The Rise of Fine-Tuned CAR-Based Therapies Against Acute Myeloid Leukemia
Simple Summary
Abstract
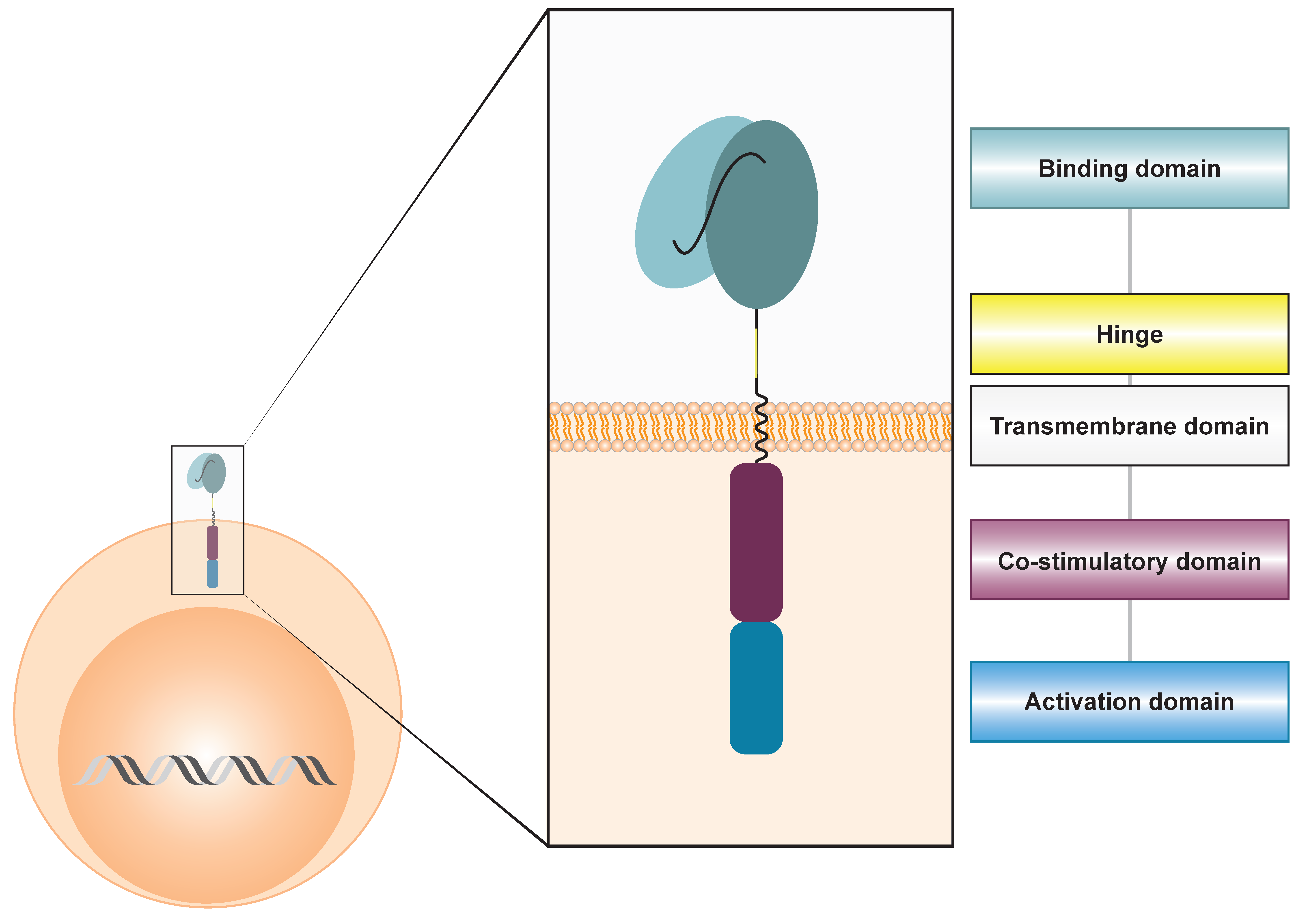
1. Introduction
2. Rationale, Scope, and Structure of the Review
- Affinity modulation to fine-tune antigen recognition and reduce toxicity.
- Logic-gated CARs (OR, AND, NOT, IF-BETTER, IF-THEN) to enhance selectivity and mitigate antigen escape.
- Modulable CAR platforms, including adapter-based systems and ON/OFF or suicide switches for dynamic control.
- Armored CARs, equipped with cytokine secretion, membrane-bound proteins, or immune engagers to boost potency.
- Gene-edited CARs, leveraging disruption or overexpression of key genes to improve function and scalability.
- Alternative immune cell platforms, such as CAR-NK, CAR-γδ T, and CAR-macrophages, to bypass limitations of αβ T cells.
3. Modulation of CAR Affinity
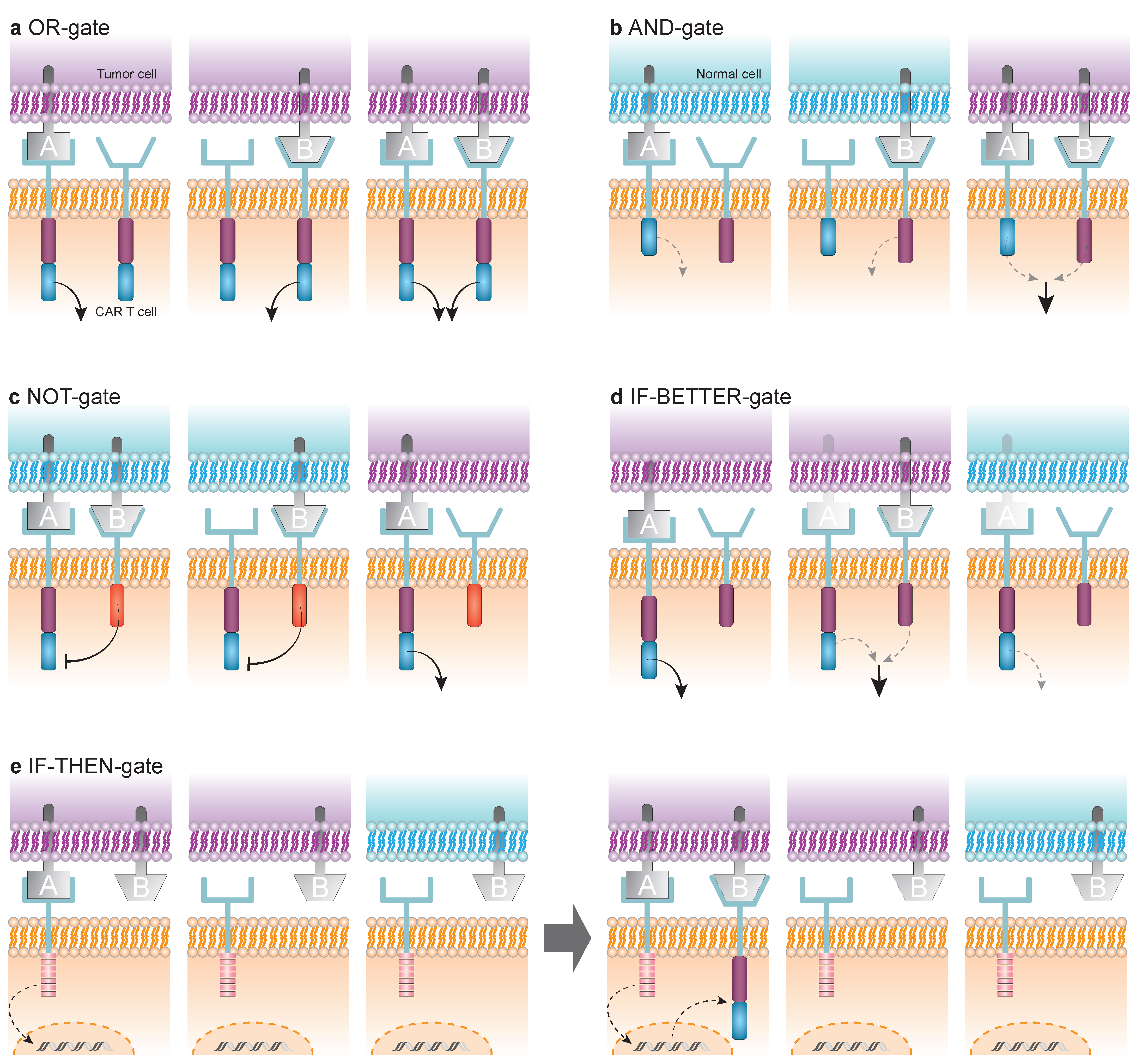
4. Logic-Gated CAR T Cells
4.1. OR-Gate
4.2. AND-Gate
4.3. NOT-Gate
4.4. IF-BETTER-Gate
4.5. IF-THEN-Gate
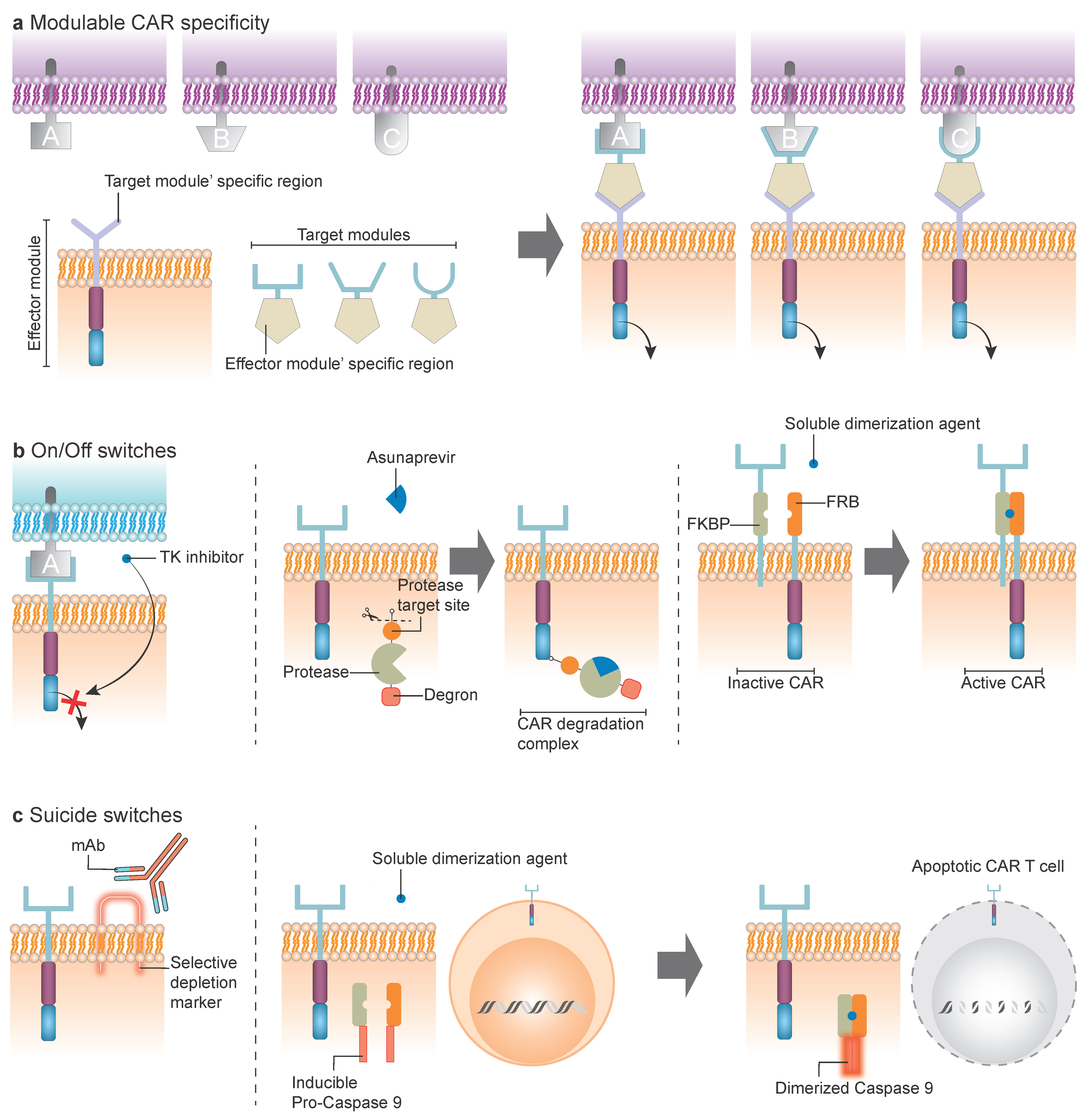
5. Modulable CAR Specificity
- Reversed CAR (RevCAR): the adapter recognizes a specific tag on the CAR extracellular domain [51].
- Split, Universal, and Programmable (SUPRA) CAR: based on leucine zipper domains, comprising a membrane-bound CAR endodomain core with a leucine zipper extracellular domain, and a soluble tumor-binding domain fused to a complementary leucine zipper [52]. This last approach has not yet been investigated in AML.
6. CAR Formats with Modifiable or Limited Activity
6.1. ON/OFF Switches
6.2. Suicide Switches
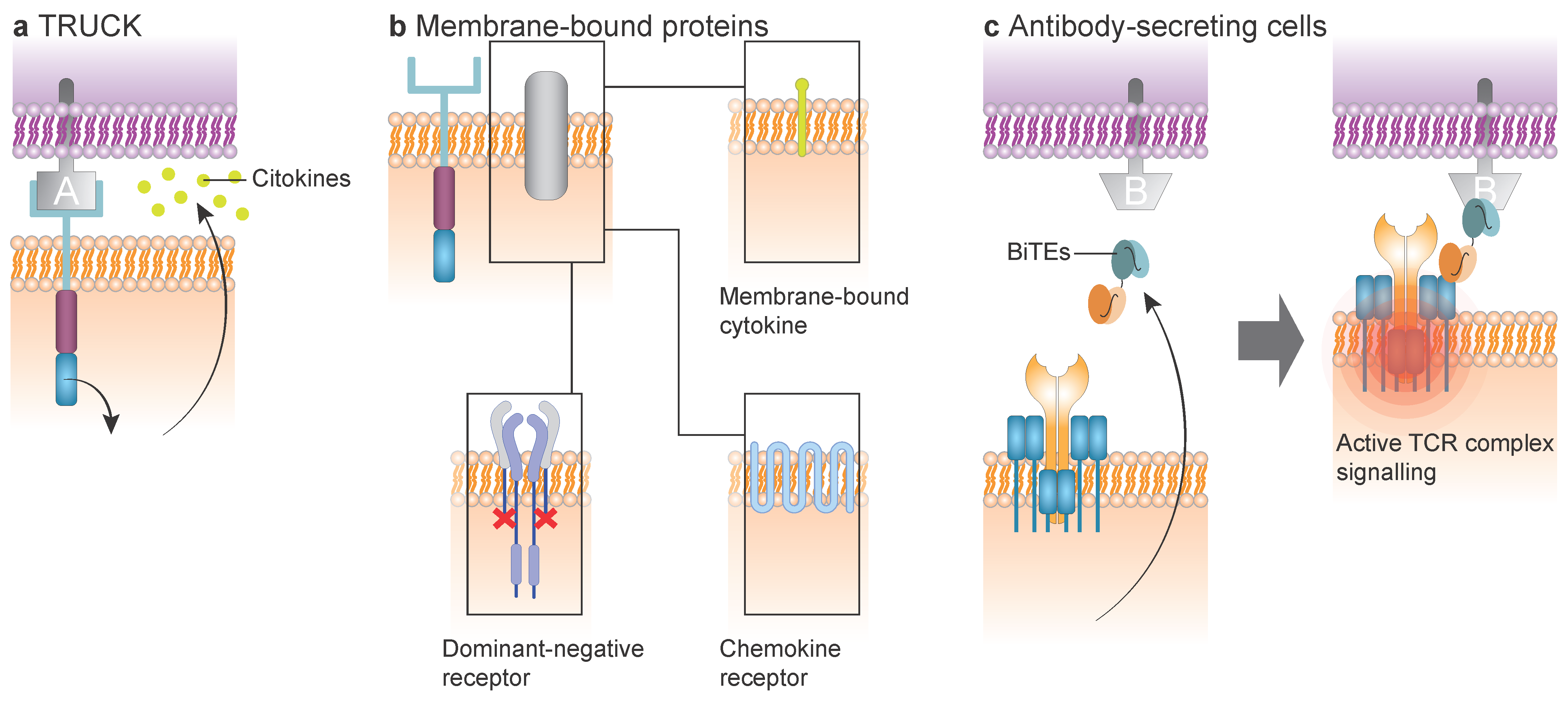
7. Armored CAR T Cell
7.1. TRUCK CAR T Cell
7.2. Membrane-Bound Protein Modulating CAR T Cell
7.3. Antibody-Secreting CAR T Cell
8. Gene-Modified CAR T Cell
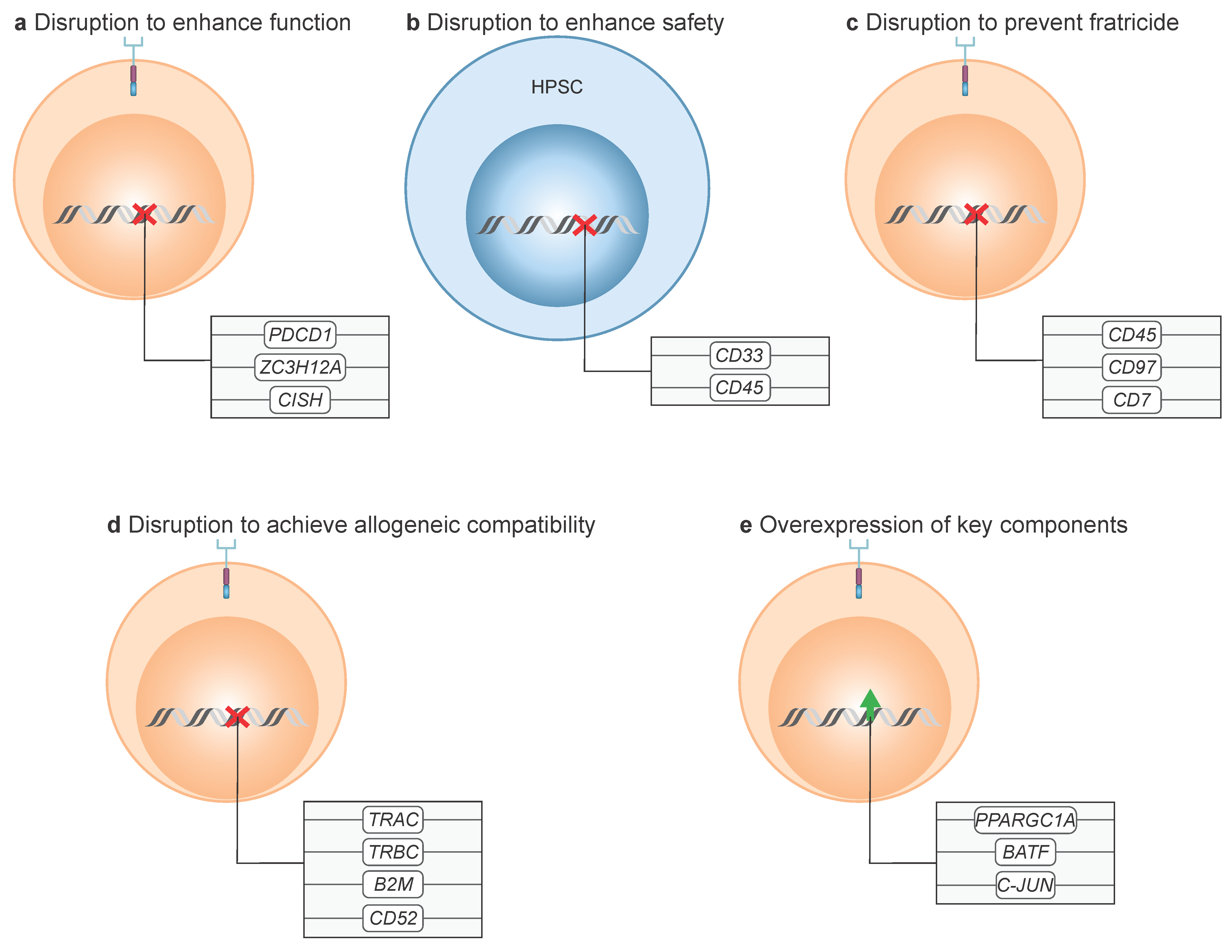
8.1. Disruption to Enhance Function
8.2. Disruption to Enhance Safety
8.3. Disruption to Prevent Fratricide
8.4. Disruption to Achieve Allogeneic Compatibility
8.5. Overexpression of Key Components
9. CAR-Non-T Cell
9.1. CAR-NK Cells
9.2. Other CAR-Non-T-Cell
- CAR-MAIT cells: Semi-invariant T cells restricted by MR1, enriched in mucosal and hepatic tissues. Their cytokine responsiveness and low GvHD risk support interest as CAR carriers, though AML applications remain unexplored [128].
- CAR-NKT cells: Invariant NKT cells share features of NK and T cells, recognizing CD1d-presented lipids. Advances in HSPC-derived CAR-NKT generation have enabled scalable products [129]. In AML models, allogeneic CD33-directed CAR-NKT cells demonstrated efficient marrow homing and cytotoxicity against CD33-high and CD33-low blasts [130].
10. Conclusions and New Horizons
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Labanieh, L.; Mackall, C.L. CAR Immune Cells: Design Principles, Resistance and the next Generation. Nature 2023, 614, 635–648. [Google Scholar] [CrossRef]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering Strategies to Overcome the Current Roadblocks in CAR T Cell Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef]
- Lim, W.A.; June, C.H. The Principles of Engineering Immune Cells to Treat Cancer. Cell 2017, 168, 724–740. [Google Scholar] [CrossRef]
- Zugasti, I.; Espinosa-Aroca, L.; Fidyt, K.; Mulens-Arias, V.; Diaz-Beya, M.; Juan, M.; Urbano-Ispizua, Á.; Esteve, J.; Velasco-Hernandez, T.; Menéndez, P. CAR-T Cell Therapy for Cancer: Current Challenges and Future Directions. Signal Transduct. Target. Ther. 2025, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Flugel, C.L.; Majzner, R.G.; Krenciute, G.; Dotti, G.; Riddell, S.R.; Wagner, D.L.; Abou-el-Enein, M. Overcoming On-Target, off-Tumour Toxicity of CAR T Cell Therapy for Solid Tumours. Nat. Rev. Clin. Oncol. 2023, 20, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Haubner, S.; Subklewe, M.; Sadelain, M. Honing CAR T Cells to Tackle Acute Myeloid Leukemia. Blood 2025, 145, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Lloret-Madrid, P.; Chorão, P.; Guerreiro, M.; Montesinos, P. CAR-T Cell Therapy for Acute Myeloid Leukemia: Where Do We Stand Now? Curr. Oncol. 2025, 32, 322. [Google Scholar] [CrossRef]
- Dunbar, A.J.; Rampal, R.K.; Levine, R. Leukemia Secondary to Myeloproliferative Neoplasms. Blood 2020, 136, 61–70. [Google Scholar] [CrossRef]
- Mazziotta, F.; Biavati, L.; Rimando, J.; Rutella, S.; Borcherding, N.; Parbhoo, S.; Mukhopadhyay, R.; Chowdhury, S.; Knaus, H.A.; Valent, P.; et al. CD8+ T-Cell Differentiation and Dysfunction Inform Treatment Response in Acute Myeloid Leukemia. Blood 2024, 144, 1168–1182. [Google Scholar] [CrossRef]
- Carturan, A.; Morè, S.; Poloni, A.; Rupoli, S.; Morsia, E. Shaping the Future of Myeloproliferative Neoplasm Therapy: Immune-Based Strategies and Targeted Innovations. Cancers 2024, 16, 4113. [Google Scholar] [CrossRef]
