The Association between Sampling and Survival in Patients with Pancreatic Ductal Adenocarcinoma Who Received Neoadjuvant Therapy and Pancreaticoduodenectomy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Pathologic Evaluation of Pancreaticoduodenectomies
2.3. Clinical and Follow-Up Data
2.4. Statistical Analyses
3. Results
3.1. Correlations of Entire Submission of the Tumor or Pancreas with Clinicopathologic Parameters
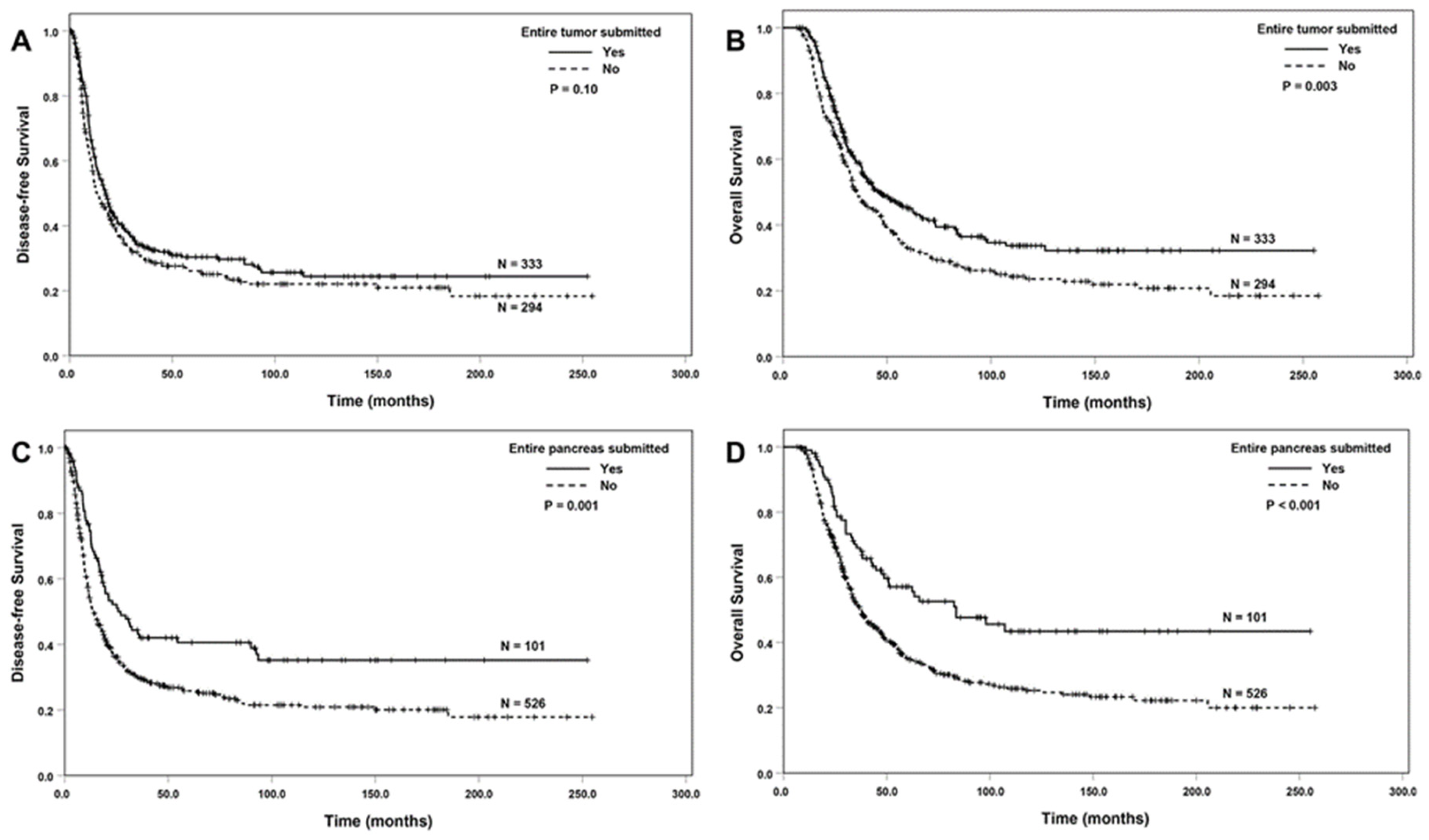
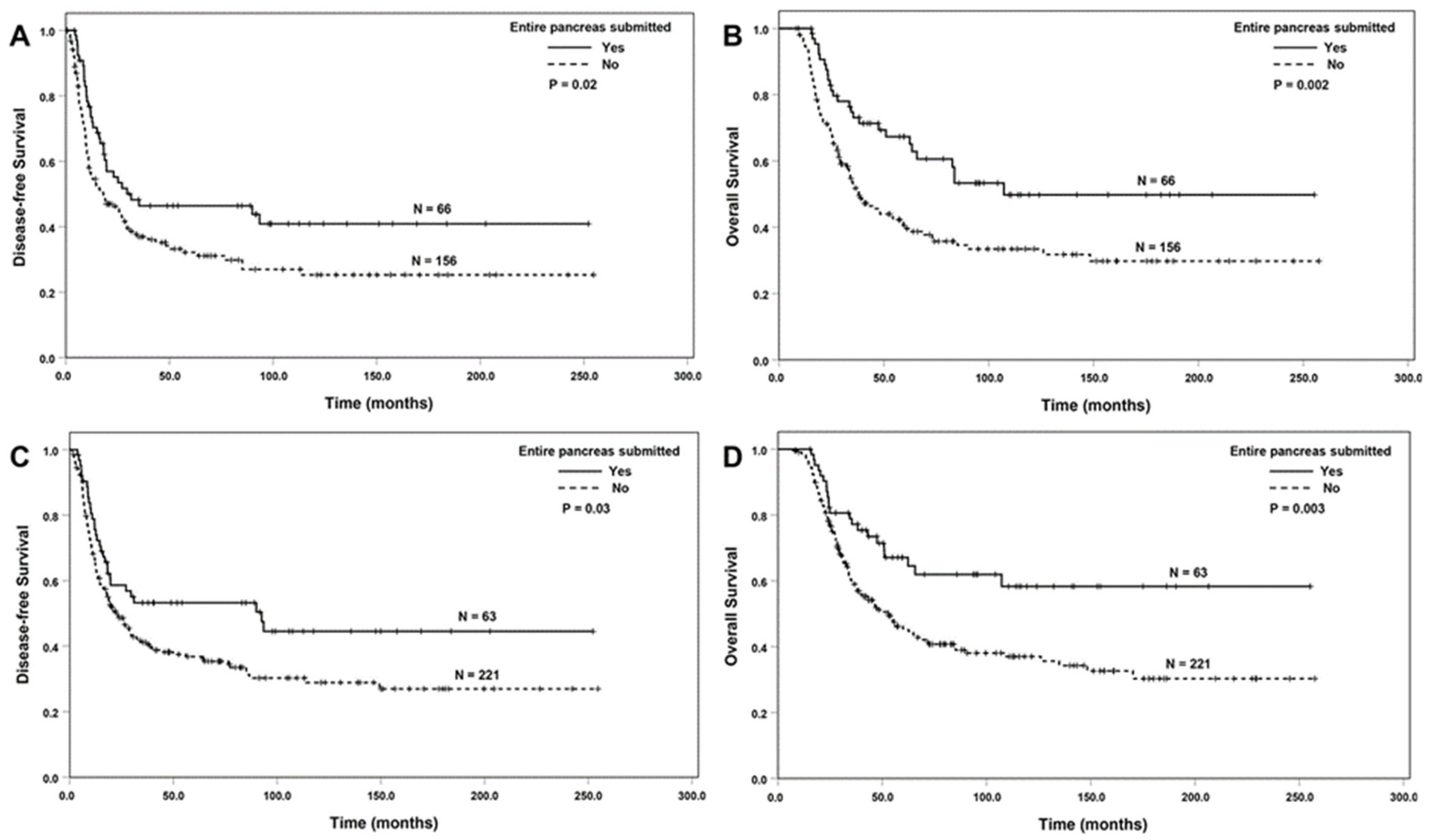
3.2. Correlation between Entire Submission of the Tumor or the Pancreas and Survival
3.3. Univariate and Multivariate Survival Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Youngwirth, L.M.; Nussbaum, D.P.; Thomas, S.; Adam, M.A.; Blazer, D.G., 3rd; Roman, S.A.; Sosa, J.A. Nationwide trends and outcomes associated with neoadjuvant therapy in pancreatic cancer: An analysis of 18 243 patients. J. Surg. Oncol. 2017, 116, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Han, Y.; Lee, H.; Kim, S.W.; Kwon, W.; Lee, K.H.; Oh, D.Y.; Chie, E.K.; Lee, J.M.; Heo, J.S.; et al. Oncological Benefits of Neoadjuvant Chemoradiation with Gemcitabine Versus Upfront Surgery in Patients with Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann. Surg. 2018, 268, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Reni, M.; Balzano, G.; Zanon, S.; Zerbi, A.; Rimassa, L.; Castoldi, R.; Pinelli, D.; Mosconi, S.; Doglioni, C.; Chiaravalli, M.; et al. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): A randomised, open-label, phase 2–3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef]
- Versteijne, E.; Vogel, J.A.; Besselink, M.G.; Busch, O.R.C.; Wilmink, J.W.; Daams, J.G.; van Eijck, C.H.J.; Groot Koerkamp, B.; Rasch, C.R.N.; van Tienhoven, G.; et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br. J. Surg. 2018, 105, 946–958. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Berson III, A.B.; Cardin, D.B.; Cha, C.; Chiorean, E.G.; Chung, V.; Czito, B.; et al. NCCN Clinical Practice Guidelines in Oncology, Pancreatic Adenocarcinoma (Version 1.2021). Available online: https://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdf (accessed on 25 January 2021).
