RET C611Y Germline Variant in Multiple Endocrine Neoplasia Type 2A in Denmark 1930–2021: A Nationwide Study
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Data Sources
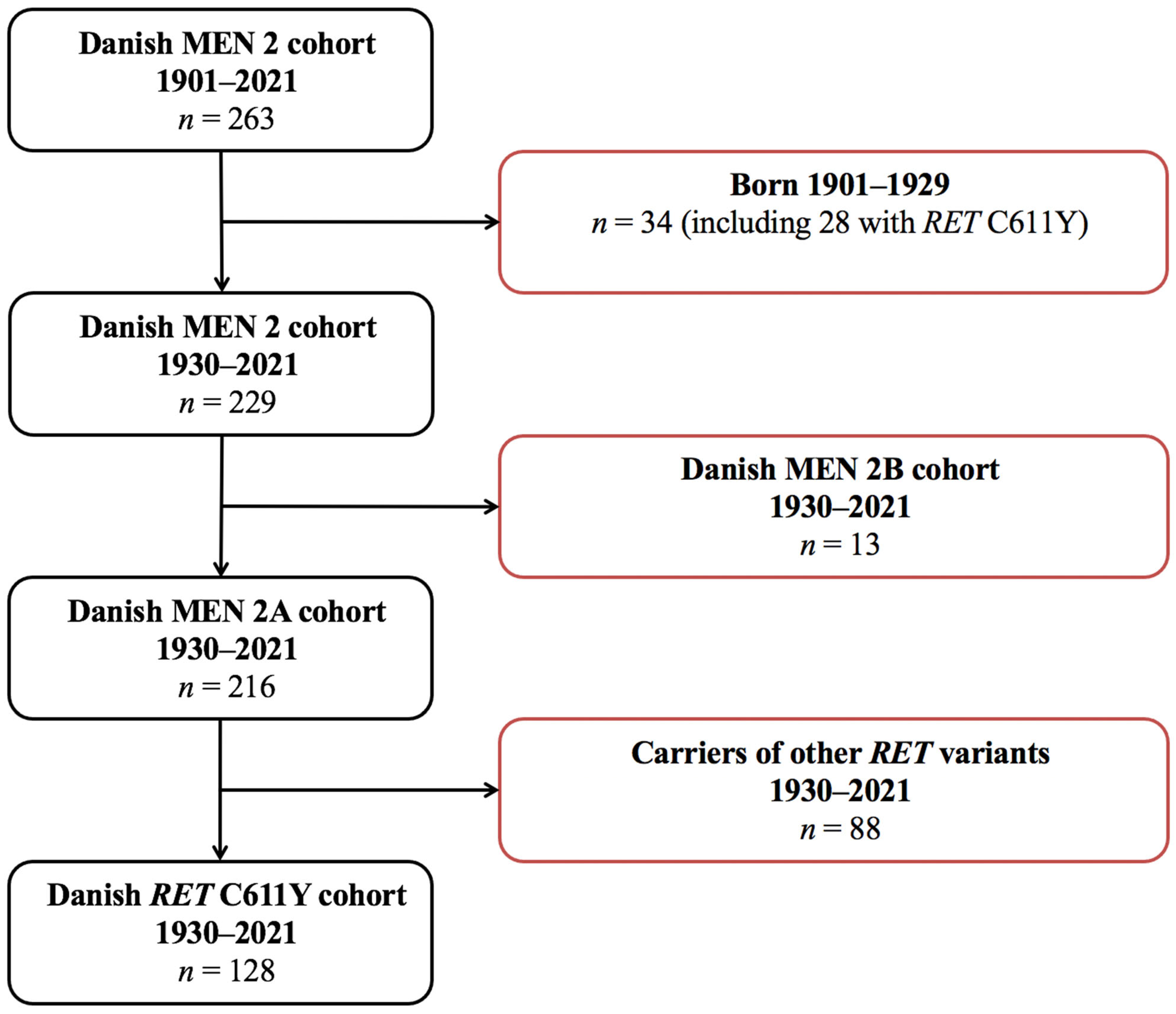
2.3. Participants
2.4. Variables
2.5. Statistical Analysis
2.6. Ethics
3. Results
3.1. Demographics
3.2. Thyroid
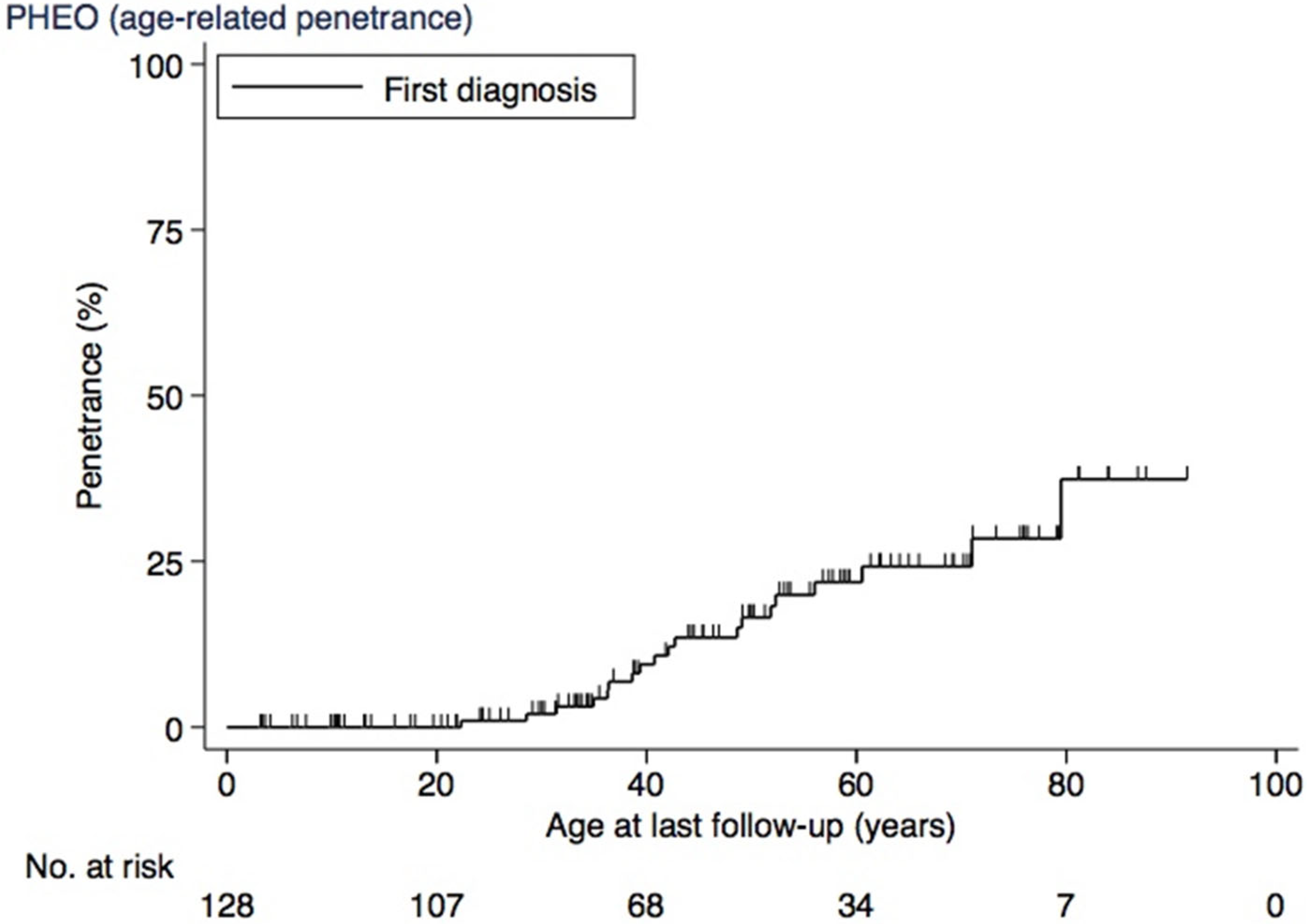
3.3. PHEO
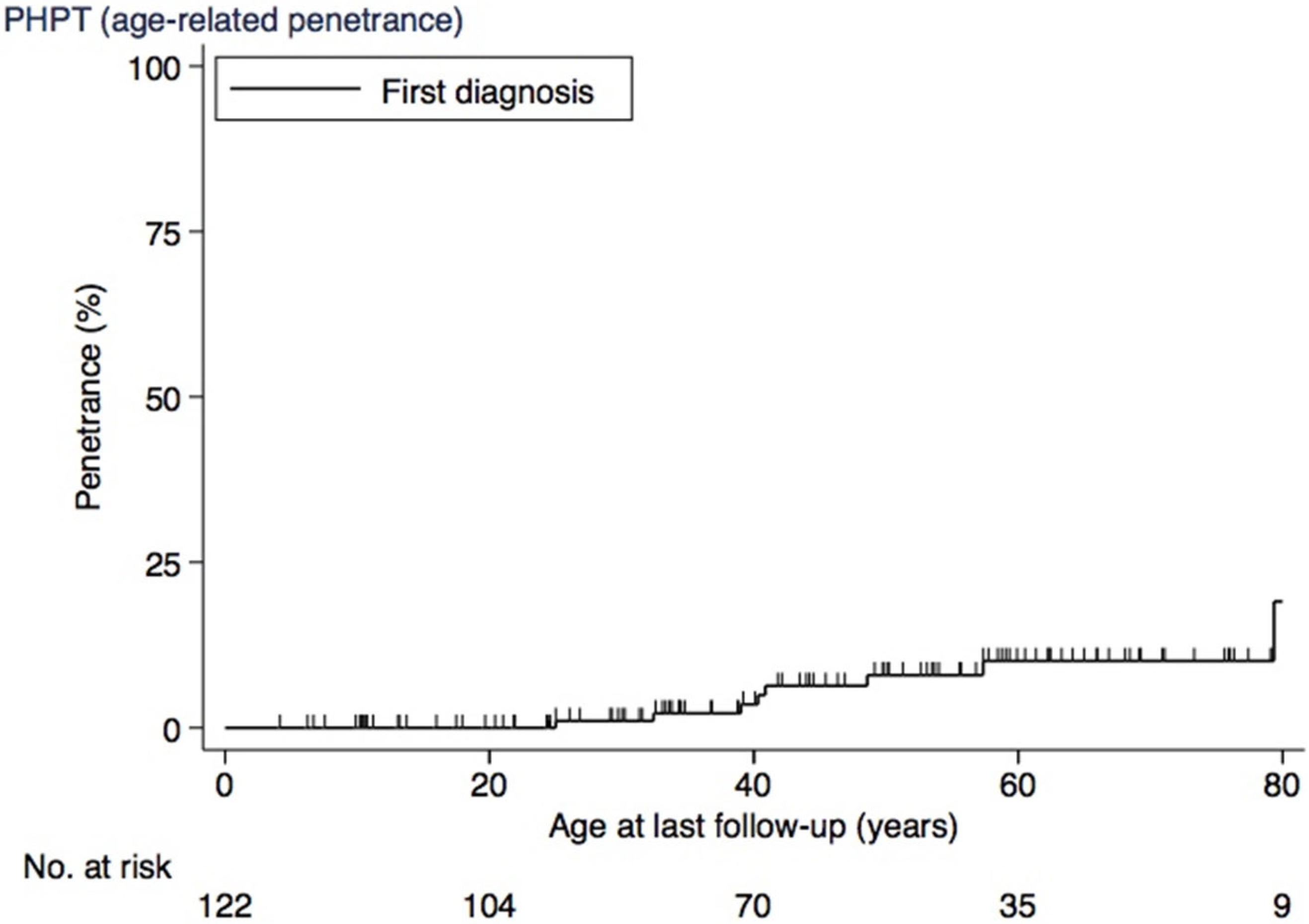
3.4. PHPT
3.5. Other Manifestations
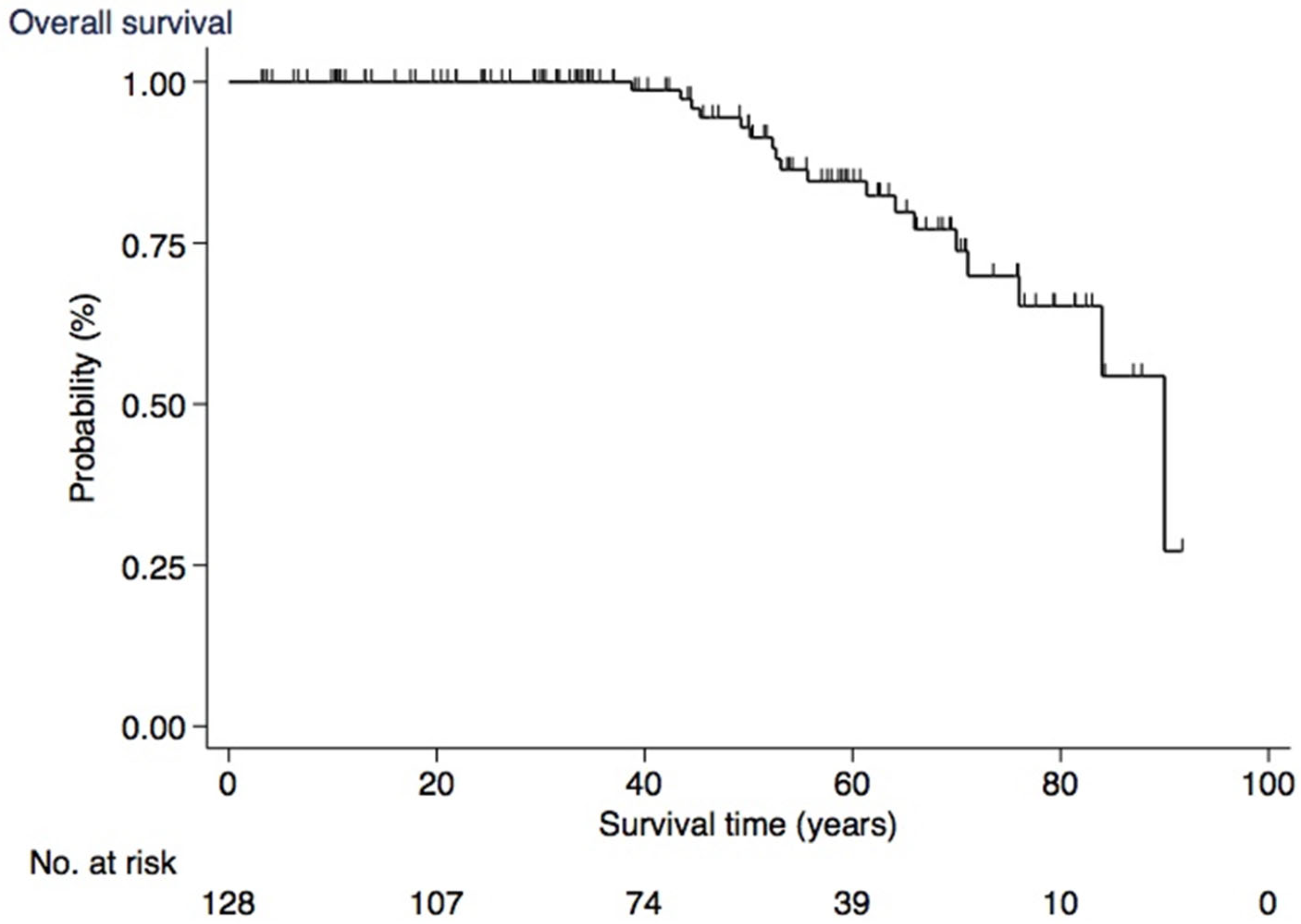
3.6. Survival
4. Discussion
4.1. Limitations
4.2. Demographics
4.3. Thyroid
4.4. PHEO
4.5. PHPT
4.6. Other Manifestations
4.7. Survival
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Opsahl, E.M.; Brauckhoff, M.; Schlichting, E.; Helset, K.; Svartberg, J.; Brauckhoff, K.; Mæhle, L.; Engebretsen, L.F.; Sigstad, E.; Grøholt, K.K.; et al. A Nationwide Study of Multiple Endocrine Neoplasia Type 2A in Norway: Predictive and Prognostic Factors for the Clinical Course of Medullary Thyroid Carcinoma. Thyroid 2016, 26, 1225–1238. [Google Scholar] [CrossRef]
- Mathiesen, J.S.; Kroustrup, J.P.; Vestergaard, P.; Stochholm, K.; Poulsen, P.L.; Rasmussen, Å.K.; Feldt-Rasmussen, U.; Schytte, S.; Pedersen, H.B.; Hahn, C.H.; et al. Incidence and prevalence of multiple endocrine neoplasia 2A in Denmark 1901–2014: A nationwide study. Clin. Epidemiol. 2018, 10, 1479–1487. [Google Scholar] [CrossRef]
- Steiner, A.L.; Goodman, A.D.; Powers, S.R. Study of a kindred with pheochromocytoma, medullary thyroid carcinoma, hyperparathyroidism and Cushing’s disease: Multiple endocrine neoplasia, type 2. Medicine 1968, 47, 371–409. [Google Scholar] [CrossRef] [PubMed]
- Donovan, D.T.; Levy, M.L.; Furst, E.J.; Alford, B.R.; Wheeler, T.; Tschen, J.A.; Gagel, R.F. Familial cutaneous lichen amyloidosis in association with multiple endocrine neoplasia type 2A: A new variant. Henry Ford Hosp. Med. J. 1989, 37, 147–150. [Google Scholar] [PubMed]
- Verdy, M.; Weber, A.M.; Roy, C.C.; Morin, C.L.; Cadotte, M.; Brochu, P. Hirschsprung’s disease in a family with multiple endocrine neoplasia type 2. J. Pediatr. Gastroenterol. Nutr. 1982, 1, 603–608. [Google Scholar] [CrossRef]
