Immunosuppressive Role of Integrin β8 in Recurrence After Bacillus Calmette–Guérin (BCG) Therapy for Non-Muscle Invasive Bladder Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Surgical Specimens
2.2. Microarray Analysis
2.3. Reagents and Cell Lines
2.4. cDNA Construction and Quantitative RT-PCR
2.5. Western Blotting Analysis
2.6. siRNA and Transfection
2.7. Adhesion Assay
2.8. ELISA
2.9. Immunohistochemistry
2.10. Statistical Analysis
3. Results
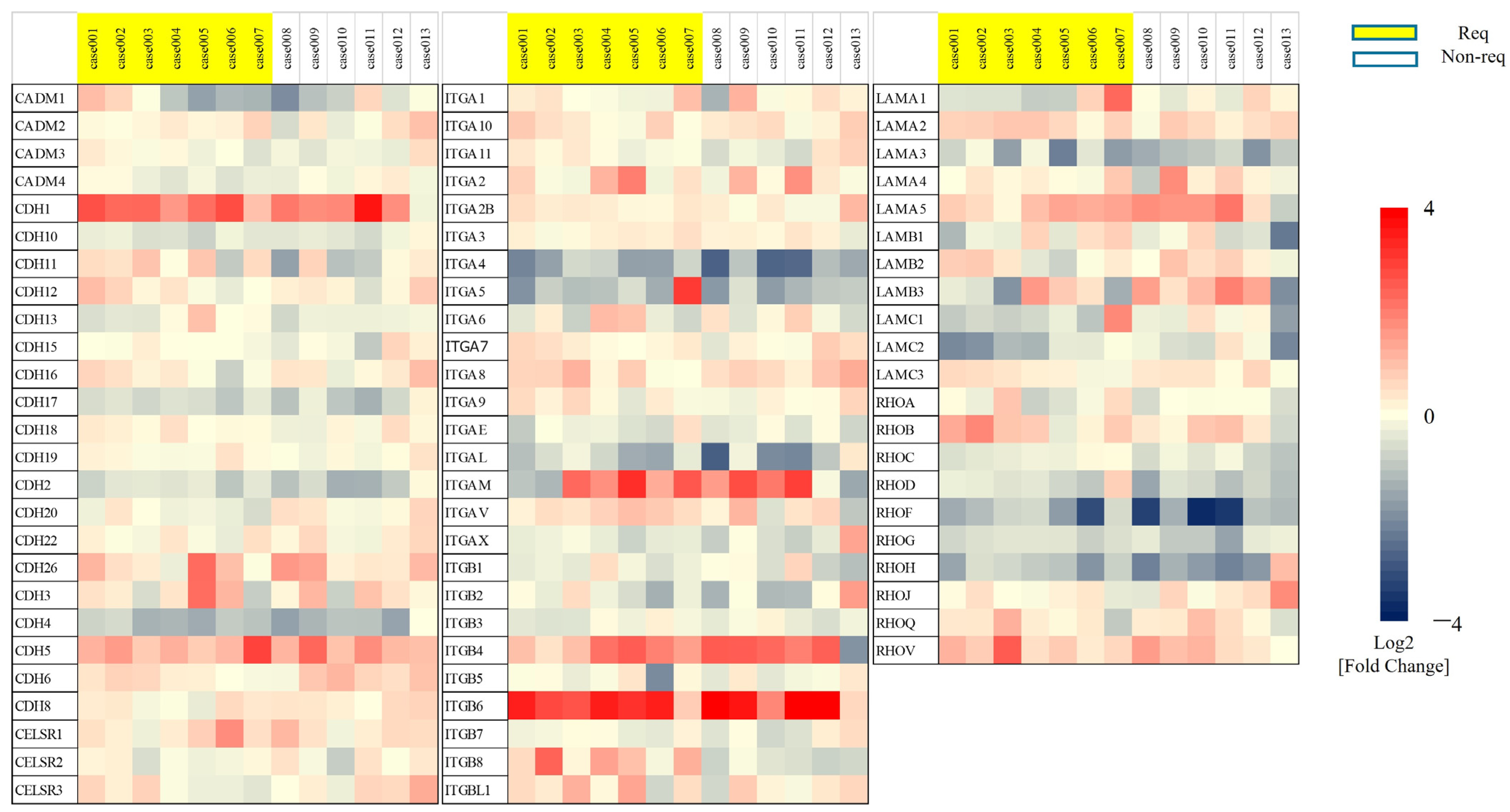
3.1. Upregulation of the Adhesion Molecule Integrin β8 in BCG-Treated Patients with Recurrent NMIBC
3.2. Suppression of ITGB8 Affects Cell Proliferation, Invasion, and Adhesion in Bladder Cancer Cells with High ITGB8 Expression
3.3. Suppression of TGF-β1 Secretion Following Knockdown of ITGB8 in Bladder Cancer Cells That Express High Levels of ITGB8
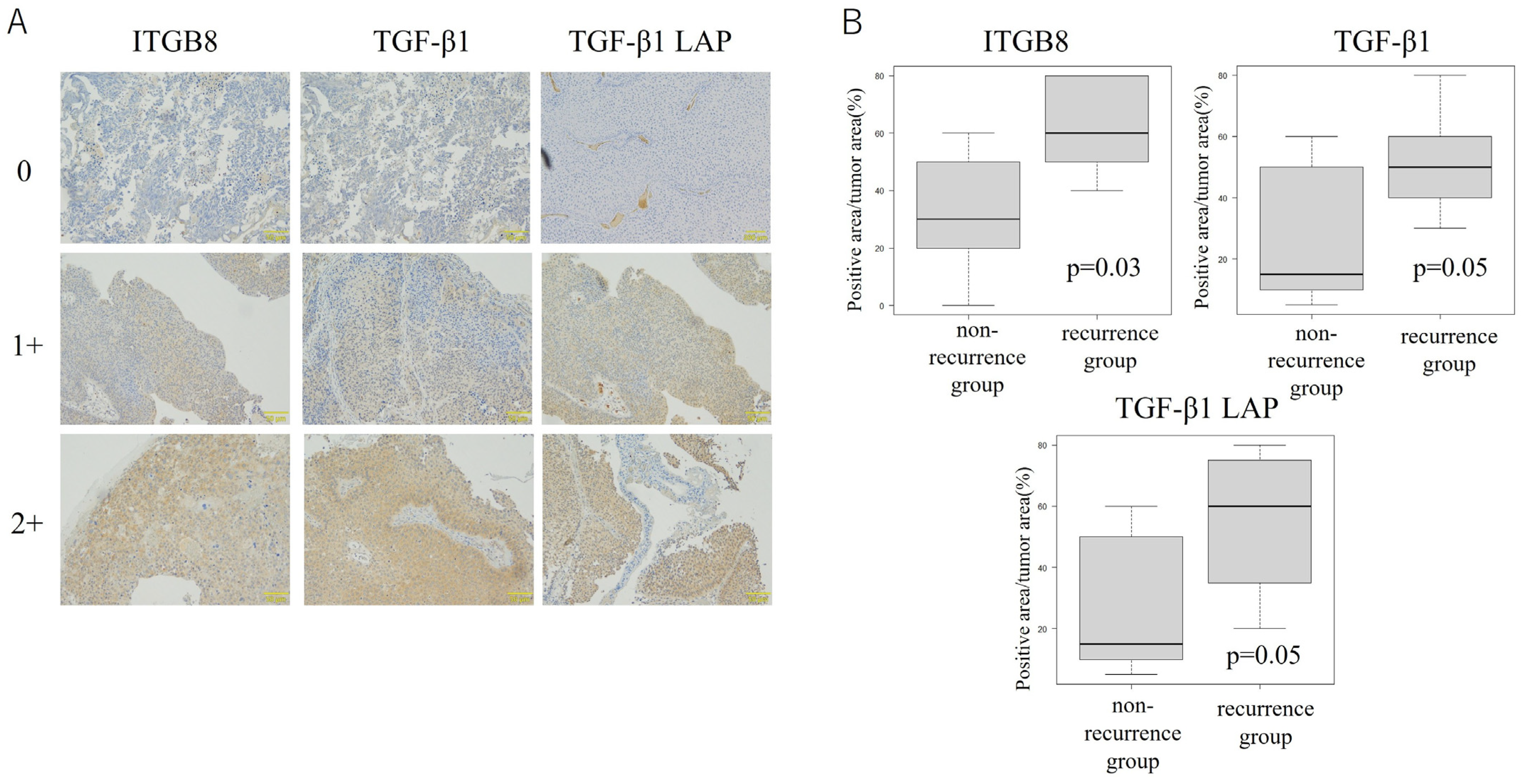
3.4. ITGB8 Expression Is Correlated with Release of the Immunosuppressive Cytokine TGF-β1 in Bladder Cancer Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCG | Bacillus Calmette–Guérin |
| cDNA | complementary DNA |
| ELISA | enzyme-linked immunosorbent assay |
| mRNA | messenger RNA |
| PD-L1 | programmed cell death ligand 1 |
| RT-PCR | reverse transcription–polymerase chain reaction |
| siRNA | small interfering RNA or short interfering RNA |
| TGF-β1 | transforming growth factor–β |
References
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 466–465; discussion 467–475. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der, M.A.; Lamm, D.L. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J. Urol. 2002, 168, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Kunath, F.; Coles, B.; Draeger, D.L.; Krabbe, L.M.; Dersch, R.; Kilian, S.; Jensen, K.; Dahm, P.; Meerpohl, J.J. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst. Rev. 2020, 1, Cd011935. [Google Scholar] [CrossRef] [PubMed]
