Diagnostic Algorithm for Secondary Extramammary Paget Disease from Institutional Cases and Literature Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Immunohistochemistry
2.3. Statistical Analyses
2.4. Literature Review
3. Results
3.1. Clinical and Histopathologic Data of 12 Secondary EMPD at Two Institutions
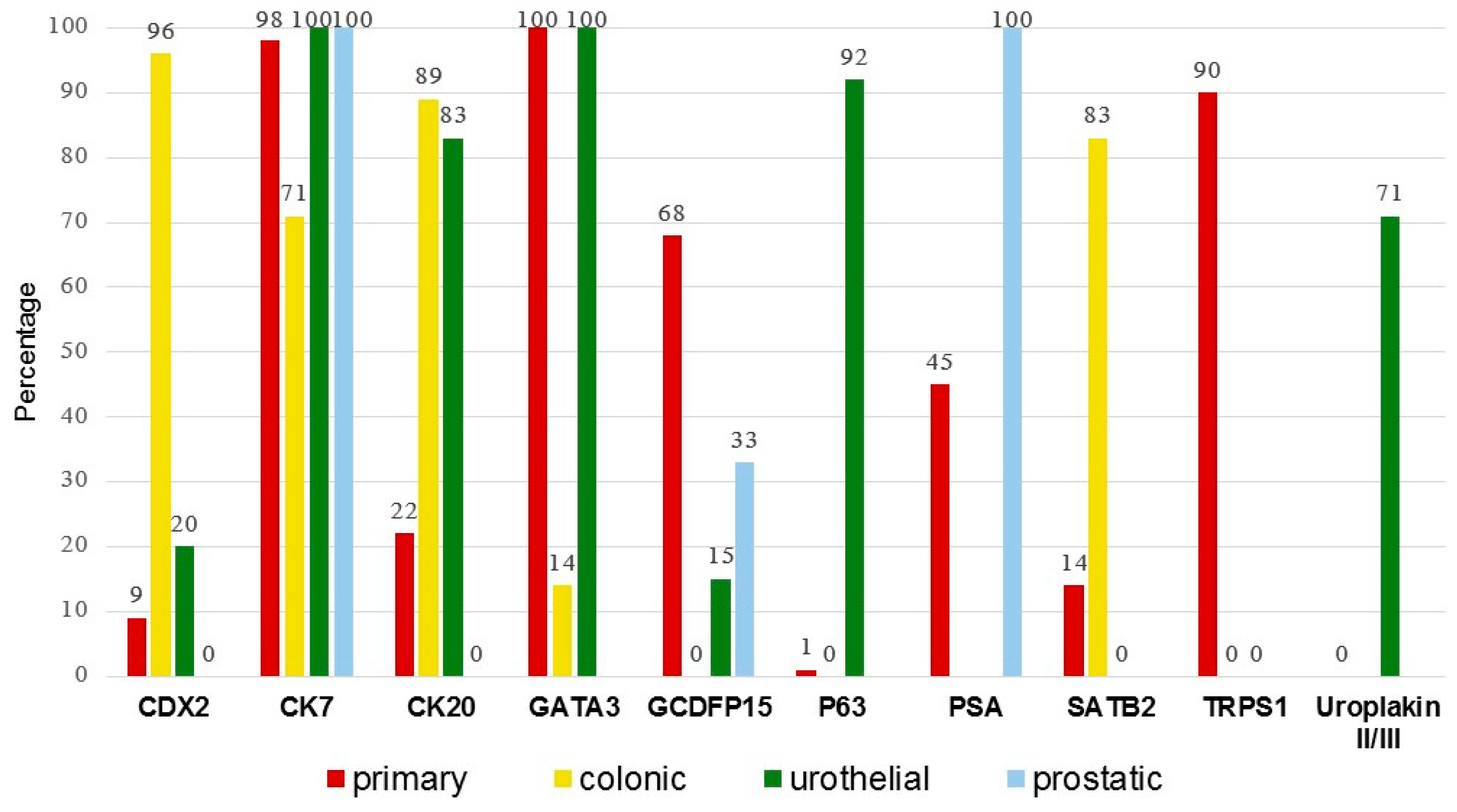
3.2. Comparison of Primary Versus Secondary EMPD
4. Discussion
4.1. TRPS1 Expression in Primary and Secondary EMPD
4.2. SATB2, CK20 and CDX2 Expression in Colonic Secondary EMPD
4.3. GATA3, p63 and Uroplakin Expression in Urothelial Secondary EMPD
4.4. PSA and NKX3.1 Expression in Prostatic Secondary EMPD
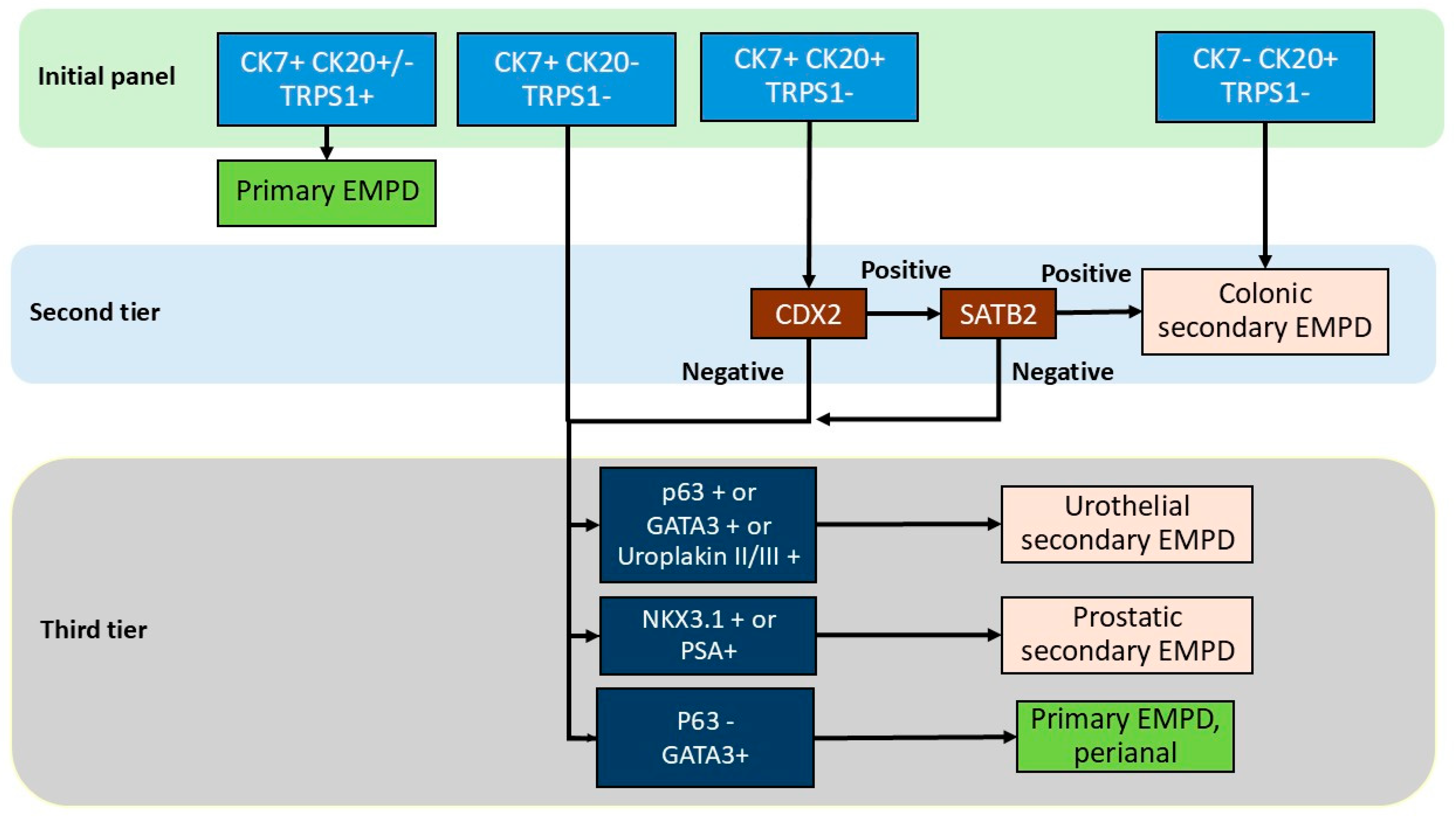
4.5. Proposed Immunohistochemical Algorithm
4.6. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDX2 | Caudal-type homeobox 2 |
| CK | Keratin |
| EMBASE | Excerpta Medica dataBASE |
| EMPD | Extramammary Paget disease |
| GATA3 | GATA binding protein 3 |
| GCDFP15 | Gross cystic disease fluid protein 15 |
| IQR | Interquartile range |
| MUC | Mucin |
| NKX3.1 | NK3 Homeobox 1 |
| PDL1 | Programmed death-ligand 1 |
| PR | Progesterone receptor |
| PSA | Prostatic-specific antigen |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| WT1 | Wilm’s tumor 1 |
| SATB2 | Special AT-rich sequence-binding protein 2 |
| SD | Standard deviation |
| TRPS1 | Trichorhinophalangeal syndrome type 1 |
References
- Chanda, J.J. Extramammary Paget’s disease: Prognosis and relationship to internal malignancy. J. Am. Acad. Dermatol. 1985, 13, 1009–1014. [Google Scholar] [CrossRef]
- Ghazawi, F.M.; Iga, N.; Tanaka, R.; Fujisawa, Y.; Yoshino, K.; Yamashita, C.; Yamamoto, Y.; Fujimura, T.; Yanagi, T.; Hata, H.; et al. Demographic and clinical characteristics of extramammary Paget’s disease patients in Japan from 2000 to 2019. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e133–e135. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.R.; Shah, K.; Wilson, B.N.; Tchack, M.; Busam, K.J.; Moy, A.; Leitao, M.M.; Cordova, M.; Neumann, N.M.; Smogorzewski, J.; et al. Extramammary Paget disease. Part I. epidemiology, pathogenesis, clinical features, and diagnosis. J. Am. Acad. Dermatol. 2024, 91, 409–418. [Google Scholar] [CrossRef]
- Hatta, N. Prognostic Factors of Extramammary Paget’s Disease. Curr. Treat. Options Oncol. 2018, 19, 47. [Google Scholar] [CrossRef]
- Lam, C.; Funaro, D. Extramammary Paget’s disease: Summary of current knowledge. Dermatol. Clin. 2010, 28, 807–826. [Google Scholar] [CrossRef]
- Karam, A.; Dorigo, O. Increased risk and pattern of secondary malignancies in patients with invasive extramammary Paget disease. Br. J. Dermatol. 2014, 170, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.R.; Shah, K.; Wilson, B.N.; Leitao, M.M.; Smogorzewski, J.; Nguyen, K.A.; Crane, C.; Funt, S.A.; Hosein, S.; Dafinone, M.; et al. Extramammary Paget disease. Part II. Evidence-based approach to management. J. Am. Acad. Dermatol. 2024, 91, 421–430. [Google Scholar] [CrossRef]
- Kibbi, N.; Owen, J.L.; Worley, B.; Wang, J.X.; Harikumar, V.; Downing, M.B.; Aasi, S.Z.; Aung, P.P.; Barker, C.A.; Bolotin, D.; et al. Evidence-Based Clinical Practice Guidelines for Extramammary Paget Disease. JAMA Oncol. 2022, 8, 618–628. [Google Scholar] [CrossRef]