- Donis-Keller, H.; Dou, S.; Chi, D.; Carlson, K.M.; Toshima, K.; Lairmore, T.C.; Howe, J.R.; Moley, J.F.; Goodfellow, P.; Wells, S.A. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum. Mol. Genet. 1993, 2, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, L.M.; Kwok, J.B.J.; Healey, C.S.; Elsdon, M.J.; Eng, C.; Gardner, E.; Love, D.R.; Mole, S.E.; Moore, J.K.; Papi, L.; et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 1993, 363, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, L.M.; Marsh, D.J.; Robinson, B.G.; Schuffenecker, I.; Zedenius, J.; Lips, C.J.M.; Gagel, R.F.; Takai, S.; Noll, W.W.; Fink, M.; et al. Genotype-phenotype correlation in multiple endocrine neoplasia type 2: Report of the International RET Mutation Consortium. J. Intern. Med. 1995, 238, 343–346. [Google Scholar] [CrossRef]
- Eng, C.; Clayton, D.; Schuffenecker, I.; Lenoir, G.; Cote, G.; Gagel, R.F.; Van Amstel, H.K.; Lips, C.J.; Nishisho, I.; Takai, S.I.; et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA 1996, 276, 1575–1579. [Google Scholar] [CrossRef]
- Frank-Raue, K.; Rybicki, L.A.; Erlic, Z.; Schweizer, H.; Winter, A.; Milos, I.; Toledo, S.P.; Toledo, R.A.; Tavares, M.R.; Alevizaki, M.; et al. Risk profiles and penetrance estimations in multiple endocrine neoplasia type 2A caused by germline RET mutations located in exon 10. Hum. Mutat. 2011, 32, 51–58. [Google Scholar] [CrossRef]
- Machens, A.; Dralle, H. Variability in Penetrance of Multiple Endocrine Neoplasia 2A with Amino Acid Substitutions in RET Codon 634. Clin. Endocrinol. 2015, 84, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Milos, I.N.; Frank-Raue, K.; Wohllk, N.; Maia, A.L.; Pusiol, E.; Patocs, A.; Robledo, M.; Biarnes, J.; Barontini, M.; Links, T.P.; et al. Age-related neoplastic risk profiles and penetrance estimations in multiple endocrine neoplasia type 2A caused by germ line RET Cys634Trp (TGC>TGG) mutation. Endocr.-Relat. Cancer 2008, 15, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Valdés, N.; Navarro, E.; Mesa, J.; Casterás, A.; Alcázar, V.; Lamas, C.; Tébar, J.; Castaño, L.; Gaztambide, S.; Forga, L. RET Cys634Arg mutation confers a more aggressive multiple endocrine neoplasia type 2A phenotype than Cys634Tyr mutation. Eur. J. Endocrinol. 2015, 172, 301–307. [Google Scholar] [CrossRef]
