HER2-Low and HER2-Ultralow Metastatic Breast Cancer and Trastuzumab Deruxtecan: Common Clinical Questions and Answers
Simple Summary
Abstract
1. Introduction
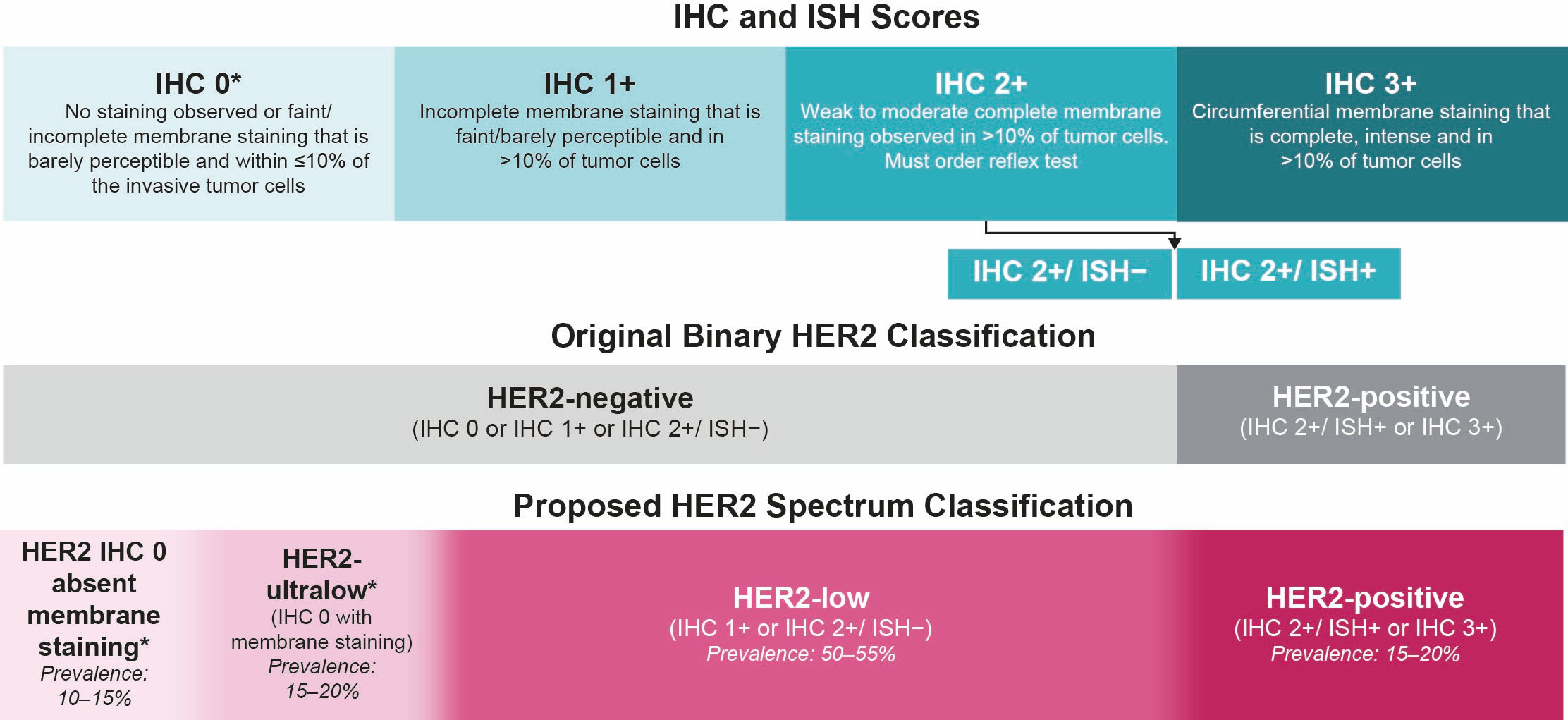
What Is the Prevalence and Unmet Need of the Patient Population with HER2-Low and HER2-Ultralow Breast Cancer?