- Kibbi, N.; Owen, J.L.; Worley, B.; Alam, M.; Extramammary Paget’s Disease Guideline Study, G. Recommended guidelines for screening for underlying malignancy in extramammary Paget’s disease based on anatomic subtype. J. Am. Acad. Dermatol. 2025, 92, 261–268. [Google Scholar] [CrossRef]
- Maloney, N.J.; Yao, H.; Aasi, S.Z.; John, E.M.; Linos, E.; Kibbi, N. Elevated Risk of Visceral Malignant Neoplasms in Extramammary Paget Disease. JAMA Dermatol. 2023, 159, 1139–1141. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Yao, J.; Yang, F.; Huo, L.; Chen, H.; Lu, W.; Soto, L.M.S.; Jiang, M.; Raso, M.G.; Wang, S.; et al. TRPS1: A highly sensitive and specific marker for breast carcinoma, especially for triple-negative breast cancer. Mod. Pathol. 2021, 34, 710–719. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.A.; Collins, K.; Aung, P.P.; Nagarajan, P.; Curry, J.L.; Prieto, V.G.; Torres-Cabala, C.A.; Cho, W.C. TRPS1 expression in primary and secondary extramammary Paget diseases: An immunohistochemical analysis of 93 cases. Hum. Pathol. 2024, 143, 5–9. [Google Scholar] [CrossRef]
- Cook, E.E.; Harrison, B.T.; Hirsch, M.S. TRPS1 expression is sensitive and specific for primary extramammary Paget disease. Histopathology 2023, 83, 104–108. [Google Scholar] [CrossRef]
- Brown, H.M.; Wilkinson, E.J. Uroplakin-III to distinguish primary vulvar Paget disease from Paget disease secondary to urothelial carcinoma. Hum. Pathol. 2002, 33, 545–548. [Google Scholar] [CrossRef]
- Goldblum, J.R.; Hart, W.R. Perianal Paget’s disease: A histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am. J. Surg. Pathol. 1998, 22, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.; Nimmo, M.; Clement, P.B.; Thomson, T.; Benedet, J.L.; Miller, D.; Gilks, C.B. Prognostic factors in Paget’s disease of the vulva: A study of 21 cases. Int. J. Gynecol. Pathol. 1999, 18, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Goldblum, J.R.; Hart, W.R. Vulvar Paget’s disease: A clinicopathologic and immunohistochemical study of 19 cases. Am. J. Surg. Pathol. 1997, 21, 1178–1187. [Google Scholar] [CrossRef]
- Ohnishi, T.; Watanabe, S. The use of cytokeratins 7 and 20 in the diagnosis of primary and secondary extramammary Paget’s disease. Br. J. Dermatol. 2000, 142, 243–247. [Google Scholar] [CrossRef]
- De Nisi, M.C.; D’Amuri, A.; Toscano, M.; Lalinga, A.V.; Pirtoli, L.; Miracco, C. Usefulness of CDX2 in the diagnosis of extramammary Paget disease associated with malignancies of intestinal type. Br. J. Dermatol. 2005, 153, 677–679. [Google Scholar] [CrossRef]
- Yanai, H.; Takahashi, N.; Omori, M.; Oda, W.; Yamadori, I.; Takada, S.; Matsuura, H.; Yoshino, T. Immunohistochemistry of p63 in primary and secondary vulvar Paget’s disease. Pathol. Int. 2008, 58, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Wieland, R.; Adhikari, P.; North, J. The utility of p63, CK7, and CAM5.2 staining in differentiating pagetoid intraepidermal carcinomas. J. Cutan. Pathol. 2023, 50, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Morbeck, D.; Tregnago, A.C.; Baiocchi, G.; Sacomani, C.; Peresi, P.M.; Osorio, C.T.; Schutz, L.; Bezerra, S.M.; de Brot, L.; Cunha, I.W. GATA3 expression in primary vulvar Paget disease: A potential pitfall leading to misdiagnosis of pagetoid urothelial intraepithelial neoplasia. Histopathology 2017, 70, 435–441. [Google Scholar] [CrossRef]
