“Modernized” en Bloc Radical Cystectomy Versus Standard Radical Cystectomy: A Nationwide Multi-Institutional Propensity Score Matched Analysis
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. The Bladder Cancer Pathway in Norway
2.2. Outcomes
2.3. Statistics
3. Results
3.1. Inclusion, Baseline, and Matching
3.2. Surgical and Perioperative Outcomes
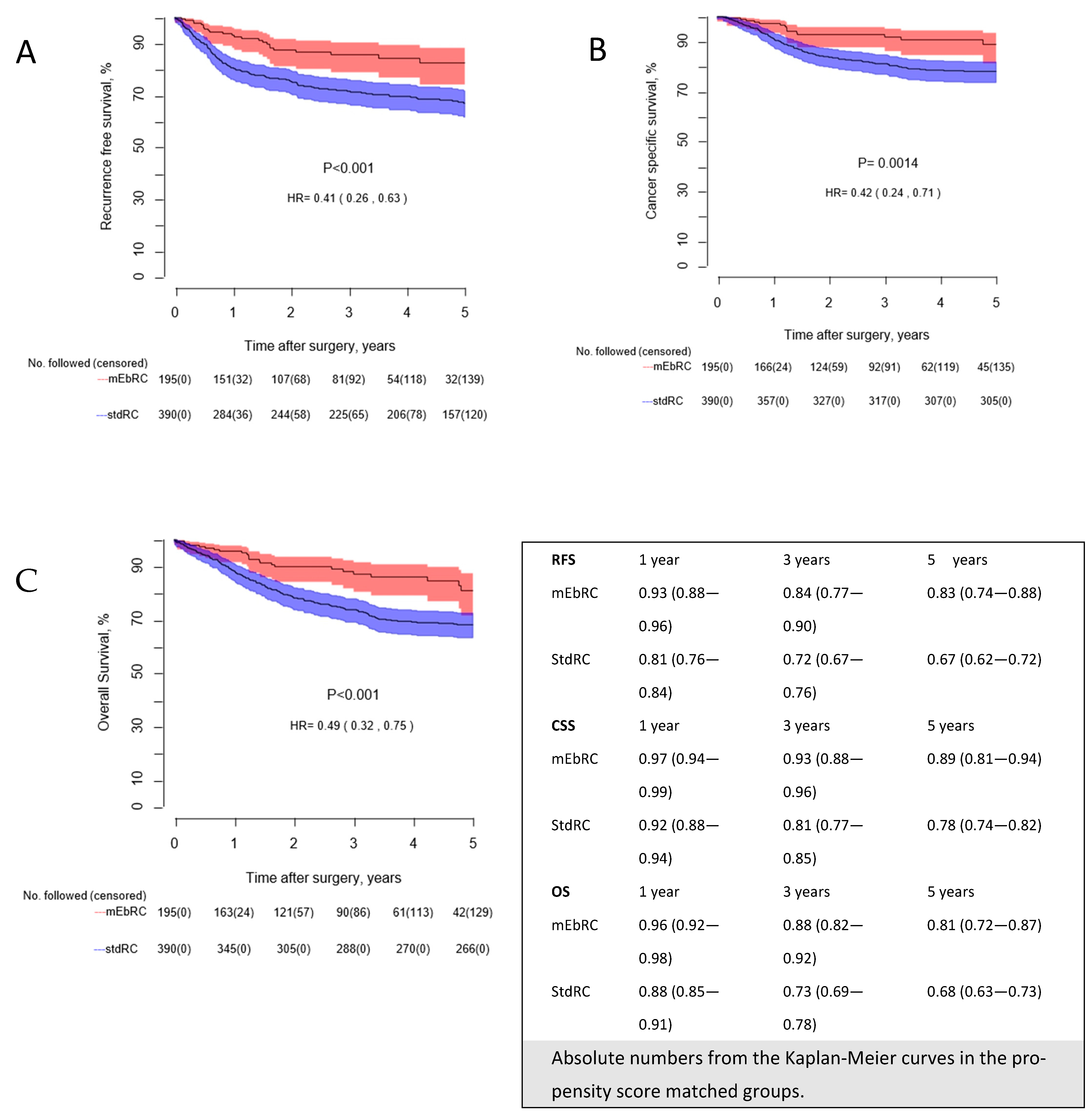
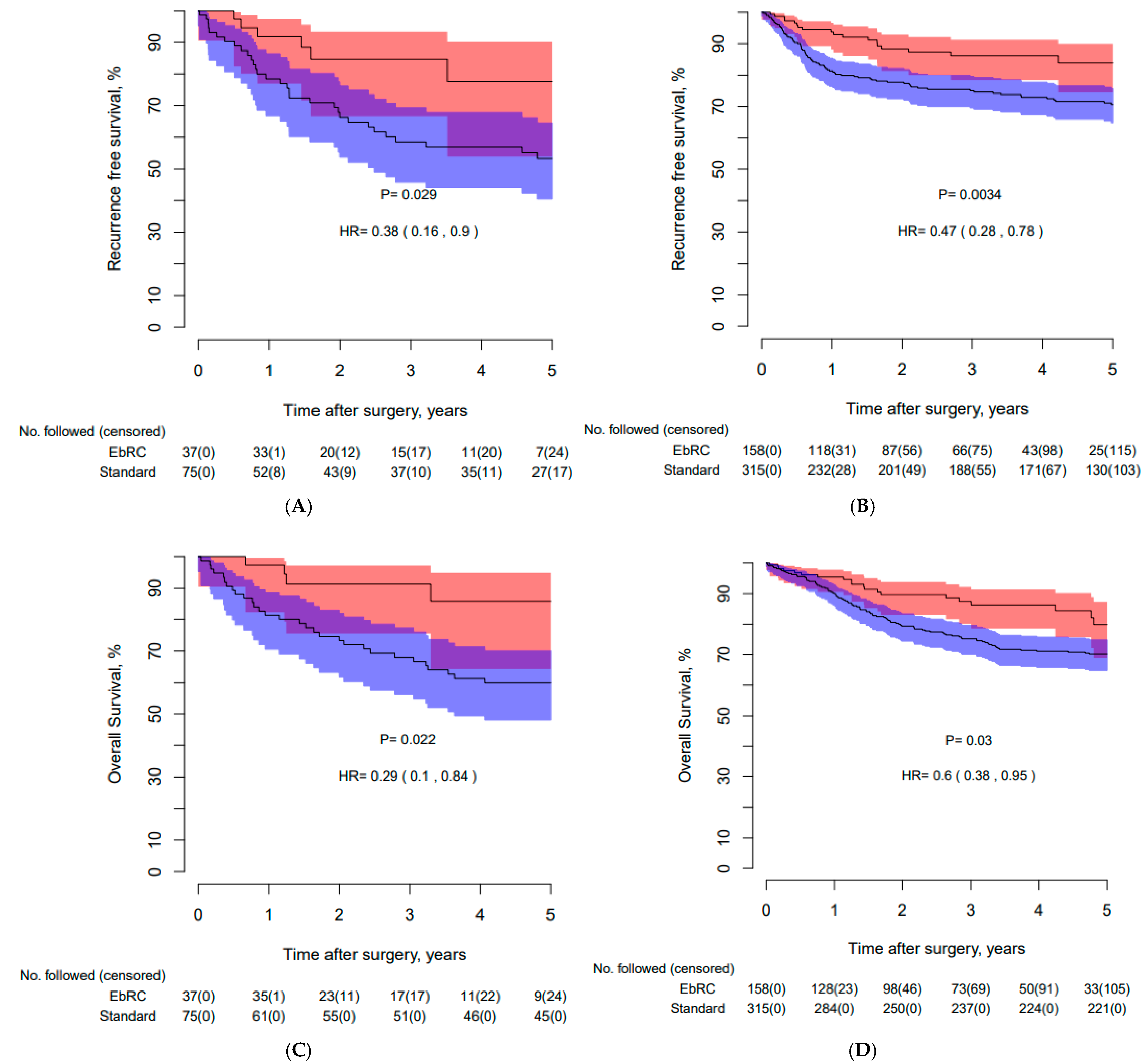
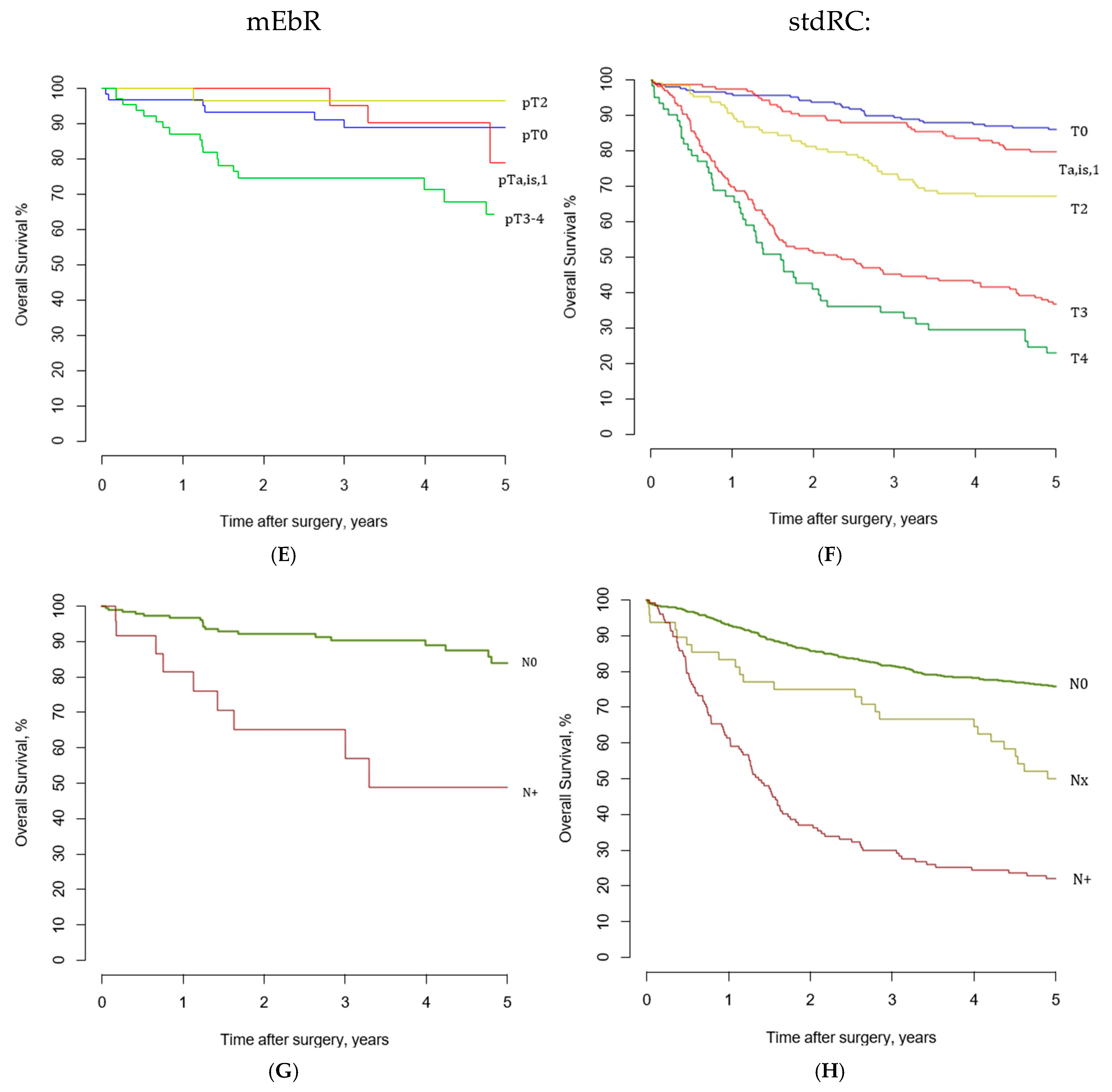
3.3. Recurrence-Free, Cancer-Specific, and Overall Survival
3.4. Treatment of Recurrent Disease
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guidellines, E. Muscle-invasive and Metastatic Bladder Cancer. In EAU Guidelines 2024, EAU Guidelines. Edn; EAU Annual Congress Paris: Paris, France, 2024; ISBN 978-94-92671-23-3. [Google Scholar]
- Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: A systematic review and meta-analysis. Lancet 2003, 361, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; deVere White, R.W.; Sarosdy, M.F.; Wood, D.P., Jr.; Raghavan, D.; et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Parekh, D.J.; Reis, I.M.; Castle, E.P.; Gonzalgo, M.L.; Woods, M.E.; Svatek, R.S.; Weizer, A.Z.; Konety, B.R.; Tollefson, M.; Krupski, T.L.; et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): An