- Bevers, R.F.; Kurth, K.H.; Schamhart, D.H. Role of urothelial cells in BCG immunotherapy for superficial bladder cancer. Br. J. Cancer 2004, 91, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Brandau, S.; Suttmann, H. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: A success story with room for improvement. Biomed. Pharmacother. 2007, 61, 299–305. [Google Scholar] [CrossRef]
- David, C.J.; Massagué, J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419–435. [Google Scholar] [CrossRef]
- Rodríguez-Izquierdo, M.; Del Cañizo, C.G.; Rubio, C.; Reina, I.A.; Hernández Arroyo, M.; Rodríguez Antolín, A.; Dueñas Porto, M.; Guerrero-Ramos, F. Immune Predictors of Response after Bacillus Calmette-Guérin Treatment in Non-Muscle-Invasive Bladder Cancer. Cancers 2023, 15, 5554. [Google Scholar] [CrossRef]
- Hinck, A.P.; Mueller, T.D.; Springer, T.A. Structural Biology and Evolution of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2016, 8, a022103. [Google Scholar] [CrossRef]
- Wells, C.A.; Ravasi, T.; Faulkner, G.J.; Carninci, P.; Okazaki, Y.; Hayashizaki, Y.; Sweet, M.; Wainwright, B.J.; Hume, D.A. Genetic control of the innate immune response. BMC Immunol. 2003, 4, 5. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, Y.; Bi, Y.; Zhang, N.; Wang, H.; Xing, T.; Bai, S.; Shen, Z.; Naz, F.; Zhang, Z.; et al. Immune escape mechanisms and immunotherapy of urothelial bladder cancer. J. Clin. Transl. Res. 2021, 7, 485–500. [Google