- Bihan, H.; Murat, A.; Fysekidis, M.; Al-Salameh, A.; Schwartz, C.; Baudin, E.; Thieblot, P.; Borson-Chazot, F.; Guillausseau, P.-J.; Cardot-Bauters, C.; et al. The clinical spectrum of RET proto-oncogene mutations in codon 790. Eur. J. Endocrinol. 2013, 169, 271–276. [Google Scholar] [CrossRef]
- Mathiesen, J.S.; Habra, M.A.; Bassett, J.H.D.; Choudhury, S.M.; Balasubramanian, S.P.; Howlett, T.A.; Robinson, B.G.; Gimenez-Roqueplo, A.-P.; Castinetti, F.; Vestergaard, P.; et al. Risk Profile of the RET A883F Germline Mutation: An International Collaborative Study. J. Clin. Endocrinol. Metab. 2017, 102, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Schulte, K.-M.; Machens, A.; Fugazzola, L.; McGregor, A.; Diaz-Cano, S.; Izatt, L.; Aylwin, S.; Talat, N.; Beck-Peccoz, P.; Dralle, H. The clinical spectrum of multiple endocrine neoplasia type 2a caused by the rare intracellular RET mutation S891A. J. Clin. Endocrinol. Metab. 2010, 95, E92–E97. [Google Scholar] [CrossRef][Green Version]
- Giacché, M.; Panarotto, A.; Tacchetti, M.C.; Tosini, R.; Campana, F.; Mori, L.; Cappelli, C.; Pirola, I.; Lombardi, D.; Pezzola, D.C.; et al. p.Ser891Ala RET gene mutations in medullary thyroid cancer: Phenotypical and genealogical characterization of 28 apparently unrelated kindreds and founder effect uncovering in Northern Italy. Hum. Mutat. 2019, 40, 926–937. [Google Scholar] [CrossRef]
- Castinetti, F.; Waguespack, S.G.; Machens, A.; Uchino, S.; Hasse-Lazar, K.; Sanso, G.; Else, T.; Dvorakova, S.; Qi, X.P.; Elisei, R.; et al. Natural history, treatment, and long-term follow up of patients with multiple endocrine neoplasia type 2B: An international, multicentre, retrospective study. Lancet Diabetes Endocrinol. 2019, 7, 213–220. [Google Scholar] [CrossRef]
- Qi, X.-P.; Peng, J.-Z.; Yang, X.-W.; Cao, Z.-L.; Yu, X.-H.; Fang, X.-D.; Zhang, D.-H.; Zhao, J.-Q. The RET C611Y mutation causes MEN 2A and associated cutaneous. Endocr. Connect. 2018, 7, 998–1005. [Google Scholar] [CrossRef]
- Machens, A.; Lorenz, K.; Weber, F.; Dralle, H. Genotype-specific progression of hereditary medullary thyroid cancer. Hum. Mutat. 2018, 39, 860–869. [Google Scholar] [CrossRef]