open-label, randomised, phase 3, non-inferiority trial. Lancet 2018, 391, 2525–2536. [Google Scholar] [CrossRef]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef]
- Gschwend, J.E.; Heck, M.M.; Lehmann, J.; Rubben, H.; Albers, P.; Wolff, J.M.; Frohneberg, D.; de Geeter, P.; Heidenreich, A.; Kalble, T.; et al. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur. Urol. 2018, 75, 604–611. [Google Scholar] [CrossRef]
- Lerner, S.P.; Tangen, C.; Svatek, R.S.; Daneshmand, S.; Pohar, K.S.; Skinner, E.; Schuckman, A.; Sagalowsky, A.I.; Smith, N.D.; Kamat, A.M.; et al. Standard or Extended Lymphadenectomy for Muscle-Invasive Bladder Cancer. N. Engl. J. Med. 2024, 391, 1206–1216. [Google Scholar] [CrossRef]
- Williams, S.B.; Huo, J.; Dafashy, T.J.; Ghaffary, C.K.; Baillargeon, J.G.; Morales, E.E.; Kim, S.P.; Kuo, Y.F.; Orihuela, E.; Tyler, D.S.; et al. Survival differences among patients with bladder cancer according to sex: Critical evaluation of radical cystectomy use and delay to treatment. Urol. Oncol. 2017, 35, e601–e602. [Google Scholar] [CrossRef]
