Treatment Sequences in Patients with Metastatic Colorectal Cancer in Japan: Real-World Evidence of First- to Fifth-Line Treatments
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
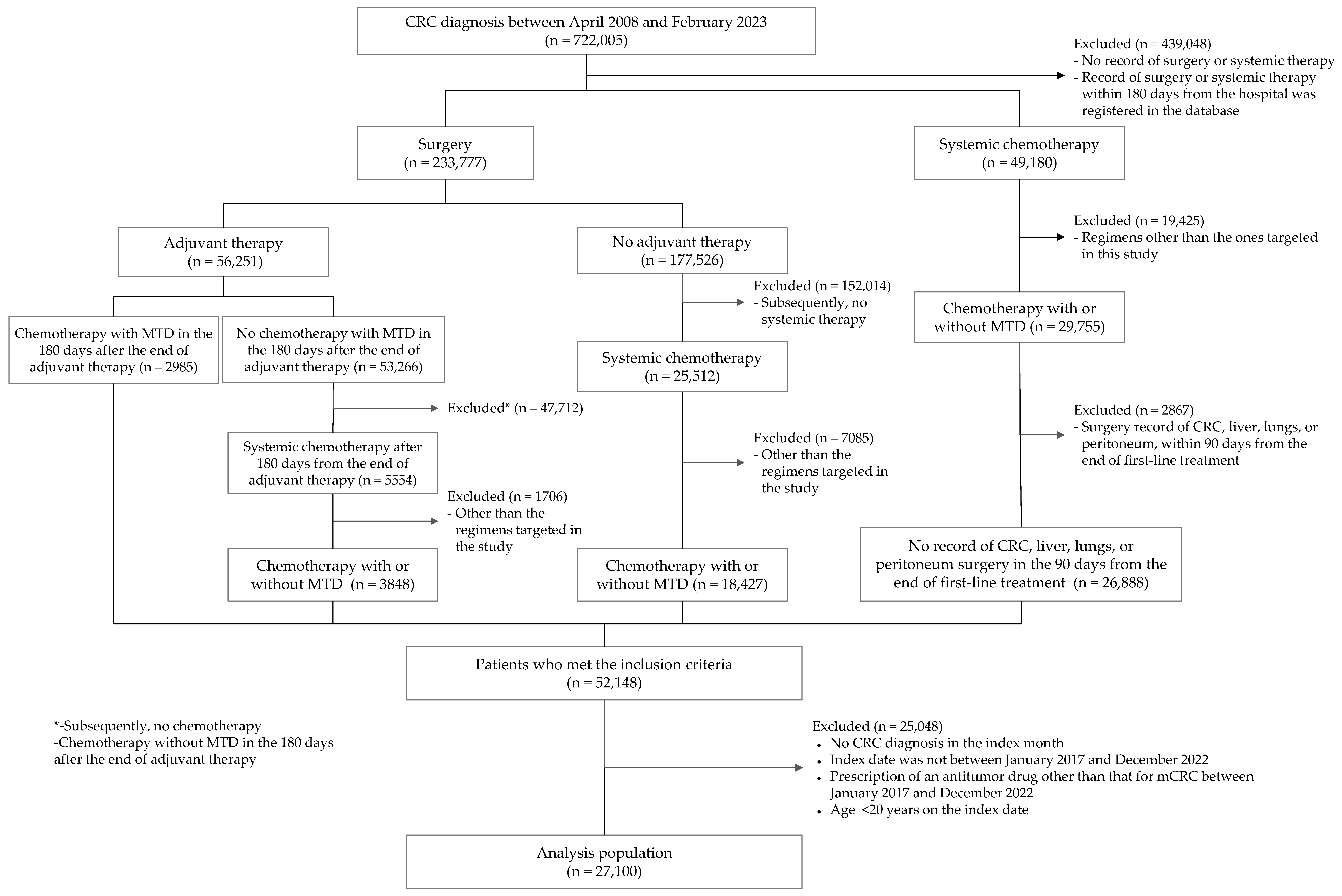
2.2. Study Population
2.3. Treatment Regimens and Sequences
2.4. Statistical Analysis
3. Results
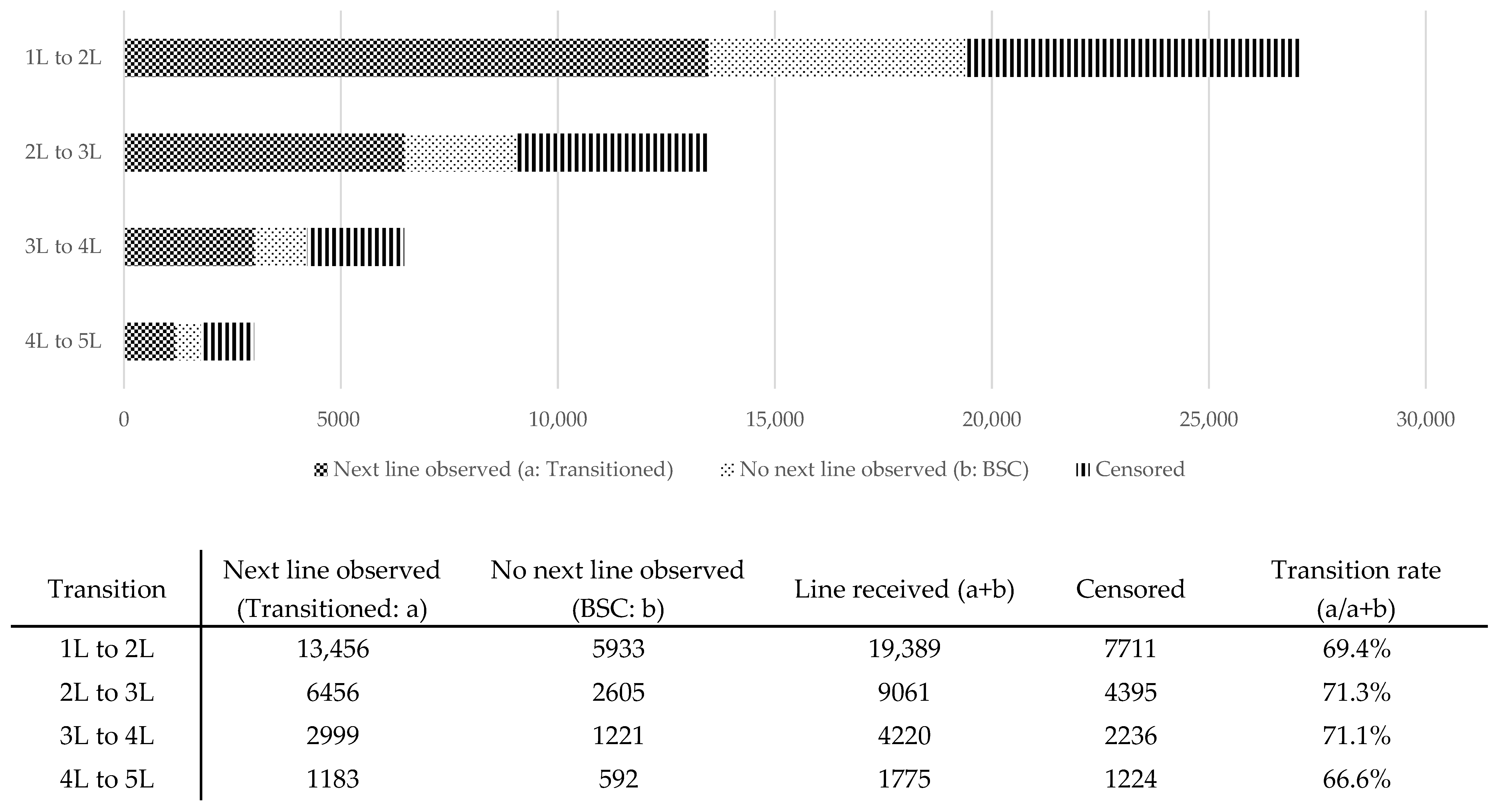
3.1. Transition Rates and Early-Line Treatment Sequences in the Overall Population
3.2. Patient Characteristics and Early-Line Treatment Sequences in Patients Who Received Second-Line Treatment
3.3. Factors Associated with Continuation to Third-Line Treatment
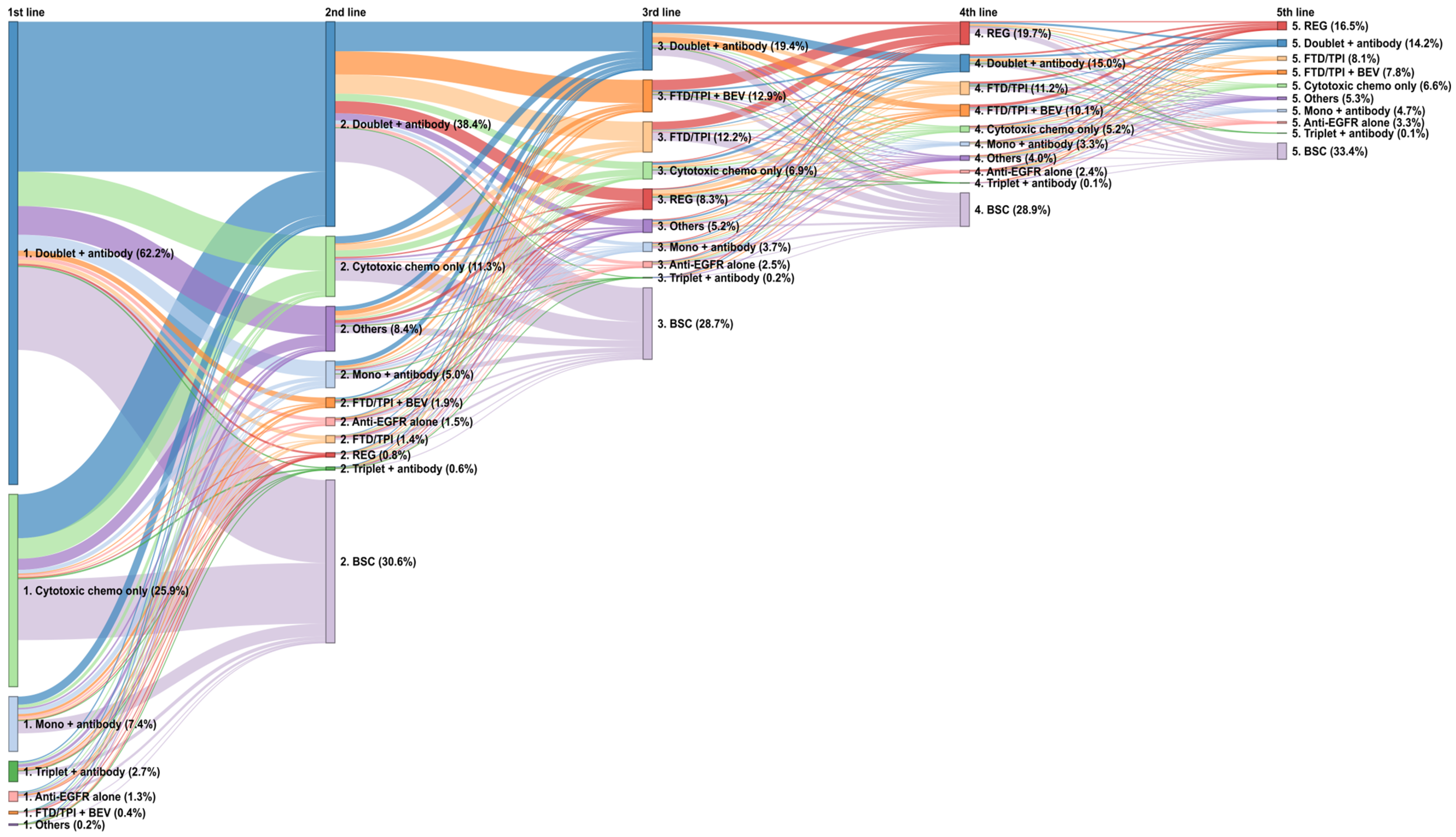
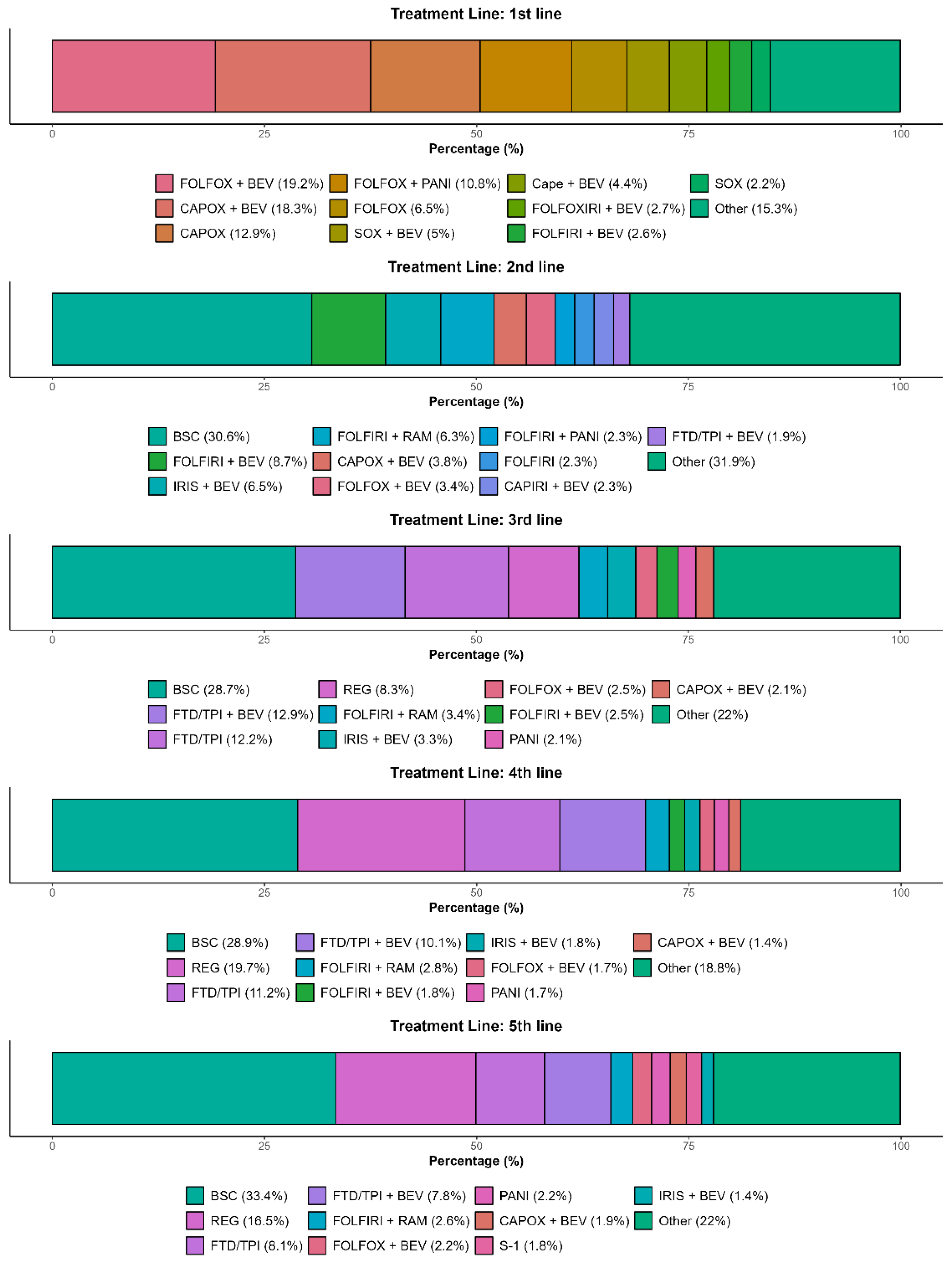
3.4. Late-Line Treatments and Sequences in the Overall Population
