Combined Tumor Cell and Lysate-Based Vaccines for Immunotherapy of Primary and Recurrent Glioblastoma (GBM)
Simple Summary
Abstract
1. Introduction
1.1. Glioblastoma and Gliomas
1.2. Standard and Recurrent GBM Management
1.3. The Immunosuppressive GBM Environment
1.4. Immunotherapeutic Strategies
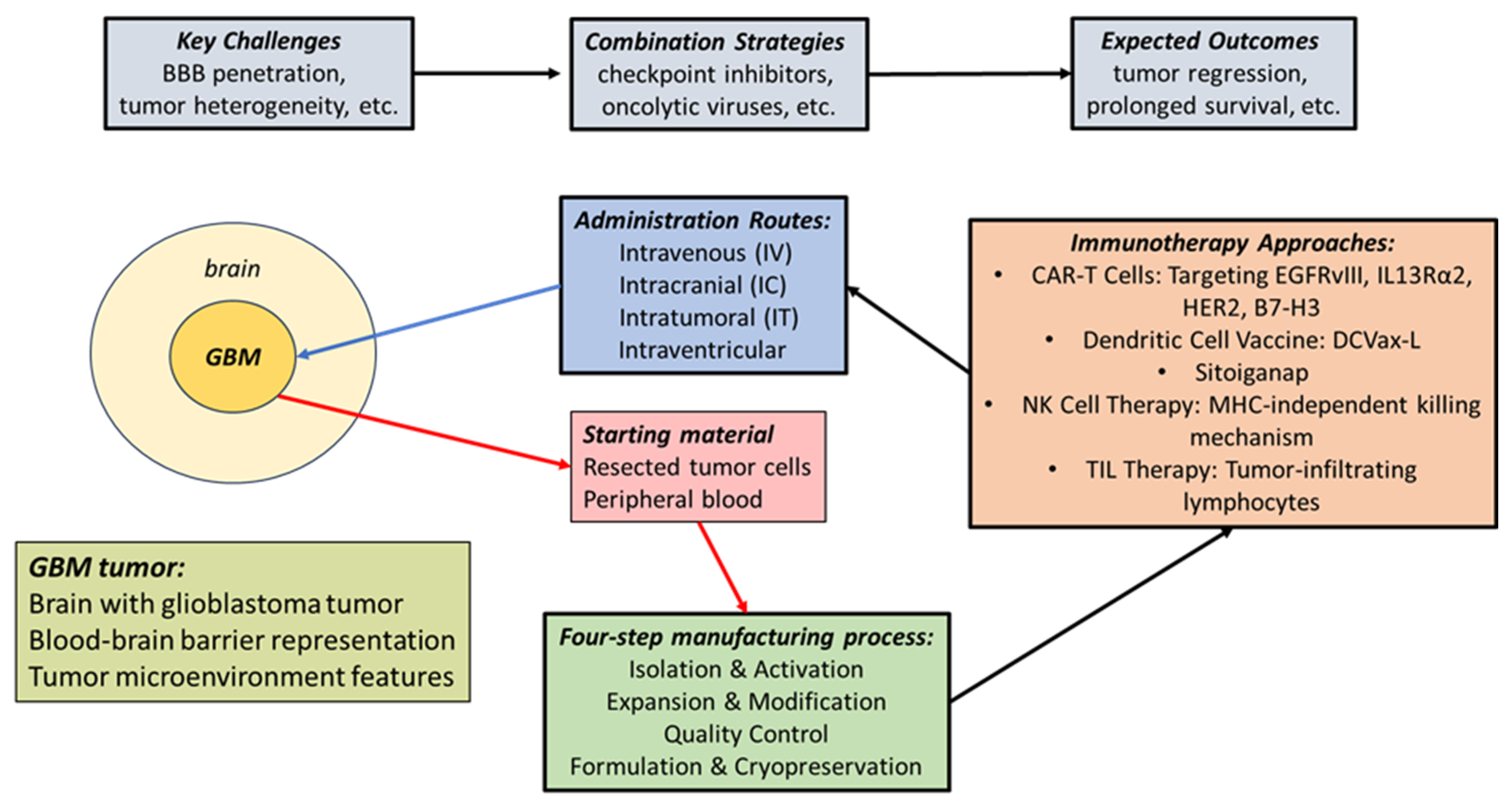
1.5. Cell-Based GBM Immunotherapy
2. Therapeutic GBM Tumor Vaccines
2.1. Anti-Tumor T Cell Responses
2.2. Responding Antigen-Presenting Cells
2.3. Tumor-Suppressive Environment (TME)
2.4. Resistance Mechanisms
2.5. Tumor-Specific Peptide Sequences in Glioblastoma
2.6. The Blood–Brain Barrier in Glioblastoma
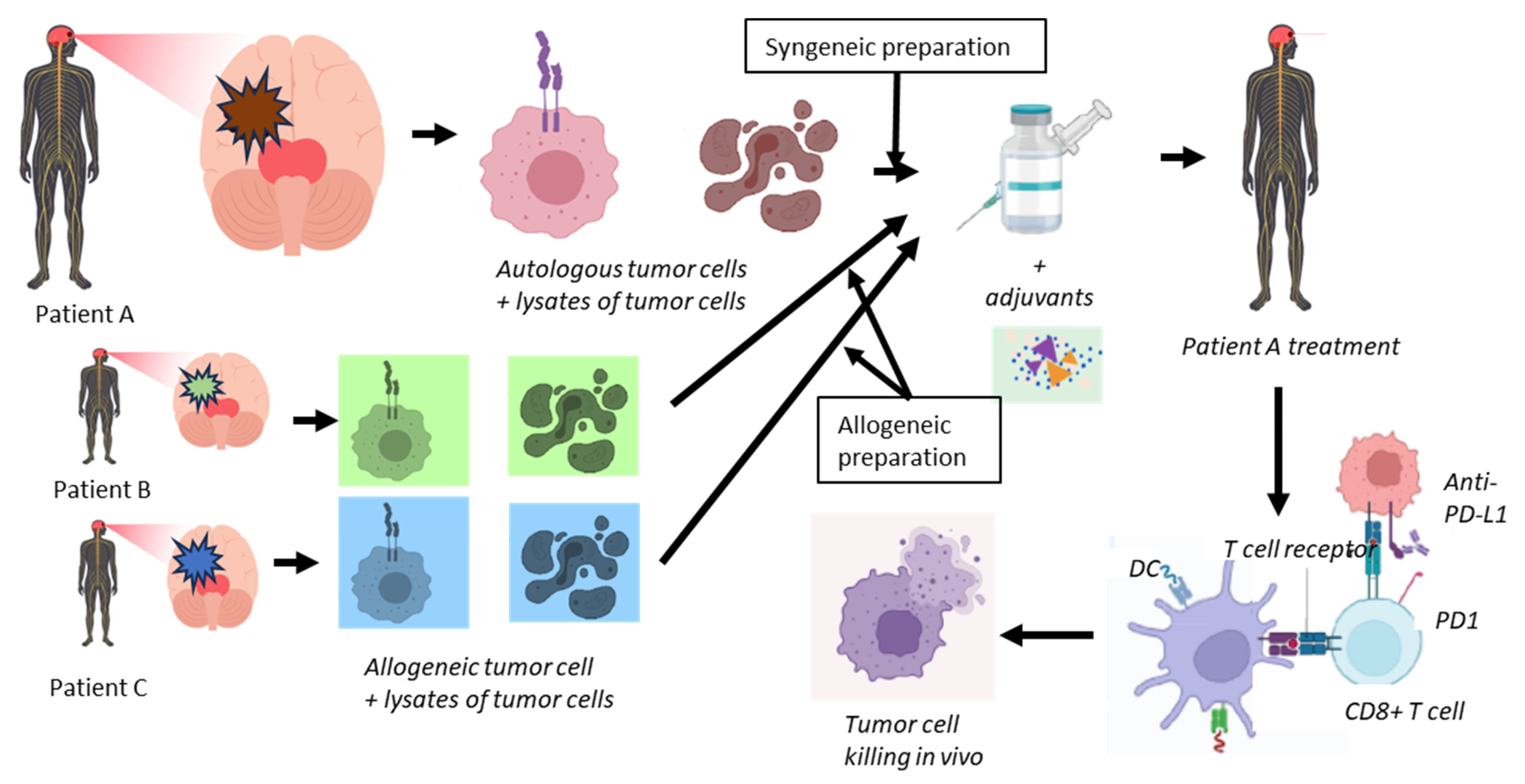
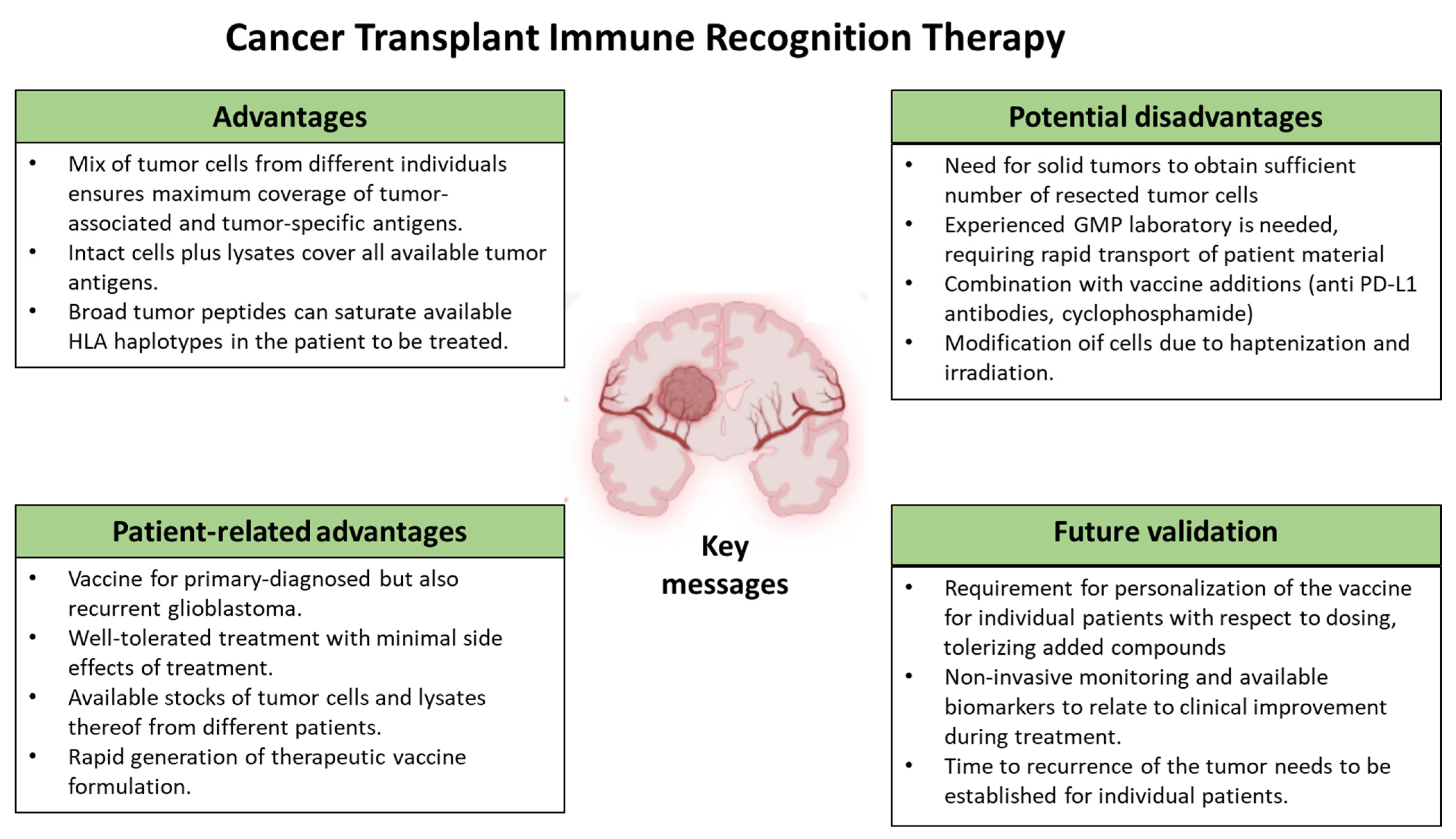
3. Rationale for Cancer Transplant Immune Recognition Therapy
3.1. Immunological Basis
3.2. Clinical Reactivity
3.3. Other Vaccine Compounds
3.4. Potential Biomarkers for GBM Immunotherapy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, R.N.; Kim, A.H. The multifaceted mechanisms of malignant glioblastoma progression and clinical implications. Cancer Metastasis Rev. 2022, 41, 871–898. [Google Scholar] [CrossRef]
- Sarantopoulos, A.; Chibawanye, E.; Aquilanti, E. Therapeutic approaches to modulate the immune microenvironment in gliomas. NPJ Precis. Oncol. 2024, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qi, Q.; Jiang, X.; Wu, J.; Li, Y.; Liu, Z.; Cai, Y.; Ran, H.; Zhang, S.; Zhang, C.; et al. Phosphocreatine Promotes Epigenetic Reprogramming to Facilitate Glioblastoma Growth Through Stabilizing BRD2. Cancer Discov. 2024, 14, 1547–1565. [Google Scholar] [CrossRef]
- Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of care for treatment of recurrent glioblastoma—Are we there yet? Neuro Oncol. 2013, 15, 4–27. [Google Scholar] [CrossRef]
- Birzu, C.; French, P.; Caccese, M.; Cerretti, G.; Idbaih, A.; Zagonel, V.; Lombardi, G. Recurrent Glioblastoma: From Molecular Landscape to New Treatment Perspectives. Cancers 2021, 13, 47. [Google Scholar] [CrossRef]
- Vaz-Salgado, M.A.; Villamayor, M.; Albarrán, V.; Alía, V.; Sotoca, P.; Chamorro, J.; Rosero, D.; Barrill, A.M.; Martín, M.; Fernandez, E.; et al. Recurrent Glioblastoma: A Review of the Treatment Options. Cancers 2023, 15, 4279. [Google Scholar] [CrossRef]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma Multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef] [PubMed]
- Ghouzlani, A.; Kandoussi, S.; Tall, M.; Reddy, K.P.; Rafii, S.; Badou, A. Immune Checkpoint Inhibitors in Human Glioma Microenvironment. Front. Immunol. 2021, 12, 679425. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, F.; Ali, H.; Lathia, J.D.; Chen, P. Immunotherapy for glioblastoma: Current state, challenges, and future perspectives. Cell. Mol. Immunol. 2024, 21, 1354–1375. [Google Scholar] [CrossRef]
- Want, M.Y.; Bashir, Z.; Najar, R.A. T Cell Based Immunotherapy for Cancer: Approaches and Strategies. Vaccines 2023, 11, 835. [Google Scholar] [CrossRef]
- Ellenbogen, Y.; Zadeh, G. A new paradigm for immunotherapy in glioblastoma. Nat. Med. 2025, 31, 1404–1405. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, G.; Wan, X. Challenges and new technologies in adoptive cell therapy. J. Hematol. Oncol. 2023, 16, 97. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shi, F.; Zhu, J.; Yuan, Y. An update on the clinical trial research of immunotherapy for glioblastoma. Front. Immunol. 2025, 16, 1582296. [Google Scholar] [CrossRef] [PubMed]
- Faust, A.C.; Andersen, B.M.; Li, Z.; Giovannoni, F.; Diebold, M.; Sanmarco, L.M.; Kilian, M.; Fehrenbacher, L.; Pernin, F.; Rone, J.M.; et al. Glioblastoma-instructed astrocytes suppress tumour-specific T cell immunity. Nature 2025, 643, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Pardoll, D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020, 367, 6477. [Google Scholar] [CrossRef]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Long, G.V.; Shklovskaya, E.; Satgunaseelan, L.; Mao, Y.; Pires da Silva, I.; Kristen, A.; Perry, K.A.; Russell, J.; Diefenbach, R.J.; Gide, T.N.; et al. Neoadjuvant triplet immune checkpoint blockade in newly diagnosed glioblastoma. Nat. Med. 2025, 31, 1557–1566. [Google Scholar] [CrossRef]
- Alexandru-Abrams, D.; Jadus, M.R.; Hsu, F.P.; Stathopoulos, A.; Bota, D.A. Therapeutic targeting of malignant glioma. Anticancer Agents Med. Chem. 2014, 14, 1075–1084. [Google Scholar] [CrossRef]
- Sarfraz, Z.; Maharaj, A.; Venur, V.A.; Lathia, J.D.; Odia, Y.; Ahluwalia, M.S. Immunotherapy in Glioblastoma: An overview of current status. Clin. Pharmacol. Adv. Appl. 2025, 17, 185–209. [Google Scholar] [CrossRef]
- Vallieri, N.; Datsi, A. Immune Cell Interplay in the Fight Against GBM. Cancers 2025, 17, 817. [Google Scholar] [CrossRef] [PubMed]
- Liau, L.M.; Ashkan, K.; Brem, S.; Campian, J.L.; Trusheim, J.E.; Iwamoto, F.M.; Tran, D.D.; Ansstas, G.; Cobbs, C.S.; Heth, J.A.; et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with glioblastoma: A phase 3, prospective, externally controlled cohort trial. JAMA Oncol. 2023, 9, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Bota, D.A.; Chung, J.; Dandekar, M.; Carrillo, J.A.; Kong, X.T.; Fu, B.D.; Hsu, F.P.; Schönthal, A.H.; Hofman, F.M.; Chen, T.C.; et al. Phase II study of ERC1671 plus bevacizumab versus bevaci-zumab plus placebo in recurrent glioblastoma: Interim results and correlations with CD4+ T-lymphocyte counts. CNS Oncol. 2018, 7, CNS22. [Google Scholar] [CrossRef] [PubMed]