- Ling, V.Y.; Heidel, F.H.; Bywater, M.J. Pathogenesis and Management of High Molecular Risk Myeloproliferative Neoplasms. Haematologica 2025, 110, 863–876. [Google Scholar] [CrossRef]
- Albakri, M.M. TP53-Mutated MDS and AML: Immune Dysregulation, Tumor Microenvironment, and Emerging Therapeutic Strategies. Front. Oncol. 2025, 15, 486. [Google Scholar] [CrossRef]
- Joshi, U.; Shallis, R.M. MECOM-Rearranged Acute Myeloid Leukemia: Pathobiology and Management Strategies. Hematol. Rep. 2025, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Kong, W.; He, Y. The Affinity of Antigen-Binding Domain on the Antitumor Efficacy of CAR T Cells: Moderate Is Better. Front. Immunol. 2022, 13, 1032403. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Chen, R.; Huang, Y.; Meng, X.; Chen, J.; Liao, C.; Tang, Y.; Zhou, C.; Gao, X.; Sun, J. Tuning the Ignition of CAR: Optimizing the Affinity of ScFv to Improve CAR-T Therapy. Cell. Mol. Life Sci. 2022, 79, 14. [Google Scholar] [CrossRef]
- Arcangeli, S.; Rotiroti, M.C.; Bardelli, M.; Simonelli, L.; Magnani, C.F.; Biondi, A.; Biagi, E.; Tettamanti, S.; Varani, L. Balance of Anti-CD123 Chimeric Antigen Receptor Binding Affinity and Density for the Targeting of Acute Myeloid Leukemia. Mol. Ther. 2017, 25, 1933–1945. [Google Scholar] [CrossRef]
- Drent, E.; Themeli, M.; Poels, R.; de Jong-Korlaar, R.; Yuan, H.; de Bruijn, J.; Martens, A.C.M.; Zweegman, S.; van de Donk, N.W.C.J.; Groen, R.W.J.; et al. A Rational Strategy for Reducing On-Target Off-Tumor Effects of CD38-Chimeric Antigen Receptors by Affinity Optimization. Mol. Ther. 2017, 25, 1946–1958. [Google Scholar] [CrossRef]
- Hamieh, M.; Mansilla-Soto, J.; Rivière, I.; Sadelain, M. Programming CAR T Cell Tumor Recognition: Tuned Antigen Sensing and Logic Gating. Cancer Discov. 2023, 13, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xie, D.; Sun, R.; Lu, W.; Xiao, X.; Yu, Y.; Meng, J.; Zhao, M. CAR-T Cells Dual-Target CD123 and NKG2DLs to Eradicate AML Cells and Selectively Target Immunosuppressive Cells. Oncoimmunology 2023, 12, 2248826. [Google Scholar] [CrossRef]
- Xie, D.; Jin, X.; Sun, R.; Zhang, M.; Lu, W.; Cao, X.; Guo, R.; Zhang, Y.; Zhao, M. Bicistronic CAR-T Cells Targeting CD123 and CLL1 for AML to Reduce the Risk of Antigen Escape. Transl. Oncol. 2023, 34, 101695. [Google Scholar] [CrossRef]
- Petrov, J.C.; Wada, M.; Pinz, K.G.; Yan, L.E.; Chen, K.H.; Shuai, X.; Liu, H.; Chen, X.; Leung, L.H.; Salman, H.; et al. Compound CAR T-Cells as a Double-Pronged Approach for Treating Acute Myeloid Leukemia. Leukemia 2018, 32, 1317–1326. [Google Scholar] [CrossRef]
- Atilla, P.A.; McKenna, M.K.; Watanabe, N.; Mamonkin, M.; Brenner, M.K.; Atilla, E. Combinatorial Antigen Targeting Strategies for Acute Leukemia: Application in Myeloid Malignancy. Cytotherapy 2022, 24, 282–290. [Google Scholar] [CrossRef]
- Zoine, J.T.; Immadisetty, K.; Ibanez-Vega, J.; Moore, S.E.; Nevitt, C.; Thanekar, U.; Tian, L.; Karouni, A.; Chockley, P.J.; Arthur, B.; et al. Peptide-ScFv Antigen Recognition Domains Effectively Confer CAR T Cell Multiantigen Specificity. Cell Rep. Med. 2024, 5, 101422. [Google Scholar] [CrossRef]
- Wang, X.Y.; Bian, M.R.; Lin, G.Q.; Yu, L.; Zhang, Y.M.; Wu, D.P. Tandem Bispecific CD123/CLL-1 CAR-T Cells Exhibit Specific Cytolytic Effector Functions against Human Acute Myeloid Leukaemia. Eur. J. Haematol. 2024, 112, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Ghamari, A.; Pakzad, P.; Majd, A.; Ebrahimi, M.; Hamidieh, A.A. Design and Production an Effective Bispecific Tandem Chimeric Antigen Receptor on T Cells against CD123 and Folate Receptor ß towards B-Acute Myeloid Leukaemia Blasts. Cell J. 2021, 23, 650–657. [Google Scholar] [CrossRef]
- Wang, H.; Feng, S.; Zhu, Y.; Zhang, Y.; Zhou, Z.; Nian, Z.; Lu, X.; Peng, P.; Wu, S.; Zhou, L. The Tandem CD33-CLL1 CAR-T as an Approach to Treat Acute Myeloid. Blood Transfus. 2025, 23, 338–347. [Google Scholar] [CrossRef]
- Ma, H.; Yan, Z.; Gu, R.; Xu, Y.; Qiu, S.; Xing, H.; Tang, K.; Tian, Z.; Rao, Q.; Wang, M.; et al. Loop33 × 123 CAR-T Targeting CD33 and CD123 against Immune Escape in Acute Myeloid Leukemia. Cancer Immunol. Immunother. 2025, 74, 20. [Google Scholar] [CrossRef]
- Schneider, D.; Xiong, Y.; Wu, D.; Hu, P.; Alabanza, L.; Steimle, B.; Mahmud, H.; Anthony-Gonda, K.; Krueger, W.; Zhu, Z.; et al. Trispecific CD19-CD20-CD22-Targeting DuoCAR-T Cells Eliminate Antigen-Heterogeneous B Cell Tumors in Preclinical Models. Sci. Transl. Med. 2021, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Han, Y.; Pan, H.-B.; Sang, C.-J.; Shi, D.-L.; Feng, C.; Xiao, H.; Zhuang, Q.-C.; Wang, P.-Y.; Fan, X.-H. Tri-Specific CD19xCD20xCD22 VHH CAR-T Cells (LCAR-AIO) Eradicate Antigen-Heterogeneous B Cell Tumors, Enhance Expansion, and Prolong Persistence in Preclinical In Vivo Models. Blood 2021, 138, 1700. [Google Scholar] [CrossRef]
