Long-Term Outcomes of Intravesical Mitomycin C Administered via Electromotive Drug Administration or Conductive Chemo-Hyperthermia in Non-Muscle-Invasive Bladder Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Interventions
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
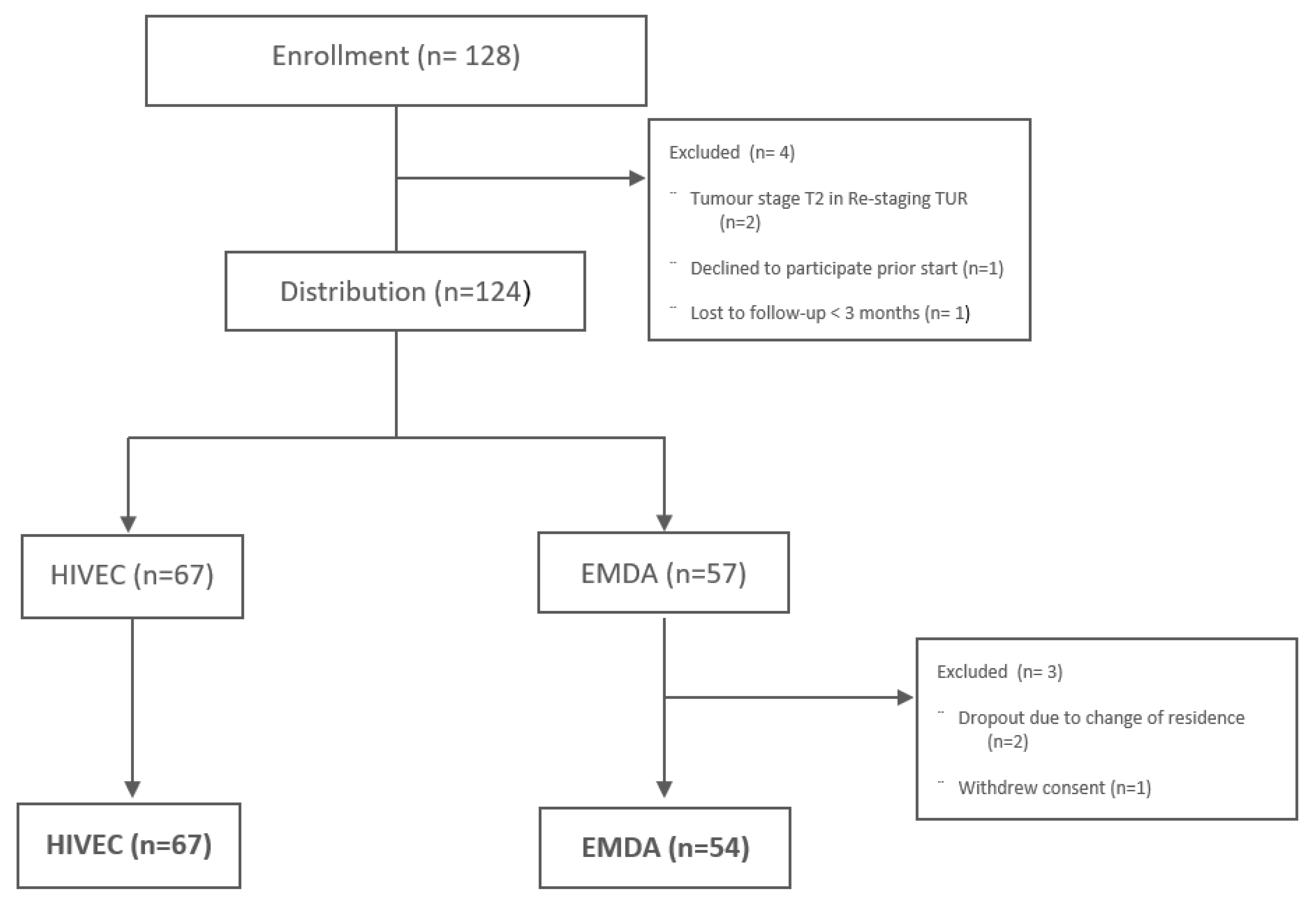
3.1. Patients
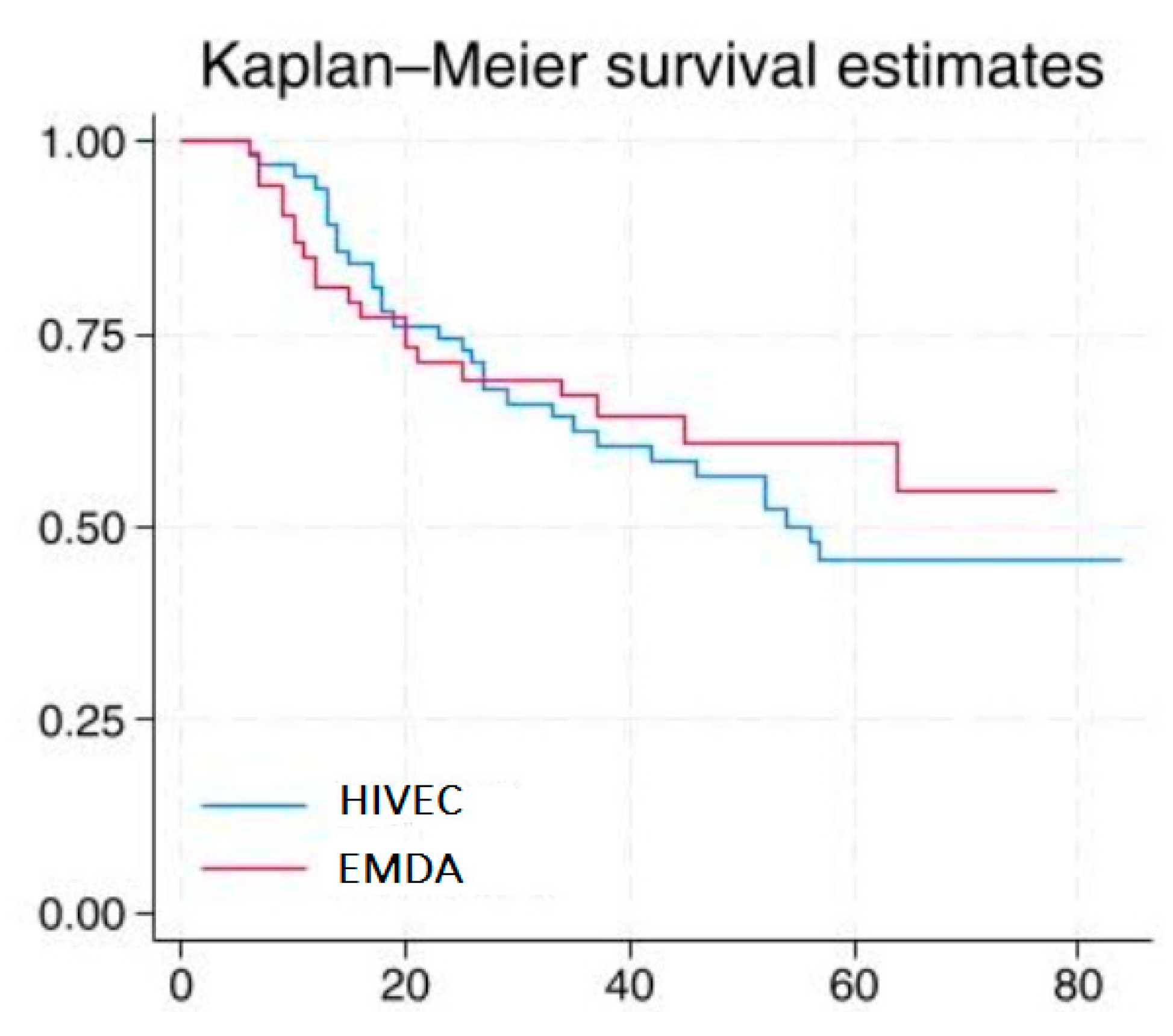
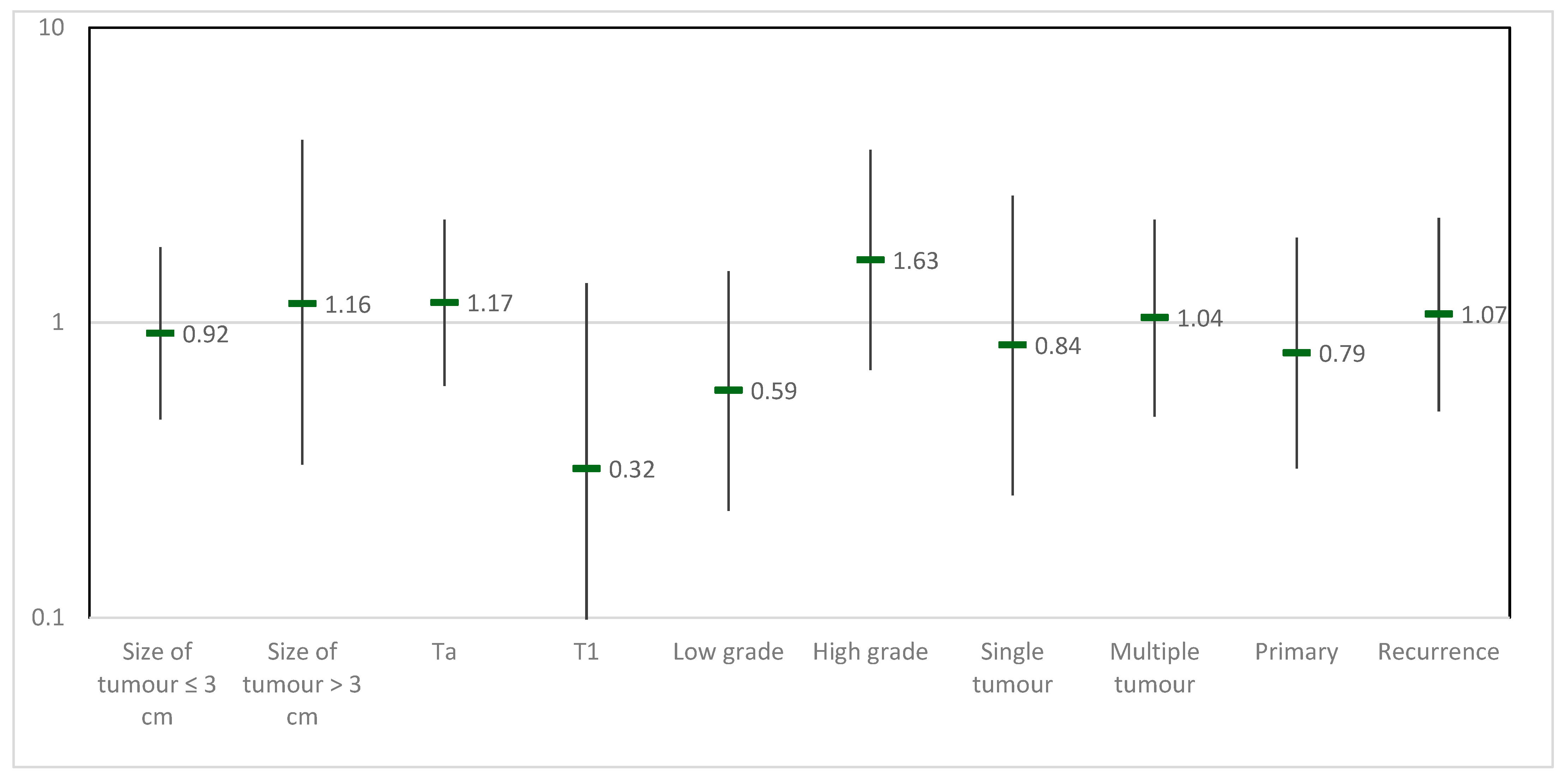
3.2. Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobo, N.; Afferi, L.; Moschini, M.; Mostafid, H.; Porten, S.; Psutka, S.P.; Gupta, S.; Smith, A.B.; Williams, S.B.; Lotan, Y. Epidemiology, Screening, and Prevention of Bladder Cancer. Eur. Urol. Oncol. 2022, 5, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, R.J.; van der Meijden, A.P.M.