- Bota, D.A.; Taylor, T.H.; Lomeli, N.; Kong, X.T.; Fu, B.D.; Schönthal, A.H.; Singer, S.; Blumenthal, D.T.; Senecal, F.M.; Linardou, H.; et al. A Prospective, Cohort Study of SITOIGANAP to Treat Glioblastoma When Given in Combination with Granulocyte-Macrophage Colony-Stimulating Fac-tor/Cyclophosphamide/Bevacizumab/Nivolumab or Granulocyte-Macrophage Colony-Stimulating ac-tor/Cyclophosphamide/Bevacizumab/Pembrolizumab in Patients Who Failed Prior Treatment With Surgical Resection, Radia-tion, and Temozolomide. Front. Oncol. 2022, 12, 934638. [Google Scholar] [CrossRef]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Iglesia, R.P.; Fernandes, C.F.L.; Coelho, B.P.; Prado, M.B.; Melo Escobar, M.I.; Almeida, G.H.D.R.; Lopes, M.H. Heat Shock Proteins in Glioblastoma Biology: Where Do We Stand? Int. J. Mol. Sci. 2019, 20, 5794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; O’Rourke, D.M.; Tran, D.D.; Fink, K.L.; Nabors, L.B.; Li, G.; Bota, D.A.; Lukas, R.V.; et al. Rindopepimut with Bevacizumab for Patients with Relapsed EGFRvIII-Expressing Glioblastoma (ReACT): Results of a Double-Blind Randomized Phase II Trial. Clin. Cancer Res. 2020, 26, 564–577. [Google Scholar] [CrossRef]
- Filley, A.C.; Henriquez, M.; Dey, M. Recurrent glioma clinical trial, CheckMate-143: The game is not over yet. Oncotarget 2017, 8, 49329–49345. [Google Scholar] [CrossRef]
- Alkayyal, A.A.; Mahmoud, A.B. A 5-Year Update on the Clinical Development of Cancer Cell-Based Vaccines for Glioblastoma Multiforme. Pharmaceuticals 2025, 18, 376. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, M.M.; Smita Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1410–1419. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.H.; Li, N. Immune evasion in cancer: Mechanisms and cutting-edge therapeutic approaches. Signal Transduct. Target. Ther. 2025, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Taal, W.; Oosterkamp, H.M.; Walenkamp, A.M.E.; Dubbink, H.J.; Beerepoot, L.V.; Hanse, M.C.J.; Buter, J.; Honkoop, A.H.; Boerman, D.; De Vos, F.Y.F.; et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled Phase II trial. Lancet Oncol. 2014, 15, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Hilf, N.; Kuttruff-Coqui, S.; Frenzel, K.; Bukur, V.; Stevanović, S.; Gouttefangeas, C.; Platten, M.; Tabatabai, G.; Dutoit, V.; van der Burg, S.H.; et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019, 565, 240–245. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef]
- Peña-Romero, A.C.; Orenes-Piñero, E. Dual Effect of Immune Cells within Tumour Microenvironment: Pro- and Anti-Tumour Effects and Their Triggers. Cancers 2022, 14, 1681. [Google Scholar] [CrossRef]
- Bota, D.A.; Alexandru-Abrams, D.; Pretto, C.; Hofman, F.M.; Chen, T.C.; Fu, B.; Carrillo, J.A.; Schijns, V.E.J.C.; Stathopoulos, A. Use of ERC-1671 Vaccine in a Patient with Recurrent Glioblastoma Multiforme after Progression during Bevacizumab Therapy: First Published Report. Perm. J. 2015, 19, 41–46. [Google Scholar] [CrossRef]
- Stathopoulos, A.; Pretto, C.; Devillers, L.; Pierre, D.; Hofman, F.M.; Kruse, C.; Jadus, M.; Chen, T.C.; Schijns, V.E.J.C. Develop-ment of immune memory to glial brain tumors after tumor regression induced by immunotherapeutic Toll-like receptor 7/8 activation. Oncoimmunology 2012, 1, 298–305. [Google Scholar] [CrossRef]
- Ravi, V.M.; Neidert, N.; Will, P.; Joseph, K.; Maier, J.P.; Kückelhaus, J.; Vollmer, L.; Goeldner, J.M.; Behringer, S.P.; Scherer, F.; et al. T-cell dysfunction in the glioblastoma microenvironment is mediated by myeloid cells releasing interleukin-10. Nat. Commun. 2022, 13, 925. [Google Scholar] [CrossRef] [PubMed]
- Green, G.B.H.; Cox-Holmes, A.N.; Potier, A.C.E.; Marlow, G.H.; McFarland, B.C. Modulation of the Immune Environment in Glioblastoma by the Gut Microbiota. Biomedicines 2024, 12, 2429. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Antonangeli, F.; Marrocco, F.; Porzia, A.; Lauro, C.; Santoni, A.; Limatola, C. Gut microbiota alterations affect glioma growth and innate immune cells involved in tumor immunosurveillance in mice. Eur. J. Immunol. 2020, 50, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Dees, K.J.; Koo, H.; Humphreys, J.F.; Hakim, J.A.; Crossman, D.K.; Crowley, M.R.; Nabors, L.B.; Benveniste, E.N.; Morrow, C.D.; McFarland, B.C. Human gut microbial communities dictate efficacy of anti-PD-1 therapy in a humanized microbiome mouse model of glioma. Neuro-Oncol. Adv. 2021, 3, vdab023. [Google Scholar] [CrossRef]
- Ruan, L.; Wang, L. Adoptive cell therapy against tumor immune evasion: Mechanisms, innovations, and future directions. Front. Oncol. 2025, 15, 1530541. [Google Scholar] [CrossRef]
- Pail, O.; Lin, M.J.; Anagnostou, T.; Brown, B.D.; Brody, J.D. Cancer vaccines and the future of immunotherapy. Lancet 2025, 406, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Hatami, M.; Ma, W.; Skutella, T. Vaccine-based immunotherapy and related preclinical models for glioma. Trends Mol. Med. 2024, 30, 965–981. [Google Scholar] [CrossRef]
- Schijns, V.E.J.C.; Pretto, C.; Strik, A.M.; Gloudemans-Rijkers, R.; Deviller, L.; Pierre, D.; Chung, J.; Dandekar, M.; Carrillo, J.A.; Kong, X.T.; et al. Therapeutic Immuniza-tion against Glioblastoma. Int. J. Mol. Sci. 2018, 19, 2540. [Google Scholar] [CrossRef]
- Kong, Z.; Wang, Y.; Ma, W. Vaccination in the immunotherapy of glioblastoma. Hum. Vaccines Immunother. 2017, 14, 255–268. [Google Scholar] [CrossRef]
- Parney, I.F.; Gustafson, M.P.; Solseth, M.; Bulur, P.; Peterson, T.E.; Smadbeck, J.B.; Johnson, S.H.; Murphy, S.J.; Vasmatzis, G.; Dietz, A.B. Novel strategy for manufacturing autologous dendritic cell/allogeneic tumor lysate vaccines for glioblastoma. Neu-Ro-Oncol. Adv. 2020, 2, vdaa105. [Google Scholar] [CrossRef]
- Schijns, V.E.J.C.; Tartour, E.; Michalek, J.; Stathopoulos, A.; Dobrovolskienė, N.T.; Strioga, M.M. Immune adjuvants as critical guides directing immunity triggered by therapeutic cancer vaccines. Cytotherapy 2014, 16, 427–439. [Google Scholar] [CrossRef]
- Dobrovolskienė, N.; Pašukonienė, V.; Darinskas, A.; Kraśko, J.A.; Žilionytė, K.; Mlynska, A.; Gudlevičienė, Ž.; Mišeikytė-Kaubrienė, E.; Schijns, V.E.J.C.; Lubitz, W.; et al. Tumor lysate-loaded Bacterial Ghosts as a tool for optimized production of therapeutic dendritic cell-based cancer vaccines. Vaccine 2018, 36, 4171–4180. [Google Scholar] [CrossRef]