2. T-DXd Efficacy in HER2-Low and HER2-Ultralow Breast Cancer
2.1. How Does T-DXd Target HER2-Low and HER2-Ultralow Breast Cancer, and How Effective Is T-DXd in These Tumors?
2.2. Evidence for T-DXd in HR-Positive, HER2-Low Breast Cancer
2.3. Evidence for T-DXd in HR-Negative, HER2-Low Breast Cancer
2.4. Evidence for T-DXd in HER2-Ultralow Breast Cancer
3. How Should T-DXd Treatment Be Sequenced for HER2-Low and HER2-Ultralow Advanced/Metastatic Breast Cancer?
3.1. What Other ADCs Can Be Considered for HER2-Negative Breast Cancer (Inclusive of HER2-Low and HER2-Ultralow)?
3.2. How Should ADCs Be Sequenced in HER2-Negative Breast Cancer (Inclusive of HER2-Low and HER2-Ultralow)?
4. What Is the Efficacy of T-DXd in Patients with HER2-Low Metastatic Breast Cancer with Brain Metastases?
5. Testing: How Do I Identify Patients Who Are Eligible for Treatment?
5.1. Which Test(s) Should I Use to Determine if a Patient Has HER2-Low or HER2-Ultralow Breast Cancer?
5.2. What Are Some of the Challenges of Testing and Identifying HER2-Low and HER2-Ultralow Breast Cancer?
5.3. How Variable Is HER2 Expression in Breast Cancer?
5.4. How Should I Evaluate HER2 Status in Patients with a HER2 IHC 0 Tumor Score on Their Most Recent Biopsy?
6. Safety: What Are the Best Practices for Preventing, Monitoring, and Managing Adverse Events with T-DXd?
6.1. What Are the Best Practices for Preventing, Monitoring, and Managing ILD/Pneumonitis with T-DXd?
6.2. What Are the Best Practices for Preventing and Managing Nausea/Vomiting with T-DXd?
6.3. What Are the Best Practices for Preventing, Monitoring, and Managing Fatigue with T-DXd?
6.4. What Are the Best Practices for Preventing, Monitoring, and Managing Alopecia with T-DXd?
6.5. How Safe Is T-DXd in Older Patients?
7. Evolving Treatment Paradigm
7.1. HR-Positive, HER2-Low Breast Cancer
7.2. HER2-Ultralow Breast Cancer
8. Future Directions
| Trial, Treatment | Study Phase | NCT | Patient Cohort | Efficacy EndPoint(s) |
|---|---|---|---|---|
| DAISY, T-DXd | 2 | NCT04132960 | HER2-positive, HER2-low, or HER2 IHC 0 metastatic breast cancer. Patients must have received ≥1 line of chemotherapy in the metastatic setting. Prior treatment should have included anthracyclines and/or taxanes. | Primary endpoint: confirmed ORR [7] |
| DEBBRAH, T-DXd | 2 | NCT04420598 | HER2-low metastatic breast cancer with CNS involvement (BM progression after local treatment or LMC). | Primary endpoints: intracranial ORR [154] |
| DESTINY-Breast08, T-DXd + combinations | 1b | NCT04556773 | HR-positive, HER2-low metastatic breast cancer | Secondary endpoints: ORR, PFS, DOR, OS [155] |
| DESTINY-Breast15, T-DXd | 3b | NCT05950945 | HR-positive or HR-negative, HER2-low or HER2 IHC 0 metastatic breast cancer. Patients must have received 1 or 2 prior lines of therapy. | Primary endpoint: TTNT [141,156] |
| DESTINY-Breast Respond HER2-low Europe | Observational, prospective | NCT05945732 | HER2-low unresectable and/or metastatic breast cancer. Patients must have received at least 1 prior chemotherapy in the metastatic setting or experienced disease recurrence within 6 months after adjuvant chemotherapy. | Primary endpoint: rwTTNT [157] |
| PONTIAC | 2 | NCT06486883 | HR-positive HER2-low or HER2-ultralow unresectable and/or metastatic breast cancer. Patients must not have received prior treatment with any systemic therapy for advanced disease. | Primary endpoint: PFS [158] |
| TUXEDO-4, T-DXd | 2 | NCT06048718 | HER2-low breast cancer with active BMs. Patients must have received ≥1 line of systemic therapy in the advanced setting. | Primary endpoint: ORR [159,160] |
| TRADE-DXd, Treatment sequences of T-DXd and Dato-DXd | 2 | NCT06533826 | Patients with HR-positive or HR-negative, HER2-low tumors. Patients must have received 0–2 lines of chemotherapy, depending on patient cohorta | Primary endpoint: ORR Secondary endpoints: PFS, OS [161] |
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT3 | Serotonin receptor |
| ADC | Antibody–drug conjugate |
| AE | Adverse events |
| ASCO | American Society of Clinical Oncology |
| BICR | Blinded independent central review |
| BMs | Brain metastases |
| CAP | College of American Pathologists |
| CDK4/6i | Cyclin-dependent kinase 4/6 inhibitor |
| CT | Computed tomography |
| Dato-DXd | Datopotamab deruxtecan |
| DCO | Data cutoff |
| DOR | Duration of response |
| DXd | Deruxtecan |
| ER | Estrogen receptor |
| ET | Endocrine therapy |
| FDA | US Food and Drug Administration |
| HER2 | Human epidermal growth factor receptor 2 |
| HR | Hormone receptor |
| IHC | Immunohistochemistry |
| ILD | Interstitial lung disease |
| ISH | In situ hybridization |
| ITT | Intention-to-treat |
| NCCN® | National Comprehensive Cancer Network® |
| ORR | Objective response rate |
| OS | Overall survival |
| PARPi | Poly (ADP-ribose) polymerase inhibitors |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death ligand 1 |
| PFS | Progression-free survival |
| PR | Progesterone receptor |
| RANO-BM | Response Assessment in Neuro-Oncology Brain Metastases |
| SG | Sacituzumab govitecan |
| SpO2 | Oxygen saturation |
| T-DM1 | Trastuzumab emtansine |
| T-DXd | Trastuzumab deruxtecan |
| TNBC | Triple-negative breast cancer |
| TPC | Treatment of physician’s choice |
| TROP2 | Trophoblast cell surface antigen 2 |
Appendix A
| IHC interpretation | Use standardized ASCO–CAP guidelines scoring criteria to examine HER2 IHC-stained slides |
| Magnification | When discriminating IHC 0 from IHC 1+ staining, examine HER2 IHC at high-power magnification (40×) |
| Additional evaluation | Consider second pathologist review when results are close to the IHC 0 versus IHC 1+ interpretive threshold (>10% of cells with incomplete membrane staining that is faint/barely perceptible) |
| Assay controls | Ensure the assay has an appropriate limit of detection by using controls with a range of protein expression (including IHC 1+) |
| Preanalytic variables | It is essential to consider preanalytic conditions of breast cancer tissue samples from both primary and metastatic sites |
References and Notes
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; McShane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 2023, 147, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Ariga, S. History and Future of HER2-Targeted Therapy for Advanced Gastric Cancer. J. Clin. Med. 2023, 12, 3391. [Google Scholar] [CrossRef]
- Tarantino, P.; Viale, G.; Press, M.F.; Hu, X.; Penault-Llorca, F.; Bardia, A.; Batistatou, A.; Burstein, H.J.; Carey, L.A.; Cortes, J.; et al. ESMO expert consensus statements (ECS) on the definition, diagnosis, and management of HER2-low breast cancer. Ann. Oncol. 2023, 34, 645–659. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Mosele, F.; Deluche, E.; Lusque, A.; Le Bescond, L.; Filleron, T.; Pradat, Y.; Ducoulombier, A.; Pistilli, B.; Bachelot, T.; Viret, F.; et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: The phase 2 DAISY trial. Nat. Med. 2023, 29, 2110–2120. [Google Scholar] [CrossRef]
- Bardia, A.; Hu, X.; Dent, R.; Yonemori, K.; Barrios, C.H.; O’Shaughnessy, J.A.; Wildiers, H.; Pierga, J.Y.; Zhang, Q.; Saura, C.; et al. Trastuzumab deruxtecan after endocrine therapy in metastatic breast cancer. N. Engl. J. Med. 2024, 391, 2110–2122. [Google Scholar] [CrossRef]
- American College of Pathologists. Reporting Template for Reporting Results of Biomarker Testing of Specimens from Patients with Carcinoma of the Breast. Available online: https://documents.cap.org/documents/New-Cancer-Protocols-March-2025/Breast.Bmk_1.6.0.0.REL.CAPCP.pdf (accessed on 23 June 2025).
- Daiichi Sankyo Inc. ENHERTU® Approved in the EU as First HER2 Directed Therapy for Patients with HR Positive, HER2 Low or HER2 Ultralow Metastatic Breast Cancer Following at Least One Endocrine Therapy. [Press Release]. 4 April 2025. 2025. Available online: https://www.daiichisankyo.com/files/news/pressrelease/pdf/202504/20250404_E.pdf (accessed on 5 November 2025).
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Morales, S.; Gasol, A.; Sanchez, D.R. Her2-positive cancers and antibody-based treatment: State of the art and future developments. Cancers 2021, 13, 5771. [Google Scholar] [CrossRef]
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-low breast cancer: Pathological and clinical landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef]
- Prat, A.; Bardia, A.; Curigliano, G.; Hammond, M.E.H.; Loibl, S.; Tolaney, S.M.; Viale, G. An overview of clinical development of agents for metastatic or advanced breast cancer without ERBB2 amplification (HER2-Low). JAMA Oncol. 2022, 8, 1676–1687. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jia, H.; Zhang, H.; Chen, L.; Zhao, P.; Zhao, J.; Fu, G.; Xing, X.; Li, Y.; Wang, C. Is HER2 ultra-low breast cancer different from HER2 null or HER2 low breast cancer? A study of 1363 patients. Breast Cancer Res. Treat. 2023, 202, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Viale, G.; Basik, M.; Niikura, N.; Tokunaga, E.; Brucker, S.; Penault-Llorca, F.; Hayashi, N.; Sohn, J.; Teixeira de Sousa, R.; Brufsky, A.M.; et al. Retrospective study to estimate the prevalence and describe the clinicopathological characteristics, treatments received, and outcomes of HER2-low breast cancer. ESMO Open 2023, 8, 101615. [Google Scholar] [CrossRef] [PubMed]
- Baez-Navarro, X.; van Bockstal, M.R.; Nawawi, D.; Broeckx, G.; Colpaert, C.; Doebar, S.C.; Hogenes, M.C.H.; Koop, E.; Lambein, K.; Peeters, D.J.E.; et al. Interobserver variation in the assessment of immunohistochemistry expression levels in HER2-negative breast cancer: Can we improve the identification of low levels of HER2 expression by adjusting the criteria? An international interobserver study. Mod. Pathol. 2023, 36, 100009. [Google Scholar] [CrossRef]
- Curigliano, G.; Dent, R.; Earle, H.; Modi, S.; Tarantino, P.; Viale, G.; Tolaney, S.M. Open questions, current challenges, and future perspectives in targeting human epidermal growth factor receptor 2-low breast cancer. ESMO Open 2024, 9, 102989. [Google Scholar] [CrossRef]
- Kang, S.; Lee, S.H.; Lee, H.J.; Jeong, H.; Jeong, J.H.; Kim, J.E.; Ahn, J.H.; Jung, K.H.; Gong, G.; Kim, H.H.; et al. Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. Eur. J. Cancer 2022, 176, 30–40. [Google Scholar] [CrossRef]
- Peiffer, D.S.; Zhao, F.; Chen, N.; Hahn, O.M.; Nanda, R.; Olopade, O.I.; Huo, D.; Howard, F.M. Clinicopathologic characteristics and prognosis of ERBB2-low breast cancer among patients in the national cancer database. JAMA Oncol. 2023, 9, 500–510. [Google Scholar] [CrossRef]
- Bae, S.Y.; Kim, S.; Lee, J.H.; Lee, H.C.; Lee, S.K.; Kil, W.H.; Kim, S.W.; Lee, J.E.; Nam, S.J. Poor prognosis of single hormone receptor- positive breast cancer: Similar outcome as triple-negative breast cancer. BMC Cancer 2015, 15, 138. [Google Scholar] [CrossRef]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef]
- Miglietta, F.; Griguolo, G.; Bottosso, M.; Giarratano, T.; Lo Mele, M.; Fassan, M.; Cacciatore, M.; Genovesi, E.; De Bartolo, D.; Vernaci, G.; et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer 2021, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Matikas, A.; Foukakis, T.; Bergh, J. Tackling endocrine resistance in ER-positive HER2-negative advanced breast cancer: A tale of imprecision medicine. Crit. Rev. Oncol. Hematol. 2017, 114, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peng, Y. Current biological, pathological and clinical landscape of HER2-low breast cancer. Cancers 2022, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.; Scatena, C.; Ghilli, M.; Bargagna, I.; Lorenzini, G.; Nicolini, A. Molecular mechanisms, biomarkers and emerging therapies for chemotherapy resistant TNBC. Int. J. Mol. Sci. 2022, 23, 1665. [Google Scholar] [CrossRef]
- European Society for Medical Oncology. ESMO Metastatic Breast Cancer Living Guidelines, v1.1. Available online: https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guideline (accessed on 23 June 2025).