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 465–466; discussion 475–477. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gomez, J.; Madero, R.; Solsona, E.; Unda, M.; Martinez-Piñeiro, L.; Gonzalez, M.; Portillo, J.; Ojea, A.; Pertusa, C.; Rodriguez-Molina, J.; et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: The CUETO scoring model. J. Urol. 2009, 182, 2195–2203. [Google Scholar] [CrossRef]
- Brausi, M.; Oddens, J.; Sylvester, R.; Bono, A.; van de Beek, C.; van Andel, G.; Gontero, P.; Turkeri, L.; Marreaud, S.; Collette, S.; et al. Side effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: Results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur. Urol. 2014, 65, 69–76. [Google Scholar]
- Ourfali, S.; Ohannessian, R.; Fassi-Fehri, H.; Pages, A.; Badet, L.; Colombel, M. Recurrence Rate and Cost Consequence of the Shortage of Bacillus Calmette-Guérin Connaught Strain for Bladder Cancer Patients. Eur. Urol. Focus 2021, 7, 111–116. [Google Scholar] [CrossRef]
- Segura, M.T.M.; Martínez, A.M.; Castillo, Y.Y.; Polo, M.Á.A.; Lechuga, P.G.; Vílchez, M.P.; Martín, M.A. Conductive hyperthermic chemotherapy versus electromotive drug administration of mitomycin C as intravesical adjuvant treatment of patients with intermediate or high-risk non-muscle invasive bladder cancer. Urol. Oncol. 2022, 41, 109.e1–109.e8. [Google Scholar] [CrossRef]
- EAU Guidelines on Non-Muscle-Invasive Bladder Cancer—Uroweb [Internet]. Available online: https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer/chapter/disease-management (accessed on 7 December 2024).