- Gupta, R.; Emens, L.A. GM-CSF-Secreting Vaccines for Solid Tumors: Moving Forward. Discov. Med. 2010, 10, 52–60. [Google Scholar] [PubMed]
- Min, L.; Isa, S.A.B.M.; Shuai, W.; Shuai, W.; Boon Piang, C.; Wee Nih, F.; Kotaka, M.; Ruedl, C. Cutting edge: Granulocyte-macrophage colony-stimulating factor is the major CD8+ T cell-derived licensing factor for dendritic cell activation. J. Immunol. 2010, 184, 4625–4629. [Google Scholar] [CrossRef] [PubMed]
- Kortleve, D.; Coelho, R.M.L.; Hammerl, D.; Debets, R. Cancer germline antigens and tumor-agnostic CD8+ T cell evasion. Trends Immunol. 2022, 43, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.E.; Gleisner, A.; Falcon-Beas, F.; Osorio1, F.; Lopez, M.N.; Salazar-Onfray, F. Tumor cell lysates as immunogenic sources for cancer vaccine design. Hum. Vaccines Immunother. 2014, 10, 3261–3269. [Google Scholar] [CrossRef]
- Ogino, H.; Taylor, J.W.; Nejo, T.; Gibson, D.; Watchmaker, P.B.; Okada, K.; Saijo, A.; Tedesco, M.R.; Shai, A.; Wong, C.M.; et al. Randomized trial of neoadjuvant vaccination with tumor-cell lysate induces T cell response in low-grade gliomas. J. Clin. Investig. 2022, 132, e151239. [Google Scholar] [CrossRef]
- Preusser, M.; Van Den Bent, M.J. Autologous tumor lysate-loaded dendritic cell vaccination (DCVax-L) in glioblastoma: Breakthrough or fata morgana? Neuro-Oncology 2023, 25, 631–634. [Google Scholar] [CrossRef]
- Bota, D.A.; Piccioni, D.; Taylor, T.H.; LaRocca, R.V.; Aiken, R.D.; Kong, X.-T.; Lopez, K.L.; Keirstead, H.S.; Nistor, G.I.; Dill-man, R.O. Final results of phase 2 trial of personal dendritic cell (DC) vaccines loaded with autologous tumor antigens (ATA) in newly diagnosed glioblastoma (GBM). J. Clin. Oncol. 2023, 41, 2047. [Google Scholar] [CrossRef]
- Wang, D.R.; Wu, X.L.; Sun, Y.L. Therapeutic targets and biomarkers of tumor immunotherapy: Response versus non-response. Signal Transduct. Target. Ther. 2022, 7, 331. [Google Scholar] [CrossRef]
- Jiang, D.; Li, Y. Unraveling the immunosuppressive microenvironment of glioblastoma and advancements in treatment. Front. Immunol. 2025, 16, 1590781. [Google Scholar] [CrossRef]
- Yeo, E.C.F.; Brown, M.P.; Gargett, T.; Ebert, L.M. The Role of Cytokines and Chemokines in Shaping the Immune Microenvironment of Glioblastoma: Implications for Immunotherapy. Cells 2021, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Latzer, P.; Zelba, H.; Battke, F.; Reinhardt, A.; Shao, B.; Bartsch, O.; Rabsteyn, A.; Harter, J.; Schulze, M.; Okech, T.; et al. A real-world observation of patients with glioblastoma treated with a personalized peptide vaccine. Nat. Commun. 2024, 15, 6870. [Google Scholar] [CrossRef]
- Desbaillets, N.; Hottinger, A.F. Immunotherapy in Glioblastoma: A Clinical Perspective. Cancers 2021, 13, 3721. [Google Scholar] [CrossRef] [PubMed]
- Ismailov, A.; Spallone, A.; Belogurov, A., Jr.; Herbert, A.; Poptsova, M. Molecular biology of the deadliest cancer—Glioblastoma: What do we know? Front. Immunol. 2025, 16, 1530305. [Google Scholar] [CrossRef] [PubMed]
- Prins, R.M.; Soto, H.; Konkankit, V.; Odesa, S.K.; Eskin, A.; Yong, W.H.; Nelson, S.F.; Liau, L.M. Gene expression profile corre-lates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin. Cancer Res. 2011, 17, 1603–1615. [Google Scholar] [CrossRef]
- Oelke, M.; Maus, M.V.; Didiano, D.; June, C.H.; Mackensen, A.; Schneck, J.P. Ex vivo induction and expansion of anti-gen-specific cytotoxic T cells by HLA-Ig–coated artificial antigen-presenting cells. Nat. Med. 2003, 9, 619–624. [Google Scholar] [CrossRef]
- Van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8+ T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef]
- Mallet, M.; Boulos, R.E.; Alcazer, V.; Bonaventura, P.; Estornes, Y.; Chuvin, N.; Depil, S. Tumour burden and antigen-specific T cell magnitude represent major parameters for clinical response to cancer vaccine and TCR-engineered T cell therapy. Eur. J. Cancer 2022, 171, 96e105. [Google Scholar] [CrossRef]
- Halle, S.; Halle, O.; Förster, R. Mechanisms and Dynamics of T Cell-Mediated Cytotoxicity In Vivo. Trends Immunol. 2017, 38, 432–443. [Google Scholar] [CrossRef]
- Pang, L.; Khan, F.; Heimberger, A.B.; Chen, P. Mechanism and therapeutic potential of tumor-immune symbiosis in glioblastoma. Trends Cancer 2022, 8, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.; Schrama, D.; Straten, P.T.; Becker, J.C. Cytotoxic T Cells. J. Investig. Dermatol. 2006, 126, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Segura-Collar, B.; Cómitre-Mariano, B.; Alcivar López, D.; Modejar-Ruescas, L.; Caamaño-Moreno, M.; Tovar Ambel, E.; Gutierrez-Martin, J.; Garín, M.; Toldos, O.; Hernández-Laín, A.; et al. The TRIB2-DNMT1 pathway generates an immune cold microenvironment in glioblastoma and its inhibition promotes immunotherapy. Cancer Immunol. Res. 2025, 13, 1022–1036. [Google Scholar] [CrossRef]
- Kruse, B.; Buzzai, A.C.; Shridhar, N.; Braun, A.D.; Gellert, S.; Knauth, K.; Pozniak, J.; Peters, J.; Dittmann, P.; Mengoni, M.; et al. CD4+ T cell-induced inflammatory cell death controls immune-evasive tumours. Nature 2023, 618, 1033–1040. [Google Scholar] [CrossRef]
- Kloosterman, D.J.; Erbani, J.; Boon, M.; Farber, M.; Handgraaf, S.M.; Ando-Kuri, M.; Sanchez-Lopez, E.; Fontein, B.; Mertz, M.; Nieuwland, M.; et al. Macrophage-mediated myelin recycling fuels brain cancer malignancy. Cell 2024, 187, 5336–5356. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Cherry, C.; Bom, S.; Dykema, A.G.; Wang, R.; Thompson, E.; Zhang, M.; Li, R.; Ji, Z.; Hou, W.; et al. Distinct myeloid-derived suppressor cell populations in human glioblastoma. Science 2025, 387, abm5214. [Google Scholar] [CrossRef] [PubMed]
- Lutsiak, M.E.; Semnani, R.T.; De Pascalis, R.; Syed, V.S.; Kashmiri, J.; Schlom, J.; Sabzevari, H. Inhibition of CD4+25+ T regula-tory cell function implicated in enhanced immune response by low- dose cyclophosphamide. Blood 2005, 105, 2862–2868. [Google Scholar] [CrossRef]