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Referenced from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.5.2025. © National Comprehensive Cancer Network, Inc, 2025. All rights reserved. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419 (accessed 3 December 2025). To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Andrahennadi, S.; Sami, A.; Manna, M.; Pauls, M.; Ahmed, S. Current landscape of targeted therapy in hormone receptor-positive and HER2-negative breast cancer. Curr. Oncol. 2021, 28, 1803–1822. [Google Scholar] [CrossRef]
- Yang, C.; Brezden-Masley, C.; Joy, A.A.; Sehdev, S.; Modi, S.; Simmons, C.; Henning, J.W. Targeting HER2-low in metastatic breast cancer: An evolving treatment paradigm. Ther. Adv. Med. Oncol. 2023, 15, 17588359231175440. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamauchi, C.; Toyama, T.; Nagai, S.; Sakai, T.; Kutomi, G.; Yoshimura, M.; Kawai, M.; Ohtani, S.; Kubota, K.; et al. The Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer, 2022 Edition: Changes from the 2018 edition and general statements on breast cancer treatment. Breast Cancer 2024, 31, 340–346. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef]
- Llombart-Cussac, A.; Harper-Wynne, C.; Perello, A.; Hennequin, A.; Fernandez, A.; Colleoni, M.; Carañana, V.; Quiroga, V.; Medioni, J.; Iranzo, V.; et al. Second-line endocrine therapy (ET) with or without palbociclib (P) maintenance in patients (pts) with hormone receptor-positive (HR[+])/human epidermal growth factor receptor 2-negative (HER2[-]) advanced breast cancer (ABC): PALMIRA trial. J. Clin. Oncol. 2023, 41, 1001. [Google Scholar] [CrossRef]
- Kalinsky, K.; Accordino, M.K.; Chiuzan, C.; Mundi, P.S.; Trivedi, M.S.; Novik, Y.; Tiersten, A.; Raptis, G.; Baer, L.N.; Young Oh, S.; et al. A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin-dependent kinase 4/6 inhibition (CDK 4/6i) in patients (pts) with unresectable or hormone receptor–positive (HR+), HER2-negative metastatic breast cancer (MBC): MAINTAIN trial. J. Clin. Oncol. 2022, 40, LBA1004. [Google Scholar] [CrossRef]
- Mayer, E.L.; Ren, Y.; Wagle, N.; Mahtani, R.; Ma, C.; DeMichele, A.; Cristofanilli, M.; Meisel, J.L.; Miller, K.D.; Jolly, T.; et al. Abstract GS3-06: GS3-06 palbociclib after CDK4/6i and endocrine therapy (PACE): A randomized phase II study of fulvestrant, palbociclib, and avelumab for endocrine pre-treated ER+/HER2- metastatic breast cancer. Cancer Res. 2023, 83, GS03-06. [Google Scholar] [CrossRef]
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(−) metastatic breast cancer. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.C.; Clarke, R.; Goetz, M.P.; Robertson, J. Cyclin-dependent kinase 4/6 inhibitors for treatment of hormone receptor-positive, ERBB2-negative breast cancer: A review. JAMA Oncol. 2023, 9, 1273–1282. [Google Scholar] [CrossRef]
- Nuzzolese, I.; Montemurro, F. Attrition in metastatic breast cancer: A metric to be reported in randomised clinical trials? Lancet Oncol. 2020, 21, 21–24. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Rugo, H.S.; Finn, R.S.; Diéras, V.; Ettl, J.; Lipatov, O.; Joy, A.A.; Harbeck, N.; Castrellon, A.; Iyer, S.; Lu, D.R.; et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 2019, 174, 719–729. [Google Scholar] [CrossRef]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019, 5, 5. [Google Scholar] [CrossRef]
- Hartkopf, A.D.; Walter, C.B.; Kolberg, H.C.; Hadji, P.; Tesch, H.; Fasching, P.A.; Ettl, J.; Lüftner, D.; Wallwiener, M.; Müller, V.; et al. Attrition in the first three therapy lines in patients with advanced breast cancer in the german real-world PRAEGNANT Registry. Geburtshilfe Frauenheilkd 2024, 84, 459–469. [Google Scholar] [CrossRef]
- Rugo, H.S.; Brufsky, A.; Liu, X.; Li, B.; McRoy, L.; Chen, C.; Layman, R.M.; Cristofanilli, M.; Torres, M.A.; Curigliano, G.; et al. Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2- metastatic breast cancer. NPJ Breast Cancer 2022, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Twelves, C.; Cheeseman, S.; Sopwith, W.; Thompson, M.; Riaz, M.; Ahat-Donker, N.; Myland, M.; Lee, A.; Przybysz, R.; Turner, S.; et al. Systemic treatment of hormone receptor positive, human epidermal growth factor 2 negative metastatic breast cancer: Retrospective analysis from Leeds Cancer Centre. BMC Cancer 2020, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, A.; Seidman, A.D. Treating triple-negative breast cancer: Where are we? J. Natl. Comp. Canc. Netw. 2015, 13, e8–e18. [Google Scholar] [CrossRef][Green Version]
- Chen, N.; Matossian, M.; Saha, P.; Rampurwala, M.; Kamaraju, S.; Hahn, O.; Howard, F.M.; Flemming, G.F.; Freeman, J.Q.; Karrison, T.; et al. A randomized pahse II trial of nab-paclitaxel with or without mifepristone for advanced triple-negative breast cancer. Breast Cancer Res. Treat. 2025, 211, 111–119. [Google Scholar] [CrossRef]
- Tolaney, S.M.; de Azambuja, E.; Kalinsky, K.; Loi, S.; Kim, S.-B.; Yam, C.; Rapoport, B.L.; Im, S.-A.; Pistilli, B.; McHayleh, W.; et al. Sacituzumab govitecan (SG) + pembrolizumab (pembro) vs chemotherapy (chemo) + pembro in previously untreated PD-L1–positive advanced triple-negative breast cancer (TNBC): Primary results from the randomized phase 3 ASCENT-04/KEYNOTE-D19 study. J. Clin. Oncol. 2025, 43, LBA109. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem. Pharm. Bull. 2019, 67, 173–185. [Google Scholar] [CrossRef]
- Han, S.; Lim, K.S.; Blackburn, B.J.; Yun, J.; Putnam, C.W.; Bull, D.A.; Won, Y.W. The potential of topoisomerase inhibitor-based antibody-drug conjugates. Pharmaceutics 2022, 14, 1707. [Google Scholar] [CrossRef]
- Tsao, L.C.; Wang, J.S.; Ma, X.; Sodhi, S.; Ragusa, J.V.; Liu, B.; McBane, J.; Wang, T.; Wei, J.; Liu, C.X.; et al. Effective extracellular payload release and immunomodulatory interactions govern the therapeutic effect of trastuzumab deruxtecan (T-DXd). Nat. Commun. 2025, 16, 3167. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Iwata, H.; Park, Y.H.; Vidal Losada, M.; Li, W.; Tsurutani, J.; Ueno, N.T.; Zaman, K.; Prat, A.; et al. Trastuzumab deruxtecan in HER2-low metastatic breast cancer: Long-term survival analysis of the randomized, phase 3 DESTINY-Breast04 trial. Nat. Med. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Daiichi Sankyo Inc. ENHERTU® (Fam-Trastuzumab Deruxtecan-Nxki) for Injection, for Intravenous Use. 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761139s028lbl.pdf (accessed on 23 June 2025).
- Gilead Sciences. TRODELVY® (Sacituzumab Govitecan-Hziy) for Injection, for Intravenous Use. 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761115s035lbl.pdf (accessed on 23 June 2025).
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortes, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Sacituzumab govitecan in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2022, 40, 3365–3376. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Gómez Pardo, P.; et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1423–1433. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Daiichi Sankyo, Inc. DATROWAY® (Datopotamab Deruxtecan-Dlnk) for Injection, for Intravenous Use. 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/761394s000lbl.pdf (accessed on 23 June 2025).
