CLDN18.2-Targeted Therapy in Gastrointestinal Cancers
Simple Summary
Abstract
1. Introduction
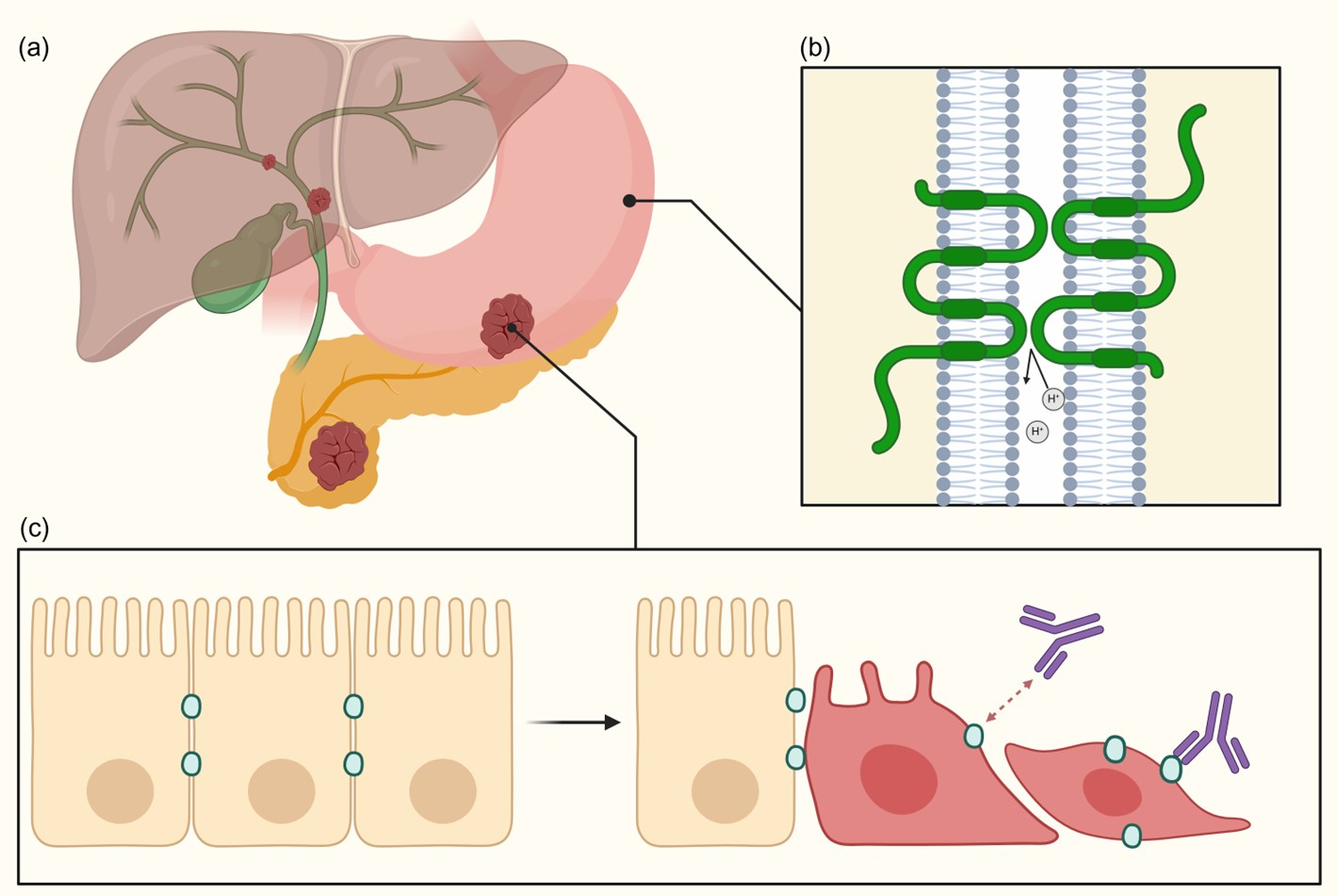
2. Background on CLDN18.2
2.1. CLDN18.2 Structure and Function
2.2. CLDN18.2 Expression and Role in Gastrointestinal Tumors
2.2.1. CLDN18.2 Expression in Gastric and Gastroesophageal Junction Cancer
2.2.2. CLDN18.2 Expression in Pancreatic Cancer
2.2.3. CLDN18.2 Expression in Biliary Tract Cancer
3. Testing CLDN18.2 Expression and Tumoral Heterogeneity
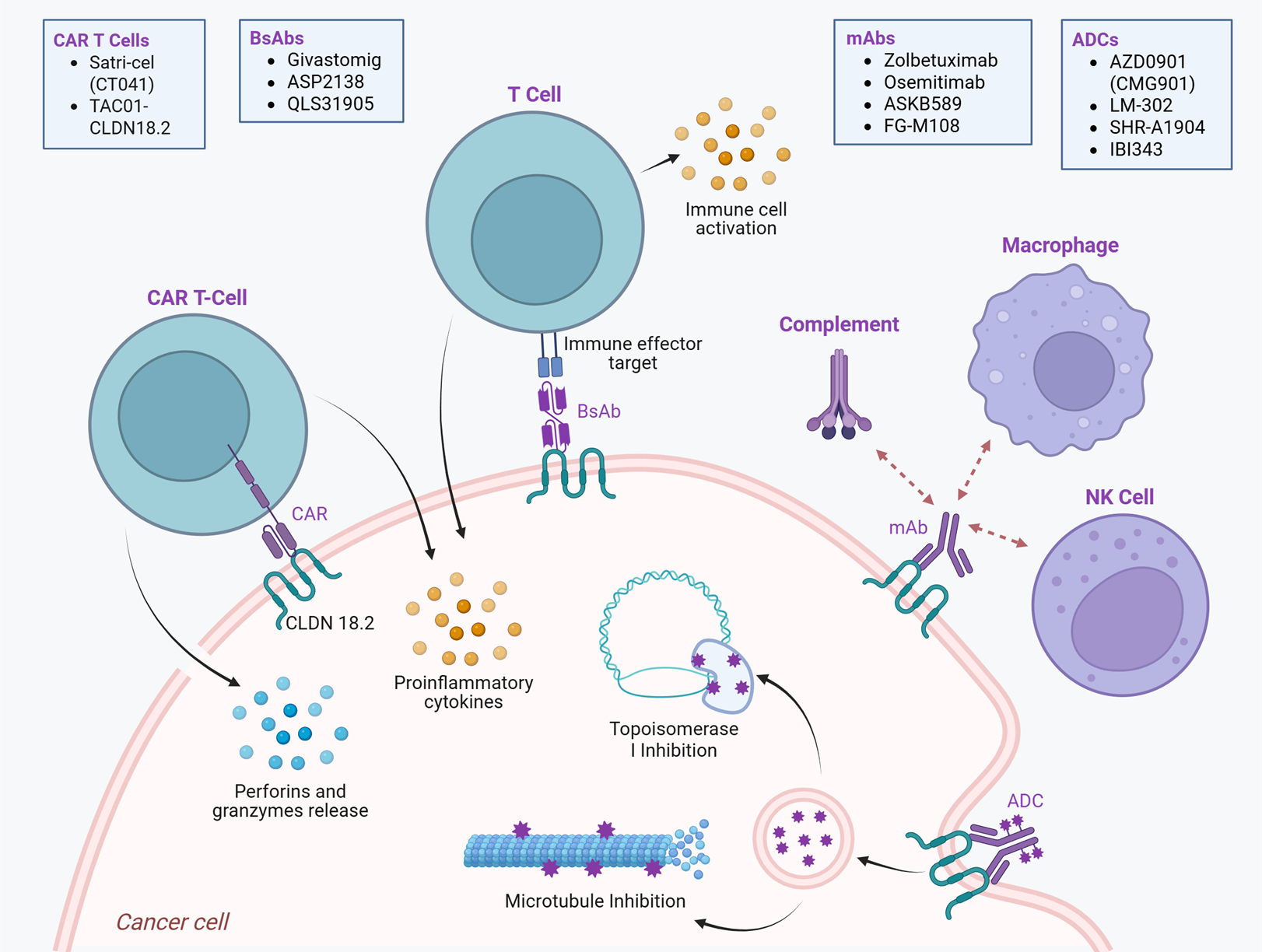
4. CLDN18.2-Targeted Therapy
4.1. Monoclonal Antibodies (mAbs)
4.1.1. Zolbetuximab (IMAB362/ClaudiXimab/Vyloy)
4.1.2. Osemitamab (TST001)
4.1.3. ASKB589
4.1.4. FG-M108 (M108)
4.2. Antibody–Drug Conjugates (ADCs)
4.2.1. AZD0901 (CMG901)
4.2.2. LM-302 (TPX4589/Tecotabart Vedotin)
4.2.3. SHR-A1904 and IBI343
4.3. Bispecific Antibodies (BsAbs)
4.3.1. Givastomig (TJ033721/ABL111)
4.3.2. ASP2138
4.3.3. QLS31905
4.4. CAR T-Cell Therapy
4.4.1. CT041/Satri-cel
4.4.2. TAC01-CLDN18.2
5. Co-Expression Patterns and Combination Therapies in Gastric/Gastroesophageal Junction Cancer
5.1. CLDN18.2 and PD-L1
5.2. CLDN18.2 and HER2
6. Possible Resistance Mechanisms to Anti-CLDN18.2 Therapy
6.1. Downregulation of CLDN18.2 Expression
6.2. Tumor Microenvironment (TME) Modulation
6.3. Poor Drug Delivery and Tumor Infiltration
7. Conclusions
Future Directions
- (1)
- How to integrate CLDN18.2 targeted therapies with ICIs in GC/GEJC, which have become standard of care. This is important given the observed co-expression of CLDN18.2 and PD-L1 in subsets of patients [94]. Prospective clinical trials evaluating the safety and efficacy of combination regimens are needed to guide sequencing and optimize outcomes.