- Sistigu, A.; Viaud, S.; Chaput, N.; Bracci, L.; Proietti, E.; Zitvogel, L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin. Immunopathol. 2011, 33, 369–383. [Google Scholar] [CrossRef]
- Olin, M.R.; Low, W.C.; McKenna, D.H.; Haines, S.J.; Dahlheimer, T.; Nascene, D.; Gustafson, M.P.; Dietz, A.B.; Clark, H.B.; Chen, W.; et al. Vaccination with dendritic cells loaded with allogeneic brain tumor cells for recur-rent malignant brain tumors induces a CD4+IL17+ response. J. Immunother. Cancer 2014, 2, 4. [Google Scholar] [CrossRef]
- Stathopoulos, A.; Pretto, C.; Devillers, L.; Pierre, D.; Hofman, F.M.; Epstein, A.L.; Farghadani, H.; Kruse, C.A.; Jadus, M.R.; Chen, T.C.; et al. Exploring the therapeutic efficacy of glioma vaccines based on allo- and syngeneic antigens and dis-tinct immunological costimulation activators. J. Clin. Cell. Immunol. 2012, S5, 004. [Google Scholar] [CrossRef]
- Schijns, V.E.J.C.; Pretto, C.; Devillers, L.; Pierre, D.; Hofman, F.M.; Chen, T.C.; Mespouille, P.; Hantos, P.; Glorieux, P.; Bota, D.A.; et al. First clinical results of a personalized immunotherapeutic vaccine against recurrent, incompletely resect-ed, treatment-resistant glioblastoma multiforme (GBM) tumors, based on combined allo- and auto-immune tumor reactivity. Vaccine 2015, 33, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, T.T.; Holle, L.M. Potential new gene therapy option with sitimagene ceradenovec for newly diagnosed patients with glioblastoma multiforme. Cancer Biol. Ther. 2014, 15, 263–265. [Google Scholar] [CrossRef]
- Zeng, R.; Spolski, R.; Finkelstein, S.E.; Oh, S.K.; Kovanen, P.E.; Hinrichs, C.S.; Pise-Masison, C.A.; Radonovich, M.F.; Brady, J.N.; Restifo, N.P.; et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 2005, 201, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Van Belzen, I.A.E.M.; Kesmir, C. Immune biomarkers for predicting response to adoptive cell transfer as cancer treatment. Immunogenetics 2019, 71, 71–86. [Google Scholar] [CrossRef]
- Suchin, E.J.; Langmuir, P.B.; Palmer, E.; Sayegh, M.H.; Wells, A.D.; Turk, L.A. Quantifying the Frequency of Alloreactive T Cells In Vivo: New Answers to an Old Question. J. Immunol. 2001, 166, 973–981. [Google Scholar] [CrossRef]
- Felix, N.J.; Allen, P.M. Specificity of T-cell alloreactivity. Nat. Rev. Immunol. 2007, 7, 942–953. [Google Scholar] [CrossRef]
- Mahajan, S.; Kortleve, D.; Debets, R.; Hammerl, D. Detection of Low-Frequency Epitope-Specific T Cells in Blood of Healthy Individuals according to an Optimized In Vitro Amplification System. J. Immunol. 2022, 209, 2239–2247. [Google Scholar] [CrossRef]
- De Boer, R.J.; Tesselaar, K.; Borghans, J.A.M. Better safe than sorry: Naive T-cell dynamics in healthy ageing. Semin. Immunol. 2023, 70, 101839. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic cancer vaccines: Advancements, challenges and prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, J.; Hustin, L.S.P.; De Boer, R.J.; Perié, L. Hematopoiesis in numbers. Trends Immunol. 2021, 42, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Kokolus, K.M.; Obermajer, N.; Kalinski, P. Quantitative evaluation of tumor-specific T cells in tumors and lymphoid tissues. Methods Enzymol. 2020, 635, 149–166. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P.; Yang, J.C.; Morgan, R.A.; Dudley, M.E. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 2008, 8, 299–308. [Google Scholar] [CrossRef]
- Jin, C.; Alia, A.; Iskantara, A.; Fotakia, G.; Wang, H.; Essanda, M.; Karlsson-Parrad, A.; Yu, D. Intratumoral administration of pro-inflammatory allogeneic dendritic cells improved the anti-tumor response of systemic anti-CTLA-4 treatment via unleash-ing a T cell-dependent response. OncoImmunology 2022, 11, e2099642. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.J.; Gartner, J.J.; Horovitz-Fried, M.; Shamalov, K.; Trebska-McGowan, K.; Bliskovsky, V.V.; Robbins, P.F. Isolation of neoan-tigen-specific T cells from tumor and peripheral lymphocytes. J. Clin. Investig. 2015, 125, 3981–3991. [Google Scholar] [CrossRef]
- Zhou, J.; Li, L.; Jia, M.; Liao, Q.; Peng, G.; Luo, G.; Zhou, Y. Dendritic cell vaccines improve the glioma microenvironment: Influence, challenges, and future directions. Cancer Med. 2023, 12, 7207–7221. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Z.; Xie, M.; Ding, F.; Zheng, X.; Sun, S.; Du, J. Exploring tumor-associated macrophages in glioblastoma: From diversity to therapy. Precis. Oncol. 2025, 9, 126. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Pellegatta, S. Immunotherapy with dendritic cells loaded with glioblastoma stem cells: From preclinical to clinical studies. Cancer Immunol. Immunother. 2016, 65, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Jeong, M.; Park, J.-H. Intratumoral adoptive transfer of inflammatory macrophages engineered by co-activating TLR and STING signaling pathways exhibits robust antitumor activity. Clin. Exp. Med. 2023, 23, 5025–5037. [Google Scholar] [CrossRef]
- Galluzzi, L.; Guilbaud, E.; Schmidt, D.; Kroemer, G.; Marincola, F.M. Targeting immunogenic cell stress and death for cancer therapy. Nat. Rev. Drug Discov. 2024, 23, 445–460. [Google Scholar] [CrossRef]
- Wang, H.; Medina, R.; Ye, J.; Zhang, Y.; Chakraborty, S.; Valenzuela, A.; Uher, O.; Hadrava Vanova, K.; Sun, M.; Sang, X.; et al. rWTC-MBTA Vaccine Induces Potent Adaptive Immune Responses Against Glioblastomas via Dynamic Activation of Dendritic. Cells Adv. Sci. 2024, 11, 2308280. [Google Scholar]
- Bastin, D.J.; Montroy, J.; Kennedy, M.A.; Martel, A.B.; Shorr, R.; Ghiasi, M.; Boucher, D.M.; Wong, B.; Gresham, L.; Diallo, J.S.; et al. Safety and efficacy of autologous cell vaccines in solid tumors: A systematic review and meta-analysis of randomized control trials. Sci. Rep. 2023, 13, 3347. [Google Scholar] [CrossRef]
