MRI-Derived Body Composition and Breast Cancer Risk in Postmenopausal Women: UK Biobank Study
Simple Summary
Abstract
1. Introduction
2. Methods
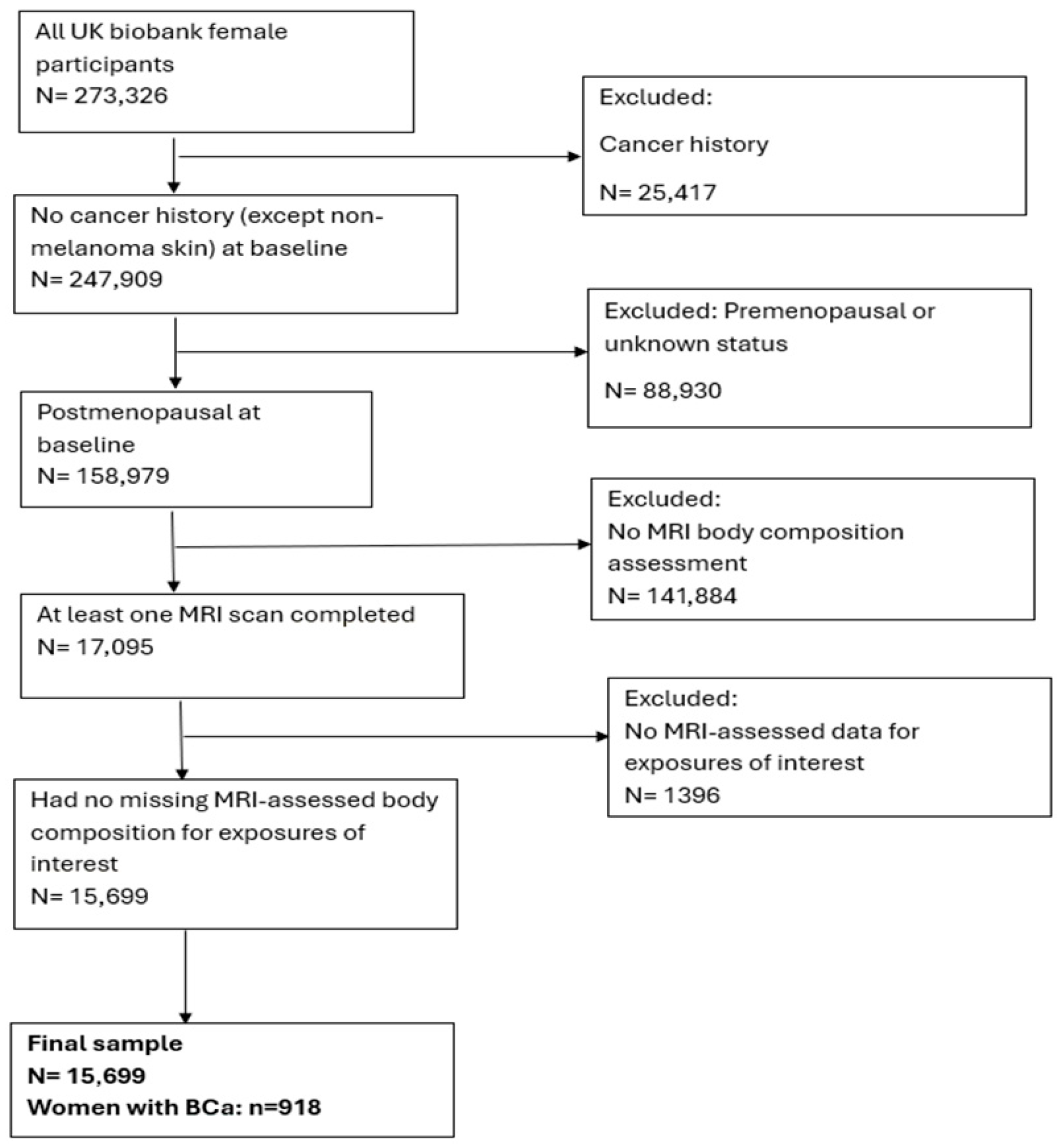
2.1. Description of Study Population and Recruitment
2.2. MRI-Body Composition Assessment
2.3. Covariate Information
2.4. Outcomes
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kelsey, J.L.; Gammon, M.D.; John, E.M. Reproductive Factors and Breast Cancer. Epidemiol. Rev. 1993, 15, 36–47. [Google Scholar] [CrossRef]
- Hunter, D.J.; Spiegelman, D.; Adami, H.O.; van den Brandt, P.A.; Folsom, A.R.; Goldbohm, R.A.; Graham, S.; Howe, G.R.; Kushi, L.H.; Marshall, J.R.; et al. Non-Dietary Factors as Risk Factors for Breast Cancer, and as Effect Modifiers of the Association of Fat Intake and Risk of Breast Cancer. Cancer Causes Control 1997, 8, 49–56. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast Cancer and Hormone Replacement Therapy: Collaborative Reanalysis of Data from 51 Epidemiological Studies of 52,705 Women with Breast Cancer and 108,411 Women without Breast Cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet 1997, 350, 1047–1059. [Google Scholar] [CrossRef]
- Hunter, D.J.; Willett, W.C. Diet, Body Size, and Breast Cancer. Epidemiol. Rev. 1993, 15, 110–132. [Google Scholar] [CrossRef]
- Arzanova, E.; Mayrovitz, H.N. The Epidemiology of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022; ISBN 978-0-6453320-3-2. [Google Scholar]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and Cancer--Mechanisms Underlying Tumour Progression and Recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular Mechanisms of Cancer Development in Obesity. Nat. Rev. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Kabat, G.C.; Xue, X.; Kamensky, V.; Lane, D.; Bea, J.W.; Chen, C.; Qi, L.; Stefanick, M.L.; Chlebowski, R.T.; Wactawski-Wende, J.; et al. Risk of Breast, Endometrial, Colorectal, and Renal Cancers in Postmenopausal Women in Association with a Body Shape Index and Other Anthropometric Measures. Cancer Causes Control 2015, 26, 219–229. [Google Scholar] [CrossRef]
- Pacholczak, R.; Klimek-Piotrowska, W.; Kuszmiersz, P. Associations of Anthropometric Measures on Breast Cancer Risk in Pre- and Postmenopausal Women—A Case-Control Study. J. Physiol. Anthropol. 2016, 35, 7. [Google Scholar] [CrossRef] [PubMed]
