1. Introduction
Glioblastoma (GBM) remains the most aggressive primary brain tumour in adults, with survival limited by poor drug penetration and rapid therapeutic resistance [
1,
2]. Standard of care therapy includes surgical resection, radiotherapy, and temozolomide chemotherapy, yet tumour recurrence is nearly universal [
3]. Multiple barriers—including the blood–brain barrier (BBB), a heterogeneous blood–tumour barrier (BTB), and a hostile tumour microenvironment (TME)—undermine delivery [
4]. Here, we outline a practical design framework for ligand-functionalised, magnetic lipid nanoparticles (MF-R-LNs) and propose clear reporting conventions for magnetic-field use in GBM.
One of the most significant obstacles to effective GBM therapy is the BBB, a tightly regulated interface that prevents many therapeutic agents from reaching the brain parenchyma [
5]. Even in areas where the BBB is compromised, referred to as the BTB, drug delivery remains highly variable and incomplete [
6]. The TME further complicates treatment due to hypoxia, acidic pH, aberrant vasculature, and a heavily immunosuppressive milieu. Interstitial fluid pressure (IFP) is typically 0–2 mmHg in normal brain but often 10–30 mmHg in GBM, with peritumoural regions reported up to 40–50 mmHg, further impeding convective drug transport. Compounding these factors are therapy-induced resistance mechanisms such as upregulation of efflux transporters, DNA repair enzymes such as O6-Methylguanine-DNA Methyltransferase (MGMT), and activation of redundant signalling pathways [
7]. Recent GBM nano approaches such as intranasal small-molecule formulations (e.g., NEO100); catheter-based radionanoliposomes (e.g., 186RNL); intralesional magnetothermal superparamagnetic iron oxide nanoparticles (SPIONs) (e.g., NanoTherm); and BBB-shuttle carriers (e.g., glutathione- or Angiopep-based), demonstrate feasibility but each addresses only part of the problem. This motivates systemically deliverable platforms that can reach tumour, be guided/monitored, and release on-target. We therefore introduce MF-R-LNs as a coherent design space built around these three aims.
To overcome these challenges, there has been growing interest in developing multifunctional nanocarrier systems that can both traverse the BBB and selectively release their payload within the GBM microenvironment [
8]. Lipid nanoparticles (LNPs) have emerged as versatile platforms due to their biocompatibility, ability to encapsulate hydrophilic and hydrophobic drugs, and potential for surface functionalisation. Magnetic nanoparticles, particularly SPIONs, offer an additional dimension of spatial control through magnetic guidance and the possibility of inducing hyperthermia under alternating magnetic fields; the resultant local heating of 42–45 °C fluidizes thermosensitive lipid bilayers and permeabilizes endosomal membranes, hence initiating on-demand drug release.
This review outlines a theoretical framework for designing ligand-functionalised, MF-R-LNs tailored to the therapeutic needs of GBM [
9,
10]. By integrating targeting ligands, PEGylation, redox- or enzyme-sensitive linkers, and magnetic cores, these multifunctional constructs aim to bypass physical barriers, enhance tumour specificity, and trigger localised drug release. The rationale for each design component is grounded in GBM pathophysiology and prior nanomedicine research, with emphasis on modularity and combinatorial synergy.
In addition to reviewing the rationale and design considerations, we explore how magnetic hyperthermia, activated remotely via SPIONs, can act as a secondary trigger to enhance intratumoural drug delivery and sensitise resistant tumour regions. We discuss potential applications of these theoretical systems in overcoming key limitations of current GBM therapy, as well as the translational hurdles that remain, including safety, ligand receptor heterogeneity, and manufacturing scalability.
By proposing a cohesive strategy that combines BBB penetration, microenvironment responsiveness, and magnetic control, this conceptual framework contributes to the growing discourse on next-generation nanotherapeutics for glioblastoma. While experimental validation remains a future goal, the principles outlined here may help guide the rational design of multifunctional delivery systems with improved therapeutic efficacy and translational potential.
2. Barriers to Drug Delivery in Glioblastoma
Despite decades of research, GBM remains one of the most treatment-refractory cancers. Its resistance to therapy arises from a confluence of structural, cellular, and molecular barriers that impair drug delivery and promote tumour survival. Understanding these barriers is essential for rational design of nanomedicines capable of penetrating the tumour, overcoming resistance mechanisms, and delivering payloads effectively. These features are illustrated in
Figure 1, which summarises the structural and physiological barriers that restrict drug delivery to GBM.
2.1. The Blood-Brain Barriers and Blood-Tumour Barrier
The BBB is a selective endothelial interface formed by tight junctions, pericytes and astrocytic end-feet that restrict the entry of most systemic drugs into the central nervous system [
11,
12]. Although the BBB may be locally disrupted in GBM, the resulting BTB remains heterogeneously permeable. This regional variability causes uneven drug distribution, with poorly perfused tumour regions remaining inaccessible to most small molecules and biologics.
Furthermore, endothelial cells in the BBB and BTB express high levels of efflux pumps such as P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), which actively transport chemotherapeutic agents back into the circulation [
13]. This severely limits the brain uptake of many systematically administered drugs and contributes to subtherapeutic concentrations in the tumour core.
2.2. Tumour Microenvironment (TME)
The GBM microenvironment presents additional biophysical and biochemical barriers. The extracellular matrix is highly dense and irregular, impeding nanoparticle penetration [
14]. Hypoxia and necrosis are common, creating an acidic and immunosuppressive milieu that supports angiogenesis, tumour invasion, and resistance to radiotherapy and chemotherapy.
Tumour-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and regulatory T cells (Treg) further contribute to immune evasion and therapeutic resistance. These immune cells can also internalise drug carriers nonspecifically, reducing delivery efficiency to glioma cells.
2.3. Therapy-Induced Resistance Mechanisms
GBM is characterised by rapid mutation rates and high intratumoural heterogeneity. As a result, subclonal populations with therapy-resistant phenotypes frequently emerge. Upregulation of DNA repair enzymes such as MGMT confers resistance to alkylating agents such as temozolomide [
12,
15]. Parallel activation of redundant signalling pathways (e.g., EGFR, PI3K/AKT/mTOR) enables escape from targeted therapies [
12,
13].
Additionally, the adaptive expression of efflux transporters and anti-apoptotic proteins under therapeutic pressure further reduces the effectiveness of chemotherapeutics [
13,
16]. The dynamic and adaptable nature of GBM requires delivery systems that can not only reach the tumour but also remain effective in the face of evolving resistance mechanisms.
3. Design Framework for Ligand-Functionalised Magnetic Lipid Nanoparticles
The design of multifunctional nanoparticles for GBM therapy requires strategic integration of features that address key delivery challenges: crossing the BBB, targeting tumour cells, avoiding immune clearance, and triggering drug release selectively within the TME [
12]. Ligand-functionalised MF-R-LNs are conceptual platforms that unite several design principles to achieve this goal.
3.1. Core Structure and Composition
The foundational structure of MF-R-LNs is a lipid-based carrier, selected for its biocompatibility, ability to encapsulate both hydrophobic and hydrophilic drugs, and ease of surface modification [
7,
17]. The lipid bilayer can be formulated to include phase-transition lipids or thermosensitive elements that respond to external triggers such as heat or magnetic fields [
18]. A representative design of a ligand-functionalised MF-R-LN is shown in
Figure 2.
In the nanoparticle core, superparamagnetic iron oxide nanoparticles (SPIONs) are embedded to enable magnetic steering and hyperthermia. SPIONs are well characterised and have been used clinically in diagnostic and therapeutic contexts [
9]. Their inclusion allows for both passive and active guidance across the BBB and for localised activation using an alternating magnetic field [
19].
Polyethylene glycol (PEG) is commonly grafted onto the nanoparticle surface to reduce opsonization by the mononuclear phagocyte system and extend systemic circulation time. While PEGylation improves stealth properties, it may reduce cell uptake, necessitating the inclusion of targeting ligands to compensate [
20,
21,
22].
3.2. Surface Functionalisation and Targeting Ligands
Surface functionalisation with targeting ligands enhances selectivity toward tumour-specific receptors overexpressed in GBM. Candidate ligands include peptides (e.g., angiopep-2, RGD) [
23,
24,
25], antibodies (e.g., anti-EGFR), aptamers, or small molecules such as folate. These ligands can be conjugated via stable or cleavable linkers depending on the desired release profile and microenvironmental triggers.