- Wong, C.E.; Chang, Y.; Chen, P.W.; Huang, Y.T.; Chang, Y.C.; Chiang, C.H.; Wang, L.C.; Lee, P.H.; Huang, C.C.; Hsu, H.J.; et al. Dendritic cell vaccine for glioblastoma: An updated meta-analysis and trial sequential analysis. J. Neurooncol. 2024, 170, 253–263. [Google Scholar] [CrossRef]
- Datsi, A.; Sorg, R.V. Dendritic Cell Vaccination of Glioblastoma: Road to Success or Dead End. Front. Immunol. 2021, 12, 770390. [Google Scholar] [CrossRef] [PubMed]
- Fotaki, G.; Jin, C.; Ramachandran, M.; Kerzeli, I.K.; Karlsson-Parra, A.; Yu, D.; Essand, M. Pro-inflammatory allogeneic DCs promote activation of bystander immune cells and thereby license antigen-specific T-cell responses. Oncoimmunology 2018, 7, e1395126. [Google Scholar] [CrossRef] [PubMed]
- Gavil, N.V.; Cheng, K.; Masopust, D. Resident memory T cells and cancer. Immunity 2024, 57, 1734–1751. [Google Scholar] [CrossRef]
- Guo, M.; Hu, K.X.; Yu, C.L.; Sun, Q.Y.; Qiao, J.H.; Wang, D.H.; Liu, G.-X.; Sun, W.-J.; Wei, L.; Sun, X.-D.; et al. Infusion of HLA-mismatched peripheral blood stem cells improves the outcome of chemo-therapy for acute myeloid leukemia in elderly patients. Blood 2011, 17, 936–941. [Google Scholar] [CrossRef]
- Rubio, M.T.; Kim, Y.M.; Sachs, T.; Mapara, M.; Zhao, G.; Sykes, M. Antitumor effect of donor marrow graft rejection induced by recipient leukocyte infusions in mixed chimeras prepared with nonmyeloablative conditioning: Critical role for recipi-ent-derived IFN-gamma. Blood 2003, 102, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, A.; Ahn, B.Y.; D’Mello, C.; Lun, X.; Menon, S.V.; Alshehri, M.M.; Szulzewsky, F.; Shen, Y.; Khan, L.; Dang, N.H.; et al. Glioma-derived IL-33 orchestrates an inflammatory brain tumor microenvironment that accelerates glioma progression. Nat. Commun. 2020, 11, 4997. [Google Scholar] [CrossRef]
- Lin, H.; Liu, C.; Hu, A.; Zhang, D.; Yang, H.; Mao, Y. Understanding the immunosuppressive microenvironment of glioma: Mechanistic insights and clinical perspectives. J. Hematol. Oncol. 2024, 17, 31. [Google Scholar] [CrossRef]
- Tripathy, D.K.; Panda, L.P.; Biswal, S.; Barhwal, K. Insights into the glioblastoma tumor microenvironment: Current and emerging therapeutic approaches. Front. Pharmacol. 2024, 15, 1355242. [Google Scholar] [CrossRef]
- Losurdo, A.; Di Muzio, A.; Cianciotti, B.C.; Dipasquale, A.; Persico, P.; Barigazzi, C.; Bono, B.; Feno, S.; Pessina, F.; Santoro, A.; et al. T Cell Features in Glioblastoma May Guide Therapeutic Strategies to Overcome Microenvironment Immunosuppression. Cancers 2024, 16, 603. [Google Scholar] [CrossRef]
- Desland, F.A.; Hormigo, A. The CNS and the Brain Tumor Microenvironment: Implications for Glioblastoma Immunothera-py. Int. J. Mol. Sci. 2020, 21, 7358. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Os-trand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Hammerl, D.; Rieder, D.; Martens, J.W.M.; Trajanoski, Z.; Debets, R. Adoptive T Cell Therapy: New Avenues Leading to Safe Targets and Powerful Allies. Trends Immunol. 2018, 39, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Agnihotri, S.; Guha, A. Targeting metabolic remodeling in glioblastoma multiforme. Oncotarget 2010, 1, 552–562. [Google Scholar] [CrossRef]
- Shen, X.; Cheng, H.; Xia, Y.; Zheng, J.; Peng, Q.; Zhang, Z.; Yin, N.; Liu, Y.; Dong, J.; Shen, Y. KIF4A Promotes Glioblastoma Malignant Progression and Transmission of Temozolomide Resistance in the Tumor Microenvironment via the HIF1A/VEGFA Axis. CNS Neurosci. Ther. 2025, 31, e70550. [Google Scholar] [CrossRef]
- Prajapati, S.; Yadav, S. Revolutionizing Glioblastoma Immunotherapy Conquering Transport and Biological Challenges, Innovating Combinatorial Approaches for Unprecedented Treatment Success. Clin. Cancer Drugs 2024, 10, E2212697X332800. [Google Scholar] [CrossRef]
- Agosti, E.; Zeppieri, M.; De Maria, L.; Tedeschi, C.; Fontanella, M.M.; Panciani, P.P.; Ius, T. Glioblastoma Immunotherapy: A Systematic Review of the Present Strategies and Prospects for Advancements. Int. J. Mol. Sci. 2023, 24, 15037. [Google Scholar] [CrossRef] [PubMed]
- Gillette, J.S.; Wang, E.J.; Dowd, R.S.; Toms, S.A. Barriers to overcoming immunotherapy resistance in glioblastoma. Front. Med. 2023, 10, 1175507. [Google Scholar] [CrossRef]
- Pérez-Baños, A.; Gleisner, M.A.; Flores, I.; Pereda, C.; Navarrete, M.; Araya, J.P.; Navarro, G.; Quezada-Monrás, C.; Tittarelli, A.; Salazar-Onfray, F. Whole tumour cell-based vaccines: Tuning the instruments to orchestrate an optimal antitumour immune response. Br. J. Cancer 2023, 129, 572. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Stockhammer, F.; Schmitt, M. Cellular-Based Immunotherapies for Patients with Glioblastoma Multiforme. Clin. Dev. Immunol. 2012, 2012, 764213. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Ozpinar, A.; Hacker, E.; Ozpinar, A. Combining locoregional CAR-T cells, autologous + allogeneic tumor ly-sate vaccination and levamisole in treatment of glioblastoma. Immunopharmacol. Immunotoxicol. 2022, 44, 797–808. [Google Scholar] [CrossRef]
- Sheykhhasan, M.; Ahmadieh-Yazdi, A.; Heidari, R.; Chamanara, M.; Akbari, M.; Poondla, N.; Yang, P.; Malih, S.; Manoochehri, H.; Tazadehpanah, H.; et al. Revolutionizing cancer treatment: The power of dendritic cell-based vaccines in immunotherapy. Biomed. Pharmacother. 2025, 184, 117858. [Google Scholar] [CrossRef]
- Tabatabai, G.; Platten, M.; Preusser, M.; Weller, M.; Wick, W.; Van den Bent, M. Treatment of glioblastoma patients with per-sonalized vaccines outside clinical trials: Lessons ignored? Neuro-Oncology 2025, 27, 302–305. [Google Scholar] [CrossRef]
- Mahajan, S.; Schmidt, M.H.H.; Schumann, U. The Glioma Immune Landscape: A Double-Edged Sword for Treatment Regimens. Cancers 2023, 15, 2024. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, M.F.; Bradshaw, T.D. Temozolomide: Mechanisms of Action, Repair and Resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef]