- Bubb, Q.R.; Balood, M.; Seir, G.E.; Swartzrock, L.; Haslett, E.; Ho, K.; Xu, P.; Wiltz, S.G.; Sotillo, E.; Gruber, T.A.; et al. Development of Multivalent CAR T Cells as Dual Immunotherapy and Conditioning Agents. Mol. Ther. Oncol. 2025, 33, 200944. [Google Scholar] [CrossRef]
- Pérez-Amill, L.; Bataller, À.; Delgado, J.; Esteve, J.; Juan, M.; Klein-González, N. Advancing CART Therapy for Acute Myeloid Leukemia: Recent Breakthroughs and Strategies for Future Development. Front. Immunol. 2023, 14, 1260470. [Google Scholar] [CrossRef]
- Tousley, A.M.; Rotiroti, M.C.; Labanieh, L.; Rysavy, L.W.; Kim, W.J.; Lareau, C.; Sotillo, E.; Weber, E.W.; Rietberg, S.P.; Dalton, G.N.; et al. Co-Opting Signalling Molecules Enables Logic-Gated Control of CAR T Cells. Nature 2023, 615, 507–516. [Google Scholar] [CrossRef]
- Dagher, O.K.; Posey, A.D. Forks in the Road for CAR T and CAR NK Cell Cancer Therapies. Nat. Immunol. 2023, 24, 1994–2007. [Google Scholar] [CrossRef]
- Perriello, V.M.; Rotiroti, M.C.; Pisani, I.; Galimberti, S.; Alberti, G.; Pianigiani, G.; Ciaurro, V.; Marra, A.; Sabino, M.; Tini, V.; et al. IL-3-Zetakine Combined with a CD33 Costimulatory Receptor as a Dual CAR Approach for Safer and Selective Targeting of AML. Blood Adv. 2023, 7, 2855–2871. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.M.; Zhao, F.; Freitas, K.A.; Parker, K.R.; Xu, P.; Fan, A.; Sotillo, E.; Daugaard, M.; Oo, H.Z.; Liu, J.; et al. NOT-Gated CD93 CAR T Cells Effectively Target AML with Minimized Endothelial Cross-Reactivity. Blood Cancer Discov. 2021, 2, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Rong, L.; Jiang, N.; Wayne, A.S.; Xie, J. Targeting HLA-DR Loss in Hematologic Malignancies with an Inhibitory Chimeric Antigen Receptor. Mol. Ther. 2022, 30, 1215–1226. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Y.; Chai, X.; Wang, Y.; Zhao, M.; Guo, S.; Zhang, Y.; Zhao, M. Modified CD15/CD16-CLL1 Inhibitory CAR-T Cells for Mitigating Granulocytopenia Toxicities in the Treatment of Acute Myeloid Leukemia. Transl. Oncol. 2025, 52, 102225. [Google Scholar] [CrossRef]
- Frankel, N.W.; Deng, H.; Yucel, G.; Gainer, M.; Leemans, N.; Lam, A.; Li, Y.; Hung, M.; Lee, D.; Lee, C.T.; et al. Precision Off-the-Shelf Natural Killer Cell Therapies for Oncology with Logic-Gated Gene Circuits. Cell Rep. 2024, 43, 114145. [Google Scholar] [CrossRef]
- DiAndreth, B.; Nesterenko, P.A.; Winters, A.G.; Flynn, A.D.; Jette, C.A.; Suryawanshi, V.; Shafaattalab, S.; Martire, S.; Daris, M.; Moore, E.; et al. Multi-Targeted, NOT Gated CAR-T Cells as a Strategy to Protect Normal Lineages for Blood Cancer Therapy. Front. Immunol. 2025, 16, 1493329. [Google Scholar] [CrossRef]
- Kondo, T.; Taylor, N. Co-Op CARs for Targeting Acute Myeloid Leukemia. Cancer Cell 2023, 41, 1841–1843. [Google Scholar] [CrossRef] [PubMed]
- Haubner, S.; Mansilla-Soto, J.; Nataraj, S.; Kogel, F.; Chang, Q.; de Stanchina, E.; Lopez, M.; Ng, M.R.; Fraser, K.; Subklewe, M.; et al. Cooperative CAR Targeting to Selectively Eliminate AML and Minimize Escape. Cancer Cell 2023, 41, 1871–1891.e6. [Google Scholar] [CrossRef] [PubMed]
- Shirzadian, M.; Moori, S.; Rabbani, R.; Rahbarizadeh, F. SynNotch CAR-T Cell, When Synthetic Biology and Immunology Meet Again. Front. Immunol. 2025, 16, 1545270. [Google Scholar] [CrossRef]
- Choe, J.H.; Watchmaker, P.B.; Simic, M.S.; Gilbert, R.D.; Li, A.W.; Krasnow, N.A.; Downey, K.M.; Yu, W.; Carrera, D.A.; Celli, A.; et al. SynNotch-CAR T Cells Overcome Challenges of Specificity, Heterogeneity, and Persistence in Treating Glioblastoma. Sci. Transl. Med. 2021, 13, 591. [Google Scholar] [CrossRef]
- Roybal, K.T.; Rupp, L.J.; Morsut, L.; Walker, W.J.; McNally, K.A.; Park, J.S.; Lim, W.A. Precision Tumor Recognition by T Cells with Combinatorial Antigen-Sensing Circuits. Cell 2016, 164, 770–779. [Google Scholar] [CrossRef]
- Jambon, S.; Sun, J.; Barman, S.; Muthugounder, S.; Bito, X.R.; Shadfar, A.; Kovach, A.E.; Wood, B.L.; Manoharan, V.T.; Morrissy, A.S.; et al. CD33–CD123 IF-THEN Gating Reduces Toxicity While Enhancing the Specificity and Memory Phenotype of AML-Targeting CAR-T Cells. Blood Cancer Discov. 2025, 6, 55–72. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, J.; Song, Y. Engineering Switchable and Programmable Universal CARs for CAR T Therapy. J. Hematol. Oncol. 2019, 12, 69. [Google Scholar] [CrossRef]
- Zarychta, J.; Kowalczyk, A.; Krawczyk, M.; Lejman, M.; Zawitkowska, J. CAR-T Cells Immunotherapies for the Treatment of Acute Myeloid Leukemia—Recent Advances. Cancers 2023, 15, 2944. [Google Scholar] [CrossRef] [PubMed]
- Atar, D.; Ruoff, L.; Mast, A.S.; Krost, S.; Moustafa-Oglou, M.; Scheuermann, S.; Kristmann, B.; Feige, M.; Canak, A.; Wolsing, K.; et al. Rational Combinatorial Targeting by Adapter CAR-T-Cells (AdCAR-T) Prevents Antigen Escape in Acute Myeloid Leukemia. Leukemia 2024, 38, 2183–2195. [Google Scholar] [CrossRef]