Receptor targets must be chosen based on expression patterns in GBM cells versus healthy brain tissue. Ideal targets include the following:
EGFR and EGFRvIII: commonly overexpressed in IDH-wildtype tumours and associated with poorer prognosis
IL-13Rα2: high specificity to GBM cells
Integrins (e.g., αvβ3, αvβ5): involved in tumour angiogenesis and invasion
Transferrin receptor (TfR): facilitates BBB crossing [
26]
Given marked inter- and intra-patient heterogeneity, ligand choice should be patient-informed wherever feasible, via biopsy immunophenotyping (e.g., receptor panels for LRP1, TfR, EGFR/EGFRvIII, IL-13Rα2) or liquid-biopsy surrogates (circulating tumour DNA/exosomes), together with imaging biomarkers. In practice, a dual-ligand design that pairs a BBB-transcytosis shuttle (e.g., Angiopep-2 or a TfR-directed binder) with ≥1 tumour-binding ligand selected from the patient’s profile helps preserve target engagement despite spatial heterogeneity and temporal evolution.
Table 1 summarises representative ligand–receptor pairs relevant to GBM. Ligand selection should balance affinity and specificity: very high-affinity binders risk off-target binding and sink effects, whereas low-affinity binders may yield insufficient uptake. Marked heterogeneity within and between tumours further motivates redundancy (e.g., dual-ligand builds) and periodic reassessment as tumours evolve
3.3. Stimuli-Responsive Release
MF-R-LNs can be engineered to release their payload in response to specific stimuli within the TME or external triggers. Common mechanisms include the following:
pH sensitive lipids or polymers: exploit acidic tumour pH for membrane destabilisation;
Redox sensitive linkers (e.g., disulfide bonds): cleaved by elevated intracellular glutathione [
54];
Enzyme-responsive elements: cleavable by matrix metalloproteinases (e.g., MMP-2/9), which are elevated in GBM during invasive growth, angiogenesis, and hypoxia-driven ECM remodelling; note that MMPs also rise in inflammatory CNS disorders (e.g., MS), so pair this trigger with tumour-targeting ligands or intracellular cues to preserve specificity [
55,
56];
These mechanisms increase spatiotemporal control of drug delivery, reducing systemic toxicity and enhancing local therapeutic effect.
3.4. Practical Constraints and Manufacturability
The modular nature of MF-R-LNs allows each functional element (core, coating, targeting ligand, release mechanism) to be tailored for a given therapeutic context. This platform can carry diverse payloads, including chemotherapeutics, gene agents (e.g., siRNA or CRISPR/Cas9 components), and immunomodulators. It also supports personalised designs for GBM heterogeneity by combining a BBB-transcytosis shuttle with at least one tumour-binding ligand and layered, stimulus-responsive release. Where profiling is limited or unavailable, a dual-ligand configuration provides a pragmatic default.
Therapeutic designs must also consider practical constraints, including the following:
Detailed good manufacturing practice/chemistry, manufacturing and controls (GMP/CMC), safety/immunogenicity, and regulatory/AMF reporting requirements are provided in
Section 6.2,
Section 6.3 and
Section 6.4 (CQAs; PEG immunogenicity; SPION characterisation and MRI/MPI tracking; AMF parameters H, f, H × f, thermometry, calibration).
With these constraints defined, we next consider magnetic hyperthermia as a trigger, how SPION-mediated heating can be harnessed to enable controlled, on-target drug release (
Section 4).
4. Magnetic Hyperthermia as a Trigger
Magnetic hyperthermia is a promising adjunctive modality for enhancing drug delivery to GBM by enabling externally controlled, localised heating within the tumour. When exposed to an AMF, SPIONs embedded in lipid nanoparticles generate heat through Néel and Brownian relaxation. This localised thermal effect can be exploited in multiple ways to improve outcomes.
4.1. Heat Induced Drug Release
Thermosensitive lipids incorporated into the MF-R-LN bilayer can be designed to undergo phase transitions slightly above physiological temperatures (e.g., 42–45 °C) [
18,
57,
58]. Upon AMF activation, SPION-mediated heating destabilises the membrane and triggers controlled drug release, enabling temporal precision so that release can be synchronised with tumour localisation (
Figure 3). Typical alternating-magnetic-field parameters for SPION hyperthermia are 100–500 kHz at <15 kA·m
−1, with safety limits applied to avoid non-specific tissue heating.
Formulation choices should minimise baseline leakage at 37 °C while preserving a sharp release transition at the target temperature. In practice, this often requires blended lipid compositions with cholesterol for bilayer stabilisation, rather than relying on a single saturated lipid (e.g., DPPC alone). Optional polymeric over-layers (e.g., PNIPAM) can narrow the thermal window but add complexity and should be justified by leakage and release assays under physiologic and hyperthermic conditions. Overall, lipid composition, cholesterol fraction, ligand density, and particle size should be jointly tuned against stability vs. triggerability criteria established in vitro before in vivo use.
4.2. Tumour Sensitisation and Direct Cytotoxicity
Magnetic hyperthermia may also contribute directly to tumour cell death via local heating. Even moderate hyperthermia (42 °C) can sensitise tumour cells to chemotherapy and radiotherapy by disrupting DNA repair pathways, increasing membrane permeability, and promoting apoptosis; inducing a local heat shock response [
58,
59]. At higher temperatures, thermal ablation can occur, although this requires more energy and may risk damaging surrounding healthy tissue.
GBM’s dense extracellular matrix and irregular vascularization often create poorly perfused regions that are resistant to therapy. Hyperthermia may improve perfusion and oxygenation in these hypoxic zones, thereby enhancing drug penetration and overall treatment efficacy [
60].
4.3. Design Considerations for Magnetic Responsiveness
Effective magnetic hyperthermia requires careful optimisation of SPION characteristics and field conditions:
Size and shape. Cores of approximately 10–20 nm remain superparamagnetic and minimise aggregation [
61,
62]; narrow polydispersity is preferred.
Field parameters. Always report the field amplitude H (kA·m−1), frequency f (kHz), their product H × f, and exposure duration, and target mild hyperthermia 42–45 °C at the lesion with verified thermometry.
Surface modification. Biocompatible coatings reduce aggregation and opsonisation and improve colloidal stability and safety.
Hybrid formulation. Embedding SPIONs within lipid matrices requires uniform dispersion and sustained stability [
62,
63]; lipid–magnetic hybrids enable guidance and stimulus-triggered release.
Magnetic-field safety. A commonly cited whole-body comfort heuristic is H × f ≤ 4.85 × 10
8 A·m
−1·s
−1 (Atkinson–Brezovich). Focal, brain-targeted applications may operate above this with careful temperature monitoring; for example, clinical SPION hyperthermia has used H ≈ 18 kA·m
−1 and f ≈ 100 kHz (H × f ≈ 1.8 × 10
9 A·m
−1·s
−1). As a practical upper bound, many groups remain below 5 × 10
9 A·m
−1·s
−1 (Hergt–Dutz heuristic). In all cases, report the measured intratumoural temperature and the measurement method; numeric heuristics never replace thermal safety monitoring [
61,
64].
4.4. Advantages of Remote Control
The ability to remotely control both nanoparticle localisation and drug release distinguishes MF-R-LNs from conventional delivery systems. By applying an external magnet or AMF, clinicians may enhance tumour accumulation via magnetic targeting and initiate payload release only after tumour localisation. This two-step strategy offers potential improvements in both selectivity and safety, reducing off-target toxicity and system side effects.
5. Translation and Clinical Outlook
The true therapeutic potential of ligand-functionalised MF-R-LNs lies not in any single design feature, but in the strategic integration of multiple functionalities that address distinct challenges in GBM treatment. By combining passive and active targeting, stimuli-responsive release, magnetic guidance, and hyperthermia, these nanoparticles can theoretically achieve a level of spatiotemporal precision unattainable with conventional therapies or single function nanocarriers.
This level of spatiotemporal control permits the cautious use of otherwise prohibitive, highly cytotoxic agents, which is attractive in a therapy-resistant disease such as GBM. Magnetic guidance provides receptor-agnostic localisation, concentrating the formulation at the lesion even when target receptors are sparse or heterogeneous, while ligands and tumour-specific triggers (e.g., MMP-sensitive linkers, pH/redox cues) retain cellular specificity and govern on-demand release. Crucially, localisation and delivery are monitorable: the magnetic core is visible on standard brain MRI (typically as focal hypointensity on T2/FLAIR) or via magnetic particle imaging (MPI); during AMF exposure, MR thermometry can titrate sessions to keep intratumoural peaks within 42–45 °C. In combination, guidance + imaging + triggered release create a pragmatic, closed-loop workflow not achievable with receptor-only “smart” drugs.
5.1. Integration of Targeting and Triggered Release
Surface ligands enhance cellular uptake by targeting overexpressed receptors on GBM cells and endothelial cells at the BBB. However, uptake alone does not guarantee therapeutic success, the cargo must be released intracellularly, ideally in response to tumour-specific cues. MF-R-LNs enable this through pH-, redox-, or enzyme-sensitive mechanisms, combined with externally applied magnetic hyperthermia to fine tune release kinetics.
The synergy between active targeting and stimuli-triggered release reduces premature leakage, improves tumour localisation, and increases intratumoural drug concentration, particularly in poorly perfused or hypoxic regions. The addition of magnetic guidance further enhances this selectivity by allowing non-invasive control over nanoparticle distribution.