- Li, H.; Wu, Y.; Chen, Y.; Lv, J.; Qu, C.; Mei, T.; Zheng, Y.; Ye, C.; Li, F.; Ge, S.; et al. Overcoming temozolomide resistance in glioma: Recent advances and mechanistic insights. Acta Neuropathol. Commun. 2025, 13, 126. [Google Scholar] [CrossRef]
- Lin, K.; Zou, C.; Hubbard, A.; Sengelmann, S.; Goudy, L.; Wang, I.-C.; Sharma, R.; Pak, J.; Foster, K.; Ozawa, T.; et al. Multi-plexed epigenetic memory editing using CRISPRoff sensitizes glioblastoma to chemotherapy. Neuro-Oncology 2025, 27, 1443–1457. [Google Scholar] [CrossRef] [PubMed]
- Abd-Aziz, N.; Laa Poh, C. Development of Peptide-Based Vaccines for Cancer. J. Oncol. 2022, 2022, 9749363. [Google Scholar] [CrossRef]
- Sotirov, S.; Dimitrov, I. Tumor-Derived Antigenic Peptides as Potential Cancer Vaccines. Int. J. Mol. Sci. 2024, 25, 4934. [Google Scholar] [CrossRef]
- Cachot, A.; Bilous, M.; Liu, Y.-C.; Li, X.; Saillard, M.; Cenerenti, M.; Rockinger, G.A.; Wyss, T.; Guillaume, P.; Schmidt, J.; et al. Tumor-specific cytolytic CD4 T cells mediate immunity against human cancer. Sci. Adv. 2021, 7, eabe3348. [Google Scholar] [CrossRef] [PubMed]
- Falkenburg, J.H.F.; Jedema, I. Allo-reactive T cells for the treatment of hematological malignancies. Mol. Oncol. 2015, 9, 1894–1903. [Google Scholar] [CrossRef]
- Dunn, G.P.; Sherpa, N.; Manyanga, J.; Johanns, T.M. Considerations for personalized neoantigen vaccination in Malignant glioma. Adv. Drug Deliv. Rev. 2022, 186, 114312. [Google Scholar] [CrossRef] [PubMed]
- Johanns, T.M.; Miller, C.A.; Liu, C.J.; Perrin, R.J.; Bender, D.; Kobayashi, D.K.; Campian, J.L.; Chicoin, M.R.; Dacey, R.G.; Huang, J.; et al. Detection of neoantigen-specific T cells following a personalized vaccine in a patient with glioblastoma. Onco-Immunology 2019, 8, e1561106. [Google Scholar] [CrossRef]
- Kim, J.M.; Potez, M.; She, C.; Huang, P.; Wu, Q.; Bao, S.; Rich, J.N.; Liu, J.K.C. Glioblastoma Stem Cell Targeting Peptide Iso-lated Through Phage Display Binds Cadherin 2. Stem Cells 2023, 41, 762–774. [Google Scholar] [CrossRef]
- Schijns, V.E.J.C.; Bota, D.A.; Stathopoulos, A. A Personalized Immunotherapeutic Vaccine (Gliovac® Or ERC1671) Against Recurrent Glioblastoma Multiforme (GBM). Brain Disord. Ther. 2015, S2, 006. [Google Scholar] [CrossRef]
- Lin, Q.; Wei, Y.; Xu, G.; Wang, L.; Ling, F.; Chen, X.; Cheng, Y.; Zhou, Y. Integrative multi-omic profiling of the neoantigen landscape of glioblastoma for the development of therapeutic vaccines reveals vast heterogeneity in immunogenic signatures. Front. Oncol. 2025, 15, 1507632. [Google Scholar] [CrossRef]
- Kinoshita, H.; Takenouchi, K.; Tsukamoto, N.; Ohnuki, K.; Suzuki, T.; Nakatsura, T. Identification of 68 HLA-A24 and -A2-restricted cytotoxic T lymphocyte-inducing peptides derived from 10 common cancer-specific antigens frequently expressed in various solid cancers. Neoplasia 2025, 61, 101135. [Google Scholar] [CrossRef]
- Wang, C.; Yu, M.; Zhang, W. Neoantigen discovery and applications in glioblastoma: An immunotherapy perspective. Cancer Lett. 2022, 550, 215945. [Google Scholar] [CrossRef]
- Raucher, D. Tumor targeting peptides: Novel therapeutic strategies in glioblastoma. Curr. Opin. Pharmacol. 2019, 47, 14–19. [Google Scholar] [CrossRef]
- Ayo, A.; Laakkonen, P. Peptide-Based Strategies for Targeted Tumor Treatment and Imaging. Pharmaceutics 2021, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Srimanee, A.; Arvanitidou, M.; Kim, K.; Hällbrink, M.; Lange, U. Cell-penetrating peptides for siRNA delivery to glioblas-tomas. Peptides 2018, 104, 62–69. [Google Scholar] [CrossRef]
- Ter Linden, E.; Abels, E.R.; van Solinge, T.S.; Neefjes, J.; Broekman, M.L.D. Overcoming Barriers in Glioblastoma—Advances in Drug Delivery Strategies. Cells 2024, 13, 998. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.; Mashal, M.; Pemminati, S.; Omole, A.; Edmondson, C.; Jones, W.; Priyadarshini, P.; Mughal, T.; Aziz, P.; Zenick, B.; et al. Cell-Based Therapy for the Treatment of Glioblastoma: An Update from Preclinical to Clinical Studies. Cells 2022, 11, 116. [Google Scholar] [CrossRef]
- Yang, M.; Oh, I.Y.; Mahanty, A.; Jin, W.-L.; Yoo, J.S. Immunotherapy for Glioblastoma: Current State, Challenges, and Future Perspectives. Cancers 2020, 12, 2334. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Q.; Hu, A.; Chen, X.; Liu, J.; Lin, J.; Xie, Y. Advances in ultrasound-mediated brain drug delivery. J. Pharm. Pharmacol. 2025, rgaf090. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Peng, Y.; Ma, W.; Wang, Y.; Li, W. Progress in phase III clinical trials of molecular targeted therapy and immunotherapy for glioblastoma. Cancer Innov. 2023, 2, 114–130. [Google Scholar] [CrossRef]
- Bloch, O.; Crane, C.A.; Fuks, Y.; Kaur, R.; Aghi, M.K.; Mitchel, S.; Berger, M.S.; Butowski, N.A.; Chang, S.M.; Clarke, J.L.; et al. Heat-shock protein peptide complex–96 vaccination for recurrent glioblastoma: A phase II, single-arm trial. Neuro-Oncology 2014, 16, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Ashley, D.M.; Ayasoufi, K.; Norberg, P.; Archer, G.; Buckley, E.D. A peptide vaccine targeting the CMV anti-gen pp65 in children and young adults with recurrent high-grade glioma and medulloblastoma: A phase 1 trial. Nat. Cancer 2025, 6, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Batich, K.A.; Reap, E.A.; Archer, G.E.; Sanchez-Perez, L.; Nair, S.K.; Schmittling, R.J.; Norberg, P.; Xie, W.; Herndon, J.E.; Healy, P.; et al. Long-term Survival in Glioblastoma with Cytomegalo-virus pp65-Targeted Vaccination. Clin. Cancer Res. 2017, 23, 1898–1909. [Google Scholar] [CrossRef] [PubMed]
- Karantalis, V.; Hernandez Schulman, I.; Balkan, W.; Hare, J.M. Allogeneic Cell Therapy: A New Paradigm in Therapeutics. Circ Res. 2015, 116, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.W.; Maus, M.V.; Mackall, C.L. The Emerging Landscape of Immune Cell Therapies. Cell 2020, 181, 46–62. [Google Scholar] [CrossRef]
- D’Avanzo, C.; Blaeschke, F.; Lysandrou, M.; Ingelfinger, F.; Zeiser, R. Advances in cell therapy: Progress and challenges in hematological and solid tumors. Trends Pharmacol. Sci. 2024, 45, 1119–1134. [Google Scholar] [CrossRef]
