Updating the Role of Carboplatin Added to Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer: A Meta-Analysis
Simple Summary
Abstract
1. Introduction
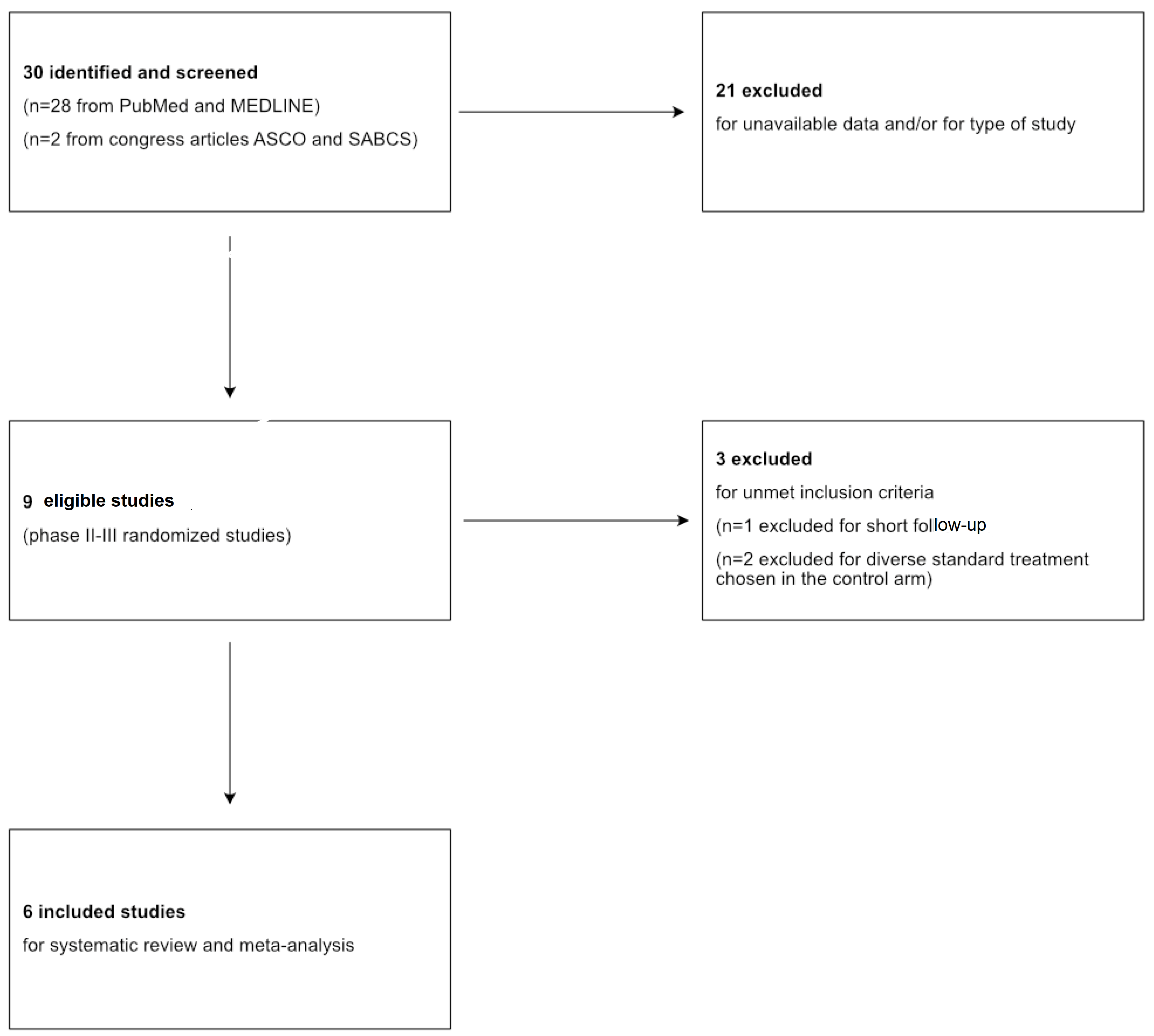
2. Materials and Methods
3. Results
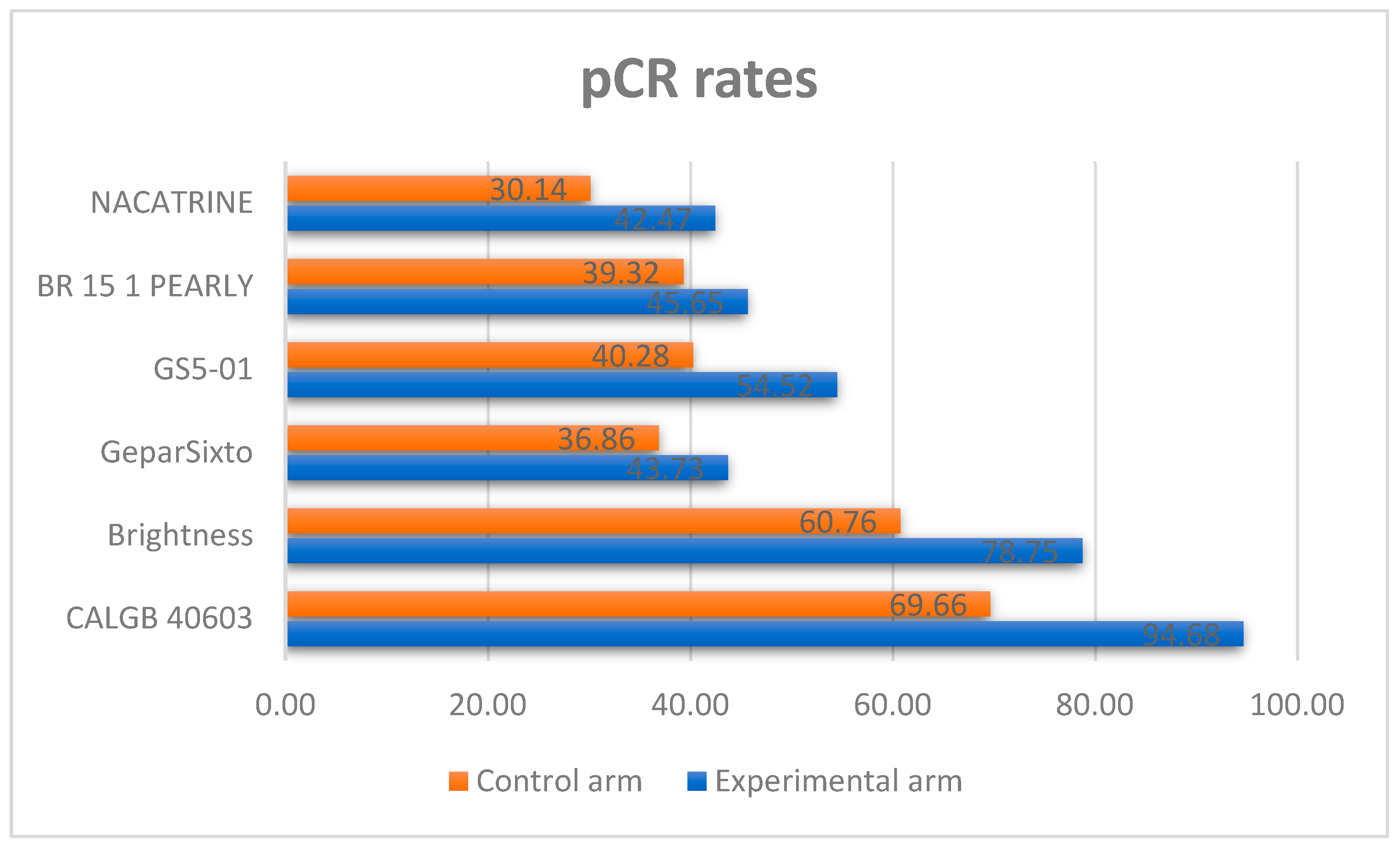
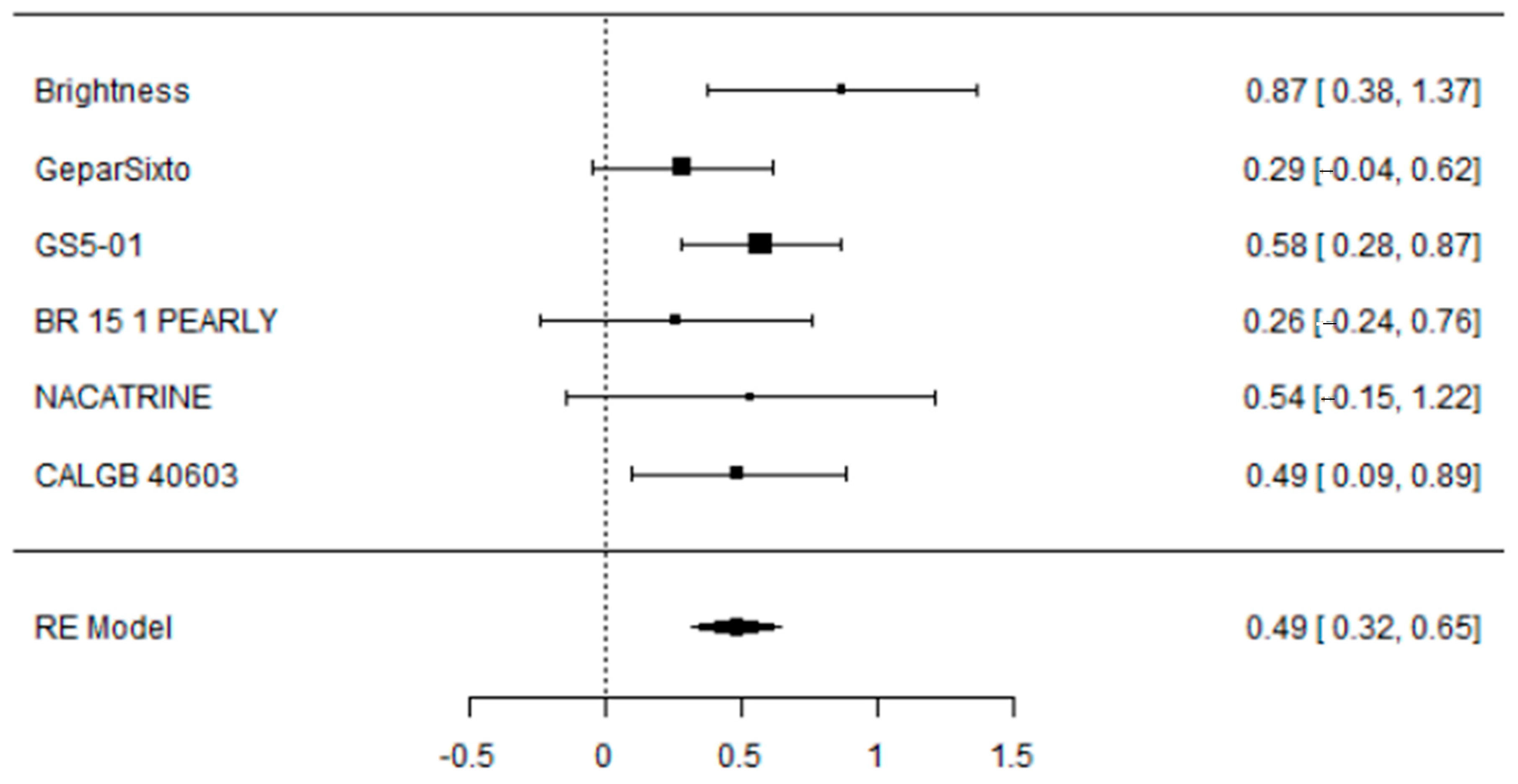
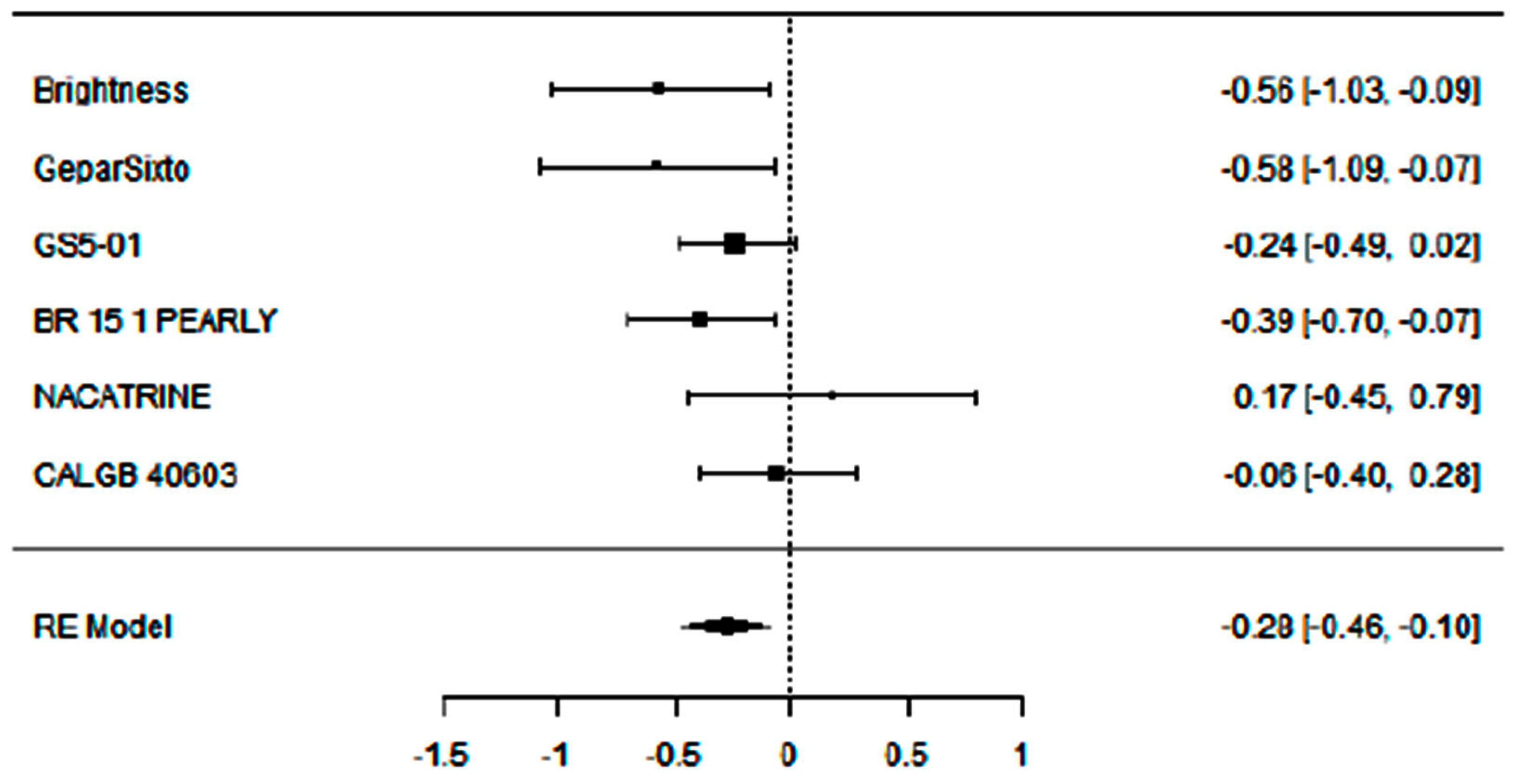
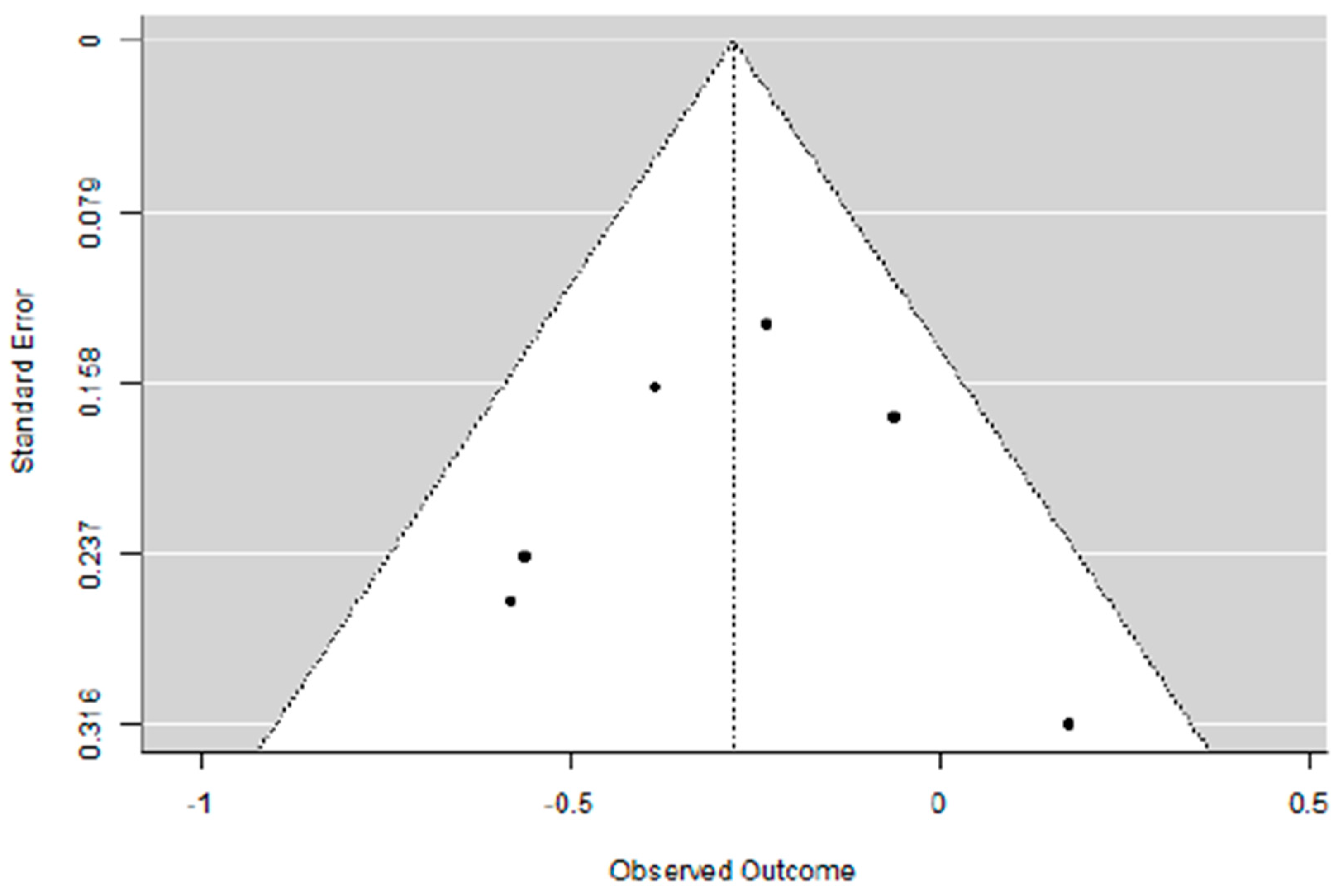
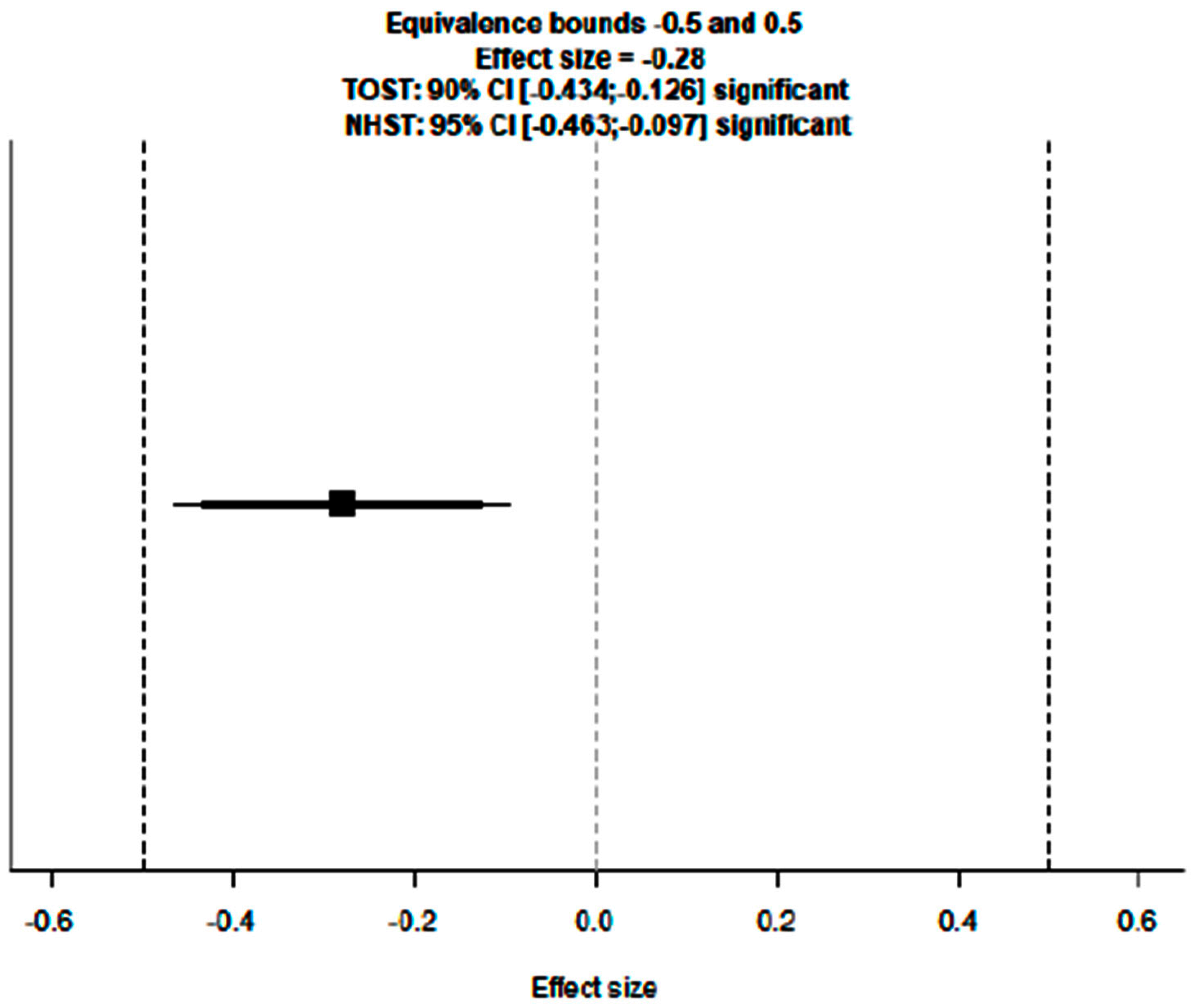
3.1. Evaluation of Adding Carboplatin to NACT in Early TNBC on pCR Rate
