholar] [CrossRef] [PubMed]
- Singha, B.; Gatla, H.R.; Phyo, S.; Patel, A.; Chen, Z.S.; Vancurova, I. IKK inhibition increases bortezomib effectiveness in ovarian cancer. Oncotarget 2015, 6, 26347–26358. [Google Scholar] [CrossRef] [PubMed]
- Gatla, H.R.; Zou, Y.; Uddin, M.M.; Singha, B.; Bu, P.; Vancura, A.; Vancurova, I. Histone Deacetylase (HDAC) Inhibition Induces IκB Kinase (IKK)-dependent Interleukin-8/CXCL8 Expression in Ovarian Cancer Cells. J. Biol. Chem. 2017, 292, 5043–5054. [Google Scholar] [CrossRef] [PubMed]
- Gatla, H.R.; Zou, Y.; Uddin, M.M.; Vancurova, I. Epigenetic regulation of interleukin-8 expression by class I HDAC and CBP in ovarian cancer cells. Oncotarget 2017, 8, 70798–70810. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, J.; Vidal, F.; Hoarau-Vechot, J.; Bonneau, C.; Darai, E.; Touboul, C.; Rafii, A. Surgical peritoneal stress creates a pro-metastatic niche promoting resistance to apoptosis via IL-8. J. Transl. Med. 2018, 16, 271. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wen, J.; Wang, L.; Wen, Q.; Wu, J.; Bie, M. Fluid shear stress-induced IL-8/CXCR signaling in human ovarian cancer cells. Transl. Cancer Res. 2019, 8, 1591–1601. [Google Scholar] [CrossRef]
- Abdollahi, T.; Robertson, N.M.; Abdollahi, A.; Litwack, G. Identification of interleukin 8 as an inhibitor of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in the ovarian carcinoma cell line OVCAR3. Cancer Res. 2003, 63, 4521–4526. [Google Scholar]
- So, J.; Navari, J.; Wang, F.Q.; Fishman, D.A. Lysophosphatidic acid enhances epithelial ovarian carcinoma invasion through the increased expression of interleukin-8. Gynecol. Oncol. 2004, 95, 314–322. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, R.C.; Zhang, X.L.; Niu, X.L.; Qu, Y.; Li, L.Z.; Meng, X.Y. Interleukin-8 secretion by ovarian cancer cells increases anchorage-independent growth, proliferation, angiogenic potential, adhesion and invasion. Cytokine 2012, 59, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Yin, Z.; Xu, H.; Li, S.; Tao, W.; Cheng, H.; Du, L.; Zhou, X.; Zhang, B. Effect of targeted silencing of IL-8 on in vitro migration and invasion of SKOV3 ovarian cancer cells. Oncol. Lett. 2017, 13, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.M.; Gaire, B.; Vancurova, I. Interleukin-8 Induces Proliferation of Ovarian Cancer Cells in 3D Spheroids. Methods Mol. Biol. 2020, 2108, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Thongchot, S.; Jamjuntra, P.; Therasakvichya, S.; Warnnissorn, M.; Ferraresi, A.; Thuwajit, P.; Isidoro, C.; Thuwajit, C. Interleukin-8 released by cancer-associated fibroblasts attenuates the autophagy and promotes the migration of ovarian cancer cells. Int. J. Oncol. 2021, 58, 5194. [Google Scholar] [CrossRef] [PubMed]
- Huldani, H.; Abdul-Jabbar Ali, S.; Al-Dolaimy, F.; Hjazi, A.; Denis Andreevich, N.; Oudaha, K.H.; Almulla, A.F.; Alsaalamy, A.; Kareem Oudah, S.; Mustafa, Y.F. The potential role of interleukins and interferons in ovarian cancer. Cytokine 2023, 171, 156379. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.I.; Castillo, M.D.; Litzinger, M.; Hamilton, D.H.; Palena, C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011, 71, 5296–5306. [Google Scholar] [CrossRef] [PubMed]