- (2)
- Identifying which levels of CLDN18.2 expression stand to benefit most from different targeting strategies. While zolbetuximab requires high expression (≥75% of tumor cells with 2+/3+ membranous staining), other agents may prove effective at intermediate or even low expression levels, as suggested with agents such as AZD901 and LM-302. This opens the door to tailoring therapeutic strategies based on expression intensity, potentially expanding eligibility to broader patient populations. Standardization of CLDN18.2 testing will be essential to identify appropriate candidates for treatment.
- (3)
- Developing novel approaches such as ADCs and BsAbs targeting CLDN18.2 to overcome resistance. Enhanced efficacy through payload delivery or dual-targeting strategies may overcome mechanisms of resistance including antigen loss, tumor heterogeneity, and immune evasion [39].
- (4)
- Expanding the application of CLDN18.2-targeted therapies to other GI malignancies, including PC and BTCs. Approximately one-third of PCs and a smaller subset of GBCs and eCCAs have been shown to express CLDN18.2 at therapeutically actionable levels [34,35,36]. This review has highlighted ongoing and emerging clinical trials that evaluate the efficacy of CLDN18.2-targeted agents in these cancers, which historically lack effective targeted treatments. The adoption of standardized testing across tumor types, accounting for intratumoral heterogeneity and primary–metastatic discordance, will be essential to identify potential patients eligible for clinical trials targeting CLDN18.2.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA A Cancer J Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.-Y.; Zhu, A.X. Biliary Tract Cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Sahin, U.; Koslowski, M.; Dhaene, K.; Usener, D.; Brandenburg, G.; Seitz, G.; Huber, C.; Türeci, Ö. Claudin-18 Splice Variant 2 Is a Pan-Cancer Target Suitable for Therapeutic Antibody Development. Clin. Cancer Res. 2008, 14, 7624–7634. [Google Scholar] [CrossRef]
- Kyuno, D.; Takasawa, A.; Takasawa, K.; Ono, Y.; Aoyama, T.; Magara, K.; Nakamori, Y.; Takemasa, I.; Osanai, M. Claudin-18.2 as a Therapeutic Target in Cancers: Cumulative Findings from Basic Research and Clinical Trials. Tissue Barriers 2022, 10, 1967080. [Google Scholar] [CrossRef]
- Shinozaki, A.; Shibahara, J.; Noda, N.; Tanaka, M.; Aoki, T.; Kokudo, N.; Fukayama, M. Claudin-18 in Biliary Neoplasms. Its Significance in the Classification of Intrahepatic Cholangiocarcinoma. Virchows Arch. 2011, 459, 73–80. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration FDA Grants Approval to Zolbetuximab with Chemotherapy for Gastric or GEJ Adenocarcinoma 2024. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zolbetuximab-clzb-chemotherapy-gastric-or-gastroesophageal-junction-adenocarcinoma (accessed on 12 June 2025).
