Comprehensive Molecular Profiling of Metastatic Pancreatic Adenocarcinomas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Pathology Specimen Processing
2.3. Tumor Molecular Testing
2.4. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics of Metastatic Pancreatic Adenocarcinoma
3.2. Specimen Adequacy for Molecular Testing
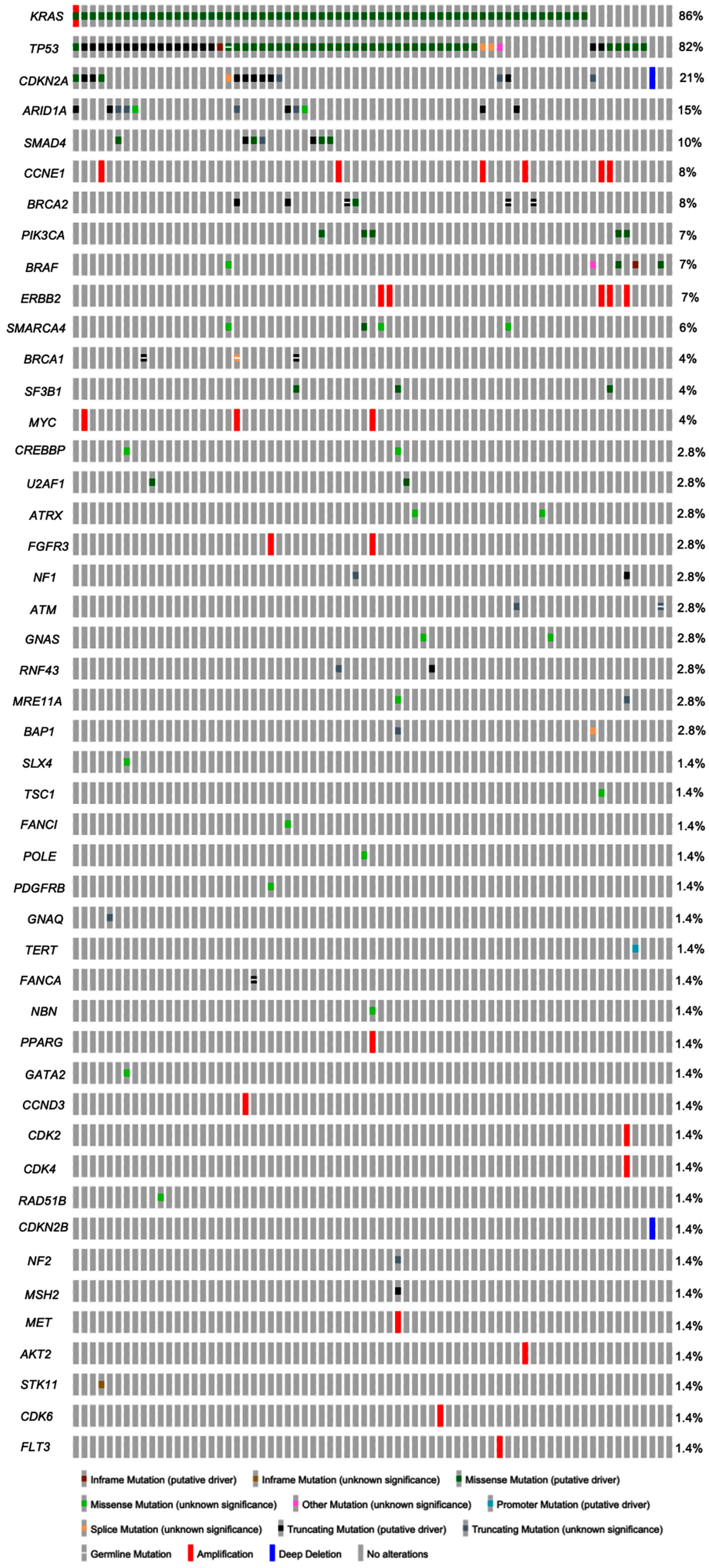
3.3. Molecular Profiles of Metastatic Pancreatic Adenocarcinoma
3.4. Molecular Alterations in Primary vs. Metastatic Pancreatic Adenocarcinoma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Waddell, N.; Pajic, M.; Patch, A.M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef]
- Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017, 32, 185–203.e113. [CrossRef] [PubMed]
- Thompson, E.D.; Roberts, N.J.; Wood, L.D.; Eshleman, J.R.; Goggins, M.G.; Kern, S.E.; Klein, A.P.; Hruban, R.H. The genetics of ductal adenocarcinoma of the pancreas in the year 2020: Dramatic progress, but far to go. Mod. Pathol. 2020, 33, 2544–2563. [Google Scholar] [CrossRef]
- Dreyer, S.B.; Upstill-Goddard, R.; Paulus-Hock, V.; Paris, C.; Lampraki, E.M.; Dray, E.; Serrels, B.; Caligiuri, G.; Rebus, S.; Plenker, D.; et al. Targeting DNA Damage Response and Replication Stress in Pancreatic Cancer. Gastroenterology 2021, 160, 362–377.e13. [Google Scholar] [CrossRef]
- Trunk, A.; Miotke, L.; Nevala-Plagemann, C.; Verdaguer, H.; Macarulla, T.; Garrido-Laguna, I. Emerging Treatment Strategies in Pancreatic Cancer. Pancreas 2021, 50, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Connor, A.A.; Gallinger, S. Pancreatic cancer evolution and heterogeneity: Integrating omics and clinical data. Nat. Rev. Cancer 2022, 22, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Yachida, S.; Jones, S.; Bozic, I.; Antal, T.; Leary, R.; Fu, B.; Kamiyama, M.; Hruban, R.H.; Eshleman, J.R.; Nowak, M.A.; et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010, 467, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulos, G.; Syrigos, K.; Saif, M.W. Genetic factors affecting patient responses to pancreatic cancer treatment. Ann. Gastroenterol. 2016, 29, 466–476. [Google Scholar] [CrossRef]
- Chiaravalli, M.; Reni, M.; O’Reilly, E.M. Pancreatic ductal adenocarcinoma: State-of-the-art 2017 and new therapeutic strategies. Cancer Treat Rev 2017, 60, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, S.B.; Chang, D.K.; Bailey, P.; Biankin, A.V. Pancreatic Cancer Genomes: Implications for Clinical Management and Therapeutic Development. Clin. Cancer Res. 2017, 23, 1638–1646. [Google Scholar] [CrossRef]