- Krimphove, M.J.; Szymaniak, J.; Marchese, M.; Tully, K.H.; D’Andrea, D.; Mossanen, M.; Lipsitz, S.R.; Kilbridge, K.; Kibel, A.S.; Kluth, L.A.; et al. Sex-specific Differences in the Quality of Treatment of Muscle-invasive Bladder Cancer Do Not Explain the Overall Survival Discrepancy. Eur. Urol. Focus. 2021, 7, 124–131. [Google Scholar] [CrossRef]
- Skinner, D.G. Technique of radical cystectomy. Urol. Clin. North. Am. 1981, 8, 353–366. [Google Scholar] [CrossRef]
- Stein, J.P.; Skinner, D.G.; Montie, J.E. Radical Cystectomy and Pelvic Lymphadenectomy in the Treatment of Infiltrative Bladder Cancer. In Bladder Cancer: Current Diagnosis and Treatment; Droller, M.J., Ed.; Humana Press: Totowa, NJ, USA, 2001; pp. 267–307. [Google Scholar]
- Stein, J.P.; Penson, D.F.; Cai, J.; Miranda, G.; Skinner, E.C.; Dunn, M.A.; Groshen, S.; Lieskovsky, G.; Skinner, D.G. Radical cystectomy with extended lymphadenectomy: Evaluating separate package versus en bloc submission for node positive bladder cancer. J. Urol. 2007, 177, 876–881; discussion 881–882. [Google Scholar] [CrossRef]