- Bardia, A.; Jhaveri, K.; Im, S.A.; Pernas, S.; De Laurentiis, M.; Wang, S.; Martínez Jañez, N.; Borges, G.; Cescon, D.W.; Hattori, M.; et al. Datopotamab deruxtecan versus chemotherapy in previously treated inoperable/metastatic hormone receptor-positive human epidermal growth factor receptor 2-negative breast cancer: Primary results from TROPION-Breast01. J. Clin. Oncol. 2024, 43, 285–296. [Google Scholar] [CrossRef]
- Pistilli, B.; Jhaveri, K.; Im, S.-A.; Pernas Simon, S.P.; De Laurentiis, M.; Wang, S.; Martinez, N.; Santos Borges, G.; Cescon, D.; Hattori, M.; et al. Datopotamab deruxtecan (Dato-DXd) vs chemotherapy (CT) in previously-treated inoperable or metastatic hormone receptor-positive, HER2-negative (HR+/HER2–) breast cancer (BC): Final overall survival (OS) from the phase III TROPION-Breast01 trial. Ann. Oncol. 2025, 36, P348. [Google Scholar] [CrossRef]
- Chen, M.; Huang, R.; Chen, R.; Pan, F.; Shen, X.; Li, H.; Rong, Q.; An, X.; Xue, C.; Shi, Y. Optimal sequential strategies for antibody-drug conjugate in metastatic breast cancer: Evaluating efficacy and cross-resistance. Oncologist 2024, 29, e957–e966. [Google Scholar] [CrossRef]
- Abelman, R.O.; Spring, L.; Fell, G.G.; Ryan, P.; Vidula, N.; Medford, A.J.; Shin, J.; Abraham, E.; Wander, S.A.; Insakoff, S.J.; et al. Sequential use of antibody-drug conjugate after antibody-drug conjugate for patients with metastatic breast cancer: ADC after ADC (A3) study. J. Clin. Oncol. 2024, 41, 1022. [Google Scholar] [CrossRef]
- Mai, N.; Klar, M.; Ferraro, E.; Bromberg, M.; Chen, Y.; Razavi, P.; Modi, S.; Chandarlapaty, S.; Walsh, E.M.; Drago, J.Z. Real world outcomes of sequential ADC therapy in metastatic breast cancer: Patients treated with sacituzumab govitecan and trastuzumab deruxtecan. J. Clin. Oncol. 2024, 42, 1085. [Google Scholar] [CrossRef]
- Poumeaud, F.; Morisseau, M.; Cabel, L.; Gonçalves, A.; Rivier, C.; Trédan, O.; Volant, E.; Frenel, J.S.; Ladoire, S.; Jacot, W.; et al. Efficacy of administration sequence: Sacituzumab Govitecan and Trastuzumab Deruxtecan in HER2-low metastatic breast cancer. Br. J. Can. 2024, 131, 702–708. [Google Scholar] [CrossRef]
- Huppert, L.A.; Mahtani, R.; Fisch, S.; Dempsey, N.; Premji, S.; Raimonde, A.; Jacob, S.; Quintal, L.; Melisko, M.; Chien, J.; et al. Multicenter retrospective cohort study of the sequential use of the antibody-drug conjugates (ADCs) trastuzumab deruxtecan (T-DXd) and sacituzumab govitecan (SG) in patients with HER2-low metastatic breast cancer (MBC). NPJ Breast Cancer 2025, 11, 34. [Google Scholar] [CrossRef]
- Collins, D.M.; Bossenmaier, B.; Kollmorgen, G.; Niederfellner, G. Acquired resistance to antibody-drug conjugates. Cancers 2019, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Abelman, R.O.; Spring, L.; Fell, G.; Davis, A.; Hensing, W.; Ryan, P.; Vidula, N.; Wander, S.; Medford, A.; Shin, J.; et al. Sequencing antibody-drug conjugate after antibody-drug conjugate in metastatic breast cancer (A3 study): Multi-institution experience and biomarker analysis. Cancer Res. 2024, 84, PS08-03. [Google Scholar] [CrossRef]
- Fenton, M.A.; Tarantino, P.; Graff, S.L. Sequencing antibody drug conjugates in breast cancer: Exploring future roles. Curr. Oncol. 2023, 30, 10211–10223. [Google Scholar] [CrossRef] [PubMed]
- Saltalamacchia, G.; Torrisi, R.; De Sanctis, R.; Masci, G.; Miggiano, C.; Gaudio, M.; Benvenuti, C.; Jacobs, F.; Gerosa, R.; Santoro, A.; et al. Charting the course in sequencing antibody-drug conjugates in breast cancer. Biomedicines 2024, 12, 500. [Google Scholar] [CrossRef]
- Guven, D.C.; Kaya, M.B.; Fedai, B.; Ozden, M.; Yildirim, H.C.; Kosemehmetoglu, K.; Kertmen, N.; Dizdar, O.; Uner, A.; Aksoy, S. HER2-low breast cancer could be associated with an increased risk of brain metastasis. Int. J. Clin. Oncol. 2022, 27, 332–339. [Google Scholar] [CrossRef]
- Jin, J.; Li, B.; Cao, J.; Li, T.; Zhang, J.; Cao, J.; Zhao, M.; Wang, L.; Wang, B.; Tao, Z.; et al. Analysis of clinical features, genomic landscapes and survival outcomes in HER2-low breast cancer. J. Transl. Med. 2023, 21, 360. [Google Scholar] [CrossRef]
- Harbeck, N.; Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Niikura, N.; et al. Trastuzumab deruxtecan vs treatment of physician’s choice in patients with HER2-low unresectable and/or metastatic breast cancer: Subgroup analyses from DESTINY-Breast04. Cancer Res. 2022, 83, P1-11-01. [Google Scholar] [CrossRef]
- Tsurutani, J.; Jacot, W.; Yamashita, T.; Riaz, F.; Yerushalmi, R.; Im, S.-A.; Niikura, N.; Halser-Strub, U.; Cortés, J.; Wennstig, A.-K.; et al. Subgroup analysis of patients (pts) with HER2-low metastatic breast cancer (mBC) with brain metastases (BMs) at baseline from DESTINY-Breast04, a randomized phase III study of trastuzumab deruxtecan (T-DXd) vs treatment of physician’s choice (TPC). Ann. Oncol. 2023, 34, S342. [Google Scholar] [CrossRef]
- Vaz Batista, M.; Pérez-García, J.M.; Cortez, P.; Garrigós, L.; Fernández-Abad, M.; Gion, M.; Martínez-Bueno, A.; Saavedra, C.; Teruel, I.; Fernandez-Ortega, A.; et al. Trastuzumab deruxtecan in patients with previously treated HER2-low advanced breast cancer and active brain metastases: The DEBBRAH trial. ESMO Open 2024, 9, 103699. [Google Scholar] [CrossRef] [PubMed]
- Hechtman, J.F.; Ross, D.S. The past, present, and future of HER2 (ERBB2) in cancer: Approaches to molecular testing and an evolving role in targeted therapy. Cancer Cytopathol. 2019, 127, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Tozbikian, G.; Bui, M.M.; Hicks, D.G.; Jaffer, S.; Khoury, T.; Wen, H.Y.; Krishnamurthy, S.; Wei, S. Best practices for achieving consensus in HER2-low expression in breast cancer: Current perspectives from practising pathologists. Histopathology 2024, 85, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Pauletti, G.; Godolphin, W.; Press, M.F.; Slamon, D.J. Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene 1996, 13, 63–72. [Google Scholar]
- Marchiò, C.; Annaratone, L.; Marques, A.; Casorzo, L.; Berrino, E.; Sapino, A. Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin. Cancer Biol. 2021, 72, 123–135. [Google Scholar] [CrossRef]
- Garrido, C.; Manoogian, M.; Ghambire, D.; Lucas, S.; Karnoub, M.; Olson, M.T.; Hicks, D.G.; Tozbikian, G.; Prat, A.; Ueno, N.T.; et al. Analytical and clinical validation of PATHWAY Anti-HER-2/neu (4B5) antibody to assess HER2-low status for trastuzumab deruxtecan treatment in breast cancer. Virchows Arch. 2024, 484, 1005–1014. [Google Scholar] [CrossRef]
- Hoffman La Roche Ltd. Roche Obtains CE Mark for First Companion Diagnostic to Identify Patients with HER2-Low Metastatic Breast Cancer Eligible for ENHERTU. [Press Release]. 10 April 2024. 2024. Available online: https://diagnostics.roche.com/global/en/news-listing/2024/roche-obtains-ce-mark-for-first-companion-diagnostic-to-identify-patients-with-her2-low-metastatic-breast-cancer-eligible-for-enhertu.html (accessed on 5 November 2025).