5.2. Multimodal Therapeutic Potential
While chemotherapy is the most obvious payload for MF-R-LNs, the platform is inherently flexible and can accommodate the following:
Gene therapies (e.g., siRNA or plasmids targeting MGMT or EGFR);
Immunomodulators (e.g., STING agonists or checkpoint inhibitors);
Photothermal or radiotherapy sensitisers [
2].
Multimodal therapy is especially relevant in GBM, where redundancy in survival pathways allows tumours to evade monotherapies. A single, modular system that co-delivers drugs and gene-silencers and releases them under tightly controlled conditions offers a rational response to tumour heterogeneity. With image guidance, otherwise prohibitive cytotoxic agents can be used more safely: magnetic guidance provides receptor-agnostic localisation, and the magnetic core is imageable (MRI/MPI), with MR thermometry during AMF to titrate exposure. See
Table 1 for ligand options that support this strategy.
5.3. Personalised and Adaptive Design
Because the MF-R-LN system is modular, its components can be personalised based on the molecular profile of an individual tumour. Ligand selection can be tailored to receptor expression, and release mechanisms can be adapted depending on microenvironmental conditions such as MMP levels or redox gradients. This adaptability could support personalised medicine approaches in GBM, where patient-to-patient variation is profound.
Future developments may also include “smart” feedback systems, in which drug release is dynamically adjusted in response to local temperatures, pH, or metabolic changes detected in real-time.
Where feasible, a minimal molecular profile—EGFR/EGFRvIII, IL-13Rα2, integrins αvβ3/αvβ5, and transcytosis targets (TfR/LRP1) by IHC or targeted RNA/NGS—can prioritise ligand selection. Map results to
Table 1; if expression is low, heterogeneous, or unavailable, proceed without delay using a dual-ligand design (BBB shuttle + tumour-binding ligand) with redundancy.
5.4. Clinical Feasibility
In practice, early studies would prioritise recurrent GBM with supratentorial, measurable lesions away from eloquent cortex, managed on stable steroids/anticonvulsants. MF-R-LN infusion could be scheduled between radiotherapy fractions or in combination with temozolomide at recurrence, with one to three AMF sessions per cycle depending on thermal readouts. Safety monitoring would include neurological exam, adverse-event surveillance, and temperature limits to keep intratumoural peaks within 42–45 °C. MR thermometry (where available) or predefined surrogate triggers (time-at-field, SAR estimates) would gate session duration. These practical constraints inform dose escalation and scheduling.
In practice, first-in-human evaluation would prioritise recurrent/refractory GBM under a Phase 0/1 framework with image-guided localisation (conventional MRI/T2–FLAIR hypointensity or MPI) and MR-thermometry–titrated AMF, with standard field reporting (H, f, H × f).
6. Translational Barriers and Limitations
While the conceptual design of MF-R-LNs offers an elegant solution to many of glioblastoma’s therapeutic challenges, several key limitations must be addressed before clinical translation can be realised. These include biological variability, engineering complexity, safety concerns, and regulatory barriers.
Clinical translation of MF-R-LNs will depend on five interconnected domains: tumour heterogeneity and personalisation, safety/immunogenicity, scalable GMP/CMC, regulatory pathway and trial design, and the limitations of current preclinical models. In this section, we outline the principal risks and practical mitigations for each domain, with emphasis on assayable quality attributes, reporting conventions for magnetic-field use, and study designs proportional to risk in recurrent GBM. These considerations are intended as design constraints and research priorities, not solved problems.
6.1. Ligand Specificity and Tumour Heterogeneity
Targeting ligands are critical for enhancing nanoparticle specificity and uptake, but their effectiveness is constrained by the heterogeneous nature of glioblastoma. Receptor expression varies widely not only between patients but also within tumour subregions, making it unlikely that a single ligand will ensure comprehensive targeting [
65]. Over reliance on one receptor system may leave large fractions of the tumour unaddressed, particularly in invasive margins where expression profiles differ from the tumour core.
Multivalent or dual-ligand strategies may mitigate this issue, but at the cost of increased formulation complexity and potential off-target interactions [
66]. Moreover, receptor saturation or downregulation following repeated exposure could reduce efficacy over time.
6.2. Manufacturing and Scale-Up
Each MF-R-LN component (lipid matrix, SPION core, PEGylation, ligand conjugation) introduces added synthesis and quality control requirements, and maintaining consistent size, surface charge, and ligand density is challenging at scale. Magnetic nanoparticles are prone to aggregation and require stabilising surface chemistries to remain colloidally stable in biological media; embedding them within lipid matrices without compromising bilayer integrity or inducing payload leakage necessitates careful optimisation. Large-scale production must ultimately meet GMP standards, which remains difficult for multifunctional, modular nanocarriers [
61,
67].
Translation will require a defined GMP/CMC package with batch-to-batch control of size/PDI/ζ-potential, ligand density (per particle), PEG density/cleavability, payload loading and release kinetics, and SPION content/relaxivity, alongside sterility/endotoxin and stability testing. CQAs and assays should be prespecified—e.g., DLS/NTA/SEC-MALS for size distributions, LC–MS for linker identity, ICP-MS/magnetometry for iron content and properties, and MRI relaxometry/MPI for tracking—while prioritising closed, scalable steps (in-line filtration, low-bioburden buffers) with complete batch records.
6.3. Safety and Immunogenicity
Although lipid-based systems and SPIONs are individually well studied, their combined use in multifunctional constructs raises additional toxicological questions. The long-term fate of iron oxide in brain tissue under repeated AMF exposure remains uncertain, with risks of oxidative stress, iron overload, and localised overheating [
68]. Surface passivation, core size/valence control, and dosing strategies should be used to mitigate Fenton-type chemistry and unintended tissue effects.
PEGylation, once considered inert, can elicit anti-PEG antibodies, accelerating clearance or provoking hypersensitivity reactions [
20]. Alternative stealth coatings, lower or cleavable PEG densities, and prospective immunosurveillance (e.g., anti-PEG titres) may reduce risk.
For uses involving alternating magnetic fields, authors should report H (kA·m−1), f (kHz), H × f, exposure duration, and in situ thermometry to manage heating risk, and include device calibration/phantom data to document field uniformity and temperature accuracy.
6.4. Regulatory and Clinical Pathways
Multifunctional nanomedicines are often regulated as combination products. Regulators will expect clear identity, purity, and stability criteria for each functional element (core, coating, linkers, ligands, payload) and for the assembled particle; validated release, immunogenicity, and safety assays; and predefined stopping rules for device-related heating. Even when components such as PEG, SPIONs, and phospholipids are individually familiar, the assembled construct carries additional burden due to potential synergy and emergent behaviour [
17,
61]. Device calibration/phantom documentation should accompany clinical use to demonstrate field uniformity and temperature accuracy.
Early clinical evaluation should prioritise recurrent/refractory GBM, where the risk–benefit is most favourable. A pragmatic Phase 0/1 approach can combine a small sentinel single-patient run-in with 3 + 3 or model-based dose escalation under Data and Safety Monitoring Board oversight. Co-primary endpoints are safety/tolerability and device/procedure feasibility; secondary readouts include intratumoural localisation (conventional MRI T2/FLAIR hypointensity or MPI), thermal-dose metrics via MR thermometry during AMF, PK/PD, and preliminary activity by Response Assessment in Neuro-Oncology (RANO).
A minimum preclinical package should precede first-in-human work: orthotopic GBM models for biodistribution, imageability, on-target heating, and efficacy; GLP toxicology (single and repeat dose), neurobehavioural testing, brain histopathology, iron fate/overload, and immunotoxicity (e.g., complement activation/anti-PEG); haemocompatibility/pyrogenicity assays; device–nanoparticle interaction testing (specific absorption rate, eddy-current effects, and H × f kept within practical heuristics); and, where feasible, large-animal studies for field dosimetry and skull-heating margins.
6.5. Magnetic-Field Applications and Clinical Infrastructure
Therapeutic use of magnetic fields for guidance and hyperthermia requires specialised equipment and trained teams. While MRI and some local-heating systems are widespread, adapting field generators for treatment remains niche and logistically demanding. Ensuring that therapeutic heating remains within safe, effective temperature ranges across heterogeneous tissues is non-trivial and may necessitate individualised calibration and on-line thermometry. Moreover, dedicated hyperthermia infrastructure is not yet standard in most oncology centres, which may limit access and scale-up, particularly in resource-limited settings [
69].
7. Future Directions
Although still theoretical, the MF-R-LN design offers a practical framework for overcoming the interlocking obstacles of GBM therapy. It points toward spatiotemporally controlled, multimodal treatment in which therapeutic agents are delivered with high specificity and released only where and when they are needed. By integrating well-established nanomedicine components into a coherent, modular platform, MF-R-LNs can guide experimental work and preclinical development. Importantly, the framework is not limited to glioblastoma: the principles of ligand targeting, magnetic responsiveness, and tumour-specific, stimuli-responsive release may extend to other solid tumours with similarly hostile microenvironments. The overall trajectory of the concept is summarised in
Figure 4.