- Volta, L.; Myburgh, R.; Pellegrino, C.; Koch, C.; Maurer, M.; Manfredi, F.; Hofstetter, M.; Kaiser, A.; Schneiter, F.; Müller, J.; et al. Efficient Combinatorial Adaptor-Mediated Targeting of Acute Myeloid Leukemia with CAR T-Cells. Leukemia 2024, 38, 2598–2613. [Google Scholar] [CrossRef]
- Nixdorf, D.; Sponheimer, M.; Berghammer, D.; Engert, F.; Bader, U.; Philipp, N.; Kazerani, M.; Straub, T.; Rohrbacher, L.; Wange, L.; et al. Adapter CAR T Cells to Counteract T-Cell Exhaustion and Enable Flexible Targeting in AML. Leukemia 2023, 37, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Benmebarek, M.R.; Cadilha, B.L.; Herrmann, M.; Lesch, S.; Schmitt, S.; Stoiber, S.; Darwich, A.; Augsberger, C.; Brauchle, B.; Rohrbacher, L.; et al. A Modular and Controllable T Cell Therapy Platform for Acute Myeloid Leukemia. Leukemia 2021, 35, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Collins, J.J.; Wong, W.W. Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses. Cell 2018, 173, 1426–1438.e11. [Google Scholar] [CrossRef]
- Mestermann, K.; Giavridis, T.; Weber, J.; Rydzek, J.; Frenz, S.; Nerreter, T.; Mades, A.; Sadelain, M.; Einsele, H.; Hudecek, M. The Tyrosine Kinase Inhibitor Dasatinib Acts as a Pharmacologic on/off Switch for CAR T Cells. Sci. Transl. Med. 2019, 11, 499. [Google Scholar] [CrossRef]
- Juillerat, A.; Tkach, D.; Busser, B.W.; Temburni, S.; Valton, J.; Duclert, A.; Poirot, L.; Depil, S.; Duchateau, P. Modulation of Chimeric Antigen Receptor Surface Expression by a Small Molecule Switch. BMC Biotechnol. 2019, 19, 44. [Google Scholar] [CrossRef]
- Appelbaum, J.; Price, A.E.; Oda, K.; Zhang, J.; Leung, W.H.; Tampella, G.; Xia, D.; So, P.P.L.; Hilton, S.K.; Evandy, C.; et al. Drug-Regulated CD33-Targeted CAR T Cells Control AML Using Clinically Optimized Rapamycin Dosing. J. Clin. Investig. 2024, 134, e162593. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, W.C.; Wong, C.L.W.; Colcher, D.; Sherman, M.; Ostberg, J.R.; Forman, S.J.; Riddell, S.R.; Jensen, M.C. A Transgene-Encoded Cell Surface Polypeptide for Selection, in Vivo Tracking, and Ablation of Engineered Cells. Blood 2011, 118, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Wei, J.; Rosser, J.M.; Kunkele, A.; Chang, C.A.; Reid, A.N.; Jensen, M.C. Rationally Designed Transgene-Encoded Cell-Surface Polypeptide Tag for Multiplexed Programming of Car t-Cell Synthetic Outputs. Cancer Immunol. Res. 2021, 9, 1047–1060. [Google Scholar] [CrossRef]
- Qasim, W.; Zhan, H.; Samarasinghe, S.; Adams, S.; Amrolia, P.; Stafford, S.; Butler, K.; Rivat, C.; Wright, G.; Somana, K.; et al. Molecular Remission of Infant B-ALL after Infusion of Universal TALEN Gene-Edited CAR T Cells. Sci. Transl. Med. 2017, 9, 374. [Google Scholar] [CrossRef]
- Philip, B.; Kokalaki, E.; Mekkaoui, L.; Thomas, S.; Straathof, K.; Flutter, B.; Marin, V.; Marafioti, T.; Chakraverty, R.; Linch, D.; et al. A Highly Compact Epitope-Based Marker/Suicide Gene for Easier and Safer T-Cell Therapy. Blood 2014, 124, 1277–1287. [Google Scholar] [CrossRef]
- Calviño, C.; Ceballos, C.; Alfonso, A.; Jauregui, P.; Calleja-Cervantes, M.E.; San Martin-Uriz, P.; Rodriguez-Marquez, P.; Martin-Mallo, A.; Iglesias, E.; Abizanda, G.; et al. Optimization of Universal Allogeneic CAR-T Cells Combining CRISPR and Transposon-Based Technologies for Treatment of Acute Myeloid Leukemia. Front. Immunol. 2023, 14, 1270843. [Google Scholar] [CrossRef]
- Minagawa, K.; Jamil, M.O.; Al-Obaidi, M.; Pereboeva, L.; Salzman, D.; Erba, H.P.; Lamb, L.S.; Bhatia, R.; Mineishi, S.; Di Stasi, A. In Vitro Pre-Clinical Validation of Suicide Gene Modified Anti-CD33 Redirected Chimeric Antigen Receptor T-Cells for Acute Myeloid Leukemia. PLoS ONE 2016, 11, e0166891. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Sauer, T.; Shum, T.; Parikh, K.; Mamonkin, M.; Omer, B.; Rouce, R.H.; Lulla, P.; Rooney, C.M.; Gottschalk, S.; et al. Treatment of Acute Myeloid Leukemia with T Cells Expressing Chimeric Antigen Receptors Directed to C-Type Lectin-like Molecule 1. Mol. Ther. 2017, 25, 2202–2213. [Google Scholar] [CrossRef]
- Magnani, C.F.; Myburgh, R.; Brunn, S.; Chambovey, M.; Ponzo, M.; Volta, L.; Manfredi, F.; Pellegrino, C.; Pascolo, S.; Miskey, C.; et al. Anti-CD117 CAR T Cells Incorporating a Safety Switch Eradicate Human Acute Myeloid Leukemia and Hematopoietic Stem Cells. Mol. Ther. Oncolytics 2023, 30, 56–71. [Google Scholar] [CrossRef]
- Bouquet, L.; Bôle-Richard, E.; Warda, W.; Neto Da Rocha, M.; Trad, R.; Nicod, C.; Haderbache, R.; Genin, D.; Ferrand, C.; Deschamps, M. RapaCaspase-9-Based Suicide Gene Applied to the Safety of IL-1RAP CAR-T Cells. Gene Ther. 2023, 30, 706–713. [Google Scholar] [CrossRef]
- Li, X.; Chen, T.; Li, X.; Zhang, H.; Li, Y.; Zhang, S.; Luo, S.; Zheng, T. Therapeutic Targets of Armored Chimeric Antigen Receptor T Cells Navigating the Tumor Microenvironment. Exp. Hematol. Oncol. 2024, 13, 96. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. TRUCKS, the Fourth-generation CAR T Cells: Current Developments and Clinical Translation. Adv. Cell Gene Ther. 2020, 3, e84. [Google Scholar] [CrossRef]