- Zeng, H.A.; Cartun, R.; Ricci, A., Jr. Potential diagnostic utility of CDX-2 immunophenotyping in extramammary Paget’s disease. Appl. Immunohistochem. Mol. Morphol. 2005, 13, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Li, A.F.-Y.; Yang, S.-H.; Ma, H.-H.; Liang, W.-Y. Perianal Paget’s Disease: The 17-Year-Experience of a Single Institution in Taiwan. Gastroenterol. Res. Pract. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Hou, P.-C.; Lee, C.-N.; Wong, T.-W.; Hsu, T.-C.; Wu, C.-L.; Lee, J.Y.-Y. A clinicopathological study of perianal paget disease. Dermatol. Sin. 2022, 40, 222–230. [Google Scholar] [CrossRef]
- Liao, X.; Liu, X.; Fan, X.; Lai, J.; Zhang, D. Perianal Paget’s disease: A clinicopathological and immunohistochemical study of 13 cases. Diagn. Pathol. 2020, 15, 29. [Google Scholar] [CrossRef]
- Nowak, M.A.; Guerriere-Kovach, P.; Pathan, A.; Campbell, T.E.; Deppisch, L.M. Perianal Paget’s disease: Distinguishing primary and secondary lesions using immunohistochemical studies including gross cystic disease fluid protein-15 and cytokeratin 20 expression. Arch. Pathol. Lab. Med. 1998, 122, 1077–1081. [Google Scholar]
- Perrotto, J.; Abbott, J.J.; Ceilley, R.I.; Ahmed, I. The role of immunohistochemistry in discriminating primary from secondary extramammary Paget disease. Am. J. Dermatopathol. 2010, 32, 137–143. [Google Scholar] [CrossRef]
- Hammer, A.; Hager, H.; Steiniche, T. Prostate-specific antigen-positive extramammary Paget’s disease—Association with prostate cancer. APMIS 2008, 116, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, K.; Byrnes, K. Utility of special AT-rich sequence-binding protein 2 (SATB2) immunohistochemistry as a marker for secondary perianal paget disease. Virchows Arch. 2024, 485, 1127–1132. [Google Scholar] [CrossRef]
- Olson, D.J.; Fujimura, M.; Swanson, P.; Okagaki, T. Immunohistochemical features of Paget’s disease of the vulva with and without adenocarcinoma. Int. J. Gynecol. Pathol. 1991, 10, 285–295. [Google Scholar] [CrossRef]
- Chang, J.; Prieto, V.G.; Sangueza, M.; Plaza, J.A. Diagnostic utility of p63 expression in the differential diagnosis of pagetoid squamous cell carcinoma in situ and extramammary Paget disease: A histopathologic study of 70 cases. Am. J. Dermatopathol. 2014, 36, 49–53. [Google Scholar] [CrossRef]
- Memezawa, A.; Okuyama, R.; Tagami, H.; Aiba, S. p63 constitutes a useful histochemical marker for differentiation of pagetoid Bowen’s disease from extramammary Paget’s disease. Acta Derm. Venereol. 2008, 88, 619–620. [Google Scholar] [CrossRef]
- Lau, J.; Kohler, S. Keratin profile of intraepidermal cells in Paget’s disease, extramammary Paget’s disease, and pagetoid squamous cell carcinoma in situ. J. Cutan. Pathol. 2003, 30, 449–454. [Google Scholar] [CrossRef]
- Fujimura, T.; Kambayashi, Y.; Kakizaki, A.; Furudate, S.; Aiba, S. RANKL expression is a useful marker for differentiation of pagetoid squamous cell carcinoma in situ from extramammary Paget disease. J. Cutan. Pathol. 2016, 43, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, N.; Matsumura, Y.; Kanazawa, N.; Morita, K.; Tachibana, T.; Sakurai, T.; Utani, A.; Miyachi, Y. Expression of prostate-specific antigen and androgen receptor in extramammary Paget’s disease and carcinoma. Clin. Exp. Dermatol. 2007, 32, 91–94. [Google Scholar] [CrossRef]