- Baez-Navarro, X.; Salgado, R.; Denkert, C.; Lennerz, J.K.; Penault-Llorca, F.; Viale, G.; Bartlett, J.M.S.; van Deurzen, C.H.M. Selecting patients with HER2-low breast cancer: Getting out of the tangle. Eur. J. Cancer 2022, 175, 187–192. [Google Scholar] [CrossRef]
- Ivanova, M.; Porta, F.M.; D’Ercole, M.; Pescia, C.; Sajjadi, E.; Cursano, G.; De Camilli, E.; Pala, O.; Mazzarol, G.; Venetis, K.; et al. Standardized pathology report for HER2 testing in compliance with 2023 ASCO/CAP updates and 2023 ESMO consensus statements on HER2-low breast cancer. Virchows Arch. 2024, 484, 3–14. [Google Scholar] [CrossRef]
- Sajjadi, E.; Guerini-Rocco, E.; De Camilli, E.; Pala, O.; Mazzarol, G.; Venetis, K.; Ivanova, M.; Fusco, N. Pathological identification of HER2-low breast cancer: Tips, tricks, and troubleshooting for the optimal test. Front. Mol. Biosci. 2023, 10, 1176309. [Google Scholar] [CrossRef]
- Fernandez, A.I.; Liu, M.; Bellizzi, A.; Brock, J.; Fadare, O.; Hanley, K.; Harigopal, M.; Jorns, J.M.; Kuba, M.G.; Ly, A.; et al. Examination of low ERBB2 protein expression in breast cancer tissue. JAMA Oncol. 2022, 8, 607–610. [Google Scholar] [CrossRef]
- Zoppoli, G.; Garuti, A.; Cirmena, G.; di Cantogno, L.V.; Botta, C.; Gallo, M.; Ferraioli, D.; Carminati, E.; Baccini, P.; Curto, M.; et al. Her2 assessment using quantitative reverse transcriptase polymerase chain reaction reliably identifies Her2 overexpression without amplification in breast cancer cases. J. Transl. Med. 2017, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Seung, B.J.; Cho, S.H.; Kim, S.H.; Lim, H.Y.; Sur, J.H. Quantitative analysis of HER2 mRNA expression by RNA in situ hybridization in canine mammary gland tumors: Comparison with immunohistochemistry analysis. PLoS ONE 2020, 15, e0229031. [Google Scholar] [CrossRef] [PubMed]
- Moutafi, M.; Robbins, C.J.; Yaghoobi, V.; Fernandez, A.I.; Martinez-Morilla, S.; Xirou, V.; Bai, Y.; Song, Y.; Gaule, P.; Krueger, J.; et al. Quantitative measurement of HER2 expression to subclassify ERBB2 unamplified breast cancer. Lab. Investig. 2022, 102, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Hicks, D.G.; Buscaglia, B.; Goda, H.; McMahon, L.; Natori, T.; Turner, B.; Soukiazian, A.; Okada, H.; Nakano, Y. A novel detection methodology for HER2 protein quantitation in formalin-fixed, paraffin embedded clinical samples using fluorescent nanoparticles: An analytical and clinical validation study. BMC Cancer 2018, 18, 1266. [Google Scholar] [CrossRef]
- Wu, S.; Yue, M.; Zhang, J.; Li, X.; Li, Z.; Zhang, H.; Wang, X.; Han, X.; Cai, L.; Shang, J.; et al. The role of artificial intelligence in accurate interpretation of HER2 immunohistochemical scores 0 and 1+ in breast cancer. Mod. Pathol. 2023, 36, 100054. [Google Scholar] [CrossRef]
- Goldsmith, J.D.; Troxell, M.L.; Roy-Chowdhuri, S.; Colasacco, C.F.; Edgerton, M.E.; Fitzgibbons, P.L.; Fulton, R.; Haas, T.; Kandalaft, P.L.; Kalicanin, T.; et al. Principles of Analytic Validation of Immunohistochemical Assays: Guideline Update. Arch. Pathol. Lab. Med. 2024, 148, e111–e153. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Approves Fam-Trastuzumab Deruxtecan-Nxki for Unresectable or Metastatic HR-Positive, HER2-Low or HER2-Ultralow Breast Cancer. [Press Release]. 6 February 2025. 2025. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-hr-positive-her2-low-or-her2 (accessed on 23 June 2025).
- Nicolò, E.; Boscolo Bielo, L.; Curigliano, G.; Tarantino, P. The HER2-low revolution in breast oncology: Steps forward and emerging challenges. Ther. Adv. Med. Oncol. 2023, 15, 17588359231152842. [Google Scholar] [CrossRef]
- Scott, M.; Vandenberghe, M.E.; Scorer, P.; Boothman, A.-M.; Barker, C. Prevalence of HER2 low in breast cancer subtypes using the VENTANA anti-HER2/neu (4B5) assay. J. Clin. Oncol. 2021, 39, 1021. [Google Scholar] [CrossRef]
- Sauter, G.; Lee, J.; Bartlett, J.M.; Slamon, D.J.; Press, M.F. Guidelines for human epidermal growth factor receptor 2 testing: Biologic and methodologic considerations. J. Clin. Oncol. 2009, 27, 1323. [Google Scholar] [CrossRef]
- Rüschoff, J.; Friedrich, M.; Nagelmeier, I.; Kirchner, M.; Andresen, L.M.; Salomon, K.; Portier, B.; Sredni, S.T.; Schildhaus, H.U.; Jasani, B.; et al. Comparison of HercepTest™ mAb pharmDx (Dako Omnis, GE001) with Ventana PATHWAY anti-HER-2/neu (4B5) in breast cancer: Correlation with HER2 amplification and HER2 low status. Virchows Arch. 2022, 481, 685–694. [Google Scholar] [CrossRef]
- Schildhaus, H.-U.; Badve, S.; D’Arrigo, C.; Farshid, G.; Lebeau, A.; Peg, V.; Penault-Llorca, F.; Rueschoff, J.; Yang, W.; Atkey, N.; et al. Concordance between the DESTINY-Breast04 clinical trial assay (4B5[CDx]) and other HER2 IHC assays for HER2-low breast cancer in real-world practice: First phase of a large-scale, multicenter global ring study. Cancer Res. 2024, 84, 1030. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Lam, C.; Luo, L.; Baverstock, R.; Pyrih, N.; Redpath, S.; Sue-Ann Woo, M.; Collin, S.M.; Davies, C.; Gannon, V.; et al. Abstract P3-09-20: Re-evaluation of human epidermal growth factor receptor 2 (HER2) immunohistochemistry (IHC) 0 or 1+ in metastatic breast cancer (mBC) samples to characterize the proportion of HER2-ultralow (IHC 0 with membrane staining). Clin. Cancer Res. 2025, 31, P3-09-20. [Google Scholar] [CrossRef]
- Hanna, W.M.; Rüschoff, J.; Bilous, M.; Coudry, R.A.; Dowsett, M.; Osamura, R.Y.; Penault-Llorca, F.; van de Vijver, M.; Viale, G. HER2 in situ hybridization in breast cancer: Clinical implications of polysomy 17 and genetic heterogeneity. Mod. Pathol. 2014, 27, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, A.; Bertaut, A.; Beltjens, F.; Charon-Barra, C.; Amet, A.; Jankowski, C.; Desmoulins, I.; Ladoire, S.; Arnould, L. Anticipating changes in the HER2 status of breast tumours with disease progression-towards better treatment decisions in the new era of HER2-low breast cancers. Br. J. Cancer 2023, 129, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Bar, Y.; Dedeoglu, A.S.; Fell, G.G.; Moffett, N.J.; Boyraz, B.; Ly, A.; Bardia, A.; Moy, B.; Ellisen, L.W.; Isakoff, S.J. Dynamic HER2-low status among patients with triple negative breast cancer (TNBC): The impact of repeat biopsies. J. Clin. Oncol. 2023, 41, 1005. [Google Scholar] [CrossRef]