Scholar] [PubMed]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Panettiere, F.J.; Goodman, P.J.; Costanzi, J.J.; Cruz, A.B.; Jr Vaitkevicius, V.K.; McCracken, J.D.; Brownlee, R.W.; Laufman, L.; Stephens, R.L.; Bonnet, J.; et al. Adjuvant therapy in large bowel adenocarcinoma: Long-term results of a Southwest Oncology Group Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1988, 6, 947–954. [Google Scholar] [CrossRef]
- Han, J.; Gu, X.; Li, Y.; Wu, Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed. Pharmacother. 2020, 129, 110393. [Google Scholar] [CrossRef]
- See, W.A.; Zhang, G.; Chen, F.; Cao, Y.; Langenstroer, P.; Sandlow, J. Bacille-Calmette Guèrin induces caspase-independent cell death in urothelial carcinoma cells together with release of the necrosis-associated chemokine high molecular group box protein 1. BJU Int. 2009, 103, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.; Avendaño-Ortiz, J.; Ruiz-Palomares, R.; Karaivanova, V.; Alberquilla, O.; Sánchez-Domínguez, R.; Casalvilla-Dueñas, J.C.; Montalbán-Hernández, K.; Lodewijk, I.; Rodríguez-Izquierdo, M.; et al. Toward Tumor Fight and Tumor Microenvironment Remodeling: PBA Induces Cell Cycle Arrest and Reduces Tumor Hybrid Cells’ Pluripotency in Bladder Cancer. Cancers 2022, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Leblond, M.M.; Zdimerova, H.; Desponds, E.; Verdeil, G. Tumor-Associated Macrophages in Bladder Cancer: Biological Role, Impact on Therapeutic Response and Perspectives for Immunotherapy. Cancers 2021, 13, 4712. [Google Scholar] [CrossRef]