3.2. Evaluation of Adding Carboplatin to NACT in Early TNBC on DFS Rate
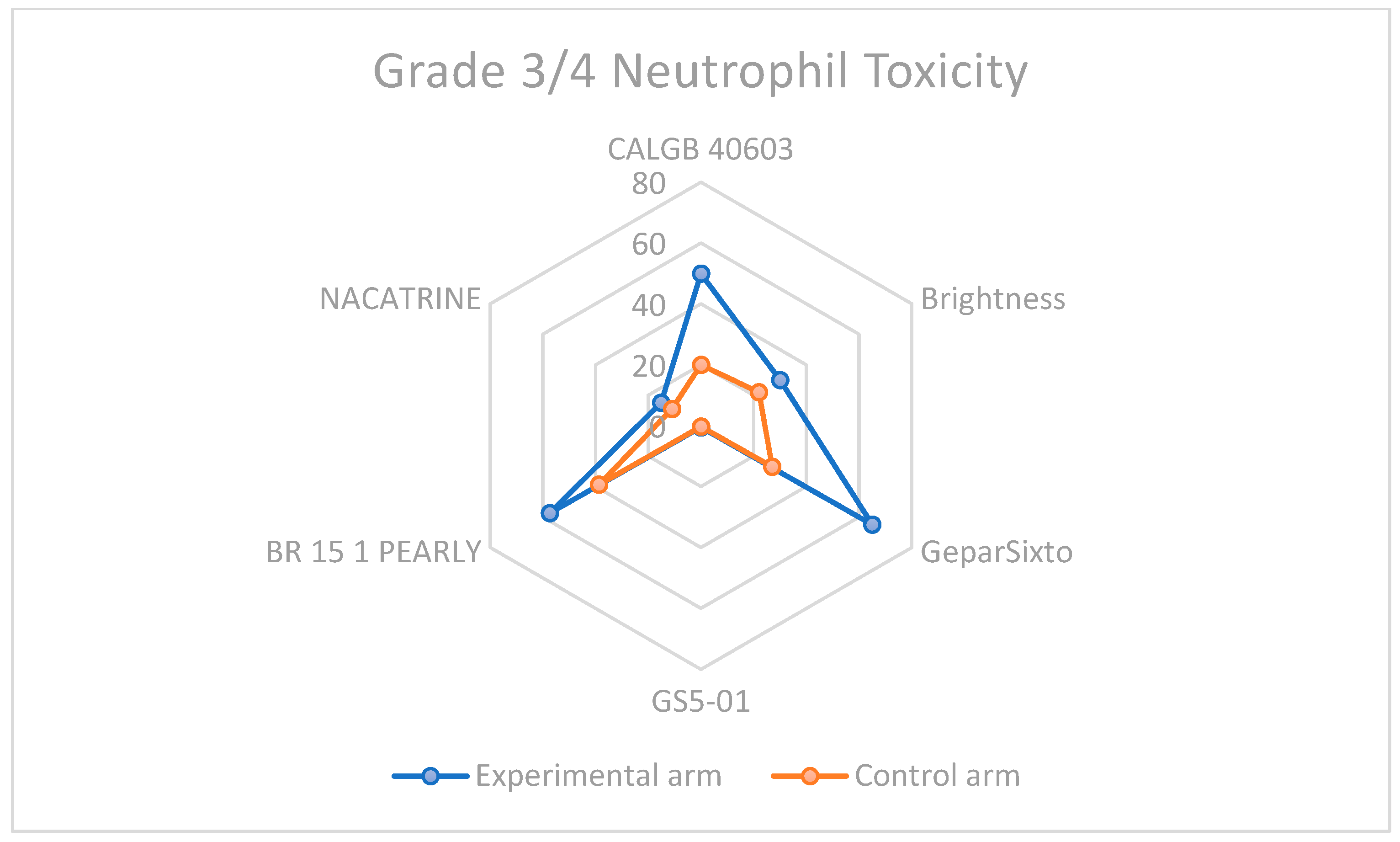
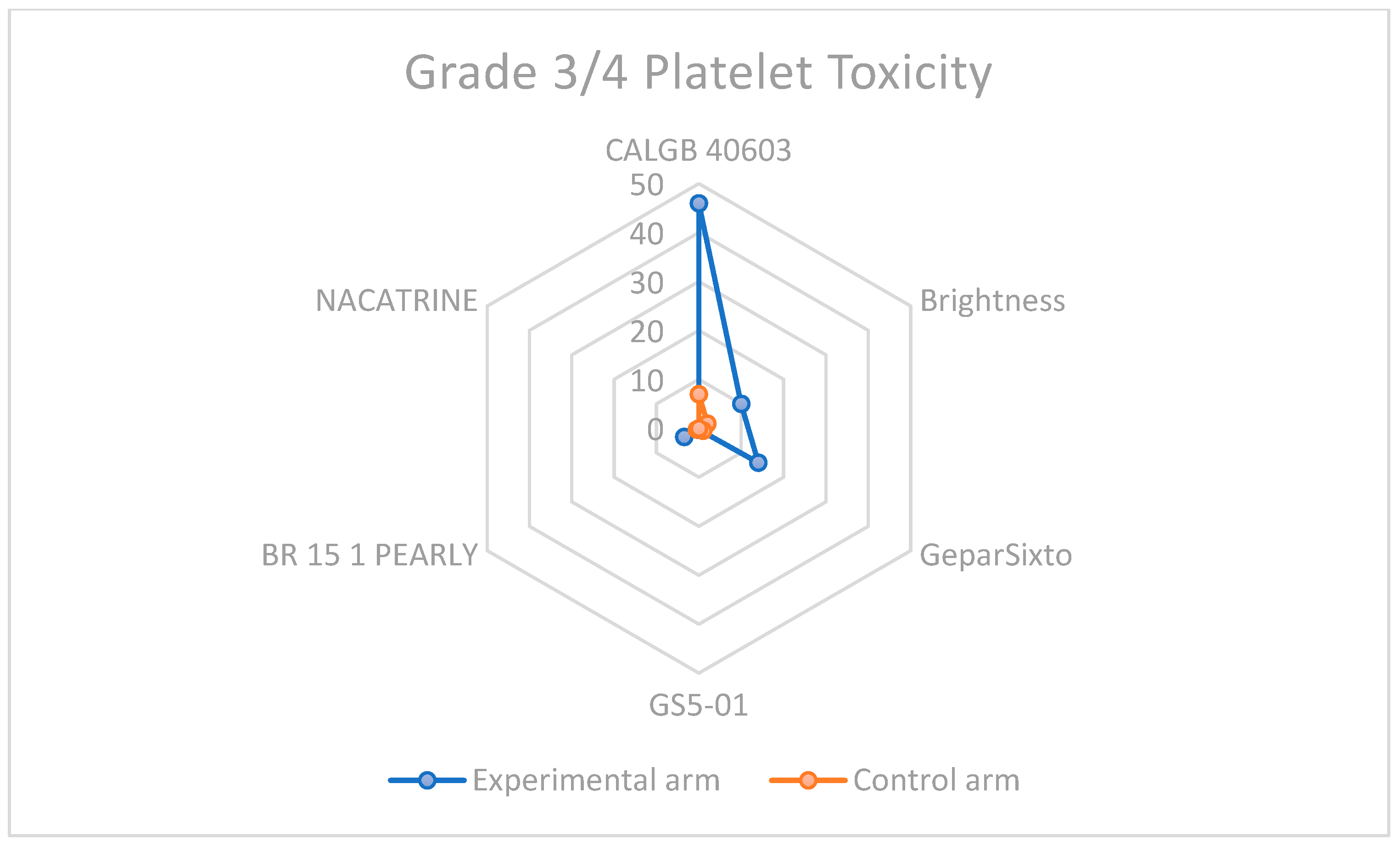
3.3. Descriptive Overview of Hematological Toxicity Among Studies
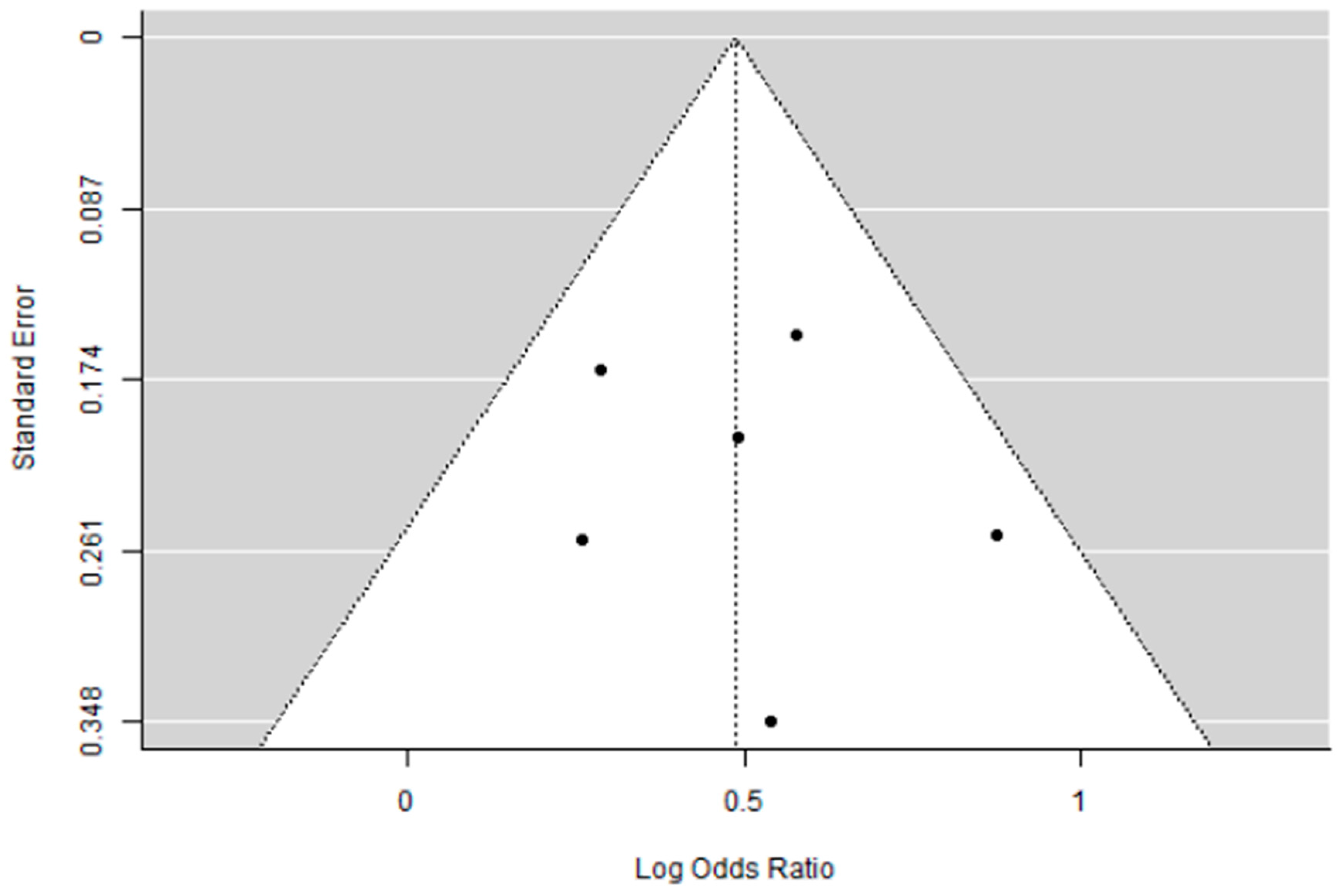
3.4. Supplementary Sensitivity Analysis Including All Eligible Trials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zattarin, E.; Taglialatela, I.; Lobefaro, R.; Leporati, R.; Fucà, G.; Ligorio, F.; Sposetti, C.; Provenzano, L.; Azzollini, J.; Vingiani, A.; et