Future development should focus on precision ligand panels matched to patient-level receptor profiles; robust, modular self-assembly with controlled ligand density and iron loading; and real-time, closed-loop control using non-invasive imaging (e.g., MR thermometry or magnetic particle imaging). Interdisciplinary collaboration among materials science, neuro-oncology, and bioengineering will be essential to translate these designs into clinically viable treatments. In parallel, early engagement with regulators to align safety reporting (H, f, H × f, temperature) and manufacturing standards will reduce uncertainty. As regulatory frameworks for nanomedicine mature, MF-R-LNs could help define a new generation of tumour-specific, multifunctional therapeutics for glioblastoma and beyond.
8. Conclusions
GBM remains one of the most formidable challenges in oncology due to its invasive nature, resistance to therapy, and the physiological barriers that limit drug delivery to the brain. This review outlines a theoretical framework for multifunctional, ligand-functionalised MF-R-LNs as a strategy to address these challenges through targeted delivery, controlled release, and localised activation.
By integrating BBB-penetrating ligands, stimuli-responsive elements, and superparamagnetic cores, MF-R-LNs represent a rational and modular approach to overcome the major therapeutic barriers in GBM. These constructs offer spatiotemporal control over drug delivery, potential for synergistic multimodal therapy, and adaptability to personalised treatment strategies.
While experimental validation remains to be seen, the principles described here may guide future design and preclinical development of nanoparticle systems aimed at brain tumours and other difficult to treat solid malignancies. As advances in materials science, targeting strategies, and magnetic field technology continue to evolve, the vision of a precisely controllable, tumour-specific nanoplatform for glioblastoma therapy becomes increasingly feasible.
Author Contributions
Conceptualization, R.C. and D.B.; methodology, R.C. and D.B.; investigation, D.B. and H.v.d.W.; resources, S.B. and R.C.; writing—original draft, D.B.; writing—review and editing, H.v.d.W., S.B. and R.C.; visualisation, D.B.; supervision, R.C.; project administration, R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Acknowledgments
Selected schematics were created with BioRender.com.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| BBB | Blood–Brain Barrier |
| BTB | Blood–Tumour Barrier |
| P-gp | P-glycoprotein |
| BCRP | Breast Cancer Resistance Protein |
| ECM | Extracellular Matrix |
| IFP | Interstitial Fluid Pressure |
| TAMs | Tumour-Associated Macrophages |
| MDSCs | Myeloid-Derived Suppressor Cells |
| Tregs | Regulatory T Cells |
| GBM | Glioblastoma |
| PEG | Polyethylene Glycol |
| SPION | Superparamagnetic Iron Oxide Nanoparticle |
| LRP1 | Low-Density Lipoprotein Receptor-Related Protein-1 |
| TfR | Transferrin Receptor |
| EGFR | Epidermal Growth Factor Receptor |
| FOLR1 | Folate Receptor-α |
| AMF | Alternating Magnetic Field |
| MR | Magnetic Resonance |
| MPI | Magnetic Particle Imaging |
| MF-R-LN | Ligand-Functionalised Magnetic Lipid Nanoparticle |
| H × f | Field–Frequency Product |
| MRI | Magnetic Resonance Imaging |
| PDI | Polydispersity Index |
References
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C.; Marino, A.; Battaglini, M.; Migliorin, S.; Brescia, R.; Scarpellini, A.; Fernández, C.D.J.; Prato, M.; Drago, F.; Ciofani, G. Stimuli-responsive lipid-based magnetic nanovectors increase apoptosis in glioblastoma cells through synergic intracellular hyperthermia and chemotherapy. Nanoscale 2019, 11, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Kondo, T. Glioblastoma-initiating cell heterogeneity generated by the cell-of-origin, genetic/epigenetic mutation and microenvironment. Semin. Cancer Biol. 2022, 82, 176–183. [Google Scholar] [CrossRef]
- Ferreira, C.; Sarmento, B.; Martins, C. In vitro models of the interplay between glioblastoma and blood-brain barrier for stratifying drug efficacy. Adv. Drug Deliv. Rev. 2025, 227, 115702. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Taylor, T.E.; Furnari, F.B.; Cavenee, W.K. Targeting EGFR for Treatment of Glioblastoma: Molecular Basis to Overcome Resistance. Curr. Cancer Drug Targets 2012, 12, 197–209. [Google Scholar] [CrossRef]
- Martins, C.; Sarmento, B. Multi-ligand functionalized blood-to-tumor sequential targeting strategies in the field of glioblastoma nanomedicine. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2023, 15, e1893. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed]
- Jnaidi, R.; Almeida, A.J.; Gonçalves, L.M. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Smart Drug Delivery Systems in the Treatment of Glioblastoma Multiforme. Pharmaceutics 2020, 12, 860. [Google Scholar] [CrossRef]
- Achar, A.; Myers, R.; Ghosh, C. Drug Delivery Challenges in Brain Disorders across the Blood–Brain Barrier: Novel Methods and Future Considerations for Improved Therapy. Biomedicines 2021, 9, 1834. [Google Scholar] [CrossRef] [PubMed]
- Dymova, M.A.; Kuligina, E.V.; Richter, V.A. Molecular Mechanisms of Drug Resistance in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 6385. [Google Scholar] [CrossRef] [PubMed]
- Mason, W.P. Blood-brain barrier-associated efflux transporters: A significant but underappreciated obstacle to drug development in glioblastoma. Neuro-Oncology 2015, 17, 1181–1182. [Google Scholar] [CrossRef][Green Version]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neuro-Oncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef]
- Butler, M.; Pongor, L.; Su, Y.-T.; Xi, L.; Raffeld, M.; Quezado, M.; Trepel, J.; Aldape, K.; Pommier, Y.; Wu, J. MGMT Status as a Clinical Biomarker in Glioblastoma. Trends Cancer 2020, 6, 380–391. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, I.; Lu, Y.; Xu, Y.; Yu, D.-G.; Song, W. Intelligent poly(l-histidine)-based nanovehicles for controlled drug delivery. J. Control. Release 2022, 349, 963–982. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. pH-Responsive Polymer Nanomaterials for Tumor Therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef]
- Busquets, M.A.; Espargaró, A.; Sabaté, R.; Estelrich, J. Magnetic Nanoparticles Cross the Blood-Brain Barrier: When Physics Rises to a Challenge. Nanomaterials 2015, 5, 2231–2248. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Harashima, H. The Polyethyleneglycol Dilemma: Advantage and Disadvantage of PEGylation of Liposomes for Systemic Genes and Nucleic Acids Delivery to Tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef]