- Tesfaye, A.A.; Kamgar, M.; Azmi, A.; Philip, P.A. The evolution into personalized therapies in pancreatic ductal adenocarcinoma: Challenges and opportunities. Expert. Rev. Anticancer. Ther. 2018, 18, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.W.; Chau, I. Emerging agents for metastatic pancreatic cancer: Spotlight on early phase clinical trials. Expert. Opin. Investig. Drugs 2021, 30, 1089–1107. [Google Scholar] [CrossRef] [PubMed]
- Dorman, K.; Heinemann, V.; Kobold, S.; von Bergwelt-Baildon, M.; Boeck, S. Novel systemic treatment approaches for metastatic pancreatic cancer. Expert. Opin. Investig. Drugs 2022, 31, 249–262. [Google Scholar] [CrossRef]
- Razzano, D.; Bouza, S.J.; Hernandez, P.V.; Wang, M.; Robert, M.E.; Walther, Z.; Cai, G. Comprehensive molecular profiling of pancreatic ductal adenocarcinoma in FNA, biopsy, and resection specimens. Cancer Cytopathol. 2022, 130, 726–734. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Notta, F.; Hahn, S.A.; Real, F.X. A genetic roadmap of pancreatic cancer: Still evolving. Gut 2017, 66, 2170–2178. [Google Scholar] [CrossRef]
- Fischer, C.G.; Wood, L.D. From somatic mutation to early detection: Insights from molecular characterization of pancreatic cancer precursor lesions. J. Pathol. 2018, 246, 395–404. [Google Scholar] [CrossRef]
- Rodríguez Gil, Y.; Jiménez Sánchez, P.; Muñoz Velasco, R.; García García, A.; Sánchez-Arévalo Lobo, V.J. Molecular Alterations in Pancreatic Cancer: Transfer to the Clinic. Int. J. Mol. Sci. 2021, 22, 2077. [Google Scholar] [CrossRef] [PubMed]
- Aldyab, M.; El Jabbour, T.; Parilla, M.; Lee, H. Benign vs malignant pancreatic lesions: Molecular insights to an ongoing debate. World J. Gastrointest. Surg. 2021, 13, 406–418. [Google Scholar] [CrossRef]
- Qian, Y.; Gong, Y.; Fan, Z.; Luo, G.; Huang, Q.; Deng, S.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. Molecular alterations and targeted therapy in pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2020, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, Y.; Yang, F.; Zhu, L.; Zhu, X.Q.; Wang, Z.F.; Wu, X.L.; Zhou, C.H.; Yan, J.Y.; Hu, B.Y.; et al. The molecular biology of pancreatic adenocarcinoma: Translational challenges and clinical perspectives. Signal Transduct. Target. Ther. 2021, 6, 249. [Google Scholar] [CrossRef] [PubMed]