- Tang, L.; Pan, S.; Wei, X.; Xu, X.; Wei, Q. Arming CAR-T Cells with Cytokines and More: Innovations in the Fourth-Generation CAR-T Development. Mol. Ther. 2023, 31, 3146–3162. [Google Scholar] [CrossRef]
- Ataca Atilla, P.; McKenna, M.K.; Tashiro, H.; Srinivasan, M.; Mo, F.; Watanabe, N.; Simons, B.W.; McLean Stevens, A.; Redell, M.S.; Heslop, H.E.; et al. Modulating Tnfα Activity Allows Transgenic Il15-Expressing Cll-1 Car t Cells to Safely Eliminate Acute Myeloid Leukemia. J. Immunother. Cancer 2020, 8, e001229. [Google Scholar] [CrossRef]
- Mu-Mosley, H.; Ostermann, L.; Muftuoglu, M.; Vaidya, A.; Bonifant, C.L.; Velasquez, M.P.; Gottschalk, S.; Andreeff, M. Transgenic Expression of IL15 Retains CD123-Redirected T Cells in a Less Differentiated State Resulting in Improved Anti-AML Activity in Autologous AML PDX Models. Front. Immunol. 2022, 13, 880108. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, I.; Ho, W.J.; Marple, A.; Ravich, J.W.; Tam, A.; Rahnama, R.; Fearnow, A.; Rietberg, C.; Yanik, S.; Solomou, E.E.; et al. Engineering CAR-NK Cells to Secrete IL-15 Sustains Their Anti-AML Functionality but Is Associated with Systemic Toxicities. J. Immunother. Cancer 2021, 9, e003894. [Google Scholar] [CrossRef]
- Webb, L.; Lofgren, M.; Patterson, T.; Watt, A.; Lajoie, J.; Zieba, A.; Fleury, M.; Liu, E.; Ding, J.; Tighe, R. Membrane-Bound IL-15 Co-Expression Powers a Potent and Persistent CD70-Targeted TRuC T-Cell Therapy. Front. Immunol. 2025, 16, 1609658. [Google Scholar] [CrossRef]
- Sallman, D.A.; Elmariah, H.; Sweet, K.L.; Talati, C.; Mishra, A.; Kelley, L.L.; Lankford, A.; Chan, T.; Shah, R.R.; Padron, E.; et al. A Phase 1/1b Safety Study of Prgn-3006 Ultracar-TTM in Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia and Higher Risk Myelodysplastic Syndrome. Blood 2020, 136, 17. [Google Scholar]
- Foeng, J.; Comerford, I.; McColl, S.R. Harnessing the Chemokine System to Home CAR-T Cells into Solid Tumors. Cell Rep. Med. 2022, 3, 100543. [Google Scholar] [CrossRef] [PubMed]
- Itoh-Nakadai, A.; Saito, Y.; Murasawa-Tomizawa, M.; Kajita, H.; Matsumoto, T.; Matsuda, M.; Watanabe, T.; Shirouzu, M.; Ohara, O.; Koseki, H.; et al. CXCR4-Expressing Anti-CD25 CAR T-Cells Effectively Eliminate Human AML Cells In Vivo. Blood 2020, 136, 35–36. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Ito, Y.; Wu, Z.; Kasuya, H.; Nakashima, T.; Okamoto, S.; Amaishi, Y.; Zhang, H.; Li, Y.; Matsukawa, T.; et al. Development of a Chimeric Cytokine Receptor That Captures IL-6 and Enhances the Antitumor Response of CAR-T Cells. Cell Rep. Med. 2024, 5, 101526. [Google Scholar] [CrossRef]
- Bell, M.; Gottschalk, S. Engineered Cytokine Signaling to Improve CAR T Cell Effector Function. Front. Immunol. 2021, 12, 684642. [Google Scholar] [CrossRef]
- Nishimoto, K.P.; Lamture, G.; Chanthery, Y.; Teague, A.G.; Verma, Y.; Au, M.; Smith-Boeck, M.; Salum, M.; Murthy, P.; Gundurao, S.R.Y.; et al. ADI-270: An Armored Allogeneic Gamma Delta T Cell Therapy Designed to Target CD70-Expressing Solid and Hematologic Malignancies. J. Immunother. Cancer 2025, 13, e011704. [Google Scholar] [CrossRef]
- Brinkmann, U.; Kontermann, R.E. The Making of Bispecific Antibodies. MAbs 2017, 9, 182–212. [Google Scholar] [CrossRef]
- Goebeler, M.E.; Bargou, R.C. T Cell-Engaging Therapies—BiTEs and Beyond. Nat. Rev. Clin. Oncol. 2020, 17, 418–434. [Google Scholar] [PubMed]
- Tapia-Galisteo, A.; Compte, M.; Álvarez-Vallina, L.; Sanz, L. When Three Is Not a Crowd: Trispecific Antibodies for Enhanced Cancer Immunotherapy. Theranostics 2023, 13, 1028–1041. [Google Scholar] [CrossRef]
- Kalos, M.; Levine, B.L.; Porter, D.L.; Katz, S.; Grupp, S.A.; Bagg, A.; June, C.H.; Zhang, M.; Jin, X.; Sun, R.; et al. CAR-T Cells Secreting BiTEs Circumvent Antigen Escape without Detectable Toxicity. Nat. Biotechnol. 2021, 12, 1049–1058. [Google Scholar]
- Díez-Alonso, L.; Falgas, A.; Arroyo-Ródenas, J.; Romencín, P.A.; Martínez, A.; Gómez-Rosel, M.; Blanco, B.; Jiménez-Reinoso, A.; Mayado, A.; Pérez-Pons, A.; et al. Engineered T Cells Secreting Anti-BCMA T Cell Engagers Control Multiple Myeloma and Promote Immune Memory In Vivo. Sci. Transl. Med. 2024, 16, eadg7962. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Ródenas, J.; Falgas, A.; Díez-Alonso, L.; Martinez-Moreno, A.; Roca-Ho, H.; Gil-Etayo, F.J.; Pérez-Pons, A.; Aguilar-Sopeña, Ó.; Velasco-Sidro, M.; Gómez-Rosel, M.; et al. CD22 CAR-T Cells Secreting CD19 T-Cell Engagers for Improved Control of B-Cell Acute Lymphoblastic Leukemia Progression. J. Immunother. Cancer 2025, 13, e009048. [Google Scholar] [CrossRef] [PubMed]