- Denman, D.S.; Dalhaimer, P. The curious case of anti-PEG antibodies. Nanoscale 2025, 17, 22594–22605. [Google Scholar] [CrossRef]
- Javid, H.; Oryani, M.A.; Rezagholinejad, N.; Esparham, A.; Tajaldini, M.; Karimi-Shahri, M. RGD peptide in cancer targeting: Benefits, challenges, solutions, and possible integrin–RGD interactions. Cancer Med. 2024, 13, e6800. [Google Scholar] [CrossRef]
- Jaen, M.; Martín-Regalado, Á.; Bartolomé, R.A.; Robles, J.; Casal, J.I. Interleukin 13 receptor alpha 2 (IL13Rα2): Expression, signaling pathways and therapeutic applications in cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188802. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Sha, X.; Jiang, X.; Chen, L.; Law, K.; Gu, J.; Chen, Y.; Wang, X.; Fang, X. The brain targeting mechanism of Angiopep-conjugated poly(ethylene glycol)-co-poly(ɛ-caprolactone) nanoparticles. Biomaterials 2012, 33, 1673–1681. [Google Scholar] [CrossRef]
- Bien-Ly, N.; Yu, Y.J.; Bumbaca, D.; Elstrott, J.; Boswell, C.A.; Zhang, Y.; Luk, W.; Lu, Y.; Dennis, M.S.; Weimer, R.M.; et al. Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J. Exp. Med. 2014, 211, 233–244. [Google Scholar] [CrossRef]
- Demeule, M.; Currie, J.; Bertrand, Y.; Ché, C.; Nguyen, T.; Régina, A.; Gabathuler, R.; Castaigne, J.; Béliveau, R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2. J. Neurochem. 2008, 106, 1534–1544. [Google Scholar] [CrossRef]
- N. R., S.; Behera, M.M.; Naik, S.K.; Das, S.K.; Gopan, S.; Ghosh, A.; Sahu, R.N.; Patra, S.; Purkait, S. Elevated expression of cholesterol transporter LRP-1 is crucially implicated in the pathobiology of glioblastoma. Front. Neurol. 2022, 13, 1003730. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Jiang, X.; Gu, J.; Sha, X.; Chen, L.; Law, K.; Chen, Y.; Wang, X.; Jiang, Y.; Fang, X. Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials 2011, 32, 4293–4305. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Shao, K.; Huang, R.; Han, L.; Liu, Y.; Li, J.; Kuang, Y.; Ye, L.; Lou, J.; Jiang, C. Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials 2009, 30, 6976–6985. [Google Scholar] [CrossRef]
- Kim, J.S.; Shin, D.H.; Kim, J. Dual-targeting immunoliposomes using angiopep-2 and CD133 antibody for glioblastoma stem cells. J. Control. Release 2018, 269, 245–257. [Google Scholar] [CrossRef]
- Zhang, W.; Refaat, A.; Li, H.; Zhu, D.; Tong, Z.; Nicolazzo, J.A.; Peng, B.; Bai, H.; Esser, L.; Voelcker, N.H. Optimizing AngiopeP-2 density on polymeric nanoparticles for enhanced Blood–Brain barrier penetration and glioblastoma targeting: Insights from in vitro and in vivo experiments. Adv. Funct. Mater. 2025, 35, 2425165. [Google Scholar] [CrossRef]
- Danhier, F.; Le Breton, A.; Préat, V. RGD-Based Strategies To Target Alpha(v) Beta(3) Integrin in Cancer Therapy and Diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Echavidre, W.; Picco, V.; Faraggi, M.; Montemagno, C. Integrin-αvβ3 as a Therapeutic Target in Glioblastoma: Back to the Future? Pharmaceutics 2022, 14, 1053. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, X.; Yu, J.; Yuan, S. Preliminary Clinical application of RGD-Containing peptides as PET radiotracers for imaging tumors. Front. Oncol. 2022, 12, 837952. [Google Scholar] [CrossRef] [PubMed]
- Murphrey, M.B.; Quaim, L.; Rahimi, N.; Varacallo, M.A. Biochemistry, Epidermal Growth Factor Receptor. In StatPearls Internet; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Razpotnik, R.; Novak, N.; Šerbec, V.Č.; Rajcevic, U. Targeting Malignant Brain Tumors with Antibodies. Front. Immunol. 2017, 8, 1181. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bent, M.; Gan, H.K.; Lassman, A.B.; Kumthekar, P.; Merrell, R.; Butowski, N.; Lwin, Z.; Mikkelsen, T.; Nabors, L.B.; Papadopoulos, K.P.; et al. Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: Results from a multi-center, international study. Cancer Chemother. Pharmacol. 2017, 80, 1209–1217. [Google Scholar] [CrossRef]
- Seo, K.; Hwang, K.; Nam, K.M.; Kim, M.J.; Song, Y.-K.; Kim, C.-Y. Nucleolin-Targeting AS1411 Aptamer-Conjugated Nanospheres for Targeted Treatment of Glioblastoma. Pharmaceutics 2024, 16, 566. [Google Scholar] [CrossRef]
- Tauser, R.-G.; Lupascu, F.-G.; Profire, B.-S.; Iacob, A.-T.; Vasincu, I.-M.; Apotrosoaei, M.; Chirliu, O.-M.; Lupascu, D.; Profire, L. Aptamer-Nanoconjugates as Potential Theranostics in Major Neuro-Oncological and Neurodegenerative Disorders. Pharmaceutics 2025, 17, 1106. [Google Scholar] [CrossRef]
- Reyes-Reyes, E.M.; Teng, Y.; Bates, P.J. A new paradigm for APTAmer therapeutic AS1411 action: Uptake by macropinocytosis and its stimulation by a Nucleolin-Dependent mechanism. Cancer Res. 2010, 70, 8617–8629. [Google Scholar] [CrossRef]
- Okada, M.; Suzuki, S.; Togashi, K.; Sugai, A.; Yamamoto, M.; Kitanaka, C. Targeting Folate Metabolism Is Selectively Cytotoxic to Glioma Stem Cells and Effectively Cooperates with Differentiation Therapy to Eliminate Tumor-Initiating Cells in Glioma Xenografts. Int. J. Mol. Sci. 2021, 22, 11633. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Dong, J.; Lu, Y. The blood–brain barriers: Novel nanocarriers for central nervous system diseases. J. Nanobiotechnol. 2025, 23, 1–29. [Google Scholar] [CrossRef]
- Elechalawar, C.K.; Bhattacharya, D.; Ahmed, M.T.; Gora, H.; Sridharan, K.; Chaturbedy, P.; Sinha, S.H.; Jaggarapu, M.M.C.S.; Narayan, K.P.; Chakravarty, S.; et al. Dual targeting of folate receptor-expressing glioma tumor-associated macrophages and epithelial cells in the brain using a carbon nanosphere–cationic folate nanoconjugate. Nanoscale Adv. 2019, 1, 3555–3567. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, C.; Chen, X.; Zhao, Y.; Liao, Y.; Huang, P.; Wu, W.; Nieto, N.S.; Li, L.; Tang, W. Development of folate receptor targeting chimeras for cancer selective degradation of extracellular proteins. Nat. Commun. 2024, 15, 8695. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Kats, D.; Rasmussen, S.; Martin, L.R.; Karki, A.; Keller, C.; Berlow, N.E. Design considerations of an IL13Rα2 antibody–drug conjugate for diffuse intrinsic pontine glioma. Acta Neuropathol. Commun. 2021, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Ulasov, I.V.; Borovjagin, A.; Laevskaya, A.; Kamynina, M.; Timashev, P.; Cerchia, L.; Rozhkova, E.A. The IL13alpha 2R paves the way for anti-glioma nanotherapy. Genes Dis. 2023, 10, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.R.; Puri, R.K. Interleukin-13 Receptor-Directed Cytotoxin for Malignant glioma therapy: From bench to bedside. J. Neuro-Oncol. 2003, 65, 37–48. [Google Scholar] [CrossRef]
- Aschmann, D.; Knol, R.A.; Kros, A. Lipid-Based Nanoparticle Functionalization with Coiled-Coil Peptides for In Vitro and In Vivo Drug Delivery. Accounts Chem. Res. 2024, 57, 1098–1110. [Google Scholar] [CrossRef]