- Palena, C.; Hamilton, D.H.; Fernando, R.I. Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncol. 2012, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zeng, F.; Wu, N.; Kang, K.; Yang, Z.; Yang, H. Interleukin-8 promotes human ovarian cancer cell migration by epithelial-mesenchymal transition induction in vitro. Clin. Transl. Oncol. 2015, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Robinson, J.B.; Deguzman, A.; Bucana, C.D.; Fidler, I.J. Blockade of NFκB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 2000, 60, 5334–5339. [Google Scholar]
- Ji, Z.; Tian, W.; Gao, W.; Zang, R.; Wang, H.; Yang, G. Cancer-Associated Fibroblast-Derived Interleukin-8 Promotes Ovarian Cancer Cell Stemness and Malignancy Through the Notch3-Mediated Signaling. Front. Cell Dev. Biol. 2021, 9, 684505. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, G.; Wang, Y.; He, M.; Xu, Q.; Lu, J.; Liu, H.; Xu, C. Tumour-associated neutrophils orchestrate intratumoural IL-8-driven immune evasion through Jagged2 activation in ovarian cancer. Br. J. Cancer 2020, 123, 1404–1416. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, H.; Osada, N.; Yoshie, O. The evolution of mammalian chemokine genes. Cytokine Growth Factor. Rev. 2010, 21, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Mukaida, N.; Shiroo, M.; Matsushima, K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J. Immunol. 1989, 143, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Mukaida, N.; Mahe, Y.; Matsushima, K. Cooperative interaction of NFκB-and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J. Biol. Chem. 1990, 265, 21128–21133. [Google Scholar] [CrossRef] [PubMed]
- Kunsch, C.; Rosen, C.A. NFκB subunit-specific regulation of the interleukin-8 promoter. Mol. Cell Biol. 1993, 13, 6137–6146. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.; Baldwin, A.S., Jr. Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NFκB. Mol. Cell Biol. 1993, 13, 7191–7198. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.C.; Mukaida, N.; Matsushima, K.; Vilcek, J. Transcriptional inhibition of the interleukin-8 gene by interferon is mediated by the NFκB site. Mol. Cell Biol. 1994, 14, 5300–5308. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Dittrich-Breiholz, O.; Holtmann, H.; Kracht, M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002, 72, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Singha, B.; Gatla, H.R.; Vancurova, I. Transcriptional regulation of chemokine expression in ovarian cancer. Biomolecules 2015, 5, 223–243. [Google Scholar] [CrossRef]
- Zerbini, L.F.; Tamura, R.E.; Correa, R.G.; Czibere, A.; Cordeiro, J.; Bhasin, M.; Simabuco, F.M.; Wang, Y.; Gu, X.; Li, L.; et al. Combinatorial effect of non-steroidal anti-inflammatory drugs and NFκB inhibitors in ovarian cancer therapy. PLoS ONE 2011, 6, e24285. [Google Scholar] [CrossRef]
- Trabert, B.; Ness, R.B.; Lo-Ciganic, W.H.; Murphy, M.A.; Goode, E.L.; Poole, E.M.; Brinton, L.A.; Webb, P.M.; Nagle, C.M.; Jordan, S.J.; et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: A pooled analysis in the Ovarian Cancer Association Consortium. J. Natl. Cancer Inst. 2014, 106, djt431. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repecka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Siu, M.K.; Jiang, Y.X.; Chan, D.W.; Cheung, A.N.; Ngan, H.Y.; Chan, K.K. Infiltration of T cells promotes the metastasis of ovarian cancer cells via the modulation of metastasis-related genes and PD-L1 expression. Cancer Immunol. Immunother. 2020, 69, 2275–2289. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Q.; Xie, Y.; Zhu, J.; Zhang, S.; Ge, Y.; Guo, J.; Luo, N.; Huang, W.; Xu, R.; et al. MUC16 stimulates neutrophils to an inflammatory and immunosuppressive phenotype in ovarian cancer. J. Ovarian Res. 2023, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Q.; Chen, B.; Zhao, Y.; Shen, B.; Wang, H.; Xu, J.; Zhu, M.; Zhao, X.; Xu, C.; et al. Gastric cancer mesenchymal stem cells derived IL-8 induces PD-L1 expression in gastric cancer cells via STAT3/mTOR-c-Myc signal axis. Cell Death Dis. 2018, 9, 928. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; He, H.; Liu, H.; Li, R.; Chen, Y.; Qi, Y.; Jiang, Q.; Chen, L.; Zhang, P.; Zhang, H.; et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut 2019, 68, 1764–1773. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y.; Duan, X.; Liu, C.; Du, Y.; Wang, X.; Luo, Y.; Cui, Y. HIF-1alpha/IL-8 axis in hypoxic macrophages promotes esophageal cancer progression by enhancing PD-L1 expression. Cancer Gene Ther. 2023, 30, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Lan, Y.; Chai, X.; Gao, S.; Zheng, J.; Huang, R.; Shi, Y.; Xiang, Y.; Guo, H.; Xi, Y.; et al. CXCL8 induces M2 macrophage polarization and inhibits CD8+ T cell infiltration to generate an immunosuppressive microenvironment in colorectal cancer. FASEB J. 2023, 37, e23173. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Perez-Gracia, J.L.; Schalper, K.A.; Fusco, J.P.; Gonzalez, A.; Rodriguez-Ruiz, M.E.; Onate, C.; Perez, G.; Alfaro, C.; Martin-Algarra, S.; et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann. Oncol. 2017, 28, 1988–1995. [Google Scholar] [CrossRef]
- Schalper, K.A.; Carleton, M.; Zhou, M.; Chen, T.; Feng, Y.; Huang, S.P.; Walsh, A.M.; Baxi, V.; Pandya, D.; Baradet, T.; et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 2020, 26, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.C.; Liu, L.F.; Gupta, V.; Madireddi, S.; Keerthivasan, S.; Li, C.; Rishipathak, D.; Williams, P.; Kadel, E.E., 3rd; Koeppen, H.; et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med. 2020, 26, 693–698. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Q.; Tan, L.; Wu, X.; Huang, R.; Zuo, Y.; Chen, L.; Yang, J.; Zhang, Z.X.; Ruan, W.; et al. Neutralizing IL-8 potentiates immune checkpoint blockade efficacy for glioma. Cancer Cell 2023, 41, 693–710 e698. [Google Scholar] [CrossRef]
- Driscoll, R.K.; Lyne, S.B.; Voce, D.J.; Maraka, S.; Gondi, V.; Chmura, S.J.; Dixit, K.S.; Kumthekar, P.U.; Karrison, T.G.; Pytel, P.; et al. A multi-institutional phase I study of acetazolamide with temozolomide in adults with newly diagnosed MGMT-methylated malignant glioma. Neurooncol. Adv. 2024, 6, vdae014. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.M.; Krimpenfort, P.; Berns, A.; Verma, I.M. Immunological defects in mice with a targeted disruption in Bcl-3. Genes Dev. 1997, 11, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Chambers, A.C.; Flanagan, D.J.; Ho, J.W.Y.; Collard, T.J.; Ngo, G.; Baird, D.M.; Timms, P.; Morgan, R.G.; Sansom, O.J.; et al. BCL-3 loss sensitises colorectal cancer cells to DNA damage by targeting homologous recombination. DNA Repair 2022, 115, 103331. [Google Scholar] [CrossRef]
- Li, C.W.; Lim, S.O.; Xia, W.; Lee, H.H.; Chan, L.C.; Kuo, C.W.; Khoo, K.H.; Chang, S.S.; Cha, J.H.; Kim, T.; et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016, 7, 12632. [Google Scholar] [CrossRef]