- Blanco, B.; Ramírez-Fernández, Á.; Bueno, C.; Argemí-Muntadas, L.; Fuentes, P.; Aguilar-Sopeña, Ó.; Gutierrez-Agüera, F.; Zanetti, S.R.; Tapia-Galisteo, A.; Díez-Alonso, L.; et al. Overcoming CAR-Mediated CD19 Downmodulation and Leukemia Relapse with T Lymphocytes Secreting Anti-CD19 T-Cell Engagers. Cancer Immunol. Res. 2022, 10, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.J.; Martin, G.; Birocchi, F.; Wehrli, M.; Kann, M.C.; Supper, V.; Parker, A.; Graham, C.; Bratt, A.; Bouffard, A.; et al. CD70 CAR T Cells Secreting an Anti-CD33/Anti-CD3 Dual-Targeting Antibody Overcome Antigen Heterogeneity in AML. Blood 2025, 145, 720–731. [Google Scholar] [CrossRef]
- Yan, Z.; Gu, R.; Ma, H.; Chen, N.; Zhang, T.; Xu, Y.; Qiu, S.; Xing, H.; Tang, K.; Tian, Z.; et al. A Dual-Targeting Approach with Anti-IL10R CAR-T Cells Engineered to Release Anti-CD33 Bispecific Antibody in Enhancing Killing Effect on Acute Myeloid Leukemia Cells. Cell. Oncol. 2024, 47, 1879–1895. [Google Scholar] [CrossRef]
- Caruana, I.; Savoldo, B.; Hoyos, V.; Weber, G.; Liu, H.; Kim, E.S.; Ittmann, M.M.; Marchetti, D.; Dotti, G. Heparanase Promotes Tumor Infiltration and Antitumor Activity of CAR-Redirected T-Lymphocytes. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef]
- Ligtenberg, M.A.; Mougiakakos, D.; Mukhopadhyay, M.; Witt, K.; Lladser, A.; Chmielewski, M.; Riet, T.; Abken, H.; Kiessling, R. Coexpressed Catalase Protects Chimeric Antigen Receptor–Redirected T Cells as Well as Bystander Cells from Oxidative Stress–Induced Loss of Antitumor Activity. J. Immunol. 2016, 196, 759–766. [Google Scholar] [CrossRef]
- Boice, M.; Salloum, D.; Mourcin, F.; Sanghvi, V.; Amin, R.; Oricchio, E.; Jiang, M.; Mottok, A.; Denis-Lagache, N.; Ciriello, G.; et al. Loss of the HVEM Tumor Suppressor in Lymphoma and Restoration by Modified CAR-T Cells. Cell 2016, 167, 405–418.e13. [Google Scholar] [CrossRef]
- Newick, K.; O’brien, S.; Sun, J.; Kapoor, V.; Maceyko, S.; Lo, A.; Puré, E.; Moon, E.; Albelda, S.M. Augmentation of CAR T-Cell Trafficking and Antitumor Efficacy by Blocking Protein Kinase a Localization. Cancer Immunol. Res. 2016, 4, 541–551. [Google Scholar] [CrossRef]
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. ‘Off-the-Shelf’ Allogeneic CAR T Cells: Development and Challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef]
- Song, P.; Zhang, Q.; Xu, Z.; Shi, Y.; Jing, R.; Luo, D. CRISPR/Cas-Based CAR-T Cells: Production and Application. Biomark. Res. 2024, 12, 54. [Google Scholar] [CrossRef]
- Lei, T.; Wang, Y.; Zhang, Y.; Yang, Y.; Cao, J.; Huang, J.; Chen, J.; Chen, H.; Zhang, J.; Wang, L.; et al. Leveraging CRISPR Gene Editing Technology to Optimize the Efficacy, Safety and Accessibility of CAR T-Cell Therapy. Leukemia 2024, 38, 2517–2543. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, Y.; Yu, L.; Wu, D. Cytotoxic Effect of CLL-1 CAR-T Cell Immunotherapy with PD-1 Silencing on Relapsed/Refractory Acute Myeloid Leukemia. Mol. Med. Rep. 2021, 23, 11847. [Google Scholar] [CrossRef]
- Tang, N.; Cheng, C.; Zhang, X.; Qiao, M.; Li, N.; Mu, W.; Wei, X.F.; Han, W.; Wang, H. TGF-β Inhibition via CRISPR Promotes the Long-Term Efficacy of CAR T Cells against Solid Tumors. JCI Insight 2020, 5, e133977. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Cheng, C.; Mu, W.; Liu, X.; Li, N.; Wei, X.; Liu, X.; Xia, C.; Wang, H. CRISPR-Cas9 Mediated LAG-3 Disruption in CAR-T Cells. Front. Med. 2017, 11, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.; Schimmer, R.R.; Koch, C.; Schneiter, F.; Fullin, J.; Lysenko, V.; Pellegrino, C.; Klemm, N.; Russkamp, N.; Myburgh, R.; et al. Targeting the Mevalonate or Wnt Pathways to Overcome CAR T-Cell Resistance in TP53-Mutant AML Cells. EMBO Mol. Med. 2024, 16, 445–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Bai, Y.; Xiong, Y.; Zhang, J.; Chen, S.; Zheng, X.; Meng, X.; Li, L.; Wang, J.; Xu, C.; et al. Potentiating the Antitumour Response of CD8+ T Cells by Modulating Cholesterol Metabolism. Nature 2016, 531, 651–655. [Google Scholar] [CrossRef]
- Gurney, M.; O’Reilly, E.; Corcoran, S.; Brophy, S.; Krawczyk, J.; Otto, N.M.; Hermanson, D.L.; Childs, R.W.; Szegezdi, E.; O’Dwyer, M.E. Concurrent Transposon Engineering and CRISPR/Cas9 Genome Editing of Primary CLL-1 Chimeric Antigen Receptor–Natural Killer Cells. Cytotherapy 2022, 24, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Borot, F.; Wang, H.; Ma, Y.; Jafarov, T.; Raza, A.; Ali, A.M.; Mukherjee, S. Gene-Edited Stem Cells Enable CD33-Directed Immune Therapy for Myeloid Malignancies. Proc. Natl. Acad. Sci. USA 2019, 116, 11978–11987. [Google Scholar] [CrossRef]