- Shitara, K.; Lordick, F.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.-H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in Patients with CLDN18.2-Positive, HER2-Negative, Untreated, Locally Advanced Unresectable or Metastatic Gastric or Gastro-Oesophageal Junction Adenocarcinoma (SPOTLIGHT): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef]
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Lordick, F.; Van Cutsem, E.; Gallego Plazas, J.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-Positive Gastric or Gastroesophageal Junction Adenocarcinoma: The Randomized, Phase 3 GLOW Trial. Nat. Med. 2023, 29, 2133–2141. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Singh, P.; Toom, S.; Huang, Y. Anti-Claudin 18.2 Antibody as New Targeted Therapy for Advanced Gastric Cancer. J. Hematol. Oncol. 2017, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Matsumoto, I.; Suzuki, K.; Tamura, A.; Shiraishi, A.; Kiyonari, H.; Kasamatsu, J.; Yamamoto, H.; Miyasaka, T.; Tanno, D.; et al. Deficiency of Lung-Specific Claudin-18 Leads to Aggravated Infection with Cryptococcus Deneoformans through Dysregulation of the Microenvironment in Lungs. Sci. Rep. 2021, 11, 21110. [Google Scholar] [CrossRef]
- LaFemina, M.J.; Sutherland, K.M.; Bentley, T.; Gonzales, L.W.; Allen, L.; Chapin, C.J.; Rokkam, D.; Sweerus, K.A.; Dobbs, L.G.; Ballard, P.L.; et al. Claudin-18 Deficiency Results in Alveolar Barrier Dysfunction and Impaired Alveologenesis in Mice. Am. J. Respir. Cell Mol. Biol. 2014, 51, 550–558. [Google Scholar] [CrossRef]
- Jovov, B.; Van Itallie, C.M.; Shaheen, N.J.; Carson, J.L.; Gambling, T.M.; Anderson, J.M.; Orlando, R.C. Claudin-18: A Dominant Tight Junction Protein in Barrett’s Esophagus and Likely Contributor to Its Acid Resistance. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 293, G1106–G1113. [Google Scholar] [CrossRef]
- Hayashi, D.; Tamura, A.; Tanaka, H.; Yamazaki, Y.; Watanabe, S.; Suzuki, K.; Suzuki, K.; Sentani, K.; Yasui, W.; Rakugi, H.; et al. Deficiency of Claudin-18 Causes Paracellular H+ Leakage, Up-Regulation of Interleukin-1β, and Atrophic Gastritis in Mice. Gastroenterology 2012, 142, 292–304. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Z.; Hu, C.; Zhang, S.; Zi, M.; Yuan, L.; Cheng, X. Targeting CLDN18.2 in Cancers of the Gastrointestinal Tract: New Drugs and New Indications. Front. Oncol. 2023, 13, 1132319. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Hu, D.; Gong, T.; Xu, R.; Gao, J. Analysis of the Expression and Genetic Alteration of CLDN18 in Gastric Cancer. Aging 2020, 12, 14271–14284. [Google Scholar] [CrossRef]
- Wu, J.; Lu, J.; Chen, Q.; Chen, H.; Zheng, Y.; Cheng, M. Pan-Cancer Analysis of CLDN18.2 Shed New Insights on the Targeted Therapy of Upper Gastrointestinal Tract Cancers. Front. Pharmacol. 2024, 15, 1494131. [Google Scholar] [CrossRef] [PubMed]
- Rohde, C.; Yamaguchi, R.; Mukhina, S.; Sahin, U.; Itoh, K.; Türeci, Ö. Comparison of Claudin 18.2 Expression in Primary Tumors and Lymph Node Metastases in Japanese Patients with Gastric Adenocarcinoma. Jpn. J. Clin. Oncol. 2019, 49, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Coati, I.; Lotz, G.; Fanelli, G.N.; Brignola, S.; Lanza, C.; Cappellesso, R.; Pellino, A.; Pucciarelli, S.; Spolverato, G.; Guzzardo, V.; et al. Claudin-18 Expression in Oesophagogastric Adenocarcinomas: A Tissue Microarray Study of 523 Molecularly Profiled Cases. Br. J. Cancer 2019, 121, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Pellino, A.; Brignola, S.; Riello, E.; Niero, M.; Murgioni, S.; Guido, M.; Nappo, F.; Businello, G.; Sbaraglia, M.; Bergamo, F.; et al. Association of CLDN18 Protein Expression with Clinicopathological Features and Prognosis in Advanced Gastric and Gastroesophageal Junction Adenocarcinomas. J. Pers. Med. 2021, 11, 1095. [Google Scholar] [CrossRef]
- Kubota, Y.; Kawazoe, A.; Mishima, S.; Nakamura, Y.; Kotani, D.; Kuboki, Y.; Bando, H.; Kojima, T.; Doi, T.; Yoshino, T.; et al. Comprehensive Clinical and Molecular Characterization of Claudin 18.2 Expression in Advanced Gastric or Gastroesophageal Junction Cancer. ESMO Open 2023, 8, 100762