- Holm, M.; Vestergaard, P.; Poulsen, M.M.; Rasmussen, Å.K.; Feldt-Rasmussen, U.; Bay, M.; Rolighed, L.; Londero, S.; Pedersen, H.B.; Hahn, C.H.; et al. Primary Hyperparathyroidism in Multiple Endocrine Neoplasia Type 2A in Denmark 1930–2021: A Nationwide Population-Based Retrospective Study in Denmark 1930–2021. Cancers 2023, 15, 2125. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, J.S.; Kroustrup, J.P.; Vestergaard, P.; Stochholm, K.; Poulsen, P.L.; Rasmussen, Å.K.; Feldt-Rasmussen, U.; Gaustadnes, M.; Ørntoft, T.F.; Hansen, T.v.O.; et al. Distribution of RET Mutations in Multiple Endocrine Neoplasia 2 in Denmark 1994–2014: A Nationwide Study. Thyroid 2017, 27, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, J.S.; Kroustrup, J.P.; Vestergaard, P.; Stochholm, K.; Poulsen, P.L.; Rasmussen, Å.K.; Feldt-Rasmussen, U.; Gaustadnes, M.; Ørntoft, T.F.; Rossing, M.; et al. Founder Effect of the RETC611Y Mutation in Multiple Endocrine Neoplasia 2A in Denmark: A Nationwide Study. Thyroid 2017, 27, 1505–1510. [Google Scholar] [CrossRef]
- Porta, M.; Greenland, S.; Hernán, M.; dos Santos Silva, I.; Last, M. A Dictionary of Epidermiology, 6th ed.; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Castinetti, F.; Moley, J.; Mulligan, L.M.; Waguespack, S.G. A comprehensive review on MEN2B. Endocrine-Related Cancer 2018, 25, T29–T39. [Google Scholar] [CrossRef]
- Raue, F.; Frank-Raue, K. Update on Multiple Endocrine Neoplasia Type 2: Focus on Medullary Thyroid Carcinoma. J. Endocr. Soc. 2018, 2, 933–943. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Schilsky, R.L.; Gaspar, L.E.; Washington, M.K.; Sullivan, D.C.; Brierley, J.D.; Balch, C.M.; Compton, C.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. AJCC Cancer Staging Manual, 7th ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Fanget, F.; Demarchi, M.S.; Maillard, L.; Lintis, A.; Decaussin, M.; Lifante, J.C. Medullary thyroid cancer outcomes in patients with undetectable versus normalized postoperative calcitonin levels. Br. J. Surg. 2021, 108, 1064–1071. [Google Scholar] [CrossRef]