- Liu, Y.A.; Aung, P.P.; Wang, Y.; Ning, J.; Nagarajan, P.; Curry, J.L.; Torres-Cabala, C.A.; Ivan, D.; Prieto, V.G.; Ding, Q.; et al. TRPS1 expression in non-melanocytic cutaneous neoplasms: An immunohistochemical analysis of 200 cases. J. Pathol. Transl. Med. 2024, 58, 72–80. [Google Scholar] [CrossRef]
- Ohnishi, T.; Watanabe, S. Immunohistochemical analysis of human milk fat globulin expression in extramammary Paget’s disease. Clin. Exp. Dermatol. 2001, 26, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Lora, V.; Kanitakis, J. CDX2 expression in cutaneous metastatic carcinomas and extramammary Paget’s Disease. Anticancer Res. 2009, 29, 5033–5037. [Google Scholar] [PubMed]
- Liu, C.F.; Wang, Q.; Kong, Y.Y.; Tu, X.Y.; Wang, J.; Zhu, X.Z. A clinicopathological study of perianal Paget’s disease associated with internal rectal adenocarcinoma. Chin. J. Pathol. 2004, 33, 11–15. [Google Scholar]
- Zuo, Z.; Wu, W.; Li, X.; Zhang, L.; Wang, J.; Guo, Z.; Hu, S.; Zhang, Q. Perianal Paget disease associated with non-invasive colorectal adenoma. J. Cutan. Pathol. 2022, 50, 35–38. [Google Scholar] [CrossRef]
- Kohler, S.; Smoller, B.R. Gross cystic disease fluid protein-15 reactivity in extramammary Paget’s disease with and without associated internal malignancy. Am. J. Dermatopathol. 1996, 18, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Harb, M.; Prince, D.S.; Bassan, M.; Mackenzie, S.; Connor, S.J.; Rutland, T. Extra-mammary Paget’s disease rising from a non-invasive rectal adenoma. Pathology 2022, 54, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, E.J.; Brown, H.M. Vulvar Paget disease of urothelial origin: A report of three cases and a proposed classification of vulvar Paget disease. Hum. Pathol. 2002, 33, 549–554. [Google Scholar] [CrossRef]
- Huh, G.; Cha, B.-B.; Lee, H.J.; Choi, Y.-J.; Kim, W.-S.; Lee, G.-Y. Extramammary Paget’s Disease of the Glans Penis Secondary to a Recurrent Urothelial Carcinoma of the Bladder. Ann. Dermatol. 2023, 35, S369. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, P.P.; Quiroga-Garza, G.M. Extramammary paget disease of the penoscrotal region. Pathol. Res. Pract. 2023, 241, 154283. [Google Scholar] [CrossRef]
- Primo, W.; Primo, G.R.P.; Basilio, D.B.; Machado, K.K.; Carvalho, J.P.; Carvalho, F.M. Vulvar Paget disease secondary to high-grade urothelial carcinoma with underlying massive vascular embolization and cervical involvement: Case report of unusual presentation. Diagn. Pathol. 2019, 14, 125. [Google Scholar] [CrossRef]
- Koyanagi, Y.; Kubo, C.; Nagata, S.; Ryu, A.; Hatano, K.; Kano, R.; Tanada, S.; Ashimura, J.-I.; Idota, A.; Kamiura, S.; et al. Detection of pagetoid urothelial intraepithelial neoplasia extending to the vagina by cervical screening cytology: A case report with renewed immunochemical summary. Diagn. Pathol. 2019, 14, 9. [Google Scholar] [CrossRef]
- Lu, B.; Liang, Y. Pagetoid Spread of Bladder Urothelial Carcinoma to the Vagina and Vulva. J. Low. Genit. Tract Dis. 2015, 19, e13–e16. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Chen, Y.-C.; Wu, C.-L. Vulvar extramammary Paget’s disease secondary to urothelial carcinoma presenting with a small painful erosion of the vulva. Indian Dermatol. Online J. 2018, 9, 471–473. [Google Scholar] [CrossRef]
- Padhy, R.R.; Nasseri-Nik, N.; Abbas, F. Poorly differentiated high-grade urothelial carcinoma presenting as Paget’s disease of the vulva with no overt urinary tract neoplasm detected. Gynecol. Oncol. Rep. 2017, 20, 70–72. [Google Scholar] [CrossRef]