- Mitra, A.P.; Cai, J.; Miranda, G.; Bhanvadia, S.; Quinn, D.I.; Schuckman, A.K.; Djaladat, H.; Daneshmand, S. Management Trends and Outcomes of Patients Undergoing Radical Cystectomy for Urothelial Carcinoma of the Bladder: Evolution of the University of Southern California Experience over 3,347 Cases. J. Urol. 2022, 207, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Wibe, A.; Eriksen, M.T.; Syse, A.; Myrvold, H.E.; Soreide, O. Total mesorectal excision for rectal cancer--what can be achieved by a national audit? Color. Dis. Off. J. Assoc. Coloproctology Great Br. Irel. 2003, 5, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, R.B., Jr.; Kyle, K.; Watson, F.R.; Spratt, J. Cancer of the colon: The influence of the no-touch isolation technic on survival rates. CA Cancer J. Clin. 1968, 18, 82–87. [Google Scholar] [CrossRef]
- Kjøbli, E.; Salvesen, Ø.; Langørgen, S.; Størkersen, Ø.; Wibe, A.; Arum, C.J. En bloc radical cystectomy: An overview of the technique and oncological results. BJUI Compass 2023, 4, 195–205. [Google Scholar] [CrossRef]
- Health, N.D.o. The Cancer Pathways. 2016. Available online: https://www.helsenorge (accessed on 9 October 2024).