- Wen, P.Y.; Reardon, D.A.; Armstrong, T.S.; Phuphanich, S.; Aiken, R.D.; Landolfi, J.C.; Curry, W.T.; Zhu, J.-J.; Glantz, M.; Peereboom, D.M.; et al. A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients with Glioblastoma. Clin. Cancer Res. 2019, 25, 5799–5807. [Google Scholar] [CrossRef]
- Qian, D.; Liu, Y.; Zheng, J.; Cai, J. Dendritic cell therapy for neurospoagioma: Immunomodulation mediated by tumor vaccine. Cell Death Discov. 2024, 10, 11. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, X.; Feng, S.; Zhu, H.; Chen, X.; Yu, X.; Shu, K.; Zhang, S. Dendritic cell vaccine of gliomas: Challenges from bench to bed. Front. Immunol. 2023, 14, 1259562. [Google Scholar] [CrossRef] [PubMed]
- Bowman-Kirigin, J.A.; Desai, R.; Saunders, B.T.; Wang, A.Z.; Schaettler, M.O.; Liu, C.J.; Livingstone, A.J.; Kobayashi, D.K.; Durai, V.; Kretzer, N.M. The Conventional Dendritic Cell 1 Subset Primes CD8+ T Cells and Traffics Tumor Antigen to Drive Antitumor Immunity in the Brain. Cancer Immunol. Res. 2023, 11, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Cerottini, J.-C.; Waanders, G.A. Novel methods to monitor antigen-specific cytotoxic T-cell responses in cancer immunotherapy. Mol. Med. Today 1998, 4, 305–312. [Google Scholar] [CrossRef] [PubMed]
- DeWolf, S.; Grinshpun, B.; Savage, T.; Lau, S.P.; Obradovic, A.; Shonts, B.; Yang, S.; Morris, H.; Zuber, J.; Winchester, R.; et al. Quantifying size and diversity of the human T cell alloresponse. JCI Insight 2018, 3, e121256. [Google Scholar] [CrossRef]
- Del Monte, U. Does the cell number 109 still really fit one gram of tumor tissue? Cell Cycle 2009, 8, 505–506. [Google Scholar] [CrossRef]
- Cozzi, S.; Najafi, M.; Gomar, M.; Ciammella, P.; Iotti, C.; Iaccarino, C.; Dominici, M.; Pavesi, G.; Chiavelli, C.; Kazemian, A.; et al. Delayed Effect of Dendritic Cells Vaccination on Survival in Glioblastoma: A Systematic Review and Meta-Analysis. Curr. Oncol. 2022, 29, 881–891. [Google Scholar] [CrossRef]
- Han, J.; Zhang, B.; Senyu Zheng, S.; Jiang, Y.; Zhang, X.; Mao, K. The Progress and Prospects of Immune Cell Therapy for the Treatment of Cancer. Cell Transplant. 2024, 33, 09636897241231892. [Google Scholar] [CrossRef]
- Tiwari, S.; Han, Z. Immunotherapy: Advancing glioblastoma treatment—A narrative review of scientific studies. Cancer Rep. 2024, 7, e1947. [Google Scholar] [CrossRef]
- Jha, R.; Spanehl, L.; Chen, J.A.; Gessler, F.A.; Arnaout, O.; Valdes, P.A.; Choi, B.D.; Peruzzi, P.P.; Bernstock, J.D.; Chiocca, E.A. Translational advancements in tumor vaccine therapies for glioblastomas. Neuro-Oncol. Adv. 2025, 7 (Suppl. 4), iv72–iv83. [Google Scholar] [CrossRef]
- Fadul, C.E.; Fisher, J.L.; Hampton, T.H.; Lallana, E.L.; Li, Z.; Gui, J.; Szczepiorkowski, Z.M.; Tosteson, T.D.; Rhodes, C.H.; Wishart, H.A.; et al. Immune Response in Patients With Newly Diagnosed Glioblastoma Multiforme Treat-ed With Intranodal Autologous Tumor Lysate-dendritic Cell Vaccination After Radiation Chemotherapy. J. Immunother. 2011, 34, 382–389. [Google Scholar] [CrossRef]
- Shah, S.; Nag, A.; Lucke-Wold, B. Autologous tumor lysate-loaded dendritic cell vaccination in glioblastoma patients: A sys-tematic review of literature. Clin. Transl. Oncol. 2025, 27, 2889–2903. [Google Scholar] [CrossRef] [PubMed]
- Seker-Polat, F.; Pinarbasi Degirmenci, N.; Solaroglu, I.; Bagci-Onder, T. Tumor Cell Infiltration into the Brain in Glioblasto-ma: From Mechanisms to Clinical Perspectives. Cancers 2022, 14, 443. [Google Scholar] [CrossRef]
- Das, S.; Sunder Dash, B.; Chen, J.-P. Immunotherapeutic Approaches for the Treatment of Glioblastoma Multiforme: Mecha-nism and Clinical Applications. Int. J. Mol. Sci. 2023, 24, 10546. [Google Scholar] [CrossRef]
- Nava, S.; Lisini, D.; Pogliani, S.; Dossena, M.; Bersano, A.; Pellegatta, S.; Parati, E.; Finocchiaro, G.; Frigerio, S. Safe and Re-producible Preparation of Functional Dendritic Cells for Immunotherapy in Glioblastoma Patients. Stem Cell Transl. Med. 2015, 4, 1164–1172. [Google Scholar] [CrossRef]
- Deuse, T.; Schrepfer, S. Progress and challenges in developing allogeneic cell therapies. Cell Stem Cell 2025, 32, 513–528. [Google Scholar] [CrossRef]
- Hofman, F.M.; Stathopoulos, A.; Kruse, C.A.; Chen, T.C.; Schijns, V.E.J.C. Immunotherapy of malignant gliomas using autolo-gous and allogeneic tissue cells. Anticancer Agents Med. Chem. 2010, 10, 462–470. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Lv, Y.; Bao, W.; Meng, Z.; Wang, S.; Wu, Y.; Li, S.; Jiao, Z.; Tian, Z.; Ma, G.; et al. Generation of whole tumor cell vaccine for on-demand manipulation of immune responses against cancer under near-infrared laser irradiation. Nat. Commun. 2023, 14, 4505. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Yang, Y.; Swift, M.; Zhang, Z.; Wu, S.; Sun, Y.; Yang, K. In situ vaccination caused by diverse irradiation-driven cell death programs. Theranostics 2024, 14, 1147–1167. [Google Scholar] [CrossRef]
- Schrand, B.; Clark, E.; Levay, A.; Capote, A.R.; Martinez, O.; Brenneman, R.; Castro, I.; Gilboa, E. Hapten-mediated recruitment of polyclonal antibodies to tumors engenders antitumor immunity. Nat. Commun. 2018, 9, 3348. [Google Scholar] [CrossRef]
- Awuah, W.; Shah, M.H.; Tan, J.K.; Ranganathan, S.; Sanker, V.; Darko, K.; Tenkorang, P.O.; Adageba, B.B.; Ahluwalia, A.; Shet, V.; et al. Immunotherapeutic advances in glioma management: The rise of vaccine-based ap-proaches. CNS Neurosci. Ther. 2024, 30, e70013. [Google Scholar] [CrossRef]
- Pour, M.E.; Moghadam, S.G.; Shirkhani, P.; Sahebkar, A.; Mosaffa, F. Therapeutic cell-based vaccines for glioblastoma mul-tiforme. Med. Oncol. 2023, 40, 354. [Google Scholar] [CrossRef]