- Brar, G.; Blais, E.M.; Joseph Bender, R.; Brody, J.R.; Sohal, D.; Madhavan, S.; Picozzi, V.J.; Hendifar, A.E.; Chung, V.M.; Halverson, D.; et al. Multi-omic molecular comparison of primary versus metastatic pancreatic tumours. Br. J. Cancer 2019, 121, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.J.; Yachida, S.; Mudie, L.J.; Stephens, P.J.; Pleasance, E.D.; Stebbings, L.A.; Morsberger, L.A.; Latimer, C.; McLaren, S.; Lin, M.L.; et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 2010, 467, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Nusrat, F.; Khanna, A.; Jain, A.; Jiang, W.; Lavu, H.; Yeo, C.J.; Bowne, W.; Nevler, A. The Clinical Implications of KRAS Mutations and Variant Allele Frequencies in Pancreatic Ductal Adenocarcinoma. J. Clin. Med. 2024, 13, 2103. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Meric-Bernstam, F.; Hong, D.; Janku, F.; Naing, A.; Piha-Paul, S.A.; Tsimberidou, A.M.; Karp, D.; Subbiah, V.; Yap, T.A.; et al. Clinical characteristics and outcomes of phase I cancer patients with CCNE1 amplification: MD Anderson experiences. Sci. Rep. 2022, 12, 8701. [Google Scholar] [CrossRef] [PubMed]
- Network NCC. NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma; NCCN.org: Plymouth Meeting, PA, USA, 2024. [Google Scholar]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Rall, C.J.; Yan, Y.X.; Graeme-Cook, F.; Beauchamp, R.; Yandell, D.W.; Povoski, S.P.; Rustgi, A.K. Ki-ras and p53 mutations in pancreatic ductal adenocarcinoma. Pancreas 1996, 12, 10–17. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, S.C.; Hong, S.M.; Kim, Y.H.; Song, K.B.; Park, K.M.; Lee, Y.J. Genetic alterations of K-ras, p53, c-erbB-2, and DPC4 in pancreatic ductal adenocarcinoma and their correlation with patient survival. Pancreas 2013, 42, 216–222. [Google Scholar] [CrossRef]
- Kimura, Y.; Fukuda, A.; Ogawa, S.; Maruno, T.; Takada, Y.; Tsuda, M.; Hiramatsu, Y.; Araki, O.; Nagao, M.; Yoshikawa, T.; et al. ARID1A Maintains Differentiation of Pancreatic Ductal Cells and Inhibits Development of Pancreatic Ductal Adenocarcinoma in Mice. Gastroenterology 2018, 155, 194–209.e192. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Sheng, W.; Wang, L.; Zhu, X.; Tan, C.; Ni, S.; Weng, W.; Huang, D.; Wang, J. SWI/SNF Complex-deficient Undifferentiated Carcinoma of the Gastrointestinal Tract: Clinicopathologic Study of 30 Cases with an Emphasis on Variable Morphology, Immune Features, and the Prognostic Significance of Different SMARCA4 and SMARCA2 Subunit Deficiencies. Am. J. Surg. Pathol. 2022, 46, 889–906. [Google Scholar] [CrossRef]