- Hou, Y.; Nitta, H.; Li, Z. HER2 intratumoral heterogeneity in breast cancer, an evolving concept. Cancers 2023, 15, 2664. [Google Scholar] [CrossRef]
- Geukens, T.; De Schepper, M.; Richard, F.; Maetens, M.; Van Baelen, K.; Mahdami, A.; Nguyen, H.L.; Isnaldi, E.; Leduc, S.; Pabba, A.; et al. Intra-patient and inter-metastasis heterogeneity of HER2-low status in metastatic breast cancer. Eur. J. Cancer 2023, 188, 152–160. [Google Scholar] [CrossRef]
- Tarantino, P.; Gandini, S.; Nicolò, E.; Trillo, P.; Giugliano, F.; Zagami, P.; Vivanet, G.; Bellerba, F.; Trapani, D.; Marra, A.; et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur. J. Cancer 2022, 163, 35–43. [Google Scholar] [CrossRef]
- Löb, S.; Linsmeier, E.; Herbert, S.L.; Schlaiß, T.; Kiesel, M.; Wischhusen, J.; Salmen, J.; Kranke, P.; Quenzer, A.; Kurz, F.; et al. Prognostic effect of HER2 evolution from primary breast cancer to breast cancer metastases. J. Cancer Res. Clin. Oncol. 2023, 149, 5417–5428. [Google Scholar] [CrossRef]
- Lin, M.; Luo, T.; Jin, Y.; Zhong, X.; Zheng, D.; Zeng, C.; Guo, Q.; Wu, J.; Shao, Z.M.; Hu, X.; et al. HER2-low heterogeneity between primary and paired recurrent/metastatic breast cancer: Implications in treatment and prognosis. Cancer 2024, 130, 851–862. [Google Scholar] [CrossRef]
- Holthuis, E.I.; Vondeling, G.T.; Kuiper, J.G.; Dezentjé, V.; Rosenlund, M.; Overbeek, J.A.; van Deurzen, C.H.M. Real-world data of HER2-low metastatic breast cancer: A population based cohort study. Breast 2022, 66, 278–284. [Google Scholar] [CrossRef]
- de Calbiac, O.; Lusque, A.; Mailliez, A.; Bachelot, T.; Uwer, L.; Mouret-Reynier, M.A.; Emile, G.; Jouannaud, C.; Gonçalves, A.; Patsouris, A.; et al. Comparison of management and outcomes in ERBB2-low vs ERBB2-Zero metastatic breast cancer in France. JAMA Netw. Open 2022, 5, e2231170. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Ngo, L.D.; Richardson, E.T.; Frangieh, A.; Mohammed-Abreu, A.; Hughes, M.E.; Zanudo, J.G.T.; Navarro, J.; Tarantino, P.; Mittendorf, E.A.; et al. Abstract HER2-10: HER2-10 Dynamics of HER2-low expression in triple-negative breast cancer. Cancer Res. 2023, 83, HER12-10. [Google Scholar] [CrossRef]
- Alvarez Lopez, I.; Guerrero Zotano, A.L.; Antolín-Novoa, S.; Tibau, A.; Falo, C.; Hernández Sosa, M.; Miguel Rodriguez, A.; Rodríguez-Lescure, A.; Martinez del Prado, P.; Chacon, J.I.; et al. Characteristics and outcomes of HER2-low (H-Low) and HER2-zero (H-0) advanced breast cancer (ABC) patients (pts) from GEICAM/2014-03 (RegistEM) registry. ESMO Breast Cancer 2023, 8, 24. [Google Scholar] [CrossRef]
- Hong, B.; Ying, F.; Zhaoqing, F.; Xichun, H.; Man, L.; Qiao, L.; Ning, L.; Ting, L.; Jianyun, N.; Yueyin, P.; et al. Consensus on clinical diagnosis and medical treatment of HER2-low breast cancer (2022 edition). J. Natl. Cancer Cent. 2023, 3, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Geukens, T.; De Schepper, M.; Richard, F.; Maetens, M.; Van Baelen, K.; Mahdami, A.; Nguyen, H.-L.; Isnaldi, E.; Leduc, S.; Pabba, A.; et al. Abstract HER2-16: HER2-16 Inter-lesion heterogeneity of HER2-status in metastatic breast cancer: Possible implications for treatment with anti-HER2 antibody-drug conjugates. Cancer Res. 2023, 83, HER12-16. [Google Scholar] [CrossRef]
- Ueno, N.T.; Jacot, W.; Yamashita, T.; Sohn, J.; Tokunaga, E.; Prat, A.; Tsurutani, J.; Park, Y.H.; Rugo, H.S.; Xu, B.; et al. Patient-reported outcomes (PROs) from DESTINY-Breast04, a randomized phase III study of trastuzumab deruxtecan (T-DXd) vs treatment of physician’s choice (TPC) in patients (pts) with HER2-low metastatic breast cancer (MBC). Ann. Oncol. 2022, 33, S632–S633. [Google Scholar] [CrossRef]
- Hu, X.; Curigliano, G.; Yonemori, K.; Bardia, A.; Barrios, C.H.E.; Sohn, J.; Levy, C.; Jacot, W.; Tsurutani, J.; K., M.; et al. Effects of trastuzumab deruxtecan (T-DXd) vs choice of chemotherapy (TPC) on patient-reported outcomes (PROs) in hormone receptor-positive, HER2-low or HER2-ultralow metastatic breast cancer (mBC): Results from DESTINY-Breast06. ESMO Congr. 2024, 35, 1–72. [Google Scholar] [CrossRef]
- Ueno, N.T.; Cottone, F.; Dunton, K.; Jacot, W.; Yamashita, T.; Sohn, J.; Tokunaga, E.; Prat, A.; Tsurutani, J.; Park, Y.H.; et al. Patient-reported outcomes from DESTINY-Breast04: Trastuzumab deruxtecan versus physician’s choice of chemotherapy in patients with HER2-low mBC. Oncologist 2025, 30, oyaf048. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bianchini, G.; Cortes, J.; Henning, J.W.; Untch, M. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. ESMO Open 2022, 7, 100553. [Google Scholar] [CrossRef] [PubMed]
- Abuhelwa, Z.; Alloghbi, A.; Alqahtani, A.; Nagasaka, M. Trastuzumab deruxtecan-induced interstitial lung disease/pneumonitis in ERBB2-positive advanced solid malignancies: A systematic review. Drugs 2022, 82, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Nishino, M.; Lancaster, L.H.; Li, B.T.; Nicholson, A.G.; Bartholmai, B.J.; Naidoo, J.; Schumacher-Wulf, E.; Shitara, K.; Tsurutani, J.; et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)–related interstitial lung disease/pneumonitis—Focus on proactive monitoring, diagnosis, and management. Cancer Treat. Rev. 2022, 106, 102378. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.A.; Modi, S.; Iwata, H.; Takahashi, S.; Smit, E.F.; Siena, S.; Chang, D.Y.; Macpherson, E.; Qin, A.; Singh, J.; et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open 2022, 7, 100554. [Google Scholar] [CrossRef]
- Park, Y.H.; Jacot, W.; Hurvitz, S.A.; Modi, S.; Yamashita, T.; Xu, B.; Tokunaga, E.; Wang, X.; Lee, K.S.; Iwata, H.; et al. Exploratory pooled safety analysis of trastuzumab deruxtecan (T-DXd) in patients With HER2+ or HER2-low unresectable and/or metastatic breast cancer (mBC) in DESTINY-Breast trials. ESMO Open 2024, 9, 103208. [Google Scholar] [CrossRef]
- Ciruelos, E.; García-Sáenz, J.; Gavilá, J.; Martín, M.; Rodríguez, C.A.; Rodríguez-Lescure, Á. Safety profile of trastuzumab deruxtecan in advanced breast cancer: Expert opinion on adverse event management. Clin. Transl. Oncol. 2024, 26, 1539–1548. [Google Scholar] [CrossRef]
- Skeoch, S.; Weatherley, N.; Swift, A.J.; Oldroyd, A.; Johns, C.; Hayton, C.; Giollo, A.; Wild, J.M.; Waterton, J.C.; Buch, M.; et al. Drug-induced interstitial lung disease: A systematic review. J. Clin. Med. 2018, 7, 356. [Google Scholar] [CrossRef]