- Carando, R.; Zazzara, M.; Cotrufo, S.; Ludovico, G.M. Intravesical Treatment with Electro-Mediated Administration of Mytomicin C as Prophylaxis for Intermediate and High-Risk Nonmuscle-Invasive Bladder Cancer: A Retrospective Multicenter Study. Urol. Int. 2019, 103, 285–290. [Google Scholar] [CrossRef]
- Carando, R.; Pradere, B.; Afferi, L.; Marra, G.; Aziz, A.; Roghmann, F.; Krajewski, W.; Di Bona, C.; Alvarez-Maestro, M.; Pagliarulo, V.; et al. The role of device-assisted therapies in the management of non-muscle invasive bladder cancer: A systematic review. Progrès Urol. 2020, 30, 322–331. [Google Scholar] [CrossRef]
- Zazzara, M.; Nazaraj, A.; Scarcia, M.; Cardo, G.; Carando, R.; Ludovico, G.M. Electromotive Drug Administration of Mitomycin C (EMDA/MMC) versus Intravesical Immunotherapy with Bacillus Calmette-Guérin (BCG) in Intermediate and High Risk Non Muscle Invasive Bladder Cancer. Urol. Int. 2021, 107, 64–71. [Google Scholar] [CrossRef]
- Di Stasi, S.M.; Giannantoni, A.; Stephen, R.L.; Capelli, G.; Navarra, P.; Massoud, R.; Vespasiani, G. Intravesical electromotive mitomycin C versus passive transport mitomycin C for high risk superficial bladder cancer: A prospective randomized study. J. Urol. 2003, 170, 777–782. [Google Scholar] [CrossRef]
- Racioppi, M.; Di Gianfrancesco, L.; Ragonese, M.; Palermo, G.; Sacco, E.; Bassi, P.F. ElectroMotive drug administration (EMDA) of Mitomycin C as first-line salvage therapy in high risk ‘BCG failure’ non muscle invasive bladder cancer: 3 years follow-up outcomes. BMC Cancer 2018, 18, 1224. [Google Scholar] [CrossRef] [PubMed]
- Sanz Gómez, I.; Huguet, J.; Bravo, A.; Robalino, J.; Rodríguez Faba, Ó.; Territo, Á.; Gaya, J.M.; Palou, J.; Breda, A. Sequential Treatment With Bacillus Calmette-Güerin (BCG) and Mitomycin C Administered With Electromotive Drug Administration (EMDA) in Patients With High-Risk Nonmuscle Invasive Bladder Cancer After BCG Failure. Clin. Genitourin. Cancer 2023, 21, e286–e290. [Google Scholar] [CrossRef] [PubMed]
- Busetto, G.M.; Finati, M.; Chirico, M.; Cinelli, F.; D’Altilia, N.; Falagario, U.G.; Sanguedolce, F.; Del Giudice, F.; De Berardinis, E.; Ferro, M.; et al. Conservative treatment for high-risk NMIBC failing BCG treatment: Who benefits from adding electromotive drug administration (EMDA) of mitomycin C (MMC) to a second BCG induction cycle? World J. Urol. 2023, 41, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.C.; Álvarez-Ossorio, J.L.; Domínguez, J.L.; Moyano, J.L.; Sousa, A.; Fernández, J.M.; Gómez-Veiga, F.; Unda, M.; Carballido, J.; Carrero, V.; et al. Hyperthermic Mitomycin C in Intermediate-risk Non-muscle-invasive Bladder Cancer: Results of the HIVEC-1 Trial. Eur. Urol. Oncol. 2022, 6, 58–66. [Google Scholar] [CrossRef]