- van der Meijden, A.P.; Sylvester, R.J.; Oosterlinck, W.; Hoeltl, W.; Bono, A.V. Maintenance Bacillus Calmette-Guerin for Ta T1 bladder tumors is not associated with increased toxicity: Results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase III Trial. Eur. Urol. 2003, 44, 429–434. [Google Scholar] [CrossRef]
- Chestnut, C.; Subramaniam, D.; Dandawate, P.; Padhye, S.; Taylor, J., 3rd; Weir, S.; Anant, S. Targeting Major Signaling Pathways of Bladder Cancer with Phytochemicals: A Review. Nutr. Cancer 2021, 73, 2249–2271. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, F.; Falagario, U.G.; Zanelli, M.; Palicelli, A.; Zizzo, M.; Busetto, G.M.; Cormio, A.; Carrieri, G.; Cormio, L. Integrating the PD-L1 Prognostic Biomarker in Non-Muscle Invasive Bladder Cancer in Clinical Practice-A Comprehensive Review on State-of-the-Art Advances and Critical Issues. J. Clin. Med. 2024, 13, 2182. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, X.; Zhao, B.; Li, J.; Lu, C.; Springer, T.A. Atypical interactions of integrin α(V)β(8) with pro-TGF-β1. Proc. Natl. Acad. Sci. USA 2017, 114, E4168–E4174. [Google Scholar] [CrossRef]
- Worthington, J.J.; Kelly, A.; Smedley, C.; Bauché, D.; Campbell, S.; Marie, J.C.; Travis, M.A. Integrin αvβ8-Mediated TGF-β Activation by Effector Regulatory T Cells Is Essential for Suppression of T-Cell-Mediated Inflammation. Immunity 2015, 42, 903–915. [Google Scholar] [CrossRef]
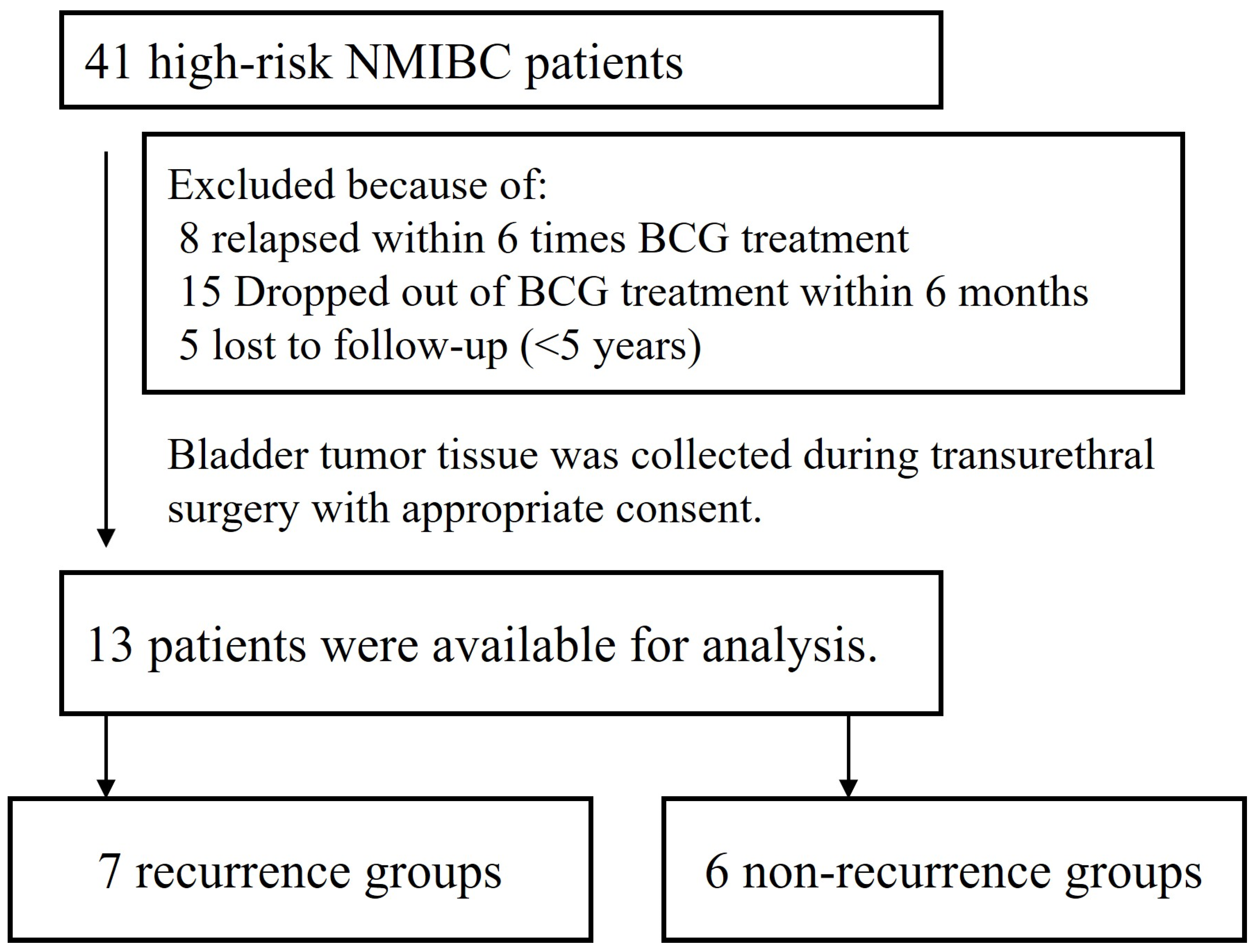



| Total (n = 13) | Recurrence Group (n = 7) | Non-Recurrence Group (n = 6) | ||
|---|---|---|---|---|
| age(average) | 73.5 ± 8.5 | 72.1 ± 9.1 | 73.5 ± 9.7 | n.s |
| sex (male:female) | 11:2 | 7:0 | 4:2 | n.s |
| cytology (positive:negative) | 9:4 | 5:2 | 3:3 | n.s |
| tumor diameter(average) | 20.0 ± 7.3 | 22.5 ± 7.4 | 22.7 ± 6.4 | n.s |