- Khorana, A.A.; Mangu, P.B.; Berlin, J.; Engebretson, A.; Hong, T.S.; Maitra, A.; Mohile, S.G.; Mumber, M.; Schulick, R.; Shapiro, M.; et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2541–2556. [Google Scholar] [CrossRef]
- Lee, S.M.; Katz, M.H.; Liu, L.; Sundar, M.; Wang, H.; Varadhachary, G.R.; Wolff, R.A.; Lee, J.E.; Maitra, A.; Fleming, J.B.; et al. Validation of a Proposed Tumor Regression Grading Scheme for Pancreatic Ductal Adenocarcinoma after Neoadjuvant Therapy as a Prognostic Indicator for Survival. Am. J. Surg. Pathol. 2016, 40, 1653–1660. [Google Scholar] [CrossRef]
- Estrella, J.S.; Rashid, A.; Fleming, J.B.; Katz, M.H.; Lee, J.E.; Wolf, R.A.; Varadhachary, G.R.; Pisters, P.W.; Abdalla, E.K.; Vauthey, J.N.; et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer 2012, 118, 268–277. [Google Scholar] [CrossRef]
- Sohn, A.J.; Taherian, M.; Katz, M.H.G.; Prakash, L.R.; Chatterjee, D.; Wang, H.; Kim, M.; Tzeng, C.D.; Lee, J.E.; Ikoma, N.; et al. Integrated Pathologic Score Effectively Stratifies Patients with Pancreatic Ductal Adenocarcinoma Who Received Neoadjuvant Therapy and Pancreaticoduodenectomy. Am. J. Surg. Pathol. 2023, 47, 421–430. [Google Scholar] [CrossRef]
- Wang, H.; Chetty, R.; Hosseini, M.; Allende, D.S.; Esposito, I.; Matsuda, Y.; Deshpande, V.; Shi, J.; Dhall, D.; Jang, K.T.; et al. Pathologic Examination of Pancreatic Specimens Resected for Treated Pancreatic Ductal Adenocarcinoma: Recommendations from the Pancreatobiliary Pathology Society. Am. J. Surg. Pathol. 2022, 46, 754–764. [Google Scholar] [CrossRef]
- Haeberle, L.; Esposito, I. Pathology of pancreatic cancer. Transl. Gastroenterol. Hepatol. 2019, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Nagaria, T.S.; Wang, H.; Chatterjee, D.; Wang, H. Pathology of Treated Pancreatic Ductal Adenocarcinoma and Its Clinical Implications. Arch. Pathol. Lab. Med. 2020, 144, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.L.; Van Decar, S.G.; McCarthy, P.M.; Valdera, F.A.; Adams, A.M.; O’Shea, A.E.; Smolinsky, T.; Thomas, K.; Clifton, G.T.; Newhook, T.E.; et al. The benefit of adjuvant chemotherapy following pancreaticoduodenectomy for pancreatic adenocarcinoma depends on response to neoadjuvant therapy. J. Surg. Oncol. 2024, 130, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Donisi, G.; Nappo, G.; Pacilli, M.; Capretti, G.L.; Spaggiari, P.; Sollai, M.; Bozzarelli, S.; Zerbi, A. Pathologic tumor response to neoadjuvant therapy in resected pancreatic cancer: Does it affect prognosis? Updates Surg. 2023, 75, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Goess, R.; Jager, C.; Perinel, J.; Pergolini, I.; Demir, E.; Safak, O.; Scheufele, F.; Schorn, S.; Muckenhuber, A.; Adham, M.; et al. Lymph node examination and survival in resected pancreatic ductal adenocarcinoma: Retrospective study. BJS Open 2024, 8, zrad125. [Google Scholar] [CrossRef]