- Reyes, M.C.; Park, K.J.; Lin, O.; Ioffe, O.; Isacson, C.; Soslow, R.A.; Reuter, V.E.; Fine, S.W. Urothelial carcinoma involving the gynecologic tract: A morphologic and immunohistochemical study of 6 cases. Am. J. Surg. Pathol. 2012, 36, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Meduri, A.R.; Tirone, B.; Lospalluti, L.; Ambrogio, F.; Cazzato, G.; Bellino, M. Beyond the Surface: Dermoscopic, Clinical, and Histopathological Insights Into Secondary Extramammary Paget Disease of the Glans Linked to Urothelial Carcinoma. Am. J. Dermatopathol. 2025, 47, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Boret, S.; Lambert, E.; Charles, V.P.; Philippe, T.; Ponette, D.; Darras, J.; Mattelaer, P.; Verbeke, S.; Lumen, N.; Decaestecker, K. Vulvar pagetoid urothelial intraepithelial neoplasia: A case report. Acta Chir. Belg. 2021, 123, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.J.; McLaren, K.; Aldridge, R.D. Paget’s disease of the scrotum: A case exhibiting positive prostate-specific antigen staining and associated prostatic adenocarcinoma. Br. J. Dermatol. 1998, 138, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Petcu, E.B.; Gonzalez-Serva, A.; Wright, R.G.; Slevin, M.; Brinzaniuc, K. Prostate carcinoma metastatic to the skin as an extrammamary Paget’s disease. Diagn. Pathol. 2012, 7, 106. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.L.; Sun, H.; Huo, L.; Wu, Y.; Chen, H.; Gan, Q.; Meis, J.M.; Maloney, N.; Lazar, A.J.; et al. Expression of TRPS1 in phyllodes tumor and sarcoma of the breast. Hum. Pathol. 2022, 121, 73–80. [Google Scholar] [CrossRef]
- Cho, W.C.; Ding, Q.; Wang, W.L.; Nagarajan, P.; Curry, J.L.; Torres-Cabala, C.A.; Ivan, D.; Albarracin, C.T.; Sahin, A.; Prieto, V.G.; et al. Immunohistochemical expression of TRPS1 in mammary Paget disease, extramammary Paget disease, and their close histopathologic mimics. J. Cutan. Pathol. 2023, 50, 434–440. [Google Scholar] [CrossRef]
- De Michele, S.; Remotti, H.E.; Del Portillo, A.; Lagana, S.M.; Szabolcs, M.; Saqi, A. SATB2 in Neoplasms of Lung, Pancreatobiliary, and Gastrointestinal Origins. Am. J. Clin. Pathol. 2021, 155, 124–132. [Google Scholar] [CrossRef]
- Berg, K.B.; Schaeffer, D.F. SATB2 as an Immunohistochemical Marker for Colorectal Adenocarcinoma: A Concise Review of Benefits and Pitfalls. Arch. Pathol. Lab. Med. 2017, 141, 1428–1433. [Google Scholar] [CrossRef]
- Brettfeld, S.M.; Ramos, B.D.; Berry, R.S.; Martin, D.R.; Hanson, J.A. SATB2 Versus CDX2: A Battle Royale for Diagnostic Supremacy in Mucinous Tumors. Arch. Pathol. Lab. Med. 2019, 143, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, A.; de Wit, M.; Johansson, C.; Uhlen, M.; Ponten, F. The role of SATB2 as a diagnostic marker for tumors of colorectal origin: Results of a pathology-based clinical prospective study. Am. J. Clin. Pathol. 2014, 141, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, C.K.; Zhou, P.; Pasolli, H.A.; Rendl, M.; Bolotin, D.; Lim, K.C.; Dai, X.; Alegre, M.L.; Fuchs, E. GATA-3: An unexpected regulator of cell lineage determination in skin. Genes Dev. 2003, 17, 2108–2122. [Google Scholar] [CrossRef]