- Johkoh, T.; Lee, K.S.; Nishino, M.; Travis, W.D.; Ryu, J.H.; Lee, H.Y.; Ryerson, C.J.; Franquet, T.; Bankier, A.A.; Brown, K.K.; et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: A position paper from the Fleischner Society. Radiology 2021, 298, 550–566. [Google Scholar] [CrossRef]
- Wekking, D.; Porcu, M.; Pellegrino, B.; Lai, E.; Mura, G.; Denaro, N.; Saba, L.; Musolino, A.; Scartozzi, M.; Solinas, C. Multidisciplinary clinical guidelines in proactive monitoring, early diagnosis, and effective management of trastuzumab deruxtecan (T-DXd)-induced interstitial lung disease (ILD) in breast cancer patients. ESMO Open 2023, 8, 102043. [Google Scholar] [CrossRef]
- Tarantino, P.; Tolaney, S.M. Detecting and managing T-DXd-related interstitial lung disease: The five “S” rules. JCO Oncol. Pract. 2023, 19, 526–527. [Google Scholar] [CrossRef]
- Rugo, H.S.; Crossno, C.L.; Gesthalter, Y.B.; Kelley, K.; Moore, H.B.; Rimawi, M.F.; Westbrook, K.E.; Buys, S.S. Real-world perspectives and practices for pneumonitis/interstitial lung disease associated with trastuzumab deruxtecan use in human epidermal growth factor receptor 2-expressing metastatic breast cancer. JCO Oncol. Pract. 2023, 19, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Natsuhara, K.H.; Blum, K.; LeVee, A.A.; Vobugari, N.; Dempsey, N.; Premji, S.K.; Moe, C.; Reddy, N.; Hoppenworth, J.E.; Vella, M.; et al. Treatment rechallenge after trastuzumab-deruxtecan–related interstitial lung disease: A multi-institution cohort study. J. Clin. Oncol. 2025, 43, 1015. [Google Scholar] [CrossRef]
- Referenced from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Antiemesis V.2.2025. ©National Comprehensive Cancer Network, Inc, 2025. All rights reserved. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1415 (accessed on 3 December 2025). To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Notini, G.; Naldini, M.M.; Sica, L.; Viale, G.; Rognone, A.; Zambelli, S.; Zucchinelli, P.; Piras, M.; Bosi, C.; Mariani, M.; et al. Management of trastuzumab deruxtecan-related nausea and vomiting in real-world practice. Front. Oncol. 2024, 14, 1374547. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Tsurutani, J.; Ozaki, Y.; Ishiguro, H.; Nozawa, K.; Yamanaka, T.; Aogi, K.; Matsumoto, K.; Iwasa, T.; Tokiwa, M.; et al. A randomized, double-blind, placebo-controlled phase II study of olanzapine based prophylactic antiemetic therapy for delayed and persistent nausea and vomiting in patients with HER2-positive or HER2-low breast cancer treated with trastuzumab deruxtecan: ERICA study (WJOG14320B). Ann. Oncol. 2024, 36, 31–42. [Google Scholar] [CrossRef]
- Bianchini, G.; Park, Y.H.; Rugo, H.S.; Licata, L.; Iihara, H.; Massagrande, M.; Dato, F.; Scotté, F.; Jordan, K.; Aapro, M.S. Perceptions of antibody drug conjugate (ADC)-induced nausea and vomiting: Results of a survey of healthcare providers at ESMO. ESMO Breast Cancer 2024, 9, 103348. [Google Scholar] [CrossRef]
- Zuo, S.; Cheng, H.; Wang, Z.; Liu, T.; Chen, S.; Tian, L.; Lin, L. Nonpharmacological interventions for cancer-related fatigue: A literature review. Asia Pac. J. Oncol. Nurs. 2023, 10, 100230. [Google Scholar] [CrossRef]
- US National Library of Medicine. Scalp Cooling in MBC. Available online: https://clinicaltrials.gov/study/NCT04986579 (accessed on 23 June 2025).
- Kruse, M.; Abraham, J. Management of chemotherapy-induced alopecia with scalp cooling. J. Oncol. Pract. 2018, 14, 149–154. [Google Scholar] [CrossRef]
- Repetto, L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J. Support. Oncol. 2003, 1, 18–24. [Google Scholar]
- Evans, N.; Anton, A.; Wong, R.; Lok, S.W.; De Boer, R.; Malik, L.; Greenberg, S.; Yeo, B.; Nott, L.; Richardson, G.; et al. Abstract PS6-35: Real world outcomes in elderly women with HER2 positive advanced breast cancer. Cancer Res. 2021, 81, PS6-35. [Google Scholar] [CrossRef]
- Krop, I.E.; Wildiers, H.; Hurvitz, S.A.; Cortes, J.; Im, S.-A.; Iwata, H.; Andre, F.; Saura, C.; Modi, S.; Kim, S.-B.; et al. An age-specific pooled analysis of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-positive (HER2+) metastatic breast cancer (mBC) from DESTINY-Breast01, -02, and -03. J. Clin. Oncol. 2023, 41, 1006. [Google Scholar] [CrossRef]
- Modi, S.; Salgado, R.; Guarneri, V.; Alagappan, D.; Dennis, N.; Newell, A.H.; Boran, A.; Morsli, O.; Llombart-Cussac, A. Abstract PO2-19-06: An open-label, interventional, multicenter study of trastuzumab deruxtecan monotherapy in patients with unresectable and/or metastatic HER2-low or HER2 immunohistochemistry 0 breast cancer: DESTINY-Breast15. Cancer Res. 2024, 84, PO2-19-06. [Google Scholar] [CrossRef]
- Moore, K.N.; Sabanathan, D.; Du, Y.; Duan, H.; Li, X.; Wang, F.; Marathe, O.; Yang, H.; Makker, V.; Growdon, W.; et al. Safety and efficacy of DB-1303 in patients with advanced/metastatic solid tumors: A multicenter, open-label, first-in-human, phase 1/2a study. J. Clin. Oncol. 2023, 41, 3023. [Google Scholar] [CrossRef]
- Bardia, A.; O’Sullivan, C.C.; Mahtani, R.L.; Blau, S.; Abdulla, N.E.; Ali, A.; Bansal, R.; Meyer, J.M.; George, M.A.; Han, H.S.; et al. An open-label, multicenter, phase 2 study to evaluate the safety and efficacy of BB-1701, a novel antibody drug conjugate (ADC) targeting human epidermal growth factor receptor 2 (HER2), in previously treated patients with HER2-positive (HER2+) or HER2-low unresectable or metastatic breast cancer (BC). J. Clin. Oncol. 2024, 42, TPS1122. [Google Scholar] [CrossRef]
- Lewis, G.D.; Li, G.; Guo, J.; Yu, S.F.; Fields, C.T.; Lee, G.; Zhang, D.; Dragovich, P.S.; Pillow, T.; Wei, B.; et al. The HER2-directed antibody-drug conjugate DHES0815A in advanced and/or metastatic breast cancer: Preclinical characterization and phase 1 trial results. Nat. Commun. 2024, 15, 466. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Zhang, Q.; Li, W.; Feng, J.; Wang, X.; Fang, J.; Han, Y.; Xu, B. Disitamab vedotin, a HER2-directed antibody-drug conjugate, in patients with HER2-overexpression and HER2-low advanced breast cancer: A phase I/Ib study. Cancer Commun. 2024, 44, 833–851. [Google Scholar] [CrossRef] [PubMed]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef]
- US National Library of Medicine. Phase II Open-Label Study of ARX788 (Anti-HER2 Antibody Drug Conjugate (ADC)) for Patients with HER2-Low Locally Advanced Unresectable or Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/study/NCT06224673 (accessed on 23 June 2025).