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Lonardi, S.; Garcia-Carbonero, R.; Elez, E.; Yoshino, T.; Sobrero, A.; Yao, J.; García-Alfonso, P.; Kocsis, J.; Cubillo Gracian, A.; et al. Fruquintinib versus Placebo in Patients with Refractory Metastatic Colorectal Cancer (FRESCO-2): An International, Multicentre, Randomised, Double-Blind, Phase 3 Study. Lancet 2023, 402, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Muro, K.; Watanabe, J.; Yamazaki, K.; Ohori, H.; Shiozawa, M.; Takashima, A.; Yokota, M.; Makiyama, A.; Akazawa, N.; et al. Baseline CtDNA Gene Alterations as a Biomarker of Survival after Panitumumab and Chemotherapy in Metastatic Colorectal Cancer. Nat. Med. 2024, 30, 730–739. [Google Scholar] [CrossRef]
- Yamada, Y.; Takahari, D.; Matsumoto, H.; Baba, H.; Nakamura, M.; Yoshida, K.; Yoshida, M.; Iwamoto, S.; Shimada, K.; Komatsu, Y.; et al. Leucovorin, Fluorouracil, and Oxaliplatin plus Bevacizumab versus S-1 and Oxaliplatin plus Bevacizumab in Patients with Metastatic Colorectal Cancer (SOFT): An Open-Label, Non-Inferiority, Randomised Phase 3 Trial. Lancet Oncol. 2013, 14, 1278–1286. [Google Scholar] [CrossRef]
- Yamazaki, K.; Nagase, M.; Tamagawa, H.; Ueda, S.; Tamura, T.; Murata, K.; Eguchi Nakajima, T.; Baba, E.; Tsuda, M.; Moriwaki, T.; et al. Randomized Phase III Study of Bevacizumab plus FOLFIRI and Bevacizumab plus MFOLFOX6 as First-Line Treatment for Patients with Metastatic Colorectal Cancer (WJOG4407G). Ann. Oncol. 2016, 27, 1539–1546. [Google Scholar] [CrossRef]
- Loupakis, F.; Cremolini, C.; Masi, G.; Lonardi, S.; Zagonel, V.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Ronzoni, M.; Spadi, R.; et al. Initial Therapy with FOLFOXIRI and Bevacizumab for Metastatic Colorectal Cancer. N. Engl. J. Med. 2014, 371, 1609–1618. [Google Scholar] [CrossRef]
- Deng, Y.; Chi, P.; Lan, P.; Wang, L.; Chen, W.; Cui, L.; Chen, D.; Cao, J.; Wei, H.; Peng, X.; et al. Neoadjuvant Modified FOLFOX6 with or without Radiation versus Fluorouracil plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese FOWARC Trial. J. Clin. Oncol. 2019, 37, 3223–3233. [Google Scholar] [CrossRef]
- Schnipper, L.E.; Davidson, N.E.; Wollins, D.S.; Tyne, C.; Blayney, D.W.; Blum, D. American Society of Clinical Oncology Statement: A Conceptual Framework to Assess the Value of Cancer Treatment Options. J. Clin. Oncol. 2015, 33, 2563–2577. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic Colorectal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up☆. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- JSCCR Guidelines 2022 for the Treatment of Colorectal Cancer. Available online: https://www.jsccr.jp/guideline/2022/index_guide.html (accessed on 24 July 2024).