- Gaspar, M.M.; Radomska, A.; Gobbo, O.L.; Bakowsky, U.; Radomski, M.W.; Ehrhardt, C. Targeted Delivery of Transferrin-Conjugated Liposomes to an Orthotopic Model of Lung Cancer in Nude Rats. J. Aerosol Med. Pulm. Drug Deliv. 2012, 25, 310–318. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Bak, M.; Kempen, P.J.; Melander, F.; Burkhart, A.; Thomsen, M.S.; Nielsen, M.S.; Moos, T.; Andresen, T.L. Antibody affinity and valency impact brain uptake of transferrin receptor-targeted gold nanoparticles. Theranostics 2018, 8, 3416–3436. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Zhang, B.; Yan, X.; Fan, K. Transferrin receptor 1 targeted nanomedicine for brain tumor therapy. Biomater. Sci. 2023, 11, 3394–3413. [Google Scholar] [CrossRef]
- Beola, L.; Iturrioz-Rodríguez, N.; Pucci, C.; Bertorelli, R.; Ciofani, G. Drug-Loaded Lipid Magnetic Nanoparticles for Combined Local Hyperthermia and Chemotherapy against Glioblastoma Multiforme. ACS Nano 2023, 17, 18441–18455. [Google Scholar] [CrossRef]
- Rempe, R.G.; Hartz, A.M.; Bauer, B. Matrix metalloproteinases in the brain and blood–brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef] [PubMed]
- Dosta, P.; Dion, M.Z.; Prado, M.; Hurtado, P.; Riojas-Javelly, C.J.; Cryer, A.M.; Soria, Y.; Interiano, N.A.; Muñoz-Taboada, G.; Artzi, N. Matrix metalloproteinase- and PH-Sensitive nanoparticle system enhances drug retention and penetration in glioblastoma. ACS Nano 2024, 18, 14145–14160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Xiao, Y.; Xie, Y.; Wang, R.; Zhang, Y.; Zhou, Q.; Liu, L.; Sun, S.; Xiao, H.; et al. Intelligent Nanoparticles With pH-Sensitive Co-Delivery of Temozolomide and siEGFR to Ameliorate Glioma Therapy. Front. Genet. 2022, 13, 921051. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Moreira, J.A.; Monteiro, F.J.; Laranjeira, M.S. Nanomaterials in cancer: Reviewing the combination of hyperthermia and triggered chemotherapy. J. Control. Release 2022, 347, 89–103. [Google Scholar] [CrossRef]
- Skandalakis, G.P.; Rivera, D.R.; Rizea, C.D.; Bouras, A.; Raj, J.G.J.; Bozec, D.; Hadjipanayis, C.G. Hyperthermia treatment advances for brain tumors. Int. J. Hyperth. 2020, 37, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Lassenberger, A.; Scheberl, A.; Stadlbauer, A.; Stiglbauer, A.; Helbich, T.; Reimhult, E. Individually Stabilized, Superparamagnetic Nanoparticles with Controlled Shell and Size Leading to Exceptional Stealth Properties and High Relaxivities. ACS Appl. Mater. Interfaces 2017, 9, 3343–3353. [Google Scholar] [CrossRef]
- Samrot, A.V.; Samanvitha, S.K.; Shobana, N.; Renitta, E.R.; Senthilkumar, P.; Kumar, S.S.; Abirami, S.; Dhiva, S.; Bavanilatha, M.; Prakash, P.; et al. The Synthesis, Characterization and Applications of Polyhydroxyalkanoates (PHAs) and PHA-Based Nanoparticles. Polymers 2021, 13, 3302. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef]
- Herrero de la Parte, B.; Rodrigo, I.; Gutiérrez-Basoa, J.; Iturrizaga Correcher, S.; Mar Medina, C.; Echevarría-Uraga, J.J.; Garcia, J.A.; Plazaola, F.; García-Alonso, I. Proposal of New Safety Limits for In Vivo Experiments of Magnetic Hyperthermia Antitumor Therapy. Cancers 2022, 14, 3084. [Google Scholar] [CrossRef]
- Torrisi, F.; Alberghina, C.; D’aprile, S.; Pavone, A.M.; Longhitano, L.; Giallongo, S.; Tibullo, D.; Di Rosa, M.; Zappalà, A.; Cammarata, F.P.; et al. The Hallmarks of Glioblastoma: Heterogeneity, Intercellular Crosstalk and Molecular Signature of Invasiveness and Progression. Biomedicines 2022, 10, 806. [Google Scholar] [CrossRef]
- Marei, H.E. Multimodal targeting of glioma with functionalized nanoparticles. Cancer Cell Int. 2022, 22, 1–17. [Google Scholar] [CrossRef]
- Gupta, S.; Smith, T.R.; Broekman, M.L. Ethical considerations of neuro-oncology trial design in the era of precision medicine. J. Neuro-Oncol. 2017, 134, 1–7. [Google Scholar] [CrossRef]
- Manescu Paltanea, V.; Antoniac, I.; Paltanea, G.; Nemoianu, I.V.; Mohan, A.G.; Antoniac, A.; Rau, J.V.; Laptoiu, S.A.; Mihai, P.; Gavrila, H.; et al. Magnetic Hyperthermia in Glioblastoma Multiforme Treatment. Int. J. Mol. Sci. 2024, 25, 10065. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jangam, A.; Shen, J.L.Y.; Ahmad, A.; Arepally, N.; Rodriguez, B.; Borrello, J.; Bouras, A.; Kleinberg, L.; Ding, K.; et al. Validation of a Temperature-Feedback Controlled Automated Magnetic Hyperthermia Therapy Device. Cancers 2023, 15, 327. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
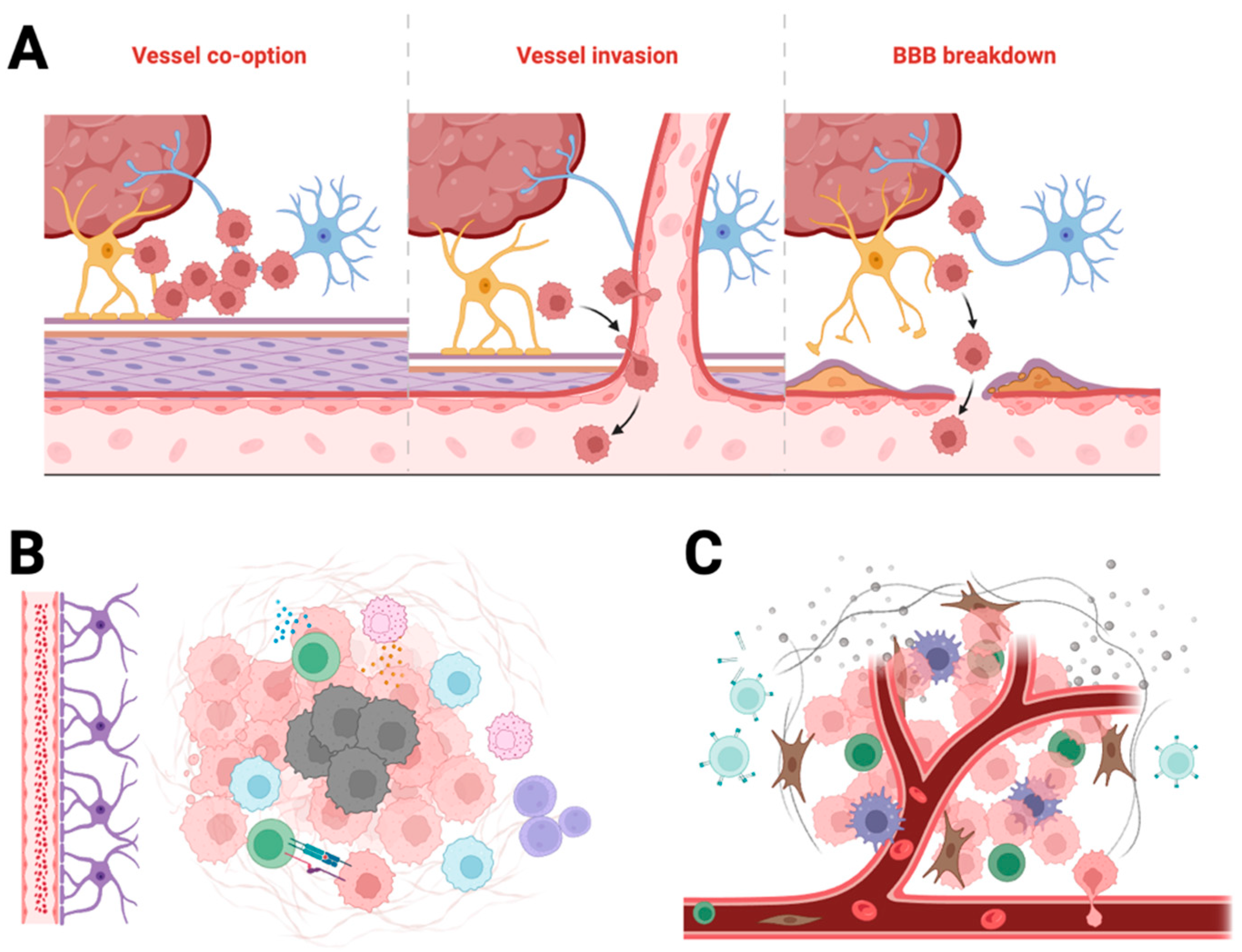
Barriers to drug delivery in glioblastoma. Schematic overview of barriers that limit therapeutic delivery to GBM. (A) Vascular barriers: the intact blood–brain barrier (BBB) and heterogeneous blood–tumour barrier (BTB) restrict parenchymal entry; high efflux via P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) further lowers intratumoural drug levels. (B) Physical and chemical microenvironment: dense extracellular matrix (ECM) and elevated interstitial fluid pressure (IFP) impede convection and diffusion, while hypoxia and acidic pH reduce drug efficacy and promote resistance. (C) Immunological and evolutionary barriers: tumour-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) sequester nanoparticles and dampen antitumour responses; genomic heterogeneity and pathway redundancy (e.g., MGMT, EGFR/PI3K/AKT) drive adaptive resistance over time. Visual conventions: neurons (blue), astrocytes (purple), endothelial cells with tight junctions (pink), pericytes (tan), red blood cells (red), cancer cells (rose) and immunosuppressive populations—TAMs/MDSCs/Tregs (green tones).
Figure 1.
Barriers to drug delivery in glioblastoma. Schematic overview of barriers that limit therapeutic delivery to GBM. (A) Vascular barriers: the intact blood–brain barrier (BBB) and heterogeneous blood–tumour barrier (BTB) restrict parenchymal entry; high efflux via P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) further lowers intratumoural drug levels. (B) Physical and chemical microenvironment: dense extracellular matrix (ECM) and elevated interstitial fluid pressure (IFP) impede convection and diffusion, while hypoxia and acidic pH reduce drug efficacy and promote resistance. (C) Immunological and evolutionary barriers: tumour-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) sequester nanoparticles and dampen antitumour responses; genomic heterogeneity and pathway redundancy (e.g., MGMT, EGFR/PI3K/AKT) drive adaptive resistance over time. Visual conventions: neurons (blue), astrocytes (purple), endothelial cells with tight junctions (pink), pericytes (tan), red blood cells (red), cancer cells (rose) and immunosuppressive populations—TAMs/MDSCs/Tregs (green tones).