- D’Andrea, V.D.; Melnick, K.; Yim, K.; Ernandez, J.; Onochie, N.; Clinton, T.N.; Steele, G.S.; Preston, M.A.; Kibel, A.S.; Mossanen, M. Evidence-Based Analysis of the Critical Steps of Radical Cystectomy for Bladder Cancer. J. Clin. Med. 2023, 12, 6845. [Google Scholar] [CrossRef]
- Brierley, J.D. (Ed.) TNM Classification of Malignant Tumours, 8th ed.; UICC: Geneva, Switzerland, 2016. [Google Scholar]
- The Cancer Registry of Norway. Available online: https://www.kreftregisteret.no/en/ (accessed on 9 October 2024).
- Koppie, T.M.; Vickers, A.J.; Vora, K.; Dalbagni, G.; Bochner, B.H. Standardization of pelvic lymphadenectomy performed at radical cystectomy: Can we establish a minimum number of lymph nodes that should be removed? Cancer 2006, 107, 2368–2374. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Mandel, P.; Tilki, D.; Eslick, G.D. Extent of lymph node dissection and recurrence-free survival after radical cystectomy: A meta-analysis. Urol. Oncol. 2014, 32, 1184–1190. [Google Scholar] [CrossRef]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Catto, J.W.F.; Khetrapal, P.; Ricciardi, F.; Ambler, G.; Williams, N.R.; Al-Hammouri, T.; Khan, M.S.; Thurairaja, R.; Nair, R.; Feber, A.; et al. Effect of Robot-Assisted Radical Cystectomy With Intracorporeal Urinary Diversion vs Open Radical Cystectomy on 90-Day Morbidity and Mortality Among Patients With Bladder Cancer: A Randomized Clinical Trial. JAMA 2022, 327, 2092–2103. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, J.; Gandaglia, G.; Larcher, A.; Sun, M.; Tian, Z.; Shariat, S.F.; McCormack, M.; Valiquette, L.; Montorsi, F.; Graefen, M.; et al. Contemporary 90-day mortality rates after radical cystectomy in the elderly. Eur. J. Surg. Oncol. 2014, 40, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Almassi, N.; Cha, E.K.; Vertosick, E.A.; Huang, C.; Wong, N.; Dason, S.; McPherson, V.; Dean, L.; Benfante, N.; Sjoberg, D.D.; et al. Trends in Management and Outcomes among Patients with Urothelial Carcinoma Undergoing Radical Cystectomy from 1995 to 2015: The Memorial Sloan Kettering Experience. J. Urol. 2020, 204, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Hautmann, R.E.; de Petriconi, R.C.; Pfeiffer, C.; Volkmer, B.G. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: Long-term results in 1100 patients. Eur. Urol. 2012, 61, 1039–1047. [Google Scholar] [CrossRef]
- Yin, M.; Joshi, M.; Meijer, R.P.; Glantz, M.; Holder, S.; Harvey, H.A.; Kaag, M.; Fransen van de Putte, E.E.; Horenblas, S.; Drabick, J.J. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist 2016, 21, 708–715. [Google Scholar] [CrossRef]
- Nikulainen, I.; Salminen, A.P.; Seikkula, H.; Högerman, M.; Perez, I.M.; Koskinen, I.; Sairanen, J.; Nikkola, J.; Murtola, T.J.; Vaarala, M.H.; et al. Nationwide analysis of survival after radical cystectomy for bladder cancer in Finland. Acta Oncol. 2023, 62, 829–835. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]