- Colorectal Cancer Facts & Figures. Available online: https://www.cancer.org/research/cancer-facts-statistics/colorectal-cancer-facts-figures.html (accessed on 5 December 2024).
- Shinozaki, E.; Makiyama, A.; Kagawa, Y.; Satake, H.; Tanizawa, Y.; Cai, Z.; Piao, Y. Treatment Sequences of Patients with Advanced Colorectal Cancer and Use of Second-Line FOLFIRI with Antiangiogenic Drugs in Japan: A Retrospective Observational Study Using an Administrative Database. PLoS ONE 2021, 16, e0246160. [Google Scholar] [CrossRef]
- Satake, H.; Kagawa, Y.; Shinozaki, E.; Tanizawa, Y.; Jin, L.; Cai, Z.; Makiyama, A. Real-World Data Analysis of Second-Line Antiangiogenic Targeted Treatments Following Anti-Epidermal Growth Factor Receptor Monoclonal Antibodies and First-Line FOLFOX for Patients with Metastatic Colorectal Cancer. Adv. Ther. 2022, 39, 2596–2613. [Google Scholar] [CrossRef]
- Yamazaki, K.; Yuki, S.; Oki, E.; Sano, F.; Makishima, M.; Aoki, K.; Hamano, T.; Yamanaka, T. Real-World Evidence on Second-Line Treatment of Metastatic Colorectal Cancer Using Fluoropyrimidine, Irinotecan, and Angiogenesis Inhibitor. Clin. Color. Cancer 2021, 20, e173–e184. [Google Scholar] [CrossRef]
- Teng, C.-L.J.; Wang, C.-Y.; Chen, Y.-H.; Lin, C.-H.; Hwang, W.-L. Optimal Sequence of Irinotecan and Oxaliplatin-Based Regimens in Metastatic Colorectal Cancer: A Population-Based Observational Study. PLoS ONE 2015, 10, e0135673. [Google Scholar] [CrossRef]
- Kagawa, Y.; Wang, C.; Piao, Y.; Jin, L.; Tanizawa, Y.; Cai, Z.; Sunakawa, Y. Real-World Evidence of FOLFIRI Combined with Anti-Angiogenesis Inhibitors or Anti-EGFR Antibodies for Patients with Early Recurrence Colorectal Cancer after Adjuvant FOLFOX/CAPOX Therapy: A Japanese Claims Database Study. Target. Oncol. 2024, 19, 575–585. [Google Scholar] [CrossRef]
- Kagawa, Y.; Shinozaki, E.; Okude, R.; Tone, T.; Kunitomi, Y.; Nakashima, M. Real-World Evidence of Trifluridine/Tipiracil plus Bevacizumab in Metastatic Colorectal Cancer Using an Administrative Claims Database in Japan. ESMO Open 2023, 8, 101614. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Takeuchi, M.; Kawakami, K. Effectiveness and Safety of Regorafenib vs. Trifluridine/Tipiracil in Unresectable Colorectal Cancer: A Retrospective Cohort Study. Clin. Color. Cancer 2020, 19, e208–e225. [Google Scholar] [CrossRef] [PubMed]
- Roset, M.; Amonkar, M.; Patel, R.; Lara, N.; Kothari, S. Real-World Treatment Patterns and Clinical Outcomes for Standard of Care Regimens in Patients with Deficient MMR or MSI-High Metastatic Colorectal and Non-Colorectal Cancer: A Retrospective Chart Review Study in France. Adv. Ther. 2022, 39, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Min, S.T.; Roohullah, A.; Tognela, A.; Jalali, A.; Lee, M.; Wong, R.; Shapiro, J.; Burge, M.; Yip, D.; Nott, L.; et al. Patient Demographics and Management Landscape of Metastatic Colorectal Cancer in the Third-Line Setting: Real-World Data in an Australian Population. Asia Pac. J. Clin. Oncol. 2022, 18, e56–e63. [Google Scholar] [CrossRef]
- MDV Database Overview. Available online: https://en.mdv.co.jp/ebm/about-mdv-database/mdv-database-overview/ (accessed on 24 July 2024).