- Hayasaki, A.; Mizuno, S.; Nagata, M.; Kaluba, B.; Maeda, K.; Shinkai, T.; Ito, T.; Gyoten, K.; Fujii, T.; Iizawa, Y.; et al. Extrapancreatic extension is a better adverse prognostic factor than tumor size in patients with localized pancreatic ductal adenocarcinoma treated with chemoradiotherapy-comparison of T category between the American Joint Committee on Cancer and Japan Pancreas Society. HPB 2023, 25, 1268–1277. [Google Scholar] [CrossRef]
- Javed, A.A.; Mahmud, O.; Fatimi, A.S.; Habib, A.; Grewal, M.; He, J.; Wolfgang, C.L.; Besselink, M.G.; Consortium, P.-P. Predictors for Long-Term Survival After Resection of Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2024, 31, 4673–4687. [Google Scholar] [CrossRef]
- Kinny-Koster, B.; Ahmad, Y.; Pfluger, M.J.; Habib, J.R.; Fujikura, K.; Hutchings, D.; Cameron, J.L.; Shubert, C.R.; Lafaro, K.J.; Burkhart, R.A.; et al. Clinical Relevance of Cancerization of Ducts in Resected Pancreatic Ductal Adenocarcinoma. Pancreas 2024, 53, e528–e536. [Google Scholar] [CrossRef]
- Murata, Y.; Mizuno, S.; Kishiwada, M.; Uchida, K.; Noguchi, D.; Gyoten, K.; Hayasaki, A.; Fujii, T.; Iizawa, Y.; Tanemura, A.; et al. Clinical significance and predictors of complete or near-complete histological response to preoperative chemoradiotherapy in patients with localized pancreatic ductal adenocarcinoma. Pancreatology 2021, 21, 1482–1490. [Google Scholar] [CrossRef]
- Neyaz, A.; Tabb, E.S.; Shih, A.; Zhao, Q.; Shroff, S.; Taylor, M.S.; Rickelt, S.; Wo, J.Y.; Fernandez-Del Castillo, C.; Qadan, M.; et al. Pancreatic ductal adenocarcinoma: Tumour regression grading following neoadjuvant FOLFIRINOX and radiation. Histopathology 2020, 77, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Han, Y.B.; Kim, J.; Kang, M.; Lee, B.; Ahn, E.S.; Han, S.; Kim, H.; Na, H.Y.; Han, H.S.; et al. Microscopic tumor mapping of post-neoadjuvant therapy pancreatic cancer specimens to predict post-surgical recurrence: A prospective cohort study. Pancreatology 2024, 24, 562–571. [Google Scholar] [CrossRef]
- Pu, N.; Wu, W.; Liu, S.; Xie, Y.; Yin, H.; Chen, Q.; He, T.; Xu, Z.; Wang, W.; Yu, J.; et al. Survival benefit and impact of adjuvant chemotherapy following systemic neoadjuvant chemotherapy in patients with resected pancreas ductal adenocarcinoma: A retrospective cohort study. Int. J. Surg. 2023, 109, 3137–3146. [Google Scholar] [CrossRef]
- Servin-Rojas, M.; Fong, Z.V.; Fernandez-Del Castillo, C.; Lionetto, G.; Bolm, L.; Fagenholz, P.J.; Ferrone, C.R.; Rocha-Castellanos, D.M.; Lillemoe, K.D.; Qadan, M. Lymph Node Yield is Associated with Improved Overall Survival and Increased Time to Recurrence in Node-Negative Pancreatic Ductal Adenocarcinoma Following Neoadjuvant Therapy. Ann. Surg. 2024, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Usui, M.; Uchida, K.; Hayasaki, A.; Kishiwada, M.; Mizuno, S.; Watanabe, M. Prognostic impact of the distance from the anterior surface to tumor cells in pancreatoduodenectomy with neoadjuvant chemoradiotherapy for pancreatic ductal adenocarcinoma. PLoS ONE 2024, 19, e0307876. [Google Scholar] [CrossRef] [PubMed]