- Phipps, A.I.; Chlebowski, R.T.; Prentice, R.; McTiernan, A.; Stefanick, M.L.; Wactawski-Wende, J.; Kuller, L.H.; Adams-Campbell, L.L.; Lane, D.; Vitolins, M.; et al. Body Size, Physical Activity, and Risk of Triple-Negative and Estrogen Receptor-Positive Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2011, 20, 454–463. [Google Scholar] [CrossRef]
- Pinheiro, R.L.; Sarian, L.O.; Pinto-Neto, A.M.; Morais, S.; Costa-Paiva, L. Waist Circumference and Waist to Hip Ratio Do Not Contribute Additional Information on Hormone Receptor Status of Breast Tumors in Obese Women. Breast J. 2010, 16, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.L.; Shaw, J.E.; Anstey, K.J.; Adams, R.; Balkau, B.; Brennan-Olsen, S.L.; Briffa, T.; Davis, T.M.E.; Davis, W.A.; Dobson, A.; et al. Comparison of Anthropometric Measures as Predictors of Cancer Incidence: A Pooled Collaborative Analysis of 11 Australian Cohorts. Int. J. Cancer 2015, 137, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-C.; Chen, S.-J.; Zhang, R.; Hidayat, K.; Qin, J.-B.; Zhang, Y.-S.; Qin, L.-Q. Central Obesity and Risks of Pre- and Postmenopausal Breast Cancer: A Dose–Response Meta-Analysis of Prospective Studies. Obes. Rev. 2016, 17, 1167–1177. [Google Scholar] [CrossRef]
- White, A.J.; Nichols, H.B.; Bradshaw, P.T.; Sandler, D.P. Overall and Central Adiposity and Breast Cancer Risk in the Sister Study. Cancer 2015, 121, 3700–3708. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Arthur, R.; Manson, J.E.; Chlebowski, R.T.; Kroenke, C.H.; Peterson, L.; Cheng, T.-Y.D.; Feliciano, E.C.; Lane, D.; Luo, J.; et al. Association of Body Fat and Risk of Breast Cancer in Postmenopausal Women with Normal Body Mass Index: A Secondary Analysis of a Randomized Clinical Trial and Observational Study. JAMA Oncol. 2019, 5, 155–163. [Google Scholar] [CrossRef]
- Arthur, R.S.; Xue, X.; Kamensky, V.; Chlebowski, R.T.; Simon, M.; Luo, J.; Shadyab, A.H.; Neuhouser, M.L.; Banack, H.; Ho, G.Y.F.; et al. The Association between DXA-Derived Body Fat Measures and Breast Cancer Risk among Postmenopausal Women in the Women’s Health Initiative. Cancer Med. 2020, 9, 1581–1599. [Google Scholar] [CrossRef]
- Cao, Y.; Xia, B.; Zhang, Z.; Hu, D.; Huang, X.; Yuan, J.; Li, F. Association of Body Fat Distribution and Risk of Breast Cancer in Pre- and Postmenopausal Women. Obes. Facts 2023, 16, 356–363. [Google Scholar] [CrossRef]
- Staunstrup, L.M.; Nielsen, H.B.; Pedersen, B.K.; Karsdal, M.; Blair, J.P.M.; Christensen, J.F.; Bager, C.L. Cancer Risk in Relation to Body Fat Distribution, Evaluated by DXA-Scans, in Postmenopausal Women—The Prospective Epidemiological Risk Factor (PERF) Study. Sci. Rep. 2019, 9, 5379. [Google Scholar] [CrossRef]