- Tsarovina, K.; Pattyn, A.; Stubbusch, J.; Muller, F.; van der Wees, J.; Schneider, C.; Brunet, J.F.; Rohrer, H. Essential role of Gata transcription factors in sympathetic neuron development. Development 2004, 131, 4775–4786. [Google Scholar] [CrossRef] [PubMed]
- Grote, D.; Souabni, A.; Busslinger, M.; Bouchard, M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 2006, 133, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Asselin-Labat, M.L.; Sutherland, K.D.; Barker, H.; Thomas, R.; Shackleton, M.; Forrest, N.C.; Hartley, L.; Robb, L.; Grosveld, F.G.; van der Wees, J.; et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol. 2007, 9, 201–209. [Google Scholar] [CrossRef]
- Liu, H.; Shi, J.; Wilkerson, M.L.; Lin, F. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: A useful immunomarker for breast and urothelial carcinomas. Am. J. Clin. Pathol. 2012, 138, 57–64. [Google Scholar] [CrossRef]
- Esheba, G.E.; Longacre, T.A.; Atkins, K.A.; Higgins, J.P. Expression of the urothelial differentiation markers GATA3 and placental S100 (S100P) in female genital tract transitional cell proliferations. Am. J. Surg. Pathol. 2009, 33, 347–353. [Google Scholar] [CrossRef]
- Higgins, J.P.; Kaygusuz, G.; Wang, L.; Montgomery, K.; Mason, V.; Zhu, S.X.; Marinelli, R.J.; Presti, J.C., Jr.; van de Rijn, M.; Brooks, J.D. Placental S100 (S100P) and GATA3: Markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am. J. Surg. Pathol. 2007, 31, 673–680. [Google Scholar] [CrossRef]
- Raspollini, M.R.; Sardi, I.; Giunti, L.; Di Lollo, S.; Baroni, G.; Stomaci, N.; Menghetti, I.; Franchi, A. Plasmacytoid urothelial carcinoma of the urinary bladder: Clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of a case series. Hum. Pathol. 2011, 42, 1149–1158. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, L.; Sun, L.; Song, Y.; Guo, Y.; Zhang, X.; Zhao, F.; Wang, P.; Yue, J.; Niu, D.; et al. GATA3 is a sensitive marker for primary genital extramammary paget disease: An immunohistochemical study of 72 cases with comparison to gross cystic disease fluid protein 15. Diagn. Pathol. 2017, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.V.; Francois, R.A.; Mully, T.W.; Sangoi, A.; LeBoit, P.E.; Simko, J.P.; Chan, E. Positive NKX3.1 as a diagnostic pitfall for prostate cancer in extramammary Paget’s disease of genitourinary sites. Histopathology 2024, 84, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Gurel, B.; Ali, T.Z.; Montgomery, E.A.; Begum, S.; Hicks, J.; Goggins, M.; Eberhart, C.G.; Clark, D.P.; Bieberich, C.J.; Epstein, J.I.; et al. NKX3.1 as a marker of prostatic origin in metastatic tumors. Am. J. Surg. Pathol. 2010, 34, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Pittman, M.E.; Milsom, J.; Yantiss, R.K. Treatment Effects Can Mimic Recurrent Extramammary Paget Disease in Perianal Skin. Am. J. Surg. Pathol. 2018, 42, 1472–1479. [Google Scholar] [CrossRef]

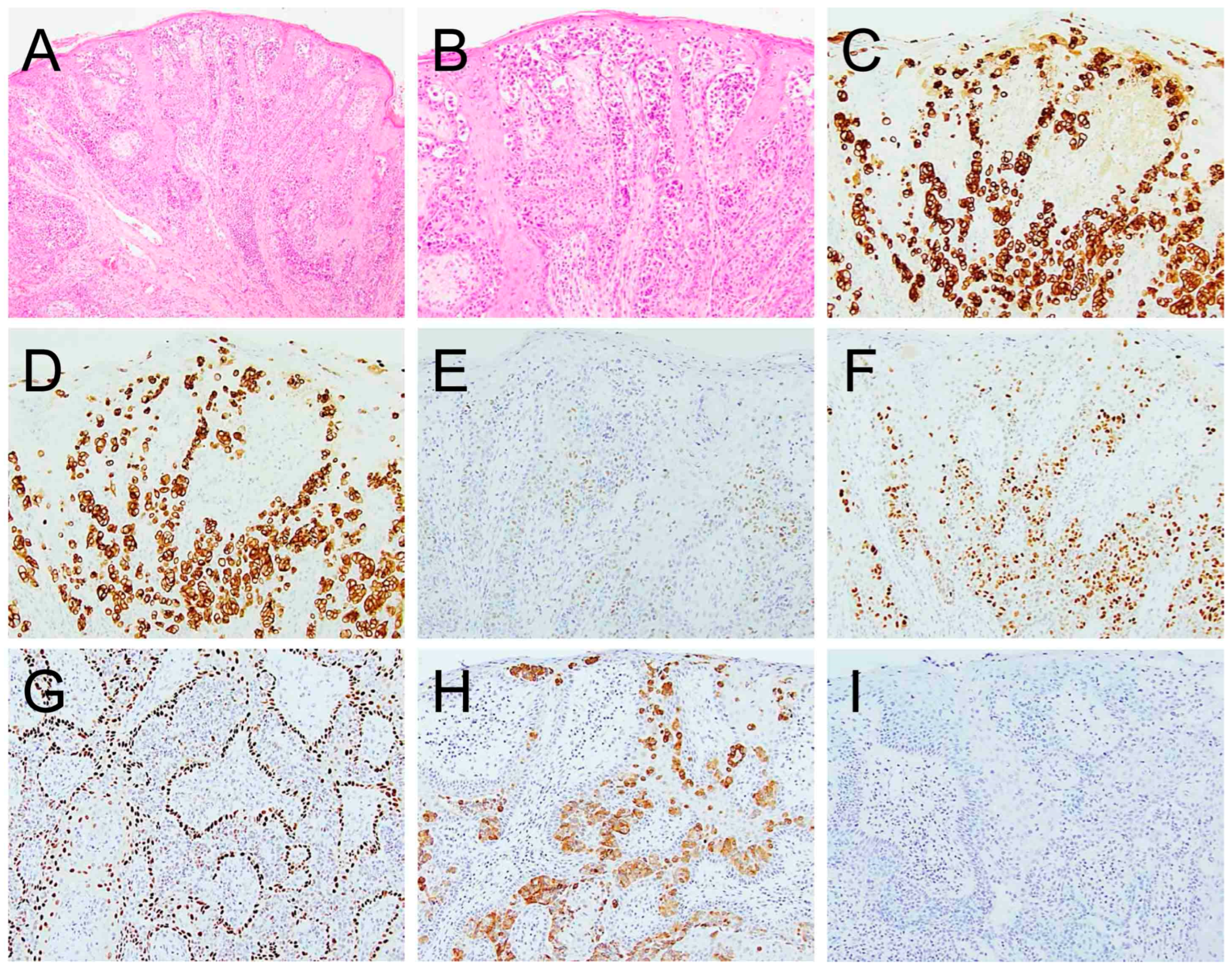
| Case | Age/Sex (Years) | EMPD Location | Primary Cancer | Duration Between EMPD and Primary Malignancy (Months) | Follow-Up Duration (Months) | Recurrence | Survival Status |
|---|---|---|---|---|---|---|---|
| 1 | 66/F | Vulva, perineum, anal/perianal | Colorectal | 84, before | 171 | yes | dead |
| 2 | 70/M | Anal/perianal | Colorectal | 84, before | 99 | yes | alive |
| 3 | 68/F | Vulva | Colorectal | 15, after | 33 | yes | alive |
| 4 | 90/M | Anal/perianal | Colorectal | 0 (concurrent) | 0 | NA | dead |