- Khan, A.A.; Hanley, D.A.; Rizzoli, R.; Bollerslev, J.; Young, J.; Rejnmark, L.; Thakker, R.; D’amour, P.; Paul, T.; Van Uum, S.; et al. Primary hyperparathyroidism: Review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos. Int. 2017, 28, 1–19. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Wang, T.S.; Ruan, D.T.; Lee, J.A.; Asa, S.L.; Duh, Q.-Y.; Doherty, G.M.; Herrera, M.F.; Pasieka, J.L.; Perrier, N.D.; et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg. 2016, 151, 959–968. [Google Scholar] [CrossRef]
- Neumann, H.P.H.; Young, W.F., Jr.; Eng, C. Pheochromocytoma and Paraganglioma. N. Engl. J. Med. 2019, 381, 552–565. [Google Scholar] [CrossRef]
- Schuffenecker, I.; Ginet, N.; Goldgar, D.; Eng, C.; Chambe, B.; Boneu, A.; Houdent, C.; Pallo, D.; Schlumberger, M.; Thivolet, C.; et al. Prevalence and parental origin of de novo RET mutations in multiple endocrine neoplasia type 2A and familial medullary thyroid carcinoma. Le Groupe d’Etude des Tumeurs a Calcitonine. Am. J. Hum. Genet. 1997, 60, 233–237. [Google Scholar]
- Imai, T.; Uchino, S.; Okamoto, T.; Suzuki, S.; Kosugi, S.; Kikumori, T.; Sakurai, A. High penetrance of pheochromocytoma in multiple endocrine neoplasia 2 caused by germ line RET codon 634 mutation in Japanese patients. Eur. J. Endocrinol. 2013, 168, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Lorenz, K.; Dralle, H. Kidney malformations and Hirschsprung’s disease in carriers of cysteine mutations in exon 10 of the RET proto-oncogene. Endocrine 2021, 73, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Hasle, H.; Correa, A.; Schendel, D.; Friedman, J.; Olsen, J.; Rasmussen, S.A. Survival among people with Down syndrome: A nationwide population-based study in Denmark. Genet. Med. 2013, 15, 64–69. [Google Scholar] [CrossRef] [PubMed]

| All | Index | Non-Index | ||
|---|---|---|---|---|
| Characteristics | (n = 128) | (n = 10) | (n = 118) | p Value |
| Sex, n (%) | 0.325 a | |||
| Female | 55 (43) | 6 (60) | 49 (42) | |
| Male | 73 (57) | 4 (40) | 69 (58) | |
| Age at MEN 2A diagnosis, | 0.053 b | |||
| median years (range) | 25 (0.1–79) c | 37 (22–68) d | 24 (0.1–79) e | |
| Thyroid, n (%) | 0.151 a | |||
| No surgery | 25 (20) | 1 (10) | 24 (20) | |
| Normal/CCH | 41 (32) | 1 (10) | 40 (34) | |
| MTC | 62 (48) | 8 (80) | 54 (46) | |