- Kim, M.Y.; Yu, K.R.; Kenderian, S.S.; Ruella, M.; Chen, S.; Shin, T.H.; Aljanahi, A.A.; Schreeder, D.; Klichinsky, M.; Shestova, O.; et al. Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell 2018, 173, 1439–1453.e19. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Schubert, M.L.; Lauk, A.; Yao, H.; Blank, M.F.; Cui, C.; Janssen, M.; Schmidt, C.; Göllner, S.; et al. CD33-Directed Immunotherapy with Third-Generation Chimeric Antigen Receptor T Cells and Gemtuzumab Ozogamicin in Intact and CD33-Edited Acute Myeloid Leukemia and Hematopoietic Stem and Progenitor Cells. Int. J. Cancer 2022, 150, 1141–1155. [Google Scholar] [CrossRef]
- Wellhausen, N.; O’Connell, R.P.; Lesch, S.; Engel, N.W.; Rennels, A.K.; Gonzales, D.; Herbst, F.; Young, R.M.; Garcia, K.C.; Weiner, D.; et al. Epitope Base Editing CD45 in Hematopoietic Cells Enables Universal Blood Cancer Immune Therapy. Sci. Transl. Med. 2023, 15, eadi1145. [Google Scholar] [CrossRef]
- Harfmann, M.; Schröder, T.; Głów, D.; Jung, M.; Uhde, A.; Kröger, N.; Horn, S.; Riecken, K.; Fehse, B.; Ayuk, F.A. CD45-Directed CAR-T Cells with CD45 Knockout Efficiently Kill Myeloid Leukemia and Lymphoma Cells In Vitro Even after Extended Culture. Cancers 2024, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Shang, K.; Huang, D.; Liu, J.; Yu, Z.; Bian, W.; Chen, J.; Zhao, Y.; Liu, L.; Jiang, J.; Wang, Y.; et al. CD97-Directed CAR-T Cells with Enhanced Persistence Eradicate Acute Myeloid Leukemia in Diverse Xenograft Models. Cell Rep. Med. 2025, 6, 102148. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, Y.; Zhang, M.; Zhao, H.; Wei, G.; Ge, W.; Cui, Q.; Mu, Q.; Chen, G.; Han, L.; et al. Genetically Modified CD7-Targeting Allogeneic CAR-T Cell Therapy with Enhanced Efficacy for Relapsed/Refractory CD7-Positive Hematological Malignancies: A Phase I Clinical Study. Cell Res. 2022, 32, 995–1007. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Wilson, N.R.; Senapati, J.; Economides, M.P.; Guzman, M.L.; Neelapu, S.S.; Kazemimood, R.; Davis, R.E.; Jain, N.; Khoury, J.D.; et al. CD123-Directed Allogeneic Chimeric-Antigen Receptor T-Cell Therapy (CAR-T) in Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN): Clinicopathological Insights. Leuk. Res. 2022, 121, 106928. [Google Scholar] [CrossRef]
- Cai, T.; Gouble, A.; Black, K.L.; Skwarska, A.; Naqvi, A.S.; Taylor, D.; Zhao, M.; Yuan, Q.; Sugita, M.; Zhang, Q.; et al. Targeting CD123 in Blastic Plasmacytoid Dendritic Cell Neoplasm Using Allogeneic Anti-CD123 CAR T Cells. Nat. Commun. 2022, 13, 2228. [Google Scholar] [CrossRef]
- Sugita, M.; Galetto, R.; Zong, H.; Ewing-Crystal, N.; Trujillo-Alonso, V.; Mencia-Trinchant, N.; Yip, W.; Filipe, S.; Lebuhotel, C.; Gouble, A.; et al. Allogeneic TCRαβ Deficient CAR T-Cells Targeting CD123 in Acute Myeloid Leukemia. Nat. Commun. 2022, 13, 2227. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Cheng, H.Y.; Nguyen, D.; Dettling, D.; Yeung, Y.A.; Sutton, J.; Hamze, M.; Valton, J.; Smith, J.; Djuretic, I.; et al. Allogeneic FLT3 CAR T Cells with an Off-Switch Exhibit Potent Activity against AML and Can Be Depleted to Expedite Bone Marrow Recovery. Mol. Ther. 2020, 28, 2237–2251. [Google Scholar] [CrossRef]
- Scharping, N.E.; Menk, A.V.; Moreci, R.S.; Whetstone, R.D.; Dadey, R.E.; Watkins, S.C.; Ferris, R.L.; Delgoffe, G.M. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity 2016, 45, 374–388. [Google Scholar] [CrossRef]
- Seo, H.; González-Avalos, E.; Zhang, W.; Ramchandani, P.; Yang, C.; Lio, C.W.J.; Rao, A.; Hogan, P.G. BATF and IRF4 Cooperate to Counter Exhaustion in Tumor-Infiltrating CAR T Cells. Nat. Immunol. 2021, 22, 983–995. [Google Scholar] [CrossRef]
- Zuo, S.; Li, C.; Sun, X.; Deng, B.; Zhang, Y.; Han, Y.; Ling, Z.; Xu, J.; Duan, J.; Wang, Z.; et al. C-JUN Overexpressing CAR-T Cells in Acute Myeloid Leukemia: Preclinical Characterization and Phase I Trial. Nat. Commun. 2024, 15, 6155. [Google Scholar] [CrossRef]
- Pan, K.; Farrukh, H.; Chittepu, V.C.S.R.; Xu, H.; Pan, C.X.; Zhu, Z. CAR Race to Cancer Immunotherapy: From CAR T, CAR NK to CAR Macrophage Therapy. J. Exp. Clin. Cancer Res. 2022, 41, 119. [Google Scholar] [CrossRef]
- Kent, A.; Crump, L.S.; Davila, E. Beyond Aβ T Cells: NK, INKT, and ΓδT Cell Biology in Leukemic Patients and Potential for off-the-Shelf Adoptive Cell Therapies for AML. Front. Immunol. 2023, 14, 1202950. [Google Scholar] [CrossRef] [PubMed]
- Albinger, N.; Pfeifer, R.; Nitsche, M.; Mertlitz, S.; Campe, J.; Stein, K.; Kreyenberg, H.; Schubert, R.; Quadflieg, M.; Schneider, D.; et al. Primary CD33-Targeting CAR-NK Cells for the Treatment of Acute Myeloid Leukemia. Blood Cancer J. 2022, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.; De Angelis, B.; Del Bufalo, F.; Ciccone, R.; Donsante, S.; Volpe, G.; Manni, S.; Guercio, M.; Pezzella, M.; Iaffaldano, L.; et al. Safe and Effective Off-the-Shelf Immunotherapy Based on CAR.CD123-NK Cells for the Treatment of Acute Myeloid Leukaemia. J. Hematol. Oncol. 2022, 15, 163. [Google Scholar] [CrossRef]
- Rahnama, R.; Kizerwetter, M.; Yang, H.; Christodoulou, I.; Guaraca, C.; Holl, N.J.; Choe, J.; Vorri, S.C.; Zinsky, M.; Jones, D.G.; et al. Single-Chain Variable Fragment Affinity Tuning Can Optimize Anti-AML CAR-NK Cell Functionality. J. Immunother. Cancer 2025, 13, e010763. [Google Scholar] [CrossRef]