- Tan, W.S.; Prendergast, A.; Ackerman, C.; Yogeswaran, Y.; Cresswell, J.; Mariappan, P.; Phull, J.; Hunter-Campbell, P.; Lazarowicz, H.; Mishra, V.; et al. Adjuvant Intravesical Chemohyperthermia Versus Passive Chemotherapy in Patients with Intermediate-risk Non-muscle-invasive Bladder Cancer (HIVEC-II): A Phase 2, Open-label, Randomised Controlled Trial. Eur. Urol. 2022, 83, 497–504. [Google Scholar] [CrossRef]
- Sachan, A.; Nayyar, R.; Pethe, S.; Singh, P.; Seth, A. A 3-arm randomized control trial to compare the efficacy of re-circulant hyperthermic intravesical chemotherapy versus conventional intravesical mitomycin C and BCG therapy for intermediate-risk non-muscle invasive bladder cancer. World J. Urol. 2024, 42, 609. [Google Scholar] [CrossRef]
- Guerrero-Ramos, F.; González-Padilla, D.A.; González-Díaz, A.; de la Rosa-Kehrmann, F.; Rodríguez-Antolín, A.; Inman, B.A.; Villacampa-Aubá, F. Recirculating hyperthermic intravesical chemotherapy with mitomycin C (HIVEC) versus BCG in high-risk non-muscle-invasive bladder cancer: Results of the HIVEC-HR randomized clinical trial. World J. Urol. 2022, 40, 999–1004. [Google Scholar] [CrossRef]
- Pazir, Y.; Esmeray, A.; Caglar, U.; Erbin, A.; Ozgor, F.; Sarilar, O.; Akbulut, F. Comparison of hyperthermic intravesical chemotherapy and Bacillus Calmette-Guerin therapy in high-risk non-muscle invasive bladder cancer: A matched-pair analysis. Int. Urol. Nephrol. 2023, 56, 957–963. [Google Scholar] [CrossRef]
- Chystiakov, R.S.; Kostyev, F.I.; Bondar, O.V.; Lysenko, V.V.; Varbanets, V.O. 3-year experience of hyperthermic intravesical chemotherapy use in patients with high risk non-muscular-invasive bladder cancer. Medicni Perspekt. 2023, 28, 64–70. [Google Scholar] [CrossRef]
- Tyson, M.D.; Morris, D.; Palou, J.; Rodriguez, O.; Mir, M.C.; Dickstein, R.J.; Guerrero-Ramos, F.; Scarpato, K.R.; Hafron, J.M.; Messing, E.M.; et al. Safety, Tolerability, and Preliminary Efficacy of TAR-200 in Patients With Muscle-invasive Bladder Cancer Who Refused or Were Unfit for Curative-intent Therapy: A Phase 1 Study. J. Urol. 2023, 209, 890–900. [Google Scholar] [CrossRef]
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; A Singer, E.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 919–930, Corrected in Lancet Oncol. 2021, 22, E347. [Google Scholar] [CrossRef] [PubMed]
- Meghani, K.; Cooley, L.F.; Choy, B.; Kocherginsky, M.; Swaminathan, S.; Munir, S.S.; Svatek, R.S.; Kuzel, T.; Meeks, J.J. First-in-human Intravesical Delivery of Pembrolizumab Identifies Immune Activation in Bladder Cancer Unresponsive to Bacillus Calmette-Guérin. Eur. Urol. 2022, 82, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, R.L.; Thomas, L.J.; Brooks, N.; Mott, S.L.; Vitale, A.; Crump, T.; Rao, M.Y.; Daniels, M.J.; Wang, J.; Nagaraju, S.; et al. Multi-Institution Evaluation of Sequential Gemcitabine and Docetaxel as Rescue Therapy for Nonmuscle Invasive Bladder Cancer. J. Urol. 2020, 203, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Catto, J.W.F.; Tran, B.; Master, V.A.; Roupret, M.; Pignot, G.; Tubaro, A.; Shimizu, N.; Vasdev, N.; Gschwend, J.E.; Loriot, Y.; et al. Phase 2 study of the efficacy and safety of erdafitinib in patients (pts) with bacillus Calmette-Guérin (BCG)-unresponsive, high-risk non–muscle-invasive bladder cancer (HR-NMIBC) with FGFR3/2 alterations (alt) in THOR-2: Cohort 2 interim analysis results. J. Clin. Oncol. 2023, 41 (Suppl. S6), 503. [Google Scholar] [CrossRef]