| PHEO, n (%) | 0.041 a | |||
| Yes | 19 (15) | 4 (40) | 15 (13) | |
| No | 109 (85) | 6 (60) | 103 (87) | |
| PHPT, n (%) c | 0.130 a | |||
| Yes | 8 (7) | 2 (20) | 6 (5) | |
| No | 114 (93) | 8 (80) | 106 (95) |
| All | Index | Non-Index | ||
|---|---|---|---|---|
| Characteristics | (n = 103) | (n = 9) | (n = 94) | p Value |
| At first thyroidectomy Sex, n (%) | 0.478 a | |||
| Female | 41 (40) | 5 (55) | 36 (38) | |
| Male | 62 (60) | 4 (44) | 58 (62) | |
| Age at thyroidectomy, | 0.039 b | |||
| median years (range) | 33 (2–79) | 38 (30–67) | 30 (2–79) | |
| Thyroidectomy, n (%) | <0.001 a | |||
| Tumorectomy | 1 (1) | 1 (11) | 0 | |
| HT | 1 (1) | 1 (11) | 0 | |
| ST | 1 (1) | 1 (11) | 0 | |
| TT | 100 (97) | 6 (67) | 94 (100) | |
| Prophylactic, n (%) | <0.001 a | |||
| Yes | 96 (93) | 3 (33) c | 93 (99) | |
| No | 7 (7) | 6 (67) | 1 (1) | |
| Lymph node surgery, n (%) | <0.001 a | |||
| None | 53 (51) | 2 (22) | 51 (54) | |
| Selective | 12 (12) | 2 (22) | 10 (11) | |
| CND | 26 (25) | 3 (33) | 23 (24) | |
| LND | 2 (2) | 1 (11) | 1 (1) | |
| CND + LND | 10 (10) | 1 (11) | 9 (10) | |
| Pathology | <0.001 a | |||
| Normal/C-cell hyperplasia | 41 (40) | 1 (11) | 40 (43) | |
| MTC N0M0 | 42 (41) | 2 (22) | 40 (43) | |
| MTC N1M0 | 18 (17) | 4 (44) | 14 (15) | |
| MTC NxM1 | 2 (2) | 2 (22) | 0 | |
| T category, n (%) | <0.001 a | |||
| Tx | 1 (1) | 1 (11) | 0 | |
| T0 | 41 (40) | 1 (11) | 40 (43) | |
| T1a | 34 (33) | 1 (11) | 33 (35) | |
| T1b | 19 (18) | 5 (56) | 14 (15) | |
| T2 | 7 (7) | 0 | 7 (7) | |
| T3 | 0 | 0 | 0 | |
| T4a | 1 (1) | 1 (11) | 0 | |
| T4b | 0 | 0 | 0 | |
| N category, n (%) | <0.001 a | |||
| N0 | 83 (81) | 3 (33) | 80 (85) | |
| N1a | 12 (12) | 2 (22) | 10 (11) | |
| N1b | 8 (8) | 4 (44) | 4 (4) | |
| M category, n (%) | 0.007 a | |||
| M0 | 101 (98) | 7 (78) | 94 (100) | |
| M1 | 2 (2) | 2 (22) | 0 | |
| At last follow-up | ||||
| Follow-up after thyroidectomy | 20 (0.5–49) | 12 (0.5–49) | 20 (1–43) | 0.248 b |
| median years (range) | ||||
| Status, n (%) | 0.010 a | |||
| Cure | 58 (56) | 1 (11) | 57 (61) | |
| No cure | 45 (44) | 8 (89) | 37 (39) |
| MTC | |||||
|---|---|---|---|---|---|
| Normal/CCH | T1-4N0M0 | TxN1M0 | TxNxM1 | ||