- Liu, L.; Li, K.; Wang, S.G.; Wang, J.; Yao, Z.; Xie, Y.; Ji, Z.; Chen, Z.; Hu, H.; Chen, H.; et al. The prognostic impact of tumor location in nonmuscle-invasive bladder cancer patients undergoing transurethral resection: Insights from a cohort study utilizing Chinese multicenter and SEER registries. Int. J. Surg. 2024, 110, 5641–5651. [Google Scholar] [CrossRef]

| Before Matching | After 1:2 Matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All mEbRC (n = 214) | All stdRC (n = 721) | p-Value | mEbRC Matched (n = 195) | stdRC Matched (n = 390) | p-Value | |||||
| Age | 70.9 | (64–77) | 70.4 | (64–75) | 0.44 | 71.5 | (65–75) | 70.0 | (63–74) | 0.57 |
| Gender | 0.93 | 1.0 | ||||||||
| Male | 169 | (79%) | 565 | (78%) | 158 | (81%) | 315 | (81%) | ||
| Female | 45 | (21%) | 156 | (22%) | 37 | (19%) | 75 | (19%) | ||
| BMI * | 25.9 | (24–30) | 25.5 | (23–28) | 0.09 | 26.2 | (24–30) | 25.9 | (23–29) | 0.20 |
| Clinical N-stage * | ||||||||||
| cN+ | 16 | (8%) | 76 | (11%) | 0.24 | 13 | (7%) | 38 | (10%) | 0.28 |
| Neoadjuvant chemotherapy | 0.32 | 1.0 | ||||||||
| Yes | 76 | (35.5%) | 230 | (32%) | 64 | (33%) | 129 | (33%) | ||
| No | 138 | (64.5%) | 491 | (68%) | 131 | (67%) | 261 | (67%) | ||
| Charlson Comorbidity Index | ||||||||||
| 0 | (0–2) | 1 | (0–2) | 0.44 | 0 | (0–1) | 0 | (0–1) | 0.78 | |
| Pathological N-stage | <0.001 | |||||||||
| pN+ | 24 | (11.2%) | 127 | (17.6%) | 0.03 | 22 | (11.3%) | 49 | (12.6%) | 0.69 |
| pN0 | 190 | (89%) | 546 | (76%) | 173 | (89%) | 341 | (87%) | ||
| pNx | 48 | (6.7%) | ||||||||
| Carcinoma in situ | 0.10 | 0.54 | ||||||||
| Yes | 108 | (51%) | 316 | (44%) | 92 | (47%) | 195 | (50%) | ||
| No | 106 | (49%) | 405 | (56%) | 103 | (53%) | 195 | (50%) | ||
| Pathological stage | 0.75 | 0.43 | ||||||||
| pT0 | 64 | (30%) | 208 | (29%) | 0.80 | 59 | (30%) | 118 | (30%) | 1.0 |
| pTis | 12 | (5.6%) | 61 | (8.5%) | 0.19 | 12 | (6%) | 39 | (10%) | 0.16 |
| pTa | 11 | (5.1%) | 26 | (3.6%) | 0.32 | 11 | (5.6%) | 12 | (3.1%) | 0.17 |
| pT1 | 25 | (12%) | 71 | (10%) | 0.44 | 24 | (12%) | 42 | (11%) | 0.58 |
| pT2 | 37 | (17%) | 128 | (18%) | 0.92 | 33 | (17%) | 59 | (15%) | 0.63 |
| pT3 | 48 | (22%) | 166 | (23%) | 0.93 | 40 | (21%) | 93 | (24%) | 0.40 |
| pT4a | 17 | (7.9%) | 61 | (8.5%) | 0.89 | 16 | (8%) | 27 | (7%) | 0.62 |
| After 1:2 Matching | |||||
|---|---|---|---|---|---|
| mEbRC Matched (n = 195) | stdRC Matched (n = 390) | p-Value | |||
| High grade cancer | 1.0 | ||||
| Yes | 192 | (99%) | 383 | (98%) | |
| No | 3 | (2%) | 5 | (1%) | |
| N/A | 2 | (1%) | |||
| PLND | <0.001 | ||||
| Superextended | 14 | (3.6%) | 0.007 | ||
| Extended | 115 | (59%) | 90 | (23%) | <0.001 |
| Standard | 80 | (41%) | 272 | (70%) | <0.001 |
| Limited | 6 | (1.5%) | |||
| None/unknown | 8 | (2.1%) | |||
| Lymph nodes in final pathology | 17 | (12–23) | 15 | (9–20) | <0.001 |
| Extended | 21 | (13–26) | 17 | (12–21) | 0.002 |
| Standard | 14 | (11–19) | 14 | (9–20) | 0.91 |
| Histologic subtype | 0.66 | ||||