al. Breast cancers arising in subjects with germline BRCA1 or BRCA2 mutations: Different biological and clinical entities with potentially diverse therapeutic opportunities. Crit. Rev. Oncol. Hematol. 2023, 190, 104109. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.R.; Willson, M.L.; Egger, S.J.; Beith, J.; Dear, R.F.; Goodwin, A. Platinum-based chemotherapy for early triple negative breast cancer. Cochrane Libr. 2023, 9, CD014805. [Google Scholar]
- Zhang, L.; Wu, Z.Y.; Li, J.; Lin, Y.; Liu, Z.; Cao, Y.; Zhang, G.; Gao, H.F.; Yang, M.; Yang, C.Q.; et al. Neoadjuvant docetaxel plus carboplatin vs epirubicin plus cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer (NeoCART): Results from a multicenter, randomized controlled, open-label phase II trial. Int. J. Cancer 2022, 150, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.; Kim, G.M.; Jung, K.H.; Jeung, H.-C.; Lee, J.; Lee, K.S.; Im, S.-A.; Kang, S.Y.; Kim, S.H.; Kim, H.J.; et al. A randomized, multicenter, open-label, phase III trial comparing anthracyclines followed by taxane versus anthracyclines followed by taxane plus carboplatin as (neo)adjuvant therapy in patients with early triple-negative breast cancer: Korean Cancer Study Group BR 15-1 PEARLY trial. J. Clin. Oncol. 2024, 42, LBA502. [Google Scholar]
- Poggio, F.; Tagliamento, M.; Ceppi, M.; Bruzzone, M.; Conte, B.; Fregatti, P.; Punie, K.; de Azambuja, E.; Del Mastro, L.; Lambertini, M. Adding a platinum agent to neoadjuvant chemotherapy for triple-negative breast cancer: The end of the debate. Ann. Oncol. 2022, 33, 347–349. [Google Scholar] [CrossRef]
- Yu, K.D.; Ye, F.G.; He, M.; Fan, L.; Ma, D.; Mo, M.; Wu, J.; Liu, G.Y.; Di, G.H.; Zeng, H.; et al. Effect of adjuvant paclitaxel and carboplatin on survival in women with triple-negative breast cancer: A phase 3 randomized clinical trial. JAMA Oncol. 2020, 6, 1390–1396. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Guidance for Industry; FDA: Silver Spring, MD, USA, 2014.