- Deng, P.; Dong, X.; Wu, Z.; Hou, X.; Mao, L.; Guo, J.; Zhao, W.; Peng, C.; Zhang, Z.; Peng, L. Development of Glycosylation-Modified DPPA-1 Compounds as Innovative PD-1/PD-L1 Blockers: Design, Synthesis, and Biological Evaluation. Molecules 2024, 29, 1898. [Google Scholar] [CrossRef]
- Oyanagi, J.; Koh, Y.; Sato, K.; Mori, K.; Teraoka, S.; Akamatsu, H.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, K.; et al. Predictive value of serum protein levels in patients with advanced non-small cell lung cancer treated with nivolumab. Lung Cancer 2019, 132, 107–113. [Google Scholar] [CrossRef]
- Kauffmann-Guerrero, D.; Kahnert, K.; Kiefl, R.; Sellmer, L.; Walter, J.; Behr, J.; Tufman, A. Systemic inflammation and pro-inflammatory cytokine profile predict response to checkpoint inhibitor treatment in NSCLC: A prospective study. Sci. Rep. 2021, 11, 10919. [Google Scholar] [CrossRef]
- Alfaro, C.; Sanmamed, M.F.; Rodriguez-Ruiz, M.E.; Teijeira, A.; Onate, C.; Gonzalez, A.; Ponz, M.; Schalper, K.A.; Perez-Gracia, J.L.; Melero, I. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat. Rev. 2017, 60, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Liao, X.; Qiu, S.; Xu, H.; Zhang, S.; Wang, S.; Ai, J.; Yang, L. CXCL8 in Tumor Biology and Its Implications for Clinical Translation. Front. Mol. Biosci. 2022, 9, 723846. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.W.; Karim, S.A.; Leach, J.D.G.; Bailey, P.; Upstill-Goddard, R.; Rishi, L.; Foth, M.; Bryson, S.; McDaid, K.; Wilson, Z.; et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell 2016, 29, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, R.; Xu, J.; Tan, Z.; Liang, C.; Meng, Q.; Lei, Y.; Hua, J.; Zhang, Y.; Liu, J.; et al. CRIP1 fosters MDSC trafficking and resets tumour microenvironment via facilitating NFκB/p65 nuclear translocation in pancreatic ductal adenocarcinoma. Gut 2023, 72, 2329–2343. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Clavijo, P.E.; Robbins, Y.; Patel, P.; Friedman, J.; Greene, S.; Das, R.; Silvin, C.; Van Waes, C.; Horn, L.A.; et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight 2019, 4, e126853. [Google Scholar] [CrossRef] [PubMed]
- Kargl, J.; Zhu, X.; Zhang, H.; Yang, G.H.Y.; Friesen, T.J.; Shipley, M.; Maeda, D.Y.; Zebala, J.A.; McKay-Fleisch, J.; Meredith, G.; et al. Neutrophil content predicts lymphocyte depletion and anti-PD1 treatment failure in NSCLC. JCI Insight 2019, 4, e130850. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.T.; Zheng, L.; Zhao, C.W.; Zhang, Y.; Xu, X.W.; Wang, X.Y.; Niu, G.P.; Man, Z.S.; Gu, F.; Chen, Y.Q. Interferon-α induces differentiation of cancer stem cells and immunosuppression in hepatocellular carcinoma by upregulating CXCL8 secretion. Cytokine 2024, 177, 156555. [Google Scholar] [CrossRef]
- Horn, L.A.; Riskin, J.; Hempel, H.A.; Fousek, K.; Lind, H.; Hamilton, D.H.; McCampbell, K.K.; Maeda, D.Y.; Zebala, J.A.; Su, Z.; et al. Simultaneous inhibition of CXCR1/2, TGF-beta, and PD-L1 remodels the tumor and its microenvironment to drive antitumor immunity. J. Immunother. Cancer 2020, 8, e000326. [Google Scholar] [CrossRef]
- Bilusic, M.; Heery, C.R.; Collins, J.M.; Donahue, R.N.; Palena, C.; Madan, R.A.; Karzai, F.; Marte, J.L.; Strauss, J.; Gatti-Mays, M.E.; et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 2019, 7, 240. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).