- Hartman, D.J.; Krasinskas, A.M. Assessing treatment effect in pancreatic cancer. Arch. Pathol. Lab. Med. 2012, 136, 100–109. [Google Scholar] [CrossRef]
- Verbeke, C.; Haberle, L.; Lenggenhager, D.; Esposito, I. Pathology assessment of pancreatic cancer following neoadjuvant treatment: Time to move on. Pancreatology 2018, 18, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Yokohira, M.; Oshima, M.; Yamakawa, K.; Ye, J.; Nakano-Narusawa, Y.; Haba, R.; Fukumura, Y.; Hirabayashi, K.; Yamaguchi, H.; Kojima, M.; et al. Adequate tissue sampling for the assessment of pathological tumor regression in pancreatic cancer. Sci. Rep. 2021, 11, 6586. [Google Scholar] [CrossRef]
- Huebner, M.; Kendrick, M.; Reid-Lombardo, K.M.; Que, F.; Therneau, T.; Qin, R.; Donohue, J.; Nagorney, D.; Farnell, M.; Sarr, M. Number of lymph nodes evaluated: Prognostic value in pancreatic adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 920–926. [Google Scholar] [CrossRef]
- Shi, S.; Hua, J.; Liang, C.; Meng, Q.; Liang, D.; Xu, J.; Ni, Q.; Yu, X. Proposed Modification of the 8th Edition of the AJCC Staging System for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019, 269, 944–950. [Google Scholar] [CrossRef]
- Vuarnesson, H.; Lupinacci, R.M.; Semoun, O.; Svrcek, M.; Julie, C.; Balladur, P.; Penna, C.; Bachet, J.B.; Resche-Rigon, M.; Paye, F. Number of examined lymph nodes and nodal status assessment in pancreaticoduodenectomy for pancreatic adenocarcinoma. Eur. J. Surg. Oncol. 2013, 39, 1116–1121. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | ESOT | p | ESOP | p | ||
|---|---|---|---|---|---|---|
| Yes (n = 333) | No (n = 294) | Yes (n = 101) | No (n = 526) | |||
| Age (Years, Mean ± SD) | 63.9 ± 9.9 | 63.5 ± 9.0 | 0.57 | 64.2 ± 9.2 | 63.6 ± 9.7 | 0.56 |
| Sex | 0.96 | 0.27 | ||||
| Female | 150 | 133 | 51 | 232 | ||
| Male | 183 | 161 | 50 | 294 | ||
| Differentiation | 0.56 | 0.02 | ||||
| Well/moderate | 213 | 181 | 74 | 320 | ||
| Poor | 120 | 113 | 27 | 206 | ||
| ypT * | <0.001 | <0.001 | ||||
| ypT0 | 17 | 0 | 14 | 3 | ||
| ypT1 | 122 | 83 | 52 | 153 | ||
| ypT2 | 146 | 179 | 20 | 305 | ||
| ypT3 | 48 | 32 | 15 | 65 | ||
| ypN stage * | 0.46 | 0.001 | ||||
| ypN0 | 156 | 128 | 63 | 221 | ||
| ypN1 | 111 | 112 | 26 | 197 | ||
| ypN2 | 66 | 54 | 12 | 108 | ||
| Lymphovascular invasion | 0.34 | 0.009 | ||||
| No | 170 | 138 | 62 | 246 | ||
| Yes | 163 | 156 | 39 | 280 | ||
| Perineural invasion | <0.001 | <0.001 | ||||
| No | 97 | 49 | 47 | 99 | ||
| Yes | 236 | 245 | 54 | 427 | ||
| CAP TRG | <0.001 | <0.001 | ||||
| Score 0 | 17 | 0 | 14 | 3 | ||
| Score 1 | 53 | 22 | 27 | 48 | ||