| 5 | 77/F | Anal/perianal | Colorectal | 2, before | 26 | no | alive |
| 6 | 83/F | Anal/perianal | Colorectal | 5, before | 41 | no | alive |
| 7 | 61/M | Anal/perianal | Colorectal | 4, before | 50 | no | dead |
| 8 | 64/M | Perineum | Urothelial | 24, after | 97 | no | alive |
| 9 | 69/M | Penis | Urothelial | 0 (concurrent) | 159 | yes | alive |
| 10 | 70/M | Penis | Urothelial | 38, after | 12 | yes | dead |
| 11 | 62/F | Stoma | Urothelial | 47, after | 146 | no | alive |
| 12 | 83/M | Lower abdomen | Urothelial | 109, after | 21 | yes | dead |
| Case | Primary Cancer | CDX2 | CK20 | CK7 | GATA3 | GCDFP15 | p63 | SATB2 | TRPS1 | Uroplakin II/III |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Colorectal | ND | pos | neg | ND | ND | ND | ND | ND | ND |
| 2 | Colorectal | ND | pos | pos | ND | ND | ND | ND | ND | ND |
| 3 | Colorectal | pos | pos | pos | ND | ND | ND | ND | ND | ND |
| 4 | Colorectal | pos | pos | neg | ND | neg | ND | ND | ND | ND |
| 5 | Colorectal | pos | pos | pos | ND | neg | neg | pos | neg | ND |
| 6 | Colorectal | pos | pos | pos | neg | neg | neg | pos | neg | ND |
| 7 | Colorectal | pos | pos | pos | ND | neg | neg | neg | neg | ND |
| 8 | Urothelial | pos | pos | pos | pos | neg | pos | neg | neg | pos |
| 9 | Urothelial | neg | pos | pos | pos | neg | pos | neg | neg | ND |
| 10 | Urothelial | pos | pos | pos | pos | neg | pos | neg | neg | ND |
| 11 | Urothelial | neg | pos | pos | ND | ND | neg | neg | neg | ND |
| 12 | Urothelial | pos | pos | pos | ND | neg | pos | neg | neg | ND |
| Total, N % | 8/10 80% | 12/12 100% | 10/12 83.3% | 3/4 75% | 0/8 0% | 4/8 50% | 2/8 25% | 0/8 0% | 1/1 100% |
| Stain | Primary, N = 480 | Secondary | p-Value § (Overall Fisher’s Exact Test) | |||
|---|---|---|---|---|---|---|
| Colonic N = 86 | Urothelial N = 41 | Prostatic N = 5 | Total Secondary N = 132 | |||
| CDX2 | 9/104 (9%) | 51/53 (96%) | 3/15 (20%) | 0/3 (0%) | 54/71 (76%) | <0.001 * |
| CK7 | 213/217 (98%) | 50/70 (71%) | 34/34 (100%) | 3/3 (100%) | 87/107 (81%) | <0.001 * |
| CK20 | 46/209 (22%) | 63/71 (89%) | 30/36 (83%) | 0/3 (0%) | 93/110 (85%) | <0.001 * |
| GATA3 | 14/14 (100%) | 1/7 (14%) | 10/10 (100%) | NA | 11/17 (65%) | <0.001 * |
| GCDFP15 | 120/177 (68%) | 0/56 (0%) | 3/20 (15%) | 1/3 (33%) | 4/79 (5%) | <0.001 * |
| NKX3.1 | NA | NA | 0/2 (0%) | NA | 0/2 (0%) | NA |
| p63 | 1/69 (1%) | 0/3 (0%) | 12/13 (92%) | NA | 12/16 (75%) | <0.001 * |
| PSA | 22/49 (45%) | NA | NA | 2/2 (100%) | 2/2 (100%) | 0.216 |
| SATB2 | 1/7 (14%) | 10/12 (83%) | 0/5 (0%) | NA | 10/17 (59%) | 0.001 * |
| TRPS1 | 91/101 (90%) | 0/14 (0%) | 0/8 (0%) | NA | 0/22 (0%) | <0.001 * |
| Uroplakin II/III | 0/14 (0%) | NA | 5/7 (71%) | NA | 5/7 (71%) | 0.001 * |
| Cam 5.2 | 25/25 (100%) | NA | 2/2 (100%) | NA | 2/2 (100%) | |
| CEA | 77/93 (83%) | 15/17 (88%) | 1/11 (9%) | 0/1 (0%) | 16/29 (55%) | |
| EMA | 56/56 (100%) | 2/2 (100%) | 2/2 (100%) | NA | 4/4 (100%) | |
| HER2 | 41/66 (62.1%) | 0/9 (0%) | 7/10 (70%) | 3/3 (100%) | 10/22 (45%) | |
| p40 | 0/3 (0%) | 0/5 (0%) | NA | NA | 0/5 (0%) | |
| B72.3 | 21/22 (95%) | NA | NA | NA | NA | |
| CD15 | 0/2 (0%) | 0/3 (0%) | NA | NA | 0/3 (0%) | |
| CK1 | 0/19 (0%) | 0/5 (0%) | 0/2 (0%) | NA | 0/7 (0%) | |
| CK1,5,10,14 | 0/19 (0%) | 0/5 (0%) | 0/2 (0%) | NA | 0/7 (0%) | |
| CK10 | 0/19 (0%) | 0/8 (0%) | 0/2 (0%) | NA | 0/10 (0%) | |