- Conforti, F.; Pala, L.; Sala, I.; Oriecuia, C.; De Pas, T.; Specchia, C.; Graffeo, R.; Pagan, E.; Queirolo, P.; Pennacchioli, E.; et al. Evaluation of pathological complete response as a surrogate endpoint for disease-free and overall survival in neoadjuvant trials of breast cancer: Systematic review and meta-analysis. BMJ 2021, 375, e066381. [Google Scholar] [CrossRef]
- Saleh, R.R.; Nadler, M.B.; Desnoyers, A.; Meti, N.; Fazelzad, R.; Amir, E. Platinum-based chemotherapy in early-stage triple negative breast cancer: A meta-analysis. Cancer Treat. Rev. 2021, 100, 102283. [Google Scholar] [CrossRef]
- Pathak, N.; Sharma, A.; Elavarasi, A.; Sankar, J.; Deo, S.; Sharma, D.N.; Mathur, S.; Kumar, S.; Prasad, C.P.; Kumar, A.; et al. Moment of truth—Adding carboplatin to neoadjuvant/adjuvant chemotherapy in triple negative breast cancer improves overall survival: An individual participant data and trial-level meta-analysis. Breast 2022, 64, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Landry, K.K.; Lyon, J.L.; Victoria, K.E.; Changizzadeh, P.N.; Cole, B.F.; Pulluri, B.; Sikov, W.M.; Wood, M.E. Weekly versus every-3-week carboplatin with weekly paclitaxel in neoadjuvant chemotherapy for triple-negative breast cancer: A retrospective analysis. Breast Cancer Res. Treat. 2022, 196, 111–121. [Google Scholar]
- Mason, S.R.E.; Willson, M.L.; Egger, S.J.; Beith, J.; Dear, R.F.; Goodwin, A. Platinum chemotherapy for early triple-negative breast cancer. Breast 2024, 75, 103712. [Google Scholar] [CrossRef] [PubMed]
- Bonadio, R.C.; de Sousa, I.M.; Balint, F.C.; Comini, A.C.M.; Tavares, M.C.; Madasi, F.; Bines, J.; Ferreira, R.D.P.; Rosa, D.D.; Santos, C.L.; et al. Dose-dense versus every-3-week platinum regimens in triple-negative breast cancer: Balancing efficacy and toxicity. J. Oncol. Pract. 2024, 20, e210–e219. [Google Scholar]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.; Bergh, J.; Burstein, H.; Cardoso, M.; Carey, L.; Dawood, S.; Del Mastro, L.; et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Pusztai, L.; Denkert, C.; O’sHaughnessy, J.; Cortes, J.; Dent, R.; McArthur, H.; Kümmel, S.; Bergh, J.; Park, Y.; Hui, R.; et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: Exploratory analysis from KEYNOTE-522. Ann. Oncol. 2024, 35, 429–436. [Google Scholar] [CrossRef]
- Guan, X.; Ma, F.; Fan, Y.; Zhu, W.; Hong, R.; Xu, B. Platinum-based chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis of randomized-controlled trials. Anticancer. Drugs 2015, 26, 894–901. [Google Scholar] [CrossRef]
- Petrelli, F.; Coinu, A.; Borgonovo, K.; Cabiddu, M.; Ghilardi, M.; Lonati, V.; Barni, S. The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2014, 144, 223–232. [Google Scholar] [CrossRef]
- Sikov, W.M. Choice of Neoadjuvant Chemotherapy for HER2-Negative Breast Cancer; UpToDate: Waltham, MA, USA, 2020. [Google Scholar]
- Huang, M.; O’SHaughnessy, J.; Zhao, J.; Haiderali, A.; Cortés, J.; Ramsey, S.D.; Briggs, A.; Hu, P.; Karantza, V.; Aktan, G.; et al. Association of pathologic complete response with long-term survival outcomes in triple-negative breast cancer: A meta-analysis. Cancer Res. 2020, 80, 5427–5434. [Google Scholar] [CrossRef]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef]
- Caramelo, O.; Silva, C.; Caramelo, F.; Frutuoso, C.; Almeida-Santos, T. The effect of neoadjuvant platinum-based chemotherapy in BRCA mutated triple negative breast cancers—Systematic review and meta-analysis. Hered. Cancer Clin. Pract. 2019, 17, 11. [Google Scholar] [CrossRef]
- Viechtbauer, W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J. Educ. Behav. Stat. 2005, 30, 261–293. [Google Scholar] [CrossRef]
- Cochran, W.G. The combination of estimates from different experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Loibl, S.; O’SHaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomized, phase 3 trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Schneeweiss, A.; Salat, C.; Rezai, M.; Zahm, D.M.; Klare, P.; Blohmer, J.U.; Tesch, H.; Khandan, F.; Jud, S.; et al. A randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto). Ann. Oncol. 2013, 31, 1004. [Google Scholar] [CrossRef]
- Gupta, S.; Nair, N.S.; Hawaldar, R.; Vanmali, V.; Parmar, V.; Gulia, S.; Ghosh, J.; Joshi, S.; Sarin, R.; Wadasadawala, T.; et al. Addition of platinum to sequential taxane-anthracycline neoadjuvant chemotherapy in patients with triple-negative breast cancer: A phase III randomized controlled trial [abstract]. In Proceedings of the 2022 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 6–10 December 2023; Abstract GS5-01. AACR: Philadelphia, PA, USA, 2023. [Google Scholar]
- de Pádua Souza, C.; Carneiro, A.S.B.; de Oliveira Lessa, A.C.; Lacerda, D.C.; Paiva, C.E.; Zorzetto, M.M.C.; de Freitas, A.J.A.; Santana, I.V.V.; de Oliveira, M.A.; Palmero, E.I.; et al. Neoadjuvant carboplatin in triple-negative breast cancer: Results from NACATRINE, a randomized phase II clinical trial. Breast Cancer Res. Treat. 2023, 202, 57–65. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Ballman, K.; Polley, M.Y.C.; Campbell, J.D.; Fan, C.; Selitsky, S.; Fernandez-Martinez, A.; Parker, J.S.; Hoadley, K.A.; Hu, Z.; et al. CALGB 40603 (Alliance): Long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. J. Clin. Oncol. 2022, 40, 1323–1334. [Google Scholar] [CrossRef]
- Zhang, P.; Yin, Y.; Mo, H.; Zhang, B.; Wang, X.; Li, Q.; Yuan, P.; Wang, J.; Zheng, S.; Cai, R.; et al. Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: A randomized phase 2 trial. Oncotarget 2016, 7, 60647. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.1, R packages retrieved from CRAN snapshot 2023-04-07; R Core Team: Vienna, Austria, 2022; Available online: https://cran.r-project.org (accessed on 10 November 2025).