- Ijaz, M.; Ullah, Z.; Aslam, B.; Khurshid, M.; Chen, P.; Guo, B. From promise to progress: The dynamic landscape of glioblastoma immunotherapy. Drug Discov. Today 2024, 29, 104188. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.F.; Ottaviani, D.; Tazare, J.; Gregson, J.; Kitchen, N.; Brandner, S.; Fersht, N.; Mulholland, P. Survival Outcomes and Prognostic Factors in Glioblastoma. Cancers 2022, 14, 3161. [Google Scholar] [CrossRef] [PubMed]
- Salvato, I.; Marchini, A. Immunotherapeutic Strategies for the Treatment of Glioblastoma: Current Challenges and Future Perspectives. Cancers 2024, 16, 1276. [Google Scholar] [CrossRef]
- Yu, M.W.; Quail, D.F. Immunotherapy for Glioblastoma: Current Progress and Challenge. Front. Immunol. 2021, 12, 676301. [Google Scholar] [CrossRef]
- Zhang, M.; Choi, J.K.; Lim, M. Advances in Immunotherapies for Gliomas. Curr. Neurol. Neurosci. Rep. 2022, 22, 1–10. [Google Scholar] [CrossRef]
- Takahashi, M.; Mukhamejanova, D.; Jasewicz, H.; Acharya, N.; Moon, J.J.; Hara, T. Opportunities to Modulate Tumor Ecosys-tem Toward Successful Glioblastoma Immunotherapy. Cancer Sci. 2025, 116, 1482–1499. [Google Scholar] [CrossRef]
- Gephart, B.D.; Coulter, D.W.; Solheim, J.C. Effects of the Alkylating Agent Cyclophospha-mide in Potentiating Anti-Tumor Immunity. Int. J. Mol. Sci. 2025, 26, 6440. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Kiesel, B.; Widhalm, G.; Rajky, O.; Ricken, G.; Wohrer, A.; Dieckmann, K.; Filipits, M.; Brandstetter, A.; Weller, M. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro-Oncology 2015, 17, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Maghrouni, A.; Givari, M.; Jalili-Nik, M.; Mollazadeh, H.; Bibak, B.; Montazami Sadeghi, M.; Afshari, A.R.; Johnston, T.P.; Saheb-kar, A. Targeting the PD-1/PD-L1 pathway in glioblastoma multiforme: Preclinical evidence and clinical interventions. Int. Immunopharmacol. 2021, 93, 107403. [Google Scholar] [CrossRef] [PubMed]
- Rustemeyer, T.; De Ligter, S.; Von Blomberg, B.M.E.; Frosch, P.J.; Sscheper, R.J. Human T lymphocyte priming in vitro by hap-tenated autologous dendritic cells. Clin. Exp. Immunol. 1999, 117, 209–216. [Google Scholar] [CrossRef]
- Kan, L.K.; Drummond, K.; Hunn, M.; Williams, D.; O'Brien, T.J.; Monif, M. Potential biomarkers and challenges in glioma diagnosis, therapy and prognosis. BMJ Neurol. Open 2020, 2, e000069. [Google Scholar] [CrossRef]
- Sareen, H.; Ma, Y.; Becker, T.M.; Roberts, T.L.; de Souza, P.; Powter, B. Molecular Biomarkers in Glioblastoma: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 8835. [Google Scholar] [CrossRef]
- Ahsan, H.; Asghar, M.; Malik, S.I. Potential diagnostic and drug target markers in glioblastoma. Sci. Rep. 2024, 14, 7292. [Google Scholar] [CrossRef]
- Peng, K.; Zhao, X.; Fu, Y.X.; Liang, Y. Eliciting antitumor immunity via therapeutic cancer vaccines. Cell Mol. Immunol. 2025, 22, 840–868. [Google Scholar] [CrossRef]
- Mensali, N.; Inderberg, E.M. Emerging Biomarkers for Immunotherapy in Glioblastoma. Cancers 2022, 14, 1940. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Karbhari, N.; Campian, J.L. Therapeutic Targets in Glioblastoma: Molecular Pathways, Emerging Strategies, and Future Directions. Cells 2025, 14, 494. [Google Scholar] [CrossRef] [PubMed]
- Savage, W.M.; Yeary, M.D.; Tang, A.J.; Sperring, C.P.; Argenziano, M.G.; Adapa, A.R.; Yoh, N.; Canoll, P.; Bruce, J.N. Bi-omarkers of immunotherapy in glioblastoma. Neuro-Oncology Pract. 2024, 11, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Squalli Houssaini, A.; Lamrabet, S.; Nshizirungu, J.P.; Senhaji, N.; Sekal, M.; Karkouri, M.; Bennis, S. Glioblastoma Vaccines as Promising Immune-Therapeutics: Challenges and Current Status. Vaccines 2024, 12, 655. [Google Scholar] [CrossRef]
- Pellegatta, S.; Poliani, P.L.; Stucchi, E.; Corno, D.; Colombo, C.A.; Orzan, F.; Ravanini, M.; Finocchiaro, G. Intra-tumoral dendritic cells increase efficacy of peripheral vaccination by modulation of glioma microenvironment. Neuro-Oncology 2010, 12, 377–388. [Google Scholar] [CrossRef]
- Gilard, V.; Tebani, A.; Dabaj, I.; Laquerrière, A.; Fontanilles, M.; Derrey, S.; Marret, S.; Bekri, S. Diagnosis and Management of Glioblastoma: A Comprehensive. Perspective. J. Pers. Med. 2021, 11, 258. [Google Scholar] [CrossRef]
| DCvax-L | Sitoiganap | |
|---|---|---|
| Donor | Mono-donor | Multi-donor |
| Monocyte-derived DC ex vivo | In vivo DC subsets | |
| Vaccine basis | Autologous tumor lysate | Allo/auto mixture of irradiated whole tumor cells plus tumor lysates |
| Adjuvants | Treg inhibitor (low-dose cyclophosphamide) + GM-CSF + VEGF blockade (bevacizumab) + PD-1 blockade (pembrolizumab) | |
| TMZ addition | +TMZ | +TMZ |
| Study phase | Phase III | Phase II |
| Newly diagnosed GBM survival | Median OS: 19.3 vs. 16.5 months | Median OS: 12 vs. 7.5 months |
| Recurrent GBM survival | Median OS: 13.2 vs. 7.8 months | Median OS: 19.6 vs. 7.5 months |
| Trials | Phase III | Phase II |
| References | [23,158] | [24,25,60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stathopoulos, A.; Glorieux, P.; Rokas, E.M.; Savelkoul, H.F.J. Combined Tumor Cell and Lysate-Based Vaccines for Immunotherapy of Primary and Recurrent Glioblastoma (GBM). Cancers 2025, 17, 3772. https://doi.org/10.3390/cancers17233772
Stathopoulos A, Glorieux P, Rokas EM, Savelkoul HFJ. Combined Tumor Cell and Lysate-Based Vaccines for Immunotherapy of Primary and Recurrent Glioblastoma (GBM). Cancers. 2025; 17(23):3772. https://doi.org/10.3390/cancers17233772
Chicago/Turabian StyleStathopoulos, Apostolos, Philippe Glorieux, Evangelos M. Rokas, and Huub F. J. Savelkoul. 2025. "Combined Tumor Cell and Lysate-Based Vaccines for Immunotherapy of Primary and Recurrent Glioblastoma (GBM)" Cancers 17, no. 23: 3772. https://doi.org/10.3390/cancers17233772
APA StyleStathopoulos, A., Glorieux, P., Rokas, E. M., & Savelkoul, H. F. J. (2025). Combined Tumor Cell and Lysate-Based Vaccines for Immunotherapy of Primary and Recurrent Glioblastoma (GBM). Cancers, 17(23), 3772. https://doi.org/10.3390/cancers17233772