- Costelloe, C.M.; Rohren, E.M.; Madewell, J.E.; Hamaoka, T.; Theriault, R.L.; Yu, T.-K.; Lewis, V.O.; Ma, J.; Stafford, R.J.; Tari, A.M.; et al. Imaging Bone Metastases in Breast Cancer: Techniques and Recommendations for Diagnosis. Lancet Oncol. 2009, 10, 606–614. [Google Scholar] [CrossRef]
- Thibault, I.; Chang, E.L.; Sheehan, J.; Ahluwalia, M.S.; Guckenberger, M.; Sohn, M.-J.; Ryu, S.; Foote, M.; Lo, S.S.; Muacevic, A.; et al. Response Assessment after Stereotactic Body Radiotherapy for Spinal Metastasis: A Report from the SPIne Response Assessment in Neuro-Oncology (SPINO) Group. Lancet Oncol. 2015, 16, e595–e603. [Google Scholar] [CrossRef] [PubMed]
- Smoking Greatly Increases Risk of Complications After Surgery. Available online: https://www.who.int/news/item/20-01-2020-smoking-greatly-increases-risk-of-complications-after-surgery (accessed on 4 December 2025).
- West, J.; Leinhard, O.D.; Romu, T.; Collins, R.; Garratt, S.; Bell, J.D.; Borga, M.; Thomas, L. Feasibility of MR-Based Body Composition Analysis in Large Scale Population Studies. PLoS ONE 2016, 11, e0163332. [Google Scholar] [CrossRef]
- Borga, M.; Thomas, E.L.; Romu, T.; Rosander, J.; Fitzpatrick, J.; Dahlqvist Leinhard, O.; Bell, J.D. Validation of a Fast Method for Quantification of Intra-Abdominal and Subcutaneous Adipose Tissue for Large-Scale Human Studies. NMR Biomed. 2015, 28, 1747–1753. [Google Scholar] [CrossRef]
- Karlsson, A.; Rosander, J.; Romu, T.; Tallberg, J.; Grönqvist, A.; Borga, M.; Dahlqvist Leinhard, O. Automatic and Quantitative Assessment of Regional Muscle Volume by Multi-Atlas Segmentation Using Whole-Body Water-Fat MRI. J. Magn. Reson. Imaging 2015, 41, 1558–1569. [Google Scholar] [CrossRef]
- Dai, L.; Huang, X.-Y.; Lu, Y.-Q.; Liu, Y.-Y.; Song, C.-Y.; Zhang, J.-W.; Li, J.; Zhang, Y.; Shan, Y.; Shi, Y. Defining Reference Values for Body Composition Indices by Magnetic Resonance Imaging in UK Biobank. J. Cachexia Sarcopenia Muscle 2023, 14, 992–1002. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and Visceral Adipose Tissue: Structural and Functional Differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Kim, M.S.; Choi, Y.-J.; Lee, Y.H. Visceral Fat Measured by Computed Tomography and the Risk of Breast Cancer. Transl. Cancer Res. 2019, 8, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Iwase, T.; Wang, X.; Shrimanker, T.V.; Kolonin, M.G.; Ueno, N.T. Body Composition and Breast Cancer Risk and Treatment: Mechanisms and Impact. Breast Cancer Res. Treat. 2021, 186, 273–283. [Google Scholar] [CrossRef]