| Score 2 | 169 | 161 | 43 | 287 | ||
| Score 3 | 94 | 111 | 17 | 188 | ||
| MD Anderson TRG | <0.001 | <0.001 | ||||
| Grade 0 | 17 | 0 | 14 | 3 | ||
| Grade 1 | 53 | 22 | 27 | 48 | ||
| Grade 2 | 263 | 272 | 60 | 475 | ||
| Margin status | 0.82 | 0.45 | ||||
| Negative | 282 | 251 | 89 | 444 | ||
| Positive | 51 | 43 | 12 | 82 | ||
| Recurrence/metastasis | <0.001 | 0.03 | ||||
| No | 115 | 81 | 42 | 154 | ||
| Local recurrence | 54 | 55 | 11 | 98 | ||
| Distant metastasis | 164 | 158 | 48 | 274 | ||
| Disease-Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|
| Characteristics (N = 627) | N | HR (95% CI) | p | HR (95% CI) | p |
| Gender | |||||
| Female (ref) | 283 | 1.00 | 1.00 | ||
| Male | 344 | 1.06 (0.88–1.28) | 0.56 | 1.09 (0.89–1.34) | 0.38 |
| Age | 627 | 1.00 (0.98–1.01) | 0.29 | 1.00 (0.99–1.01) | 0.61 |
| Tumor differentiation | |||||
| Well/Moderate (ref) | 394 | 1.00 | 1.00 | ||
| Poor | 233 | 1.33 (1.09–1.61) | 0.004 | 1.32 (1.07–1.62) | 0.009 |
| Margin status | |||||
| Negative (ref) | 533 | 1.00 | 1.00 | ||
| Positive | 94 | 1.35 (1.04–1.74) | 0.02 | 1.14 (0.85–1.53) | 0.39 |
| ypT | 0.003 | 0.01 | |||
| ypT0/T1 (ref) | 222 | 1.00 | 1.00 | ||
| ypT2 | 325 | 1.36 (1.10–1.68) | 0.005 | 1.35 (1.08–1.69) | 0.009 |
| ypT3 | 80 | 1.60 (1.18–2.17) | 0.003 | 1.49 (1.06–2.08) | 0.02 |
| ypN stage | <0.001 | <0.001 | |||
| ypN0 (ref) | 284 | 1.00 | 1.00 | ||
| ypN1 | 223 | 1.54 (1.24–1.91) | <0.001 | 1.62 (1.28–2.04) | <0.001 |
| ypN2 | 120 | 2.52 (1.97–3.23) | <0.001 | 2.48 (1.90–3.23) | <0.001 |
| MDA TRG | |||||
| Grade 0/1 (ref) | 92 | 1.00 | 1.00 | ||
| Grade 2 | 535 | 1.94 (1.44–2.63) | <0.001 | 2.25 (1.61–3.15) | <0.001 |
| Entire submission of tumor | |||||
| No (ref) | 294 | 1.00 | 1.00 | ||
| Yes | 333 | 0.85 (0.71–1.03) | 0.10 | 0.74 (0.61–0.91) | 0.004 |
| Entire submission of pancreas | |||||
| No (ref) | 526 | 1.00 | 1.00 | ||
| Yes | 101 | 0.63 (0.48–0.83) | 0.001 | 0.56 (0.41–0.76) | <0.001 |
| Disease-Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|
| Characteristics (N = 627) | N | HR (95% CI) | p | HR (95% CI) | p |
| Tumor differentiation | |||||
| Well/moderate (ref) | 394 | 1.00 | 1.00 | ||
| Poor | 233 | 1.27 (1.05–1.54) | 0.02 | 1.28 (1.04–1.58) | 0.02 |
| Margin status | |||||
| Negative (ref) | 533 | 1.00 | 1.00 | ||
| Positive | 94 | 1.09 (0.83–1.42) | 0.53 | 0.88 (0.65–1.19) | 0.88 |
| ypT | 0.73 | 0.84 | |||
| ypT0/T1 (ref) | 222 | 1.00 | 1.00 | ||
| ypT2 | 325 | 1.03 (0.82–1.29) | 0.81 | 0.97 (0.77–1.24) | 0.83 |
| ypT3 | 80 | 1.14 (0.82–1.58) | 0.44 | 1.07 (0.75–1.53) | 0.70 |
| ypN stage | <0.001 | <0.001 | |||
| ypN0 (ref) | 284 | 1.00 | 1.00 | ||
| ypN1 | 223 | 1.43 (1.15–1.78) | 0.001 | 1.48 (1.17–1.87) | 0.001 |
| ypN2 | 120 | 2.32 (1.81–2.99) | <0.001 | 2.32 (1.77–3.03) | <0.001 |
| MDA TRG | |||||
| Grade 0/1 (ref) | 92 | 1.00 | 1.00 | ||