- Laurent, T.; Simeone, J.; Kuwatsuru, R.; Hirano, T.; Graham, S.; Wakabayashi, R.; Phillips, R.; Isomura, T. Context and Considerations for Use of Two Japanese Real-World Databases in Japan: Medical Data Vision and Japanese Medical Data Center. Drugs Real World Outcomes 2022, 9, 175–187. [Google Scholar] [CrossRef]
- Hess, L.M.; Li, X.; Wu, Y.; Goodloe, R.J.; Cui, Z.L. Defining Treatment Regimens and Lines of Therapy Using Real-World Data in Oncology. Future Oncol. 2021, 17, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Nishina, T.; Shinozaki, E.; Yamazaki, K.; Shitara, K.; Okamoto, W.; Kajiwara, T.; Matsumoto, T.; Tsushima, T.; Mochizuki, N.; et al. TAS-102 plus Bevacizumab for Patients with Metastatic Colorectal Cancer Refractory to Standard Therapies (C-TASK FORCE): An Investigator-Initiated, Open-Label, Single-Arm, Multicentre, Phase 1/2 Study. Lancet Oncol. 2017, 18, 1172–1181. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Yilmaz, M.; Möller, S.; Zitnjak, D.; Krogh, M.; Petersen, L.N.; Poulsen, L.Ø.; Winther, S.B.; Thomsen, K.G.; Qvortrup, C. TAS-102 with or without Bevacizumab in Patients with Chemorefractory Metastatic Colorectal Cancer: An Investigator-Initiated, Open-Label, Randomised, Phase 2 Trial. Lancet Oncol. 2020, 21, 412–420. [Google Scholar] [CrossRef]
- Grothey, A.; Sargent, D. Overall Survival of Patients With Advanced Colorectal Cancer Correlates With Availability of Fluorouracil, Irinotecan, and Oxaliplatin Regardless of Whether Doublet or Single-Agent Therapy Is Used First Line. J. Clin. Oncol. 2005, 23, 9441–9442. [Google Scholar] [CrossRef]
- Jain, R.K. Normalizing Tumor Vasculature with Anti-Angiogenic Therapy: A New Paradigm for Combination Therapy. Nat. Med. 2001, 7, 987–989. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Nilsson, M.B.; Le, X.; Cascone, T.; Jain, R.K.; Heymach, J.V. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin. Cancer Res. 2023, 29, 30–39. [Google Scholar] [CrossRef]
- Watanabe, J.; Muro, K.; Shitara, K.; Yamazaki, K.; Shiozawa, M.; Ohori, H.; Takashima, A.; Yokota, M.; Makiyama, A.; Akazawa, N.; et al. Panitumumab vs Bevacizumab Added to Standard First-Line Chemotherapy and Overall Survival among Patients with RAS Wild-Type, Left-Sided Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2023, 329, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus Cetuximab versus FOLFIRI plus Bevacizumab as First-Line Treatment for Patients with Metastatic Colorectal Cancer (FIRE-3): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.-J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined with Cetuximab or Bevacizumab on Overall Survival in Patients with KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef]
- Baltussen, J.C.; de Glas, N.A.; Liefers, G.-J.; Slingerland, M.; Speetjens, F.M.; van den Bos, F.; Cloos-van Balen, M.; Verschoor, A.J.; Jochems, A.; Spierings, L.E.A.M.M.; et al. Time Trends in Treatment Patterns and Survival of Older Patients with Synchronous Metastatic Colorectal Cancer in the Netherlands: A Population-Based Study. Int. J. Cancer 2023, 152, 2043–2051. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, R.; Yau, T.C.C.; Ma, B.; Pan, H.; Xu, J.; Bai, Y.; Chi, Y.; Wang, L.; et al. Regorafenib plus Best Supportive Care versus Placebo plus Best Supportive Care in Asian Patients with Previously Treated Metastatic Colorectal Cancer (CONCUR): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2015, 16, 619–629. [Google Scholar] [CrossRef]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Prager, G.W.; Taieb, J.; Fakih, M.; Ciardiello, F.; Van Cutsem, E.; Elez, E.; Cruz, F.M.; Wyrwicz, L.; Stroyakovskiy, D.; Pápai, Z.; et al. Trifluridine-Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2023, 388, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Nevala-Plagemann, C.; Sama, S.; Ying, J.; Shen, J.; Haaland, B.; Florou, V.; Garrido-Laguna, I. A Real-World Comparison of Regorafenib and Trifluridine/Tipiracil in Refractory Metastatic Colorectal Cancer in the United States. J. Natl. Compr. Canc. Netw. 2023, 21, 257–264. [Google Scholar] [CrossRef]
- Strickler, J.H.; Cercek, A.; Siena, S.; André, T.; Ng, K.; Van Cutsem, E.; Wu, C.; Paulson, A.S.; Hubbard, J.M.; Coveler, A.L.; et al. Tucatinib plus Trastuzumab for Chemotherapy-Refractory, HER2-Positive, RAS Wild-Type Unresectable or Metastatic Colorectal Cancer (MOUNTAINEER): A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2023, 24, 496–508. [Google Scholar] [CrossRef]
- Fakih, M.G.; Salvatore, L.; Esaki, T.; Modest, D.P.; Lopez-Bravo, D.P.; Taieb, J.; Karamouzis, M.V.; Ruiz-Garcia, E.; Kim, T.-W.; Kuboki, Y.; et al. Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 389, 2125–2139. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Weiss, J.; Pelster, M.S.; Spira, A.I.; Barve, M.; Ou, S.-H.I.; Leal, T.A.; Bekaii-Saab, T.S.; Paweletz, C.P.; Heavey, G.A.; et al. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 388, 44–54. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Salvatore, L.; Esaki, T.; Modest, D.P.; Lopez-Bravo, D.P.; Taieb, J.; Karamouzis, M.V.; Ruiz-Garcia, E.; Kim, T.W.; Kuboki, Y.; et al. Overall Survival Analysis of the Phase III CodeBreaK 300 Study of Sotorasib plus Panitumumab versus Investigator’s Choice in Chemorefractory KRAS G12C Colorectal Cancer. J. Clin. Oncol. 2025, 43, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Saeed, A.; Parikh, A.; Wang, Y.; Wang, Z.; Schwickart, M.; Curran, D.; Hecht, J. P-35 Zanzalintinib (XL092) in Combination with Atezolizumab for Previously Treated Metastatic Colorectal Cancer. Ann. Oncol. 2023, 34, S25–S26. [Google Scholar] [CrossRef]
- Hecht, J.R.; Park, Y.S.; Tabernero, J.; Lee, M.-A.; Lee, S.; Virgili, A.C.; Van den Eynde, M.; Fontana, E.; Fakih, M.; Asghari, G.; et al. Zanzalintinib plus Atezolizumab versus Regorafenib in Refractory Colorectal Cancer (STELLAR-303): A Randomised, Open-Label, Phase 3 Trial. Lancet 2025, 406, 2360–2370. [Google Scholar] [CrossRef] [PubMed]
| Transited to Third Line | Transited to BSC | p Value a | |
|---|---|---|---|
| (n = 6456) | (n = 2605) | ||
| Age, years, median (IQR) | 69 (61, 74) | 71 (64, 77) | <0.001 |
| Sex, n (%) | 0.638 | ||
| Male | 3937 (61.0%) | 1574 (60.4%) | |
| Female | 2519 (39.0%) | 1031 (39.6%) | |
| Index year, n (%) | 0.411 | ||
| 2017 | 1205 (18.7%) | 467 (17.9%) | |
| 2018 | 1284 (19.9%) | 557 (21.4%) | |
| 2019 | 1430 (22.1%) | 545 (20.9%) | |
| 2020 | 1349 (20.9%) | 534 (20.5%) | |
| 2021 | 1006 (15.6%) | 420 (16.1%) | |
| 2022 | 182 (2.8%) | 82 (3.1%) | |
| Primary tumor location, n (%) | 0.952 | ||
| Right side | 1810 (28.0%) | 729 (28.0%) | |
| Left side | 4340 (67.2%) | 1745 (67.0% | |
| Both | 162 (2.5%) | 69 (2.6%) | |
| Unknown | 144 (2.2%) | 62 (2.4%) | |
| Metastatic sites, n (%) | |||
| Liver | 3302 (51.1%) | 1095 (42.0%) | <0.001 |
| Lung | 1834 (28.4%) | 582 (22.3%) | <0.001 |
| Peritoneal | 1088 (16.9%) | 422 (16.2%) | <0.001 |
| Lymph node | 891 (13.8%) | 338 (13.0%) | <0.001 |