- Dent, R.A.; Cescon, D.W.; Bachelot, T.; Jung, K.H.; Shao, Z.M.; Saji, S.; Traina, T.A.; Vukovic, P.; Mapiye, D.; Maxwell, M.J.; et al. TROPION-Breast02: Datopotamab deruxtecan for locally recurrent inoperable or metastatic triple-negative breast cancer. Future Oncol. 2023, 19, 2349–2359. [Google Scholar] [CrossRef]
- Schmid, P.; Wysocki, P.J.; Ma, C.X.; Park, Y.H.; Fernandes, R.; Lord, S.; Baird, R.D.; Prady, C.; Jung, K.H.; Asselah, J.; et al. 379MO Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): Updated results from BEGONIA, a phase Ib/II study. Ann. Oncol. 2023, 34, S337. [Google Scholar] [CrossRef]
- Bardia, A.; Pusztai, L.; Albain, K.; Ciruelos, E.M.; Im, S.A.; Hershman, D.; Kalinsky, K.; Isaacs, C.; Loirat, D.; Testa, L.; et al. TROPION-Breast03: A randomized phase III global trial of datopotamab deruxtecan ± durvalumab in patients with triple-negative breast cancer and residual invasive disease at surgical resection after neoadjuvant therapy. Ther. Adv. Med. Oncol. 2024, 16, 17588359241248336. [Google Scholar] [CrossRef]
- McArthur, H.L.; Tolaney, S.M.; Dent, R.; Schmid, P.; Asselah, J.; Liu, Q.; Meisel, J.L.; Niikura, N.; Park, Y.H.; Werutsky, G.; et al. TROPION-Breast04: A randomized phase III study of neoadjuvant datopotamab deruxtecan (Dato-DXd) plus durvalumab followed by adjuvant durvalumab versus standard of care in patients with treatment-naïve early-stage triple negative or HR-low/HER2- breast cancer. Ther. Adv. Med. Oncol. 2025, 17, 17588359251316176. [Google Scholar] [CrossRef]
- Schmid, P.; Oliveira, M.; O’Shaughnessy, J.; Cristofanilli, M.; Graff, S.L.; Im, S.A.; Loi, S.; Saji, S.; Wang, S.; Cescon, D.W.; et al. TROPION-Breast05: A randomized phase III study of Dato-DXd with or without durvalumab versus chemotherapy plus pembrolizumab in patients with PD-L1-high locally recurrent inoperable or metastatic triple-negative breast cancer. Ther. Adv. Med. Oncol. 2025, 17, 17588359251327992. [Google Scholar] [CrossRef]
- Rugo, H.; Cortés, J.; Curigliano, G.; Barrios, C.; Punie, K.; Park, Y.; Iwata, H.; Young, A.; Ren, X.; Cinar, P.; et al. ASCENT-07: A phase 3, randomized, open-label study of sacituzumab govitecan versus treatment of physician’s choice in patients with HR+/HER2– inoperable, locally advanced, or metastatic breast cancer post-endocrine therapy. Cancer Res. 2024, 84, PO01-05-09. [Google Scholar] [CrossRef]
- Vaz Batista, M.; Pérez-García, J.M.; Garrigós, L.; García-Sáenz, J.; Cortez, P.; Racca, F.; Blanch, S.; Ruiz-Borrego, M.; Fernández-Ortega, A.; Fernández-Abad, M.; et al. The DEBBRAH trial: Trastuzumab deruxtecan in HER2-positive and HER2-low breast cancer patients with leptomeningeal carcinomatosis. Med 2025, 6, 100502. [Google Scholar] [CrossRef]
- Jhaveri, K.; André, F.; Hamilton, E.; Schmid, P.; Anders, C.; Testa, L.; Ganshina, I.; Lu, Y.-S.; Im, S.-A.; Young, R.; et al. Trastuzumab deruxtecan (T-DXd) in combination with anastrozole or fulvestrant in patients with HER2-low HR+ advanced/metastatic breast cancer: A Phase 1b, open-label, multicenter, dose-expansion study (DESTINY-Breast08). Cancer Res. 2024, 84, RF02-033. [Google Scholar] [CrossRef]
- US National Library of Medicine. Trastuzumab Deruxtecan (T-DXd) in Patients Who Have Hormone Receptor-Negative and Hormone Receptor-Positive HER2-Low or HER2 IHC 0 Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/study/NCT05950945 (accessed on 12 June 2025).
- Guarneri, V.; Passos Coelho, J.L.; Duhoux, F.P.; Egle, D.; García-Sáenz, J.; Penault-Llorca, F.; Selander, K.; Wildiers, H.; Zaman, K.; Laeis, P.; et al. Study design for DESTINY-Breast Respond HER2-low Europe: T-DXd in patients with HER2-low advanced breast cancer. Future Oncol. 2024, 20, 1237–1250. [Google Scholar] [CrossRef]
- US National Library of Medicine. A Randomized Phase II Study to Evaluate the Safety and Efficacy of Trastuzumab Deruxtecan Versus CDK4/6 Inhibitor-Based Endocrine Therapy as First-Line Therapy of HR-Positive and HER2-Low/Ultralow Advanced Breast Cancer Patients Classified as Non-Luminal Subtype According to Gene Expression Profiling. Available online: https://clinicaltrials.gov/study/NCT06486883 (accessed on 4 June 2025).
- US National Library of Medicine. T-DXd Therapy for HER2-Low Breast Cancer Patients with Brain Metastases (TUXEDO-4). Available online: https://clinicaltrials.gov/study/NCT06048718 (accessed on 12 June 2025).
- Marhold, M.; Vaz Batista, M.; Blancas, I.; Morales, C.; Saura-Manich, C.; Saavedra, C.; Ruíz-Borrego, M.; Cortez, P.; Slebe, F.; Campolier, M.; et al. TUXEDO-4: Phase II study of trastuzumab-deruxtecan in HER2-low breast cancer with new or progressing brain metastases. Future Oncol. 2025, 21, 1065–1073. [Google Scholar] [CrossRef]
- US National Library of Medicine. A phase II Non-Comparative Trial of Datopotamab Deruxtecan (Dato-DXd) or Trastuzumab Deruxtecan (T-DXd) in Patients with Metastatic HER2-Low Breast Cancer After Progression on Prior Antibody Drug Conjugate therapy. Available online: https://clinicaltrials.gov/study/NCT06533826 (accessed on 13 February 2025).


| DESTINY-Breast04 a,b | HR-Positive Cohort n = 494 | HR-Negative Cohort n = 58 | All Patients n = 557 | ||||
|---|---|---|---|---|---|---|---|
| Efficacy Endpoint | T-DXd | TPC | T-DXd | TPC | T-DXd | TPC | |
| Primary analysis [6] DCO, 11 January 2022 | N | 331 | 163 | 40 | 18 | 373 | 184 |
| ORR by BICR c (95% CI), % | 52.6 (47.0–58.0) | 16.3 (11.0–22.8) | 50.0 (33.8–66.2) | 16.7 (3.6–41.4) | 52.3 (47.1–57.4) | 16.3 (11.3–22.5) | |
| PFS by BICR, median (95% CI), months | 10.1 (9.5–11.5) | 5.4 (4.4–7.1) | 8.5 (4.3–11.7) | 2.9 (1.4–5.1) | 9.9 (9.0–11.3) | 5.1 (4.2–6.8) | |
| Hazard ratio (95% CI) p value | 0.51 (0.40–0.64) p < 0.001 | 0.46 (0.24–0.89) c | 0.50 (0.40–0.63) p < 0.001 | ||||
| OS, median (95% CI), months | 23.9 (20.8–24.8) | 17.5 (15.2–22.4) | 18.2 (13.6–NE) | 8.3 (5.6–20.6) | 23.4 (20.0–24.8) | 16.8 (14.5–20.0) | |
| Hazard ratio (95% CI) p value | 0.64 (0.48–0.86) p = 0.003 | 0.48 (0.24–0.95) d | 0.64 (0.49–0.84) p = 0.001 | ||||
| DESTINY-Breast06 | HER2-Low Population n = 713 | HER2-Ultralow Population n = 152 | ITT Population e n = 866 | ||||
| Efficacy Endpoint | T-DXd | TPC | T-DXd | TPC | T-DXd | TPC | |
| Primary analysis [8] DCO, 18 March 2024 | N | 359 | 354 | 76 | 76 | 436 | 430 |
| ORR by BICR, (95% CI), % | 56.5 (51.2–61.7) | 32.2 (27.4–37.3) | 61.8 (50.0–72.8) | 26.3 (16.9–37.7) | 57.3 (52.5–62.0) | 31.2 (26.8–35.8) | |
| PFS by BICR, median (95% CI), months | 13.2 (11.4–15.2) | 8.1 (7.0–9.0) | 13.2 (9.8–17.3) | 8.3 (5.8–15.2) | 13.2 (12.0–15.2) | 8.1 (7.0–9.0) | |
| Hazard ratio (95% CI) p value | 0.62 (0.52–0.75) p < 0.001 | 0.78 (0.50–1.21)d | 0.64 (0.54–0.76) p < 0.001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagegni, N.A.; Giridhar, K.V.; Stewart, D. HER2-Low and HER2-Ultralow Metastatic Breast Cancer and Trastuzumab Deruxtecan: Common Clinical Questions and Answers. Cancers 2025, 17, 4021. https://doi.org/10.3390/cancers17244021
Bagegni NA, Giridhar KV, Stewart D. HER2-Low and HER2-Ultralow Metastatic Breast Cancer and Trastuzumab Deruxtecan: Common Clinical Questions and Answers. Cancers. 2025; 17(24):4021. https://doi.org/10.3390/cancers17244021
Chicago/Turabian StyleBagegni, Nusayba A., Karthik V. Giridhar, and Daphne Stewart. 2025. "HER2-Low and HER2-Ultralow Metastatic Breast Cancer and Trastuzumab Deruxtecan: Common Clinical Questions and Answers" Cancers 17, no. 24: 4021. https://doi.org/10.3390/cancers17244021
APA StyleBagegni, N. A., Giridhar, K. V., & Stewart, D. (2025). HER2-Low and HER2-Ultralow Metastatic Breast Cancer and Trastuzumab Deruxtecan: Common Clinical Questions and Answers. Cancers, 17(24), 4021. https://doi.org/10.3390/cancers17244021