| pathological grade (high:low) | 4:9 | 3:4 | 1:5 | n.s |
| pT stage (pTa:pT1) | 11:2 | 6:1 | 5:1 | n.s |
| Cis (with or without) | 1:12 | 1:6 | 0:6 | n.s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoshi, S.; Meguro, S.; Makabe, S.; Onagi, A.; Kayama, E.; Yaginuma, K.; Imai, H.; Tanji, R.; Honda-Takinami, R.; Matsuoka, K.; et al. Immunosuppressive Role of Integrin β8 in Recurrence After Bacillus Calmette–Guérin (BCG) Therapy for Non-Muscle Invasive Bladder Cancer. Cancers 2025, 17, 3964. https://doi.org/10.3390/cancers17243964
Hoshi S, Meguro S, Makabe S, Onagi A, Kayama E, Yaginuma K, Imai H, Tanji R, Honda-Takinami R, Matsuoka K, et al. Immunosuppressive Role of Integrin β8 in Recurrence After Bacillus Calmette–Guérin (BCG) Therapy for Non-Muscle Invasive Bladder Cancer. Cancers. 2025; 17(24):3964. https://doi.org/10.3390/cancers17243964
Chicago/Turabian StyleHoshi, Seiji, Satoru Meguro, Syunta Makabe, Akifumi Onagi, Emina Kayama, Kei Yaginuma, Hitomi Imai, Ryo Tanji, Ruriko Honda-Takinami, Kanako Matsuoka, and et al. 2025. "Immunosuppressive Role of Integrin β8 in Recurrence After Bacillus Calmette–Guérin (BCG) Therapy for Non-Muscle Invasive Bladder Cancer" Cancers 17, no. 24: 3964. https://doi.org/10.3390/cancers17243964
APA StyleHoshi, S., Meguro, S., Makabe, S., Onagi, A., Kayama, E., Yaginuma, K., Imai, H., Tanji, R., Honda-Takinami, R., Matsuoka, K., Hata, J., Sato, Y., Akaihata, H., Ogawa, S., Uemura, M., & Kojima, Y. (2025). Immunosuppressive Role of Integrin β8 in Recurrence After Bacillus Calmette–Guérin (BCG) Therapy for Non-Muscle Invasive Bladder Cancer. Cancers, 17(24), 3964. https://doi.org/10.3390/cancers17243964