- Yavas, A.; Ozcan, K.; Adsay, N.V.; Balci, S.; Tarcan, Z.C.; Hechtman, J.F.; Luchini, C.; Scarpa, A.; Lawlor, R.T.; Mafficini, A.; et al. SWI/SNF Complex-Deficient Undifferentiated Carcinoma of the Pancreas: Clinicopathologic and Genomic Analysis. Mod. Pathol. 2024, 37, 100585. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.N.; Maher, M.E.; Tran, N.H.; Van De Hey, D.R.; Foley, T.M.; Yueh, A.E.; Leystra, A.A.; Pasch, C.A.; Jeffrey, J.J.; Clipson, L.; et al. PIK3CA mutations can initiate pancreatic tumorigenesis and are targetable with PI3K inhibitors. Oncogenesis 2015, 4, e169. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, T.; Sunami, K.; Tahara, M.; Nishiwaki, S.; Tanaka, S.; Baba, E.; Kanai, M.; Kinoshita, I.; Shirota, H.; Hayashi, H.; et al. Dabrafenib and trametinib administration in patients with BRAF V600E/R or non-V600 BRAF mutated advanced solid tumours (BELIEVE, NCCH1901): A multicentre, open-label, and single-arm phase II trial. eClinicalMedicine 2024, 69, 102447. [Google Scholar] [CrossRef] [PubMed]
- Kamel, D.; Gray, C.; Walia, J.S.; Kumar, V. PARP Inhibitor Drugs in the Treatment of Breast, Ovarian, Prostate and Pancreatic Cancers: An Update of Clinical Trials. Curr. Drug Targets 2018, 19, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wei, M.; Xu, J.; Hua, J.; Liang, C.; Meng, Q.; Zhang, Y.; Liu, J.; Zhang, B.; Yu, X.; et al. PARP inhibitors in pancreatic cancer: Molecular mechanisms and clinical applications. Mol. Cancer 2020, 19, 49. [Google Scholar] [CrossRef] [PubMed]
| Age, Years | |
| Mean | 67 |
| Range | 36–91 |
| Gender, n (%) | |
| Male | 64 (56%) |
| Female | 51 (44%) |
| Interval between primary and metastasis, months | |
| Mean | 11 |
| Range | 0–143 |
| Specimen type, n (%) | |
| Core biopsy | 92 (80%) |
| Cytology | 13 (11%) |
| Resection | 10 (9%) |
| Metastatic site, n (%) | |
| Liver | 77 (67%) |
| Lymph node | 11 (10%) |
| Omentum/peritoneum | 8 (7%) |
| Lung | 7 (6%) |
| Soft tissue | 6 (5%) |
| Biliary tract | 2 (2%) |
| Bone | 1 (1%) |
| Duodenum | 1 (1%) |
| Ascitic fluid | 1 (1%) |
| Pleural fluid | 1 (1%) |
| Total (n = 115) | Biopsy (n = 92) | Cytology (n = 13) | Resection (n = 10) | p | |
|---|---|---|---|---|---|
| Microdissection, n (%) | 0.0085 * | ||||
| Yes | 58 (51%) | 49 (53%) | 1 (8%) | 8 (80%) | |
| No | 13 (11%) | 11 (12%) | 1 (8%) | 1 (10%) | |
| Inadequate | 44 (38%) | 32 (35%) | 11 (84%) | 1 (10%) | |
| Tumor proportion (%) | 0.6613 | ||||
| Mean (SD) | 49 (20) | 50 (20) | 45 (7) | 41 (24) | |
| Range | 10–90 | 15–90 | 40–50 | 10–75 | |
| DNA analysis, n (%) | 0.0012 * | ||||
| Yes | 71 (62%) | 60 (65%) | 2 (15%) | 9 (90%) | |
| No | 44 (38%) | 32 (34%) | 11 (85%) | 1 (10%) | |