| QHT 6 + 6 N = 67 | EMDA 6 + 6 N = 54 | p | ||
|---|---|---|---|---|
| Sex n, (%) | Men | 52 (77.6%) | 43 (79.6%) | 0.072 |
| Women | 15 (22.4%) | 11 (20.4%) | ||
| Age n, (%) | ≤70 years | 31 (46.3%) | 26 (48.1%) | 0.837 |
| >70 years | 36 (53.7%) | 28 (51.9%) | ||
| Tumour Status n, (%) | Primary | 38 (56.7%) | 32 (56.3%) | 0.778 |
| Recurrent | 29 (43.3%) | 22 (30.7%) | ||
| Multiplicity n, (%) | No | 27 (40.3%) | 24 (44.4%) | 0.646 |
| Múltiple | 40 (59.7%) | 30 (55.6%) | ||
| Size n, (%) | ≤3 cm | 52 (77.6%) | 39 (72.2%) | 0.495 |
| >3 cm | 15 (22.4%) | 15 (27.8%) | ||
| Stage n, (%) | Ta | 41 (61.2%) | 34 (63%) | 0.842 |
| T1 | 26 (38.8%) | 20 (37%) | ||
| WHO Grade 2004/2016 n, (%) | Low | 35 (52.2%) | 25 (46.3%) | 0.516 |
| High | 32 (47.8%) | 29 (53.7%) | ||
| EAU Risk n, (%) | Intermediate | 44 (65.7%) | 32 (59.2%) | 0.468 |
| High | 23 (34.33%) | 22 (40.7%) | ||
| Time (Months) | 10 | 20 | 30 | 40 |
|---|---|---|---|---|
| Patients at risk of HIVEC | 63 | 48 | 38 | 33 |
| Patients at risk of EMDA | 48 | 39 | 34 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melgarejo-Segura, M.T.; Zambudio-Munuera, A.; Arrabal-Polo, M.Á.; Lardelli-Claret, P.; Pareja-Vilchez, M.; Arrabal-Martín, M. Long-Term Outcomes of Intravesical Mitomycin C Administered via Electromotive Drug Administration or Conductive Chemo-Hyperthermia in Non-Muscle-Invasive Bladder Cancer. Cancers 2025, 17, 453. https://doi.org/10.3390/cancers17030453
Melgarejo-Segura MT, Zambudio-Munuera A, Arrabal-Polo MÁ, Lardelli-Claret P, Pareja-Vilchez M, Arrabal-Martín M. Long-Term Outcomes of Intravesical Mitomycin C Administered via Electromotive Drug Administration or Conductive Chemo-Hyperthermia in Non-Muscle-Invasive Bladder Cancer. Cancers. 2025; 17(3):453. https://doi.org/10.3390/cancers17030453
Chicago/Turabian StyleMelgarejo-Segura, Maria Teresa, Alberto Zambudio-Munuera, Miguel Ángel Arrabal-Polo, Pablo Lardelli-Claret, Manuel Pareja-Vilchez, and Miguel Arrabal-Martín. 2025. "Long-Term Outcomes of Intravesical Mitomycin C Administered via Electromotive Drug Administration or Conductive Chemo-Hyperthermia in Non-Muscle-Invasive Bladder Cancer" Cancers 17, no. 3: 453. https://doi.org/10.3390/cancers17030453
APA StyleMelgarejo-Segura, M. T., Zambudio-Munuera, A., Arrabal-Polo, M. Á., Lardelli-Claret, P., Pareja-Vilchez, M., & Arrabal-Martín, M. (2025). Long-Term Outcomes of Intravesical Mitomycin C Administered via Electromotive Drug Administration or Conductive Chemo-Hyperthermia in Non-Muscle-Invasive Bladder Cancer. Cancers, 17(3), 453. https://doi.org/10.3390/cancers17030453