| Bone | 304 (4.7%) | 123 (4.7%) | <0.001 |
| Brain | 45 (0.7%) | 28 (1.1%) | 0.024 |
| Other | 292 (4.5%) | 123 (4.7%) | <0.001 |
| Comorbidity, n (%) | |||
| Hypertension | 3188 (49.4%) | 1228 (47.1%) | <0.001 |
| Peripheral neuropathy | 1606 (24.9%) | 601 (23.1%) | <0.001 |
| Hand–foot syndrome | 2390 (37.0%) | 841 (32.3%) | <0.001 |
| Anemia | 467 (7.2%) | 280 (10.7%) | <0.001 |
| Leukopenia | 342 (5.3%) | 110 (4.2%) | <0.001 |
| Interstitial pneumonitis | 110 (1.7%) | 59 (2.3%) | 0.002 |
| Proteinuria | 96 (1.5%) | 26 (1.0%) | 0.071 |
| Size of hospital, n (%) | <0.001 | ||
| <200 beds | 302 (4.7%) | 170 (6.5%) | |
| 200–499 beds | 3576 (55.4%) | 1470 (56.4%) | |
| ≥500 beds | 2578 (39.9%) | 965 (37.0%) | |
| Designated cancer hospital, n (%) | 5108 (79.1%) | 1974 (75.8%) | <0.001 |
| Treatment duration, median (IQR) | |||
| First line | 196 (116, 314) | 169 (98, 288) | <0.001 |
| Second line | 143 (71, 254) | 99 (42, 204) | <0.001 |
| Previous treatment, n (%) | |||
| OX and IRI plus VEGF inhibitors and/or anti-EGFR antibodies | 2753 (42.6%) | 843 (32.4%) | <0.001 |
| OR | 95% CI | p Value | ||
|---|---|---|---|---|
| Age | <65 years | 1.50 | (1.36, 1.67) | <0.001 |
| Sex | Female | 1.00 | (0.91, 1.10) | 0.977 |
| Primary tumor location | ||||
| Right side | 1.07 | (0.96, 1.19) | 0.200 | |
| Both left and right sides | 1.06 | (0.79, 1.43) | 0.711 | |
| Unknown | 0.98 | (0.72, 1.34) | 0.893 | |
| Treatment duration | ||||
| First line | ≥180 days | 1.24 | (1.13, 1.37) | <0.001 |
| Second line | ≥120 days | 1.70 | (1.55, 1.86) | <0.001 |
| Previous treatment | ||||
| OX and IRI plus VEGF inhibitors and/or anti-EGFR antibodies | 1.41 | (1.27, 1.56) | <0.001 | |
| Metastatic sites | ||||
| Liver | 1.42 | (1.29, 1.56) | <0.001 | |
| Lung | 1.14 | (1.01, 1.29) | 0.031 | |
| Peritoneal | 1.04 | (0.90, 1.20) | 0.613 | |
| Lymph node * | 0.96 | (0.83, 1.11) | 0.553 | |
| Treatment Lines and Regimens | n | Median | IQR |
| (Days) | (Days) | ||
| Third-line | 4220 | 87 | (43–175) |
| FTD/TPI | 682 | 65 | (38–101) |
| FTD/TPI + EV | 708 | 92 | (50–173) |
| REG | 446 | 49 | (21–98) |
| Fourth-line | 1775 | 78 | (38–147) |
| FTD/TPI | 282 | 66 | (38–102.75) |
| FTD/TPI + BEV | 247 | 108 | (71–192) |
| REG | 434 | 49 | (21–84) |
| Fifth-line | 713 | 71 | (35–140) |
| FTD/TPI | 85 | 60 | (23–94) |
| FTD/TPI + BEV | 78 | 96.5 | (50–179) |
| REG | 164 | 49 | (21–98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kagawa, Y.; Osaka, T.; Imamura, T.; Kuwabara, H. Treatment Sequences in Patients with Metastatic Colorectal Cancer in Japan: Real-World Evidence of First- to Fifth-Line Treatments. Cancers 2025, 17, 3962. https://doi.org/10.3390/cancers17243962
Kagawa Y, Osaka T, Imamura T, Kuwabara H. Treatment Sequences in Patients with Metastatic Colorectal Cancer in Japan: Real-World Evidence of First- to Fifth-Line Treatments. Cancers. 2025; 17(24):3962. https://doi.org/10.3390/cancers17243962
Chicago/Turabian StyleKagawa, Yoshinori, Tsuyoshi Osaka, Toshiki Imamura, and Hiroyo Kuwabara. 2025. "Treatment Sequences in Patients with Metastatic Colorectal Cancer in Japan: Real-World Evidence of First- to Fifth-Line Treatments" Cancers 17, no. 24: 3962. https://doi.org/10.3390/cancers17243962
APA StyleKagawa, Y., Osaka, T., Imamura, T., & Kuwabara, H. (2025). Treatment Sequences in Patients with Metastatic Colorectal Cancer in Japan: Real-World Evidence of First- to Fifth-Line Treatments. Cancers, 17(24), 3962. https://doi.org/10.3390/cancers17243962