| RNA analysis, n (%) | 0.0175 * | ||||
| Yes | 64 (56%) | 55 (60%) | 2 (15%) | 7 (70%) | |
| No | 51 (44%) | 37 (40%) | 11 (85%) | 3 (30%) |
| Molecular Alterations, per Case | |
| Mean | 3.4 |
| Range | 0–9 |
| Type of molecular alterations, n (%) | 239 (total) |
| Gene mutation | 214 (89.5) |
| Gene copy number alteration | 25 (10.5) |
| Gene fusion | 0 (0) |
| Common gene mutations, n (%) * | |
| KRAS | 61 (85.9) |
| TP53 ** | 59 (83.1) |
| CDKN2A | 15 (21.1) |
| ARID1A | 11 (15.5) |
| SMAD4 | 7 (9.9) |
| BRCA2 *** | 6 (8.5) |
| PIK3CA | 5 (7.0) |
| BRAF | 5 (7.0) |
| SMARCA4 | 4 (5.6) |
| SF3B1 | 3 (4.2) |
| BRCA1 **** | 3 (4.2) |
| KRAS mutations, n (%) | 61 (total) |
| G12D | 27 (44.3) |
| G12V | 15 (24.6) |
| G12R | 13 (21.3) |
| G12C | 1 (1.6) |
| G12S | 1 (1.6) |
| G13D | 1 (1.6) |
| Q61H | 1 (1.6) |
| Q61R | 1 (1.6) |
| Q61L | 1 (1.6) |
| Common Mutated Genes | TCGA Cohort (N = 772) | Primary Cohort * (N = 61) | Metastatic Cohort (N = 71) | p-Values |
|---|---|---|---|---|
| KRAS | 709/772 (91.8%) | 55/61 (90.2%) | 61/71 (85.9%) | >0.05 |
| TP53 | 495/772 (64.1%) | 39/61 (63.9%) | 59/71 (83.1%) | 0.003 |
| SMAD4 | 163/772 (21.1%) | 9/61 (14.8%) | 7/71 (9.9%) | 0.041 |
| CDKN2A | 124/772 (16.1%) | 15/61 (24.6%) | 15/71 (21.1%) | >0.05 |
| ARID1A | 49/772 (6.3%) | 2/61 (3.3%) | 11/71 (15.5%) | 0.013 |
| BRCA2 | 11/772 (1.4%) | 4/61(6.6%) | 6/71 (8.5%) | 0.0003 |
| PIK3CA | 12/772 (1.6%) | 1/61 (1.6%) | 5/71 (7.0%) | 0.0196 |
| BRAF | 7/772 (0.9%) | 1/61 (1.6%) | 5/71 (7.0%) | 0.0026 |
| SMARCA4 | 16/772 (2.1%) | 1/61 (1.6%) | 4/71 (5.6%) | >0.05 |
| SF3B1 | 16/772 (2.1%) | 0/61 (0) | 3/71 (4.2%) | >0.05 |
| BRCA1 | 8/772 (1.0%) | 0/61 (0) | 3/71 (4.2%) | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antony, V.; Sun, T.; Dolezal, D.; Cai, G. Comprehensive Molecular Profiling of Metastatic Pancreatic Adenocarcinomas. Cancers 2025, 17, 335. https://doi.org/10.3390/cancers17030335
Antony V, Sun T, Dolezal D, Cai G. Comprehensive Molecular Profiling of Metastatic Pancreatic Adenocarcinomas. Cancers. 2025; 17(3):335. https://doi.org/10.3390/cancers17030335
Chicago/Turabian StyleAntony, Vijay, Tong Sun, Darin Dolezal, and Guoping Cai. 2025. "Comprehensive Molecular Profiling of Metastatic Pancreatic Adenocarcinomas" Cancers 17, no. 3: 335. https://doi.org/10.3390/cancers17030335
APA StyleAntony, V., Sun, T., Dolezal, D., & Cai, G. (2025). Comprehensive Molecular Profiling of Metastatic Pancreatic Adenocarcinomas. Cancers, 17(3), 335. https://doi.org/10.3390/cancers17030335