| (n = 41) | (n = 42 b) | (n = 20 c) | (n = 7) | p Value | |
| At first diagnosis | |||||
| Age, | <0.001 a | ||||
| mean years (CI) | 16 (12–20) | 38 (34–42) | 45 (38–53) | 49 (36–61) | |
| All | Index | Non-Index | ||
|---|---|---|---|---|
| Characteristics | (n = 19) | (n = 4) | (n = 15) | p Value |
| At PHEO diagnosis | ||||
| Sex, n (%) | 1 a | |||
| Female | 9 (47) | 2 (50) | 7 (47) | |
| Male | 10 (53) | 2 (50) | 8 (53) | |
| Age, median years (range) | 42 (22–79) | 38 (22–49) | 43 (29–79) | 0.194 b |
| Symptoms, n (%) | 0.255 a | |||
| Yes | 13 (68) | 4 (100) | 9 (60) | |
| No | 6 (32) | 0 | 6 (40) | |
| Hypertensive crisis, n (%) | 0.386 a | |||
| Yes | 2 (11) | 1 (25) | 1 (7) | |
| No | 17 (89) | 3 (75) | 14 (93) | |
| ADX, n (%) | 0.648 a | |||
| None c | 2 (11) | 1 (25) | 1 (7) | |
| Cortical sparing | 2 (11) | 0 | 2 (13) | |
| Total | 15 (79) | 3 (75) | 12 (80) | |
| Largest tumor size, | 0.029 b | |||
| median mm (range) | 26 (13–150) d | 50 (30–150) | 22 (13–65) e | |
| Laterality, n (%) | 1a | |||
| Unilateral | 18 (95) | 4 (100) | 14 (93) | |
| Bilateral | 1 (5) | 0 | 1 (7) | |
| Metastatic, n (%) | 0.211 a | |||
| Yes | 1 (5) | 1 (25) | 0 | |
| No | 18 (95) | 3 (75) | 15 (100) | |
| At last follow-up f | ||||
| Follow-up after first ADX, | 0.457 b | |||
| median years (range) | 12 (0.5–55) | 17 (0.6–30) | 11 (0.5–55) | |
| Status, n (%) | 1 a | |||
| No further surgery | 16 (89) | 4 (100) | 12 (86) | |
| Contralateral PHEO | 2 (11) g | 0 | 2 (14) g | |
| Ipsilateral recurrence | 0 | 0 | 0 |
| All | Index | Non-Index | ||
|---|---|---|---|---|
| Characteristics | (n = 8) | (n = 2) | (n = 6) | p Value |
| At PHPT diagnosis | ||||
| Sex, n (%) | 0.464 a | |||
| Female | 2 (75) | 1 (50) | 1 (17) | |
| Male | 6 (25) | 1 (50) | 5 (83) | |
| Age, median years (range) | 41 (24–79) | 44 (39–49) | 41 (24–79) | 1 b |
| Ionized calcium level, | 0.051 b | |||
| median mmol/L (range) | 1.37 (1.34–1.65) c | 1.59 (1.53–1.65) | 1.37 (1.34–1.38) d | |
| PTH level, | 0.046 b | |||
| median pmol/L (range) | 12 (3–19) | 18 (16–19) | 9 (3–15) | |
| Symptoms, n (%) | 1 a | |||
| Yes | 3 (38) | 1 (50) | 2 (33) | |
| No | 5 (63) | 1 (50) | 4 (67) | |
| Hypercalcemic crisis, n (%) | ||||
| Yes | 0 | 0 | 0 | |