| CK13 | 0/19 (0%) | 0/8 (0%) | 0/2 (0%) | NA | 0/10 (0%) | |
| CK15 | 0/16 (0%) | NA | NA | NA | NA | |
| CK18 | not done | NA | 1/1 (100%) | NA | 1/1 (100%) | |
| CK19 | 16/16 (100%) | NA | 1/1 (100%) | NA | 1/1 (100%) | |
| CK5/6 | 0/10 (0%) | 0/1 (0%) | NA | NA | 0/1 (0%) | |
| CK8 | not done | 4/4 (100%) | 1/1 (100%) | NA | 5/5 (100%) | |
| Cyclin D1 | 36/43 (84%) | 3/6 (50%) | 2/6 (33%) | 3/3 (100%) | 8/15 (53%) | |
| ER | 0/42 (0%) | 0/1 (0%) | 0/1 (0%) | NA | 0/2 (0%) | |
| Lysozyme | 0/2 (0%) | 0/3 (0%) | NA | NA | 0/3 (0%) | |
| MUC1 | 3/3 (100%) | 2/6 (33%) | NA | NA | 2/6 (33%) | |
| MUC2 | 3/3 (100%) | 6/7 (96%) | NA | NA | 6/7 (96%) | |
| MUC5AC | NA | 0/1 (0%) | NA | NA | 0/1 (0%) | |
| MUC6 | NA | 0/1 (0%) | NA | NA | 0/1 (0%) | |
| p16 | not done | NA | 6/8 (75%) | NA | 6/8 (75%) | |
| P501S | 0/16 (0%) | NA | NA | NA | NA | |
| p53 | 16/20 (80%) | NA | 0/1 (0%) | NA | 0/1 (0%) | |
| Pan-keratin | NA | 3/3 (100%) | NA | NA | 3/3 (100%) | |
| PD-L1 | 3/6 (50%) | NA | NA | NA | NA | |
| PR | not done | NA | 0/1 (0%) | NA | 0/1 (0%) | |
| RANKL | 6/6 (100%) | NA | NA | NA | NA | |
| S100 | 0/22 (0%) | 0/1 (0%) | 0/2 (0%) | NA | 0/3 (0%) | |
| Thrombomodulin | NA | NA | 1/1 (100%) | NA | 1/1 (100%) | |
| WT1 | NA | NA | 0/1 (0%) | NA | 0/1 (0%) | |
| References | [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] | [13,14,16,19,20,24,25,26,27,28,29,31,38,39,40,41,42,43,44] | [13,14,18,19,21,23,29,38,43,45,46,47,48,49,50,51,52,53,54,55] | [29,30,56,57] | ||
| Immunostain | Bonferroni Adjusted p-Value | |||||
|---|---|---|---|---|---|---|
| Primary vs. Colonic | Primary vs. Urothelial | Primary vs. Prostatic | Colonic vs. Urothelial | Colonic vs. Prostatic | Urothelial vs. Prostatic | |
| CDX2 | <0.001 * | 1 | 1 | <0.001 * | <0.001 * | 1 |
| CK7 | <0.001 * | 1 | 1 | <0.001 * | 1 | - |
| CK20 | <0.001 * | <0.001 * | 1 | 1 | <0.018 * | 0.054 |
| GATA3 | <0.001 * | - | NA | 0.003 * | NA | NA |
| GCDFP15 | <0.001 * | <0.001 * | 1 | 0.096 | 0.306 | 1 |
| p63 | 1 | <0.001 * | NA | 0.042 * | NA | NA |
| PSA | NA | NA | 0.216 | NA | NA | NA |
| SATB2 | 0.036 * | NA | NA | 0.018 * | NA | NA |
| TRPS1 | <0.001 * | <0.001 * | NA | - | NA | NA |
| Uroplakin II/III | NA | 0.001 * | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiratikanon, S.; Fukui, A.; Hirata, M.; Moran, J.M.T.; Fujimoto, M.; Hoang, M.P. Diagnostic Algorithm for Secondary Extramammary Paget Disease from Institutional Cases and Literature Review. Cancers 2025, 17, 4014. https://doi.org/10.3390/cancers17244014
Kiratikanon S, Fukui A, Hirata M, Moran JMT, Fujimoto M, Hoang MP. Diagnostic Algorithm for Secondary Extramammary Paget Disease from Institutional Cases and Literature Review. Cancers. 2025; 17(24):4014. https://doi.org/10.3390/cancers17244014
Chicago/Turabian StyleKiratikanon, Salin, Ayaka Fukui, Masahiro Hirata, Jakob M. T. Moran, Masakazu Fujimoto, and Mai P. Hoang. 2025. "Diagnostic Algorithm for Secondary Extramammary Paget Disease from Institutional Cases and Literature Review" Cancers 17, no. 24: 4014. https://doi.org/10.3390/cancers17244014
APA StyleKiratikanon, S., Fukui, A., Hirata, M., Moran, J. M. T., Fujimoto, M., & Hoang, M. P. (2025). Diagnostic Algorithm for Secondary Extramammary Paget Disease from Institutional Cases and Literature Review. Cancers, 17(24), 4014. https://doi.org/10.3390/cancers17244014