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Lakens, D. Equivalence tests: A practical primer for t-tests, correlations, and meta-analyses. Soc. Psychol. Personal. Sci. 2017, 8, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Hahnen, E.; Lederer, B.; Hauke, J.; Loibl, S.; Kröber, S.; Schneeweiss, A.; Denkert, C.; Fasching, P.A.; Blohmer, J.U.; Jackisch, C.; et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: Secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 2017, 3, 1378–1385. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Han, M.; Li, A.; Ruan, M.; Tong, Y.; Yang, C.; Zhang, X.; Zhu, C.; Wang, C.; et al. Association between homologous recombination deficiency status and carboplatin treatment response in early triple-negative breast cancer. Breast Cancer Res. Treat. 2024, 208, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.P.; Sevilimedu, V.; Barrio, A.V.; Tadros, A.B.; Mamtani, A.; Robson, M.E.; Morrow, M.; Lee, M.K. Pathologic complete response after neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers. npj Breast Cancer 2024, 10, 63. [Google Scholar] [CrossRef]
- Sokolenko, A.; Gorodnova, T.; Enaldieva, D.; Shestakova, A.; Ivantsov, A.; Nyuganen, A.; Berlev, I.; Krivorotko, P.; Belyaev, A.; Imyanitov, E. Comparison of outcomes of neoadjuvant chemotherapy in BRCA1- versus BRCA2-associated breast and ovarian cancers. Explor. Target. Anti-Tumor Ther. 2025, 6, 1002325. [Google Scholar] [CrossRef]
- Bae, S.J.; Kim, J.H.; Kim, M.J.; Kook, Y.; Baek, S.H.; Kim, J.H.; Moon, S.; Lee, S.E.; Jeong, J.; Cha, Y.J.; et al. Stromal tumor-infiltrating lymphocytes and pathologic response to neoadjuvant chemotherapy with the addition of platinum and pembrolizumab in TNBC: A single-center real-world study. Breast Cancer Res. 2024, 26, 182. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Chen, A.X.; Chen, X.; Li, X.X.; Guo, Z.Y.; Cao, X.C.; Wang, X.; Zhang, B. Impacts of tumor stage at diagnosis and adjuvant therapy on long-term survival outcomes in patients with triple-negative breast cancer achieving pathologic complete response after neoadjuvant chemotherapy. Clin. Breast Cancer 2025, 25, e30–e39. [Google Scholar] [CrossRef] [PubMed]
| Study | Publishing Year | Phase | Treatment Arms | CBDCA Schedule | eTNBC Patients (n) | Primary Endpoints | Secondary Endpoints | pCR Definition |
|---|---|---|---|---|---|---|---|---|
| Brightness | 2022 | III R | ddAC *+paclitaxel ddAC+paclitaxel+carboplatin ddAC+paclitaxel/carboplatin+veliparib | AUC 6 every 3 weeks ×4 cycles | 634 | pCR | EFS, OS | ypT0/is ypN0 |
| GeparSixto | 2017 | II R | ddAC+paclitaxel+bevacizumab+carboplatin ddAC+paclitaxel+bevacizumab | AUC 1.5 weekly × 12 administrations | 588 | DFS | pCR, OS | ypT0 ypN0 |
| GS5-01 | 2023 | III R | AC+paclitaxel AC+carboplatin+paclitaxel | AUC 2 weekly × 12 administrations | 720 | DFS | pCR, OS | ypT0 ypN0 |
| BR15-1 PEARLY | 2024 | III R | AC+paclitaxel AC+carboplatin+paclitaxel | AUC 5 every 3 weeks × 4 cycles | 868 | EFS | pCR, OS | ypT0/is ypN0 |
| NACATRINE | 2023 | II R | docetaxel+carboplatin EC+docetaxel | AUC 1.5 Weekly × 12 administrations | 146 | pCR | DFS, OS | ypT0ypN0 |
| CALGB 40603 | 2022 | II R | ddAC+carboplatin ddAC+carboplatin+bevacizumab ddAC ddAC+bevacizumab | AUC 6 Every three weeks × 4 cycles | 446 | pCR | EFS, OS | ypT0/is ypN0 |
| Study | CBDCA Arm/s Neutropenia | Non-CBDCA Arm/s Neutropenia | CBDCA Arm/s Thrombocytopenia | Non-CBDCA Arm/s Thrombocytopenia |
|---|---|---|---|---|
| CALGB 40603 | >50 | >20 | 46 | 7 |
| Brightness | 30 | 22 | 10 | 2 |
| GeparSixto | 65 | 27 | 14 | <1 |
| GS5-01 | 0.55 | 0.28 | 0.27 | 0.2 |
| BR 15 1 PEARLY | 57.4 | 38.7 | 3.5 | 0.5 |
| NACATRINE | 15.1 | 11 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taglialatela, I.; Ruffilli, B.; Conte, B.; D’Avanzo, F.; Rossi, V.; Nardin, S.; Gennari, A. Updating the Role of Carboplatin Added to Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer: A Meta-Analysis. Cancers 2025, 17, 3961. https://doi.org/10.3390/cancers17243961
Taglialatela I, Ruffilli B, Conte B, D’Avanzo F, Rossi V, Nardin S, Gennari A. Updating the Role of Carboplatin Added to Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer: A Meta-Analysis. Cancers. 2025; 17(24):3961. https://doi.org/10.3390/cancers17243961
Chicago/Turabian StyleTaglialatela, Ida, Beatrice Ruffilli, Benedetta Conte, Francesca D’Avanzo, Valentina Rossi, Simone Nardin, and Alessandra Gennari. 2025. "Updating the Role of Carboplatin Added to Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer: A Meta-Analysis" Cancers 17, no. 24: 3961. https://doi.org/10.3390/cancers17243961
APA StyleTaglialatela, I., Ruffilli, B., Conte, B., D’Avanzo, F., Rossi, V., Nardin, S., & Gennari, A. (2025). Updating the Role of Carboplatin Added to Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer: A Meta-Analysis. Cancers, 17(24), 3961. https://doi.org/10.3390/cancers17243961