- Klaihmon, P.; Luanpitpong, S.; Kang, X.; Issaragrisil, S. Anti-TIM3 Chimeric Antigen Receptor-Natural Killer Cells from Engineered Induced Pluripotent Stem Cells Effectively Target Acute Myeloid Leukemia Cells. Cancer Cell Int. 2023, 23, 297. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.G.; Teng, K.Y.; Li, Z.; Zhu, Z.; Chen, H.; Tian, L.; Ali, A.; Zhang, J.; Lu, T.; Ma, S.; et al. Off-the-Shelf CAR–Engineered Natural Killer Cells Targeting FLT3 Enhance Killing of Acute Myeloid Leukemia. Blood Adv. 2023, 7, 6225–6239. [Google Scholar] [CrossRef]
- Bexte, T.; Albinger, N.; Al Ajami, A.; Wendel, P.; Buchinger, L.; Gessner, A.; Alzubi, J.; Särchen, V.; Vogler, M.; Rasheed, H.M.; et al. CRISPR/Cas9 Editing of NKG2A Improves the Efficacy of Primary CD33-Directed Chimeric Antigen Receptor Natural Killer Cells. Nat. Commun. 2024, 15, 8439. [Google Scholar] [CrossRef]
- Ureña-Bailén, G.; Dobrowolski, J.M.; Hou, Y.; Dirlam, A.; Roig-Merino, A.; Schleicher, S.; Atar, D.; Seitz, C.; Feucht, J.; Antony, J.S.; et al. Preclinical Evaluation of CRISPR-Edited CAR-NK-92 Cells for Off-the-Shelf Treatment of AML and B-ALL. Int. J. Mol. Sci. 2022, 23, 12828. [Google Scholar] [CrossRef]
- Sedloev, D.; Chen, Q.; Unglaub, J.M.; Schanda, N.; Hao, Y.; Besiridou, E.; Neuber, B.; Schmitt, A.; Raffel, S.; Liu, Y.; et al. Proteasome Inhibition Enhances the Anti-Leukemic Efficacy of Chimeric Antigen Receptor (CAR) Expressing NK Cells against Acute Myeloid Leukemia. J. Hematol. Oncol. 2024, 17, 85. [Google Scholar] [CrossRef]
- Ikeda, S.; Hasegawa, K.; Kogue, Y.; Arimori, T.; Kawamoto, R.; Wibowo, T.; Yaga, M.; Inada, Y.; Uehara, H.; Matsubara, M.; et al. CAR T or NK Cells Targeting Mismatched HLA-DR Molecules in Acute Myeloid Leukemia after Allogeneic Hematopoietic Stem Cell Transplant. Nat. Cancer 2025, 6, 595–611. [Google Scholar] [CrossRef]
- Huang, T.; Bei, C.; Hu, Z.; Li, Y. CAR-Macrophage: Breaking New Ground in Cellular Immunotherapy. Front. Cell Dev. Biol. 2024, 12, 1464218. [Google Scholar] [CrossRef]
- Branella, G.M.; Lee, J.Y.; Okalova, J.; Parwani, K.K.; Alexander, J.S.; Arthuzo, R.F.; Fedanov, A.; Yu, B.; McCarty, D.; Brown, H.C.; et al. Ligand-Based Targeting of c-Kit Using Engineered Γδ T Cells as a Strategy for Treating Acute Myeloid Leukemia. Front. Immunol. 2023, 14, 1294555. [Google Scholar] [CrossRef]
- Martínez, D.S.; Tirado, N.; Mensurado, S.; Martínez-Moreno, A.; Romecín, P.; Agüera, F.G.; Correia, D.V.; Silva-Santos, B.; Menéndez, P. Generation and Proof-of-Concept for Allogeneic CD123 CAR-Delta One T (DOT) Cells in Acute Myeloid Leukemia. J. Immunother. Cancer 2022, 10, e005400. [Google Scholar] [CrossRef]
- Li, Y.R.; Zhou, K.; Wilson, M.; Kramer, A.; Zhu, Y.; Dawson, N.; Yang, L. Mucosal-Associated Invariant T Cells for Cancer Immunotherapy. Mol. Ther. 2023, 31, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Zhou, Y.; Yu, J.; Kim, Y.J.; Li, M.; Lee, D.; Zhou, K.; Chen, Y.; Zhu, Y.; Wang, Y.C.; et al. Generation of Allogeneic CAR-NKT Cells from Hematopoietic Stem and Progenitor Cells Using a Clinically Guided Culture Method. Nat. Biotechnol. 2025, 43, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Fang, Y.; Niu, S.; Zhu, Y.; Chen, Y.; Lyu, Z.; Zhu, E.; Tian, Y.; Huang, J.; Rezek, V.; et al. Allogeneic CD33-Directed CAR-NKT Cells for the Treatment of Bone Marrow-Resident Myeloid Malignancies. Nat. Commun. 2025, 16, 1248. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segura Tudela, A.; Geller, R.; Paiva, B.; Torres Sánchez, S.C.; González Romero, E.; Lloret Madrid, P.; Chorão, P.; de la Rubia, J.; Montesinos, P.; Guerreiro, M. The Rise of Fine-Tuned CAR-Based Therapies Against Acute Myeloid Leukemia. Cancers 2025, 17, 3892. https://doi.org/10.3390/cancers17243892
Segura Tudela A, Geller R, Paiva B, Torres Sánchez SC, González Romero E, Lloret Madrid P, Chorão P, de la Rubia J, Montesinos P, Guerreiro M. The Rise of Fine-Tuned CAR-Based Therapies Against Acute Myeloid Leukemia. Cancers. 2025; 17(24):3892. https://doi.org/10.3390/cancers17243892
Chicago/Turabian StyleSegura Tudela, Alejandro, Ron Geller, Bruno Paiva, Sara Carmen Torres Sánchez, Elisa González Romero, Pilar Lloret Madrid, Pedro Chorão, Javier de la Rubia, Pau Montesinos, and Manuel Guerreiro. 2025. "The Rise of Fine-Tuned CAR-Based Therapies Against Acute Myeloid Leukemia" Cancers 17, no. 24: 3892. https://doi.org/10.3390/cancers17243892
APA StyleSegura Tudela, A., Geller, R., Paiva, B., Torres Sánchez, S. C., González Romero, E., Lloret Madrid, P., Chorão, P., de la Rubia, J., Montesinos, P., & Guerreiro, M. (2025). The Rise of Fine-Tuned CAR-Based Therapies Against Acute Myeloid Leukemia. Cancers, 17(24), 3892. https://doi.org/10.3390/cancers17243892