- Park, J.W.; Han, K.; Shin, D.W.; Yeo, Y.; Chang, J.W.; Yoo, J.E.; Jeong, S.-M.; Lee, S.-K.; Ryu, J.M.; Park, Y.-M. Obesity and Breast Cancer Risk for Pre- and Postmenopausal Women among over 6 Million Korean Women. Breast Cancer Res. Treat. 2021, 185, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.M.; Abar, L.; Cariolou, M.; Nanu, N.; Greenwood, D.C.; Bandera, E.V.; McTiernan, A.; Norat, T. World Cancer Research Fund International: Continuous Update Project-Systematic Literature Review and Meta-Analysis of Observational Cohort Studies on Physical Activity, Sedentary Behavior, Adiposity, and Weight Change and Breast Cancer Risk. Cancer Causes Control 2019, 30, 1183–1200. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased Visceral Fat and Decreased Energy Expenditure during the Menopausal Transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef]
- Liedtke, S.; Schmidt, M.E.; Vrieling, A.; Lukanova, A.; Becker, S.; Kaaks, R.; Zaineddin, A.K.; Buck, K.; Benner, A.; Chang-Claude, J.; et al. Postmenopausal Sex Hormones in Relation to Body Fat Distribution. Obesity 2012, 20, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Baglietto, L.; Severi, G.; English, D.R.; Krishnan, K.; Hopper, J.L.; McLean, C.; Morris, H.A.; Tilley, W.D.; Giles, G.G. Circulating Steroid Hormone Levels and Risk of Breast Cancer for Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 2010, 19, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D. The Proliferating Role of Insulin and Insulin-like Growth Factors in Cancer. Trends Endocrinol. Metab. 2010, 21, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Novosyadlyy, R.; Lann, D.E.; Vijayakumar, A.; Rowzee, A.; Lazzarino, D.A.; Fierz, Y.; Carboni, J.M.; Gottardis, M.M.; Pennisi, P.A.; Molinolo, A.A.; et al. Insulin-Mediated Acceleration of Breast Cancer Development and Progression in a Nonobese Model of Type 2 Diabetes. Cancer Res. 2010, 70, 741–751. [Google Scholar] [CrossRef]
- Divella, R.; De Luca, R.; Abbate, I.; Naglieri, E.; Daniele, A. Obesity and Cancer: The Role of Adipose Tissue and Adipo-Cytokines-Induced Chronic Inflammation. J. Cancer 2016, 7, 2346–2359. [Google Scholar] [CrossRef]
- Trayhurn, P. Hypoxia and Adipose Tissue Function and Dysfunction in Obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef]
- Gilbert, C.A.; Slingerland, J.M. Cytokines, Obesity, and Cancer: New Insights on Mechanisms Linking Obesity to Cancer Risk and Progression. Annu. Rev. Med. 2013, 64, 45–57. [Google Scholar] [CrossRef]