| Urothelial carcinoma | 186 | (95%) | 375 | (96%) | |
| Squamous cell carcinoma | 4 | (2.1%) | 7 | (1.8%) | |
| Adenocarcinoma | 2 | (1.0%) | 4 | (1.0%) | |
| Small cell carcinoma | 2 | (0.5%) | |||
| Sarcoma | 3 | (1.5%) | 2 | (0.5%) | |
| Positive surgical margin | |||||
| Yes | 1 | (0.5%) | 19 | (4.9%) | <0.001 |
| No | 194 | (99%) | 371 | (95%) | |
| Complications | |||||
| CL-D 2 | 72 | (37%) | 146 | (37%) | 0.93 |
| CL-D 3 | 22 | (11%) | 48 | (12%) | 0.79 |
| CL-D 4 | 1 | (0.5%) | 8 | (2.1%) | 0.28 |
| Perioperative blood loss (mL) | 300 | (150–450) | 400 | (250–600) | <0.001 |
| 30-day readmission rate | 23 | (11.8%) | 34 | (8.7) | 0.24 |
| Mortality rates | |||||
| 30-day | 1 | (0.5%) | 4 | (1%) | 0.67 |
| 90-day | 5 | (2.6%) | 12 | (3.1%) | 0.80 |
| After 1:2 Matching | |||||
|---|---|---|---|---|---|
| mEbRC Matched (n = 195) | stdRC Matched (n = 390) | p-Value | |||
| Treatment recurrence | n = 24 | n = 116 | |||
| None | 4 | (17%) | 18 | (16%) | 1.0 |
| Palliative | 5 | (21%) | 55 | (47%) | 0.02 |
| Surgery | 0 | 18 | (16%) | 0.04 | |
| Radiation | 4 | (17%) | 39 | (34%) | 0.14 |
| Chemotherapy | 12 | (50%) | 54 | (47%) | 0.82 |
| Immunotherapy | 8 | (33%) | 11 | (9%) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kjøbli, E.; Haug, E.S.; Salvesen, Ø.; Arstad, C.; Bergesen, A.K.; Brennhovd, B.; Carlsen, B.; Gharib-Alhaug, B.; Gudbrandsdottir, G.; Juliebø-Jones, P.; et al. “Modernized” en Bloc Radical Cystectomy Versus Standard Radical Cystectomy: A Nationwide Multi-Institutional Propensity Score Matched Analysis. Cancers 2025, 17, 404. https://doi.org/10.3390/cancers17030404
Kjøbli E, Haug ES, Salvesen Ø, Arstad C, Bergesen AK, Brennhovd B, Carlsen B, Gharib-Alhaug B, Gudbrandsdottir G, Juliebø-Jones P, et al. “Modernized” en Bloc Radical Cystectomy Versus Standard Radical Cystectomy: A Nationwide Multi-Institutional Propensity Score Matched Analysis. Cancers. 2025; 17(3):404. https://doi.org/10.3390/cancers17030404
Chicago/Turabian StyleKjøbli, Eirik, Erik Skaaheim Haug, Øyvind Salvesen, Christian Arstad, Anne Kvaale Bergesen, Bjørn Brennhovd, Birgitte Carlsen, Bita Gharib-Alhaug, Gigja Gudbrandsdottir, Patrick Juliebø-Jones, and et al. 2025. "“Modernized” en Bloc Radical Cystectomy Versus Standard Radical Cystectomy: A Nationwide Multi-Institutional Propensity Score Matched Analysis" Cancers 17, no. 3: 404. https://doi.org/10.3390/cancers17030404
APA StyleKjøbli, E., Haug, E. S., Salvesen, Ø., Arstad, C., Bergesen, A. K., Brennhovd, B., Carlsen, B., Gharib-Alhaug, B., Gudbrandsdottir, G., Juliebø-Jones, P., Haugland, J. N., Karlsvik, A.-K., Larsen, M., Lilleaasen, G. M., Mûller, S., Plathan, M. L., Roaldsen, M., Roth, I., Schwenke, B. L. L., ... Beisland, C. (2025). “Modernized” en Bloc Radical Cystectomy Versus Standard Radical Cystectomy: A Nationwide Multi-Institutional Propensity Score Matched Analysis. Cancers, 17(3), 404. https://doi.org/10.3390/cancers17030404