![Cancers 17 03905 g001 Cancers 17 03905 g001]()
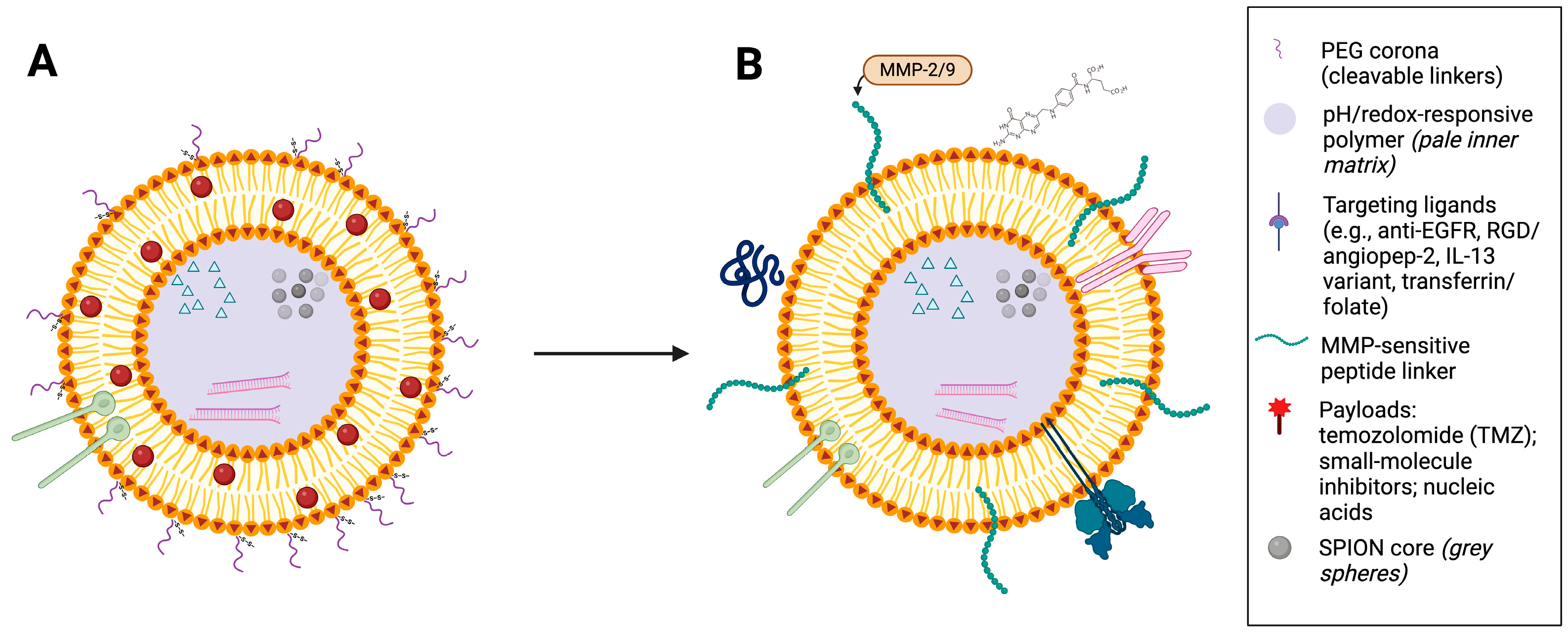
Figure 2.
Stealth-to-activated ligand-functionalised magnetic lipid nanoparticle (MF-R-LN). (A) Stealth state: lipid-bilayer carrier with a PEG corona, embedded superparamagnetic iron oxide nanoparticle (SPION) core, targeting ligands, and encapsulated payload. (B) Activated state after tumour accumulation: tumour-associated enzymes (e.g., MMP-2/9) and intracellular pH/redox cues reduce PEG shielding and promote stimulus-triggered release; the SPION core enables magnetic guidance and mild hyperthermia. In (B), surface features are shown generically—targeting ligands attached via MMP-sensitive linkers with partially shed PEG—while a pale inner matrix denotes a pH/redox-responsive polymer; grey spheres indicate the SPION core, and payloads are depicted inside.
Figure 2.
Stealth-to-activated ligand-functionalised magnetic lipid nanoparticle (MF-R-LN). (A) Stealth state: lipid-bilayer carrier with a PEG corona, embedded superparamagnetic iron oxide nanoparticle (SPION) core, targeting ligands, and encapsulated payload. (B) Activated state after tumour accumulation: tumour-associated enzymes (e.g., MMP-2/9) and intracellular pH/redox cues reduce PEG shielding and promote stimulus-triggered release; the SPION core enables magnetic guidance and mild hyperthermia. In (B), surface features are shown generically—targeting ligands attached via MMP-sensitive linkers with partially shed PEG—while a pale inner matrix denotes a pH/redox-responsive polymer; grey spheres indicate the SPION core, and payloads are depicted inside.
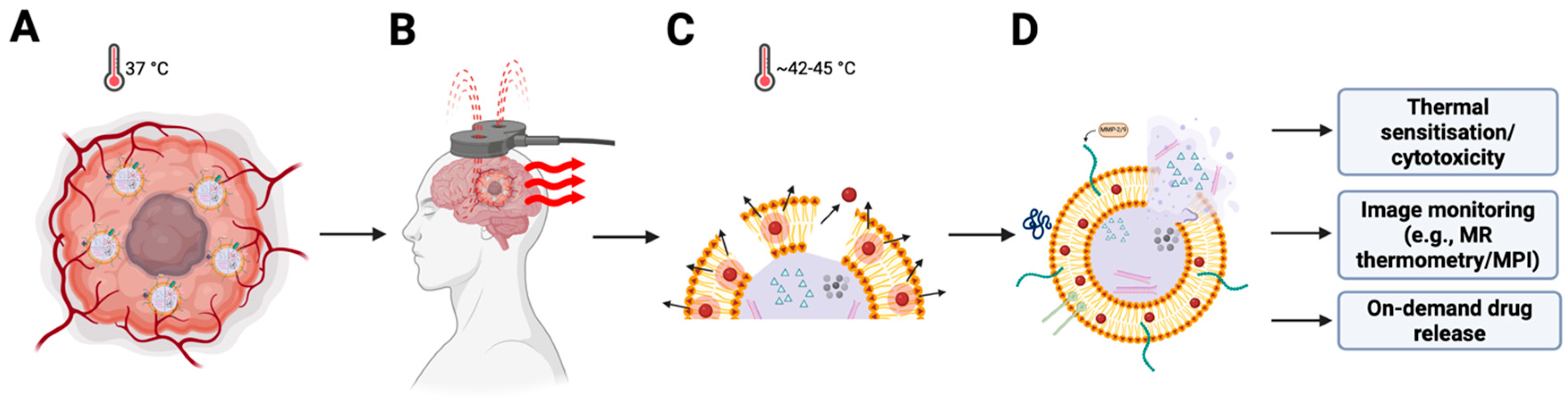
Figure 3.
Alternating-magnetic-field triggering of heating and release from MF-R-LNs. (A) Intratumoural accumulation of heat-responsive, ligand-functionalised magnetic lipid nanoparticles (MF-R-LNs) at physiological temperature (37 °C). (B) Application of an alternating magnetic field (AMF) over the tumour region. (C) Superparamagnetic iron oxide nanoparticle (SPION) cores dissipate heat (42–45 °C) via Néel/Brownian relaxation, transiently perturbing the lipid bilayer and promoting membrane permeabilisation/endosomal escape. (D) Stimulus-triggered payload release into tumour cells and the microenvironment enables thermal sensitisation/cytotoxicity, image monitoring with conventional MRI (e.g., T2/FLAIR) or magnetic particle imaging (MPI), and dose-titration with MR thermometry during AMF, with on-demand drug release.
Figure 3.
Alternating-magnetic-field triggering of heating and release from MF-R-LNs. (A) Intratumoural accumulation of heat-responsive, ligand-functionalised magnetic lipid nanoparticles (MF-R-LNs) at physiological temperature (37 °C). (B) Application of an alternating magnetic field (AMF) over the tumour region. (C) Superparamagnetic iron oxide nanoparticle (SPION) cores dissipate heat (42–45 °C) via Néel/Brownian relaxation, transiently perturbing the lipid bilayer and promoting membrane permeabilisation/endosomal escape. (D) Stimulus-triggered payload release into tumour cells and the microenvironment enables thermal sensitisation/cytotoxicity, image monitoring with conventional MRI (e.g., T2/FLAIR) or magnetic particle imaging (MPI), and dose-titration with MR thermometry during AMF, with on-demand drug release.