- Borga, M. MRI Adipose Tissue and Muscle Composition Analysis—A Review of Automation Techniques. Br. J. Radiol. 2018, 91, 20180252. [Google Scholar] [CrossRef]
- Al Saedi, A.; Debruin, D.A.; Hayes, A.; Hamrick, M. Lipid Metabolism in Sarcopenia. Bone 2022, 164, 116539. [Google Scholar] [CrossRef]
- Biltz, N.K.; Collins, K.H.; Shen, K.C.; Schwartz, K.; Harris, C.A.; Meyer, G.A. Infiltration of Intramuscular Adipose Tissue Impairs Skeletal Muscle Contraction. J. Physiol. 2020, 598, 2669–2683. [Google Scholar] [CrossRef]
- Wang, L.; Valencak, T.G.; Shan, T. Fat Infiltration in Skeletal Muscle: Influential Triggers and Regulatory Mechanism. iScience 2024, 27, 109221. [Google Scholar] [CrossRef] [PubMed]
- Correa-de-Araujo, R.; Addison, O.; Miljkovic, I.; Goodpaster, B.H.; Bergman, B.C.; Clark, R.V.; Elena, J.W.; Esser, K.A.; Ferrucci, L.; Harris-Love, M.O.; et al. Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging. Front. Physiol. 2020, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Aduse-Poku, L.; Bian, J.; Gopireddy, D.R.; Hernandez, M.; Lall, C.; Falzarano, S.M.; Masood, S.; Jo, A.; Cheng, T.-Y.D. Associations of Computed Tomography Image-Assessed Adiposity and Skeletal Muscles with Triple-Negative Breast Cancer. Cancers 2022, 14, 1846. [Google Scholar] [CrossRef] [PubMed]
- Smotherman, C.; Sprague, B.; Datta, S.; Braithwaite, D.; Qin, H.; Yaghjyan, L. Association of Air Pollution with Postmenopausal Breast Cancer Risk in UK Biobank. Breast Cancer Res. 2023, 25, 83. [Google Scholar] [CrossRef]

| Characteristics | Overall | Normal | High Adiposity/High Muscle | Low Adiposity/Low Muscle | Adverse Body Composition Phenotype (High Adiposity/Low Muscle) |
|---|---|---|---|---|---|
| N | 15,669 | 2517 | 2247 | 8289 | 2616 |
| Mean (SD) | |||||
| Age at recruitment, years | 58.58 (5.18) | 57.41 (5.18) | 57.41 (5.12) | 59.08 (5.10) | 59.10 (5.13) |
| Age at menarche, years | 12.63 (2.57) | 12.78 (2.59) | 12.54 (2.56) | 12.66 (2.57) | 12.46 (2.57) |
| Age of menopause, years | 47.29 (12.24) | 48.05 (10.94) | 47.88 (11.03) | 47.06 (12.75) | 46.80 (12.72) |
| Index of multiple Deprivation | 15.01 (11.69) | 13.92 (10.74) | 16.16 (11.94) | 14.41 (11.28) | 16.98 (13.21) |
| Body mass index (BMI) | 26.07 (3.87) | 24.93 (2.76) | 31.05 (4.57) | 23.96 (2.85) | 29.61 (3.87) |
| Waist circumference (cm) | 82.43 (10.77) | 79.85 (7.20) | 77.17 (7.70) | 94.66 (10.58) | 89.61 (9.08) |