| No | 8 (100) | 2 (100) | 6 (100) | |
| PTX, n (%) | 0.429 a | |||
| None | 2 (25) | 0 | 2 (33) | |
| Selective | 2 (25) | 0 | 2 (33) | |
| Subtotal | 4 (50) | 2 (100) | 2 (33) | |
| Histology, n (%) e | 0.467 a | |||
| Adenoma | 4 (67) | 2 (100) | 2 (50) | |
| Hyperplasia | 2 (33) | 0 | 2 (50) | |
| Carcinoma | 0 | 0 | 0 | |
| At last follow-up e | ||||
| Follow-up after first PTX, | 0.165 b | |||
| median years (range) | 20 (0.3–28) | 6 (0.3–12) | 28 (3–28) | |
| Status, n (%) | ||||
| Cure | 6 (100) | 2 (100) | 4 (100) f | |
| Persistence | 0 | 0 | 0 | |
| Recurrence | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, A.W.; Vestergaard, P.; Poulsen, M.M.; Rasmussen, Å.K.; Feldt-Rasmussen, U.; Madsen, M.; Næraa, R.W.; Hansen, D.; Main, K.; Pedersen, H.B.; et al. RET C611Y Germline Variant in Multiple Endocrine Neoplasia Type 2A in Denmark 1930–2021: A Nationwide Study. Cancers 2025, 17, 374. https://doi.org/10.3390/cancers17030374
Hansen AW, Vestergaard P, Poulsen MM, Rasmussen ÅK, Feldt-Rasmussen U, Madsen M, Næraa RW, Hansen D, Main K, Pedersen HB, et al. RET C611Y Germline Variant in Multiple Endocrine Neoplasia Type 2A in Denmark 1930–2021: A Nationwide Study. Cancers. 2025; 17(3):374. https://doi.org/10.3390/cancers17030374
Chicago/Turabian StyleHansen, Anders Würgler, Peter Vestergaard, Morten Møller Poulsen, Åse Krogh Rasmussen, Ulla Feldt-Rasmussen, Mette Madsen, Rune Weis Næraa, Dorte Hansen, Katharina Main, Henrik Baymler Pedersen, and et al. 2025. "RET C611Y Germline Variant in Multiple Endocrine Neoplasia Type 2A in Denmark 1930–2021: A Nationwide Study" Cancers 17, no. 3: 374. https://doi.org/10.3390/cancers17030374
APA StyleHansen, A. W., Vestergaard, P., Poulsen, M. M., Rasmussen, Å. K., Feldt-Rasmussen, U., Madsen, M., Næraa, R. W., Hansen, D., Main, K., Pedersen, H. B., Londero, S. C., Rolighed, L., Hahn, C. H., Rask, K. B., Maare, C., Nielsen, H. H., Gaustadnes, M., Rossing, M., Hermann, P., & Mathiesen, J. S., on behalf of Danish Thyroid Cancer Group (DATHYRCA). (2025). RET C611Y Germline Variant in Multiple Endocrine Neoplasia Type 2A in Denmark 1930–2021: A Nationwide Study. Cancers, 17(3), 374. https://doi.org/10.3390/cancers17030374