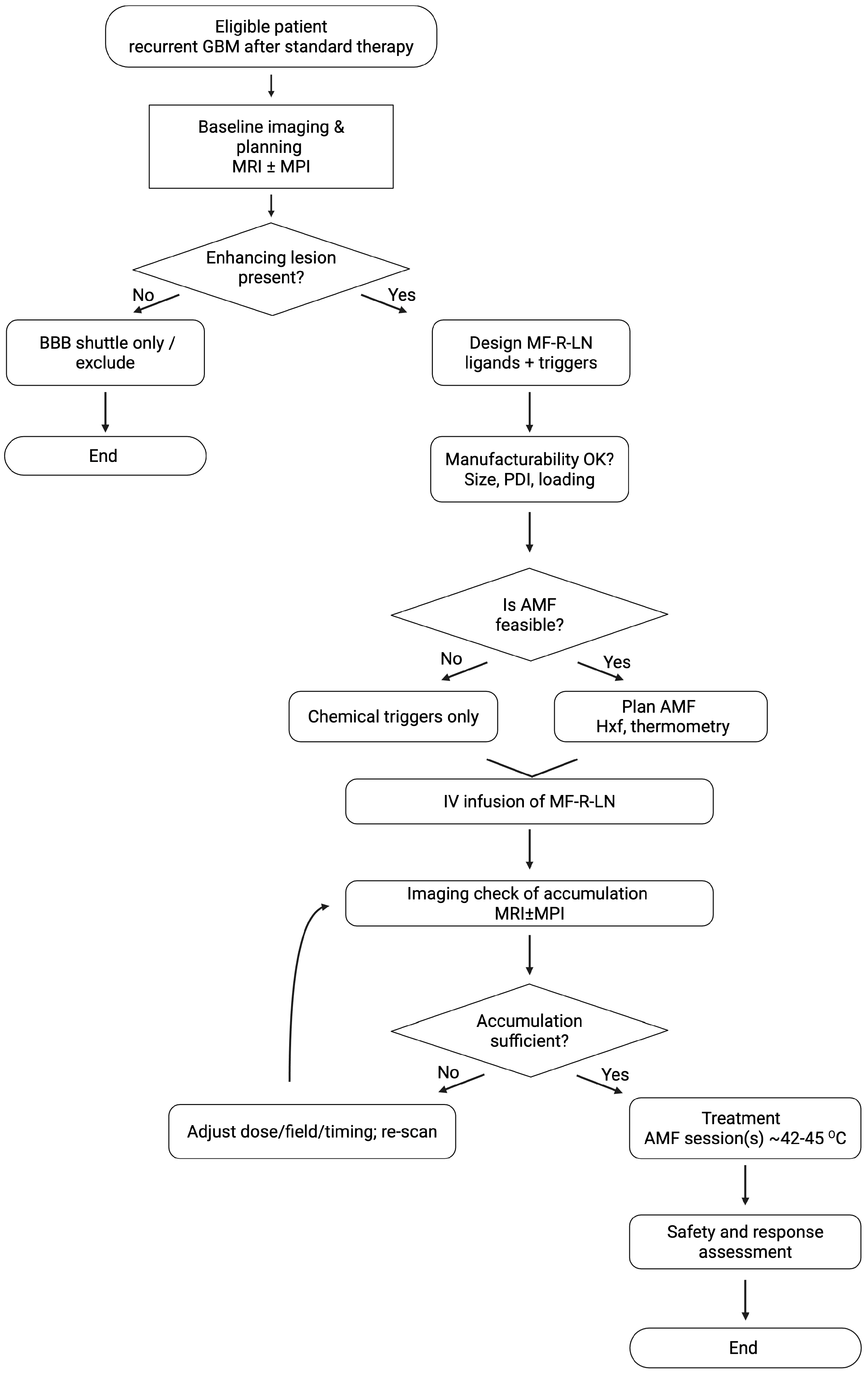
Figure 4.
Clinical decision flow for MF-R-LNs in glioblastoma. Eligible patients (recurrent GBM) undergo baseline imaging and planning (MRI ± MPI). If a contrast-enhancing lesion is present, an MF-R-LN is designed (ligands by profile or dual strategy; pH/redox/MMP triggers) and its manufacturability confirmed (size, PDI, loading). Centre resources determine whether AMF can be used (within H × f comfort ranges with thermometry); if not, proceed with chemical triggers only. After IV infusion, an imaging check confirms intratumoural accumulation (MRI/MPI). If insufficient, adjust dose/field/timing and re-scan; if sufficient, proceed to treatment execution targeting mild hyperthermia (42–45 °C) with predefined monitoring and abort criteria, followed by safety and response assessment. A non-enhancing lesion may be managed with a BBB-shuttle-only approach or excluded from AMF-based protocols.
Figure 4.
Clinical decision flow for MF-R-LNs in glioblastoma. Eligible patients (recurrent GBM) undergo baseline imaging and planning (MRI ± MPI). If a contrast-enhancing lesion is present, an MF-R-LN is designed (ligands by profile or dual strategy; pH/redox/MMP triggers) and its manufacturability confirmed (size, PDI, loading). Centre resources determine whether AMF can be used (within H × f comfort ranges with thermometry); if not, proceed with chemical triggers only. After IV infusion, an imaging check confirms intratumoural accumulation (MRI/MPI). If insufficient, adjust dose/field/timing and re-scan; if sufficient, proceed to treatment execution targeting mild hyperthermia (42–45 °C) with predefined monitoring and abort criteria, followed by safety and response assessment. A non-enhancing lesion may be managed with a BBB-shuttle-only approach or excluded from AMF-based protocols.
Table 1.
Ligand–receptor options for GBM targeting and practical considerations. Representative ligands with primary receptor/target, selectivity (GBM vs. normal brain), evidence (BBB/uptake), and pros/risks (practical). Conjugation chemistries and caveats are given in footnotes [a–c]. Payload examples are indicated by superscripts p1–p7 immediately after each ligand name in column 1. Clinical status/biocompatibility badges appear in the same cell as the ligand (e.g., [CNS-H], [Non-CNS-H], [APP-Non-CNS], [Pre-clin], [Low-immuno], [Immuno-flag]); see key below. Supporting references for each ligand are listed in the “References by ligand” table footer block.
Table 1.
Ligand–receptor options for GBM targeting and practical considerations. Representative ligands with primary receptor/target, selectivity (GBM vs. normal brain), evidence (BBB/uptake), and pros/risks (practical). Conjugation chemistries and caveats are given in footnotes [a–c]. Payload examples are indicated by superscripts p1–p7 immediately after each ligand name in column 1. Clinical status/biocompatibility badges appear in the same cell as the ligand (e.g., [CNS-H], [Non-CNS-H], [APP-Non-CNS], [Pre-clin], [Low-immuno], [Immuno-flag]); see key below. Supporting references for each ligand are listed in the “References by ligand” table footer block.
| Ligand | Primary
Receptor/Target | Selectivity (GBM vs. Normal Brain) | Evidence (BBB/Uptake) | Pros/Risks (Practical) |
|---|
Angiopep-2 p1 [CNS-H]
[Low- immuno] | LRP1 | High on brain endothelium; variable on GBM cells; low on neurons | Preclinical BBB transcytosis; human CNS data | Good shuttle; peripheral LRP1 “sink”—tune ligand density; see [a] |
| Transferrin p2 [Non-CNS-H] [Immuno-flag] | TfR/CD71 | High on BBB endothelium and many tumours; present in normal tissues | Receptor-mediated BBB transcytosis (preclinical); human imaging/targeting outside CNS | Widely used shuttle; competition with endogenous Tf; avoid very high affinity/valency; see [a] |
anti-EGFR (IgG/fragment) p3
[APP-Non-CNS] [CNS-H] [Immuno-flag] | EGFR/EGFRvIII | Overexpressed in GBM (heterogeneous); minimal in normal brain | ↑ cellular uptake; full IgG BBB-limited; fragments improve penetration | Potent binder; off-tumour EGFR (skin/gut); consider fragments/bispecifics; see [b] |
IL-13 (variant/peptide) p4
[CNS-H]
[Immuno-flag] | IL-13Rα2 | Frequent in GBM; minimal in normal brain; heterogeneous | Receptor-mediated internalisation; CNS human experience (selected agents) | GBM-selective axis; minimise IL-13Rα1 binding; species differences; monitor immunogenicity; see [b] |
RGD/cRGD (peptide) p5
[CNS-H]
[Low-immuno] | Integrins αvβ3/αvβ5 | Enriched on GBM cells/vasculature; low on normal brain endothelium | ↑ tumour binding/penetration; not a BBB shuttle | Solid tumour targeting; platelet/vascular binding risk; prefer cyclic RGD; control multivalency; see [b] |
Aptamers (e.g., AS1411) p6
[Non-CNS-H]
[Low-immuno] | Nucleolin (cell-surface) | Overexpressed on many tumour cells incl. GBM; low on normal brain surface | Receptor-mediated uptake in glioma models; BBB limited without shuttle | Small and engineerable; nuclease sensitivity—use 2′-mods/PEGylation; rapid renal clearance if small; see [c] |
Folate (small molecule) p7
[Non-CNS-H]
[Low-immuno] | FOLR1
(± FRβ on macrophages) | Variable in GBM; low in normal parenchyma | Strong uptake in FR-positive cells; not a BBB shuttle | Simple chemistry; kidney uptake; competition with endogenous folate; heterogeneous expression; human experience outside CNS (late-phase trials), GBM use remains preclinical/early-phase; see [c]. |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).