| Hip circumference | 101.71 (8.95) | 100.70 (5.94) | 111.49 (9.18) | 97.42 (6.27) | 107.86 (8.36) |
| Waist-hip ratio (WHR) | 1.25 (0.10) | 1.26 (0.09) | 1.28 (0.09) | 1.18 (0.09) | 1.20 (0.09) |
| Summed MET minutes per week | 2662.04 (2377.84) | 3056.31 (2715.68) | 2766.05 (2323.21) | 2408.40 (2316.24) | 2171.27 (2124.07) |
| N (%) | |||||
| Race White Other races | 14,296 (91.4) 1340 (8.6) | 2288 (91.2) 222 (8.8) | 2054 (91.7) 185 (8.3) | 7564 (91.4) 709 (8.6) | 2390 (91.4) 224 (8.6) |
| Smoking status Never Former Current | 9612 (61.5) 5296 (33.9) 724 (4.6) | 1567 (62.4) 844 (33.6) 99 (3.9) | 1297 (57.9) 814 (36.3) 131 (5.8) | 5234 (63.2) 2681 (32.4) 361 (4.4) | 1514 (58.1) 957 (36.8) 133 (5.1) |
| Alcohol drinker status Never Former Current | 515 (3.3) 345 (2.2) 14,802 (94.5) | 52 (2.1) 40 (1.6) 2423 (96.3) | 74 (3.3) 56 (2.5) 2115 (94.2) | 271 (3.3) 174 (2.1) 7842 (94.6) | 118 (4.5) 75 (2.9) 2422 (92.6) |
| Fruit intake No Yes | 273 (11.3) 2140 (88.7 | 41 (9.5) 391 (90.5) | 53 (14.9) 302 (85.1) | 119 (9.3) 1159 (90.7) | 60 (17.2) 288 (82.8) |
| Vegetable intake No Yes | 299 (12.4) 2114 (87.6) | 53 (12.3) 379 (87.7) | 44 (12.4) 311 (87.6) | 149 (11.7) 1129 (88.3) | 53 (15.2) 295 (84.8) |
| Hormone replacement therapy No Yes | 8611 (55.0) 7041 (45.0) | 1547 (61.5) 968 (38.5) | 1306 (58.2) 938 (41.8) | 4450 (53.8) 3829 (46.2) | 1308 (50.0) 1306 (50.0) |
| Bilateral oophorectomy No Yes | 14,946 (95.9) 646 (4.1) | 2427 (96.9) 79 (3.1) | 7937 (96.2) 312 (3.8) | 2129 (95.1) 110 (4.9) | 2443 (94.4) 145 (5.6) |
| Diabetes No Yes | 15,359 (98.1) 299 (1.9) | 2487 (98.9) 28 (1.1) | 8193 (98.9) 93 (1.1) | 2168 (96.6) 77 (3.4) | 2511 (96.1) 101 (3.9) |
| Hypertension No Yes | 10,683 (68.2) 4986 (31.18) | 1930 (76.7) 587 (23.3) | 6046 (72.9) 2243 (27.1) | 1273 (56.7) 974 (43.3) | 1434 (54.8) 1182 (45.2) |
| Body Composition Measures | Cases/Person-Years | Unadjusted HR (95% CI) Model 1 | Age-Adjusted (95% CI) Model 2 | aHR (95% CI) Model 3 |
|---|---|---|---|---|
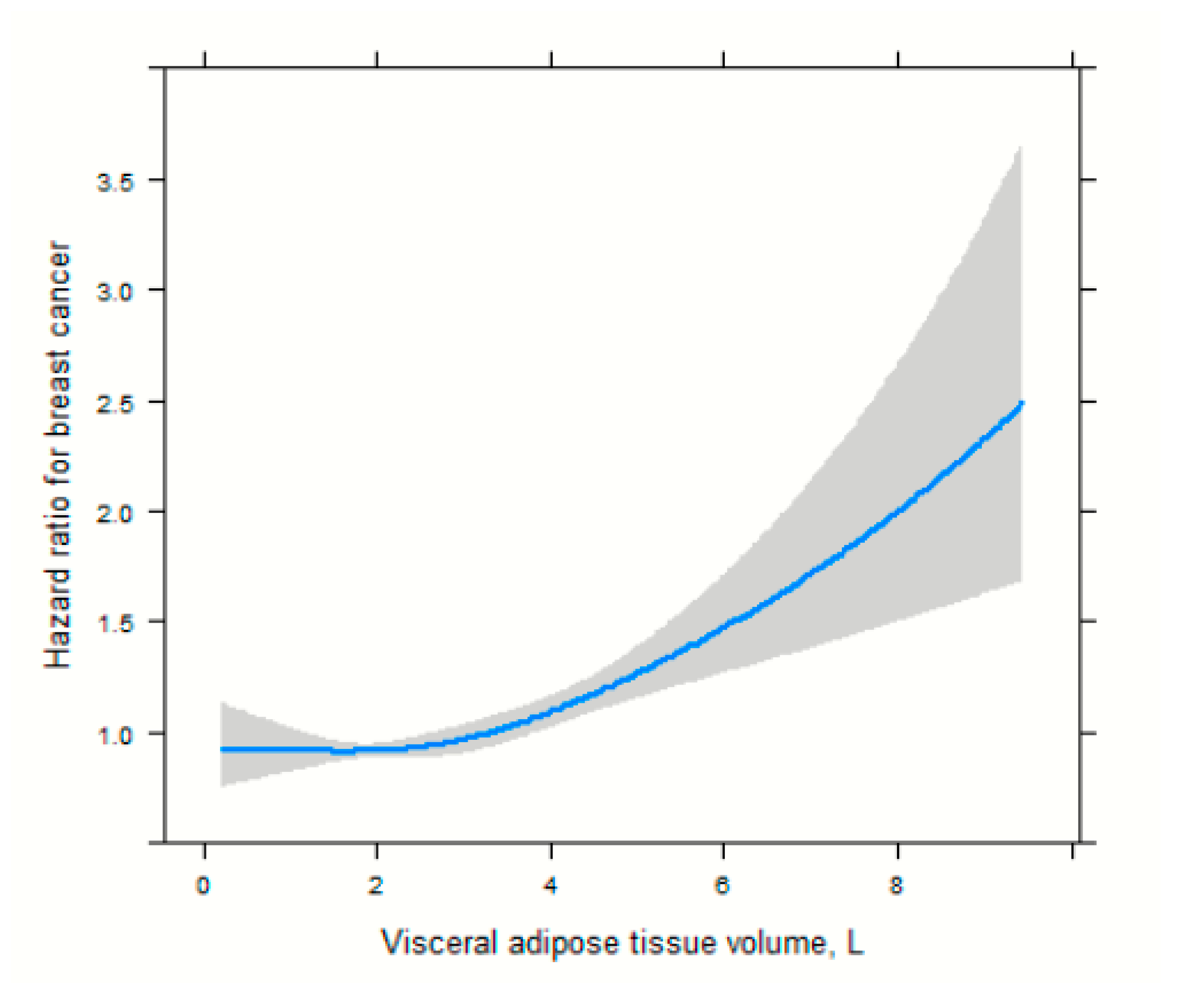
| Visceral adipose tissue (VAT) Low Medium High | 277/2286.03 291/2213.35 350/2815.24 | Ref 1.04 (0.88–1.23) 1.27 (1.09–1.49) | Ref 1.03 (0.88–1.22) 1.26 (1.10–1.47) | Ref 1.04 (0.88–1.22) 1.24 (1.10–1.45) |
| Subcutaneous adipose tissue (SAT) Low Medium High | 298/2469.67 293/2362.22 327/2482.72 | Ref 0.97 (0.83–1.14) 1.09 (0.93–1.27) | Ref 0.97 (0.82–1.14) 1.10 (0.94–1.28) | Ref 0.96 (0.81–1.13) 1.08 (0.92–1.26) |