| Grade 2 | 535 | 1.66 (1.22–2.25) | 0.001 | 1.79 (1.26–2.52) | 0.001 |
| Entire submission of tumor | |||||
| No (ref) | 294 | 1.00 | 1.00 | ||
| Yes | 333 | 0.89 (0.73–1.08) | 0.23 | 0.80 (0.65–0.98) | 0.03 |
| Disease-Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|
| Characteristics | N | HR (95% CI) | p | HR (95% CI) | p |
| Tumor differentiation | |||||
| Well/moderate (ref) | 394 | 1.00 | 1.00 | ||
| Poor | 233 | 1.26 (1.04–1.54) | 0.02 | 1.27 (1.03–1.56) | 0.02 |
| Margin status | |||||
| Negative (ref) | 533 | 1.00 | 1.00 | ||
| Positive | 94 | 1.09 (0.83–1.42) | 0.54 | 0.88 (0.65–1.17) | 0.41 |
| ypT | 0.74 | 0.79 | |||
| ypT0/T1 (ref) | 222 | 1.00 | 1.00 | ||
| ypT2 | 325 | 1.00 (0.80–1.25) | 0.99 | 0.95 (0.75–1.21) | 0.70 |
| ypT3 | 80 | 1.12 (0.81–1.55) | 0.49 | 1.06 (0.74–1.51) | 0.75 |
| ypN stage | <0.001 | <0.001 | |||
| ypN0 (ref) | 284 | 1.00 | 1.00 | ||
| ypN1 | 223 | 1.42 (1.14–1.77) | 0.002 | 1.47 (1.17–1.86) | 0.001 |
| ypN2 | 120 | 2.31 (1.79–2.97) | <0.001 | 2.24 (1.71–2.93) | <0.001 |
| MDA TRG | |||||
| Grade 0/1 (ref) | 92 | 1.00 | 1.00 | ||
| Grade 2 | 535 | 1.53 (1.12–2.10) | 0.008 | 1.73 (1.21–2.45) | 0.002 |
| Entire pancreas submitted | |||||
| No (ref) | 526 | 1.00 | 1.00 | ||
| Yes | 101 | 0.77 (0.58–1.02) | 0.07 | 0.72 (0.53–0.99) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taherian, M.; Katz, M.H.G.; Prakash, L.R.; Wei, D.; Tong, Y.T.; Lai, Z.; Chatterjee, D.; Wang, H.; Kim, M.; Tzeng, C.-W.D.; et al. The Association between Sampling and Survival in Patients with Pancreatic Ductal Adenocarcinoma Who Received Neoadjuvant Therapy and Pancreaticoduodenectomy. Cancers 2024, 16, 3312. https://doi.org/10.3390/cancers16193312
Taherian M, Katz MHG, Prakash LR, Wei D, Tong YT, Lai Z, Chatterjee D, Wang H, Kim M, Tzeng C-WD, et al. The Association between Sampling and Survival in Patients with Pancreatic Ductal Adenocarcinoma Who Received Neoadjuvant Therapy and Pancreaticoduodenectomy. Cancers. 2024; 16(19):3312. https://doi.org/10.3390/cancers16193312
Chicago/Turabian StyleTaherian, Mehran, Matthew H. G. Katz, Laura R. Prakash, Dongguang Wei, Yi Tat Tong, Zongshan Lai, Deyali Chatterjee, Hua Wang, Michael Kim, Ching-Wei D. Tzeng, and et al. 2024. "The Association between Sampling and Survival in Patients with Pancreatic Ductal Adenocarcinoma Who Received Neoadjuvant Therapy and Pancreaticoduodenectomy" Cancers 16, no. 19: 3312. https://doi.org/10.3390/cancers16193312
APA StyleTaherian, M., Katz, M. H. G., Prakash, L. R., Wei, D., Tong, Y. T., Lai, Z., Chatterjee, D., Wang, H., Kim, M., Tzeng, C.-W. D., Ikoma, N., Wolff, R. A., Zhao, D., Koay, E. J., Maitra, A., & Wang, H. (2024). The Association between Sampling and Survival in Patients with Pancreatic Ductal Adenocarcinoma Who Received Neoadjuvant Therapy and Pancreaticoduodenectomy. Cancers, 16(19), 3312. https://doi.org/10.3390/cancers16193312