| Total adipose tissue (TAT) Low Medium High | 310/2567.22 290/2265.15 318.2482.24 | Ref 0.99 (0.84–1.16) 1.17 (1.01–1.37) | Ref 0.98 (0.84–1.16) 1.18 (1.01–1.38) | Ref 0.98 (0.81–1.15) 1.17 (1.01–1.37) |
| Fat-free muscle volume Low Medium High | 221/1755.41 205/1492.76 275/2343.76 | Ref 0.93 (0,77–1.13) 0.98 (0.81–1.19) | Ref 0.95 (0.78–1.15) 1.02 (0.85–1.24) | Ref 0.94 (0.78–1.14) 1.02 (0.84–1.23) |
| Muscle fat infiltration Low Medium High | 163/1228.47 223/1714.05 276/2344.79 | Ref 1.38 (1.12–1.68) 1.61 (1.32–2.00) | Ref 1.34 (1.09–1.64) 1.54 (1.26–1.89) | Ref 1.34 (1.09–1.64) 1.53 (1.25–1.87) |
| Body composition phenotypes Normal Low adiposity/low muscle High adiposity/high muscle Adverse body composition | 135/1097.00 465/3735.38 138/1158.84 180/1323.41 | Ref 1.15 (0.91–1.46) 1.05 (0.87–1.27) 1.29 (1.03–1.61) | Ref 1.01 (0.84–1.23) 1.15 (0.90–1.45) 1.24 (1.02–1.55) | Ref 1.02 (0.854–1.24) 1.14 (0.90–1.44) 1.23 (0.98–1.54) |
| Body Composition Measures | Cases/Person-Years | Unadjusted HR (95% CI) Model 1 | Age-Adjusted (95% CI) Model 2 | aHR (95% CI) Model 3 |
|---|---|---|---|---|
| Visceral adipose tissue (VAT) Low Medium High | 237/2242.72 257/2179.19 300/2767.74 | Ref 1.13 (0.95–1.35) 1.22 (1.03–1.45) | Ref 1.13 (0.95–1.35) 1.22 (1.02–1.44) | Ref 1.15 (0.96–1.37) 1.24 (1.05–1.48) |
| Subcutaneous adipose tissue (SAT) Low Medium High | 269/2437.29 248/2319.75 277/2432.61 | Ref 0.94 (0.79–1.11) 1.06 (0.90–1.26) | Ref 0.94 (0.79–1.11) 1.08 (0.91–1.28) | Ref 0.94 (0.79–1.11) 1.10 (0.93–1.30) |
| Total adipose tissue (TAT) Low Medium High | 272/2526.59 253/2228.36 269/2434.70 | Ref 0.99 (0.83–1.17) 1.12 (0.95–1.33) | Ref 0.99 (0.83–1.17) 1.13 (0.96–1.34) | Ref 0.99 (0.83–1.17) 1.15 (0.97–1.37) |
| Fat-free muscle volume Low Medium High | 184/1720.81 174/1461.98 188/1693.44 | Ref 0.96 (0.78–1.19) 1.02 (0.83–1.25) | Ref 0.98 (0.80–1.21) 1.06 (0.86–1.30) | Ref 0.96 (0.78–1.19) 1.04 (0.85–1.28) |
| Muscle fat infiltration Low Medium High | 143/1207.26 192/1679.80 211/1989.18 | Ref 1.35 (1.09–1.68) 1.39 (1.12–1.72) | Ref 1.32 (1.06–1.64) 1.33 (1.07–1.65) | Ref 1.33 (1.06–1.65) 1.35 (1.08–1.68) |
| Body composition phenotypes Normal Low adiposity/low muscle High adiposity/high muscle Adverse body composition | 121/1081.23 404/3673.72 120/1144.43 149/1290.27 | Ref 1.02 (0.83–1.25) 1.08 (0.84–1.40) 1.19 (0.94–1.52) | Ref 0.99 (0.80–1.21) 1.09 (0.85–1.41) 1.16 (0.91–1.48) | Ref 1.00 (0.81–1.22) 1.12 (0.87–1.44) 1.20 (0.94–1.52) |
| Anthropometric Measures | HR (95% CI) | p-Interaction |
|---|---|---|
| BMI (<25), kg/m2 Low VAT Medium VAT High VAT | Ref 1.12 (0.90–1.40) 1.22 (0.89–1.67) | 0.187 |
| BMI (≥25), kg/m2 Low VAT Medium VAT High VAT | Ref 0.88 (0.66–1.17) 0.99 (0.76–1.29) | 0.863 |
| Waist circumference (<80), cm Low VAT Medium VAT High VAT | Ref 1.13 (0.90–1.41) 1.03 (0.70–1.52) | 0.504 |
| Waist circumference (≥80), cm Low VAT Medium VAT High VAT | Ref 0.87 (0.66–1.14) 0.96 (0.75–1.23 | 0.273 |
| Waist-hip ratio (<0.80) Low VAT Medium VAT High VAT | Ref 0.96 (0.72–1.28) 1.27 (1.39–1.65) | 0.002 |
| Waist-hip ratio (≥0.80) Low VAT Medium VAT High VAT | Ref 1.20 (0.98–1.48) 0.88 (0.66–1.18) | 0.066 |
| Anthropometric Measures | HR (95% CI) | p-Interaction |
|---|---|---|
| BMI (<25), kg/m2 Low MFI Medium MFI High MFI | Ref 1.47 (1.13–1.90) 1.21 (0.88–1.68) | 0.010 |
| BMI (≥25), kg/m2 Low MFI Medium MFI High MFI | Ref 1.35 (0.96–1.90) 1.69 (1.23–2.32) | 0.047 |
| Waist circumference (<80), cm Low MFI Medium MFI High MFI | Ref 1.39 (1.06–1.82) 1.10 (0.77–1.56) | 0.050 |
| Waist circumference (≥80), cm Low MFI Medium MFI High MFI | Ref 1.36 (0.99–1.86) 1.61 (1.21–2.16) | 0.038 |
| Waist-hip ratio (<0.80) Low MFI Medium MFI High MFI | Ref 1.39 (1.02–1.90) 1.55 (1.16–2.08) | 0.012 |
| Waist-hip ratio (≥0.80) Low MFI Medium MFI High MFI | Ref 1.36 (1.04–1.77) 1.34 (1.00–1.79) | 0.054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aduse-Poku, L.; Yaghjyan, L.; Kimmel, S.E.; Datta, S.; Karanth, S.D.; Yang, J.J.; Washington, C.; Braithwaite, D. MRI-Derived Body Composition and Breast Cancer Risk in Postmenopausal Women: UK Biobank Study. Cancers 2025, 17, 4036. https://doi.org/10.3390/cancers17244036
Aduse-Poku L, Yaghjyan L, Kimmel SE, Datta S, Karanth SD, Yang JJ, Washington C, Braithwaite D. MRI-Derived Body Composition and Breast Cancer Risk in Postmenopausal Women: UK Biobank Study. Cancers. 2025; 17(24):4036. https://doi.org/10.3390/cancers17244036
Chicago/Turabian StyleAduse-Poku, Livingstone, Lusine Yaghjyan, Stephen E. Kimmel, Susmita Datta, Shama D. Karanth, Jae Jeong Yang, Caretia Washington, and Dejana Braithwaite. 2025. "MRI-Derived Body Composition and Breast Cancer Risk in Postmenopausal Women: UK Biobank Study" Cancers 17, no. 24: 4036. https://doi.org/10.3390/cancers17244036
APA StyleAduse-Poku, L., Yaghjyan, L., Kimmel, S. E., Datta, S., Karanth, S. D., Yang, J. J., Washington, C., & Braithwaite, D. (2025). MRI-Derived Body Composition and Breast Cancer Risk in Postmenopausal Women: UK Biobank Study. Cancers, 17(24), 4036. https://doi.org/10.3390/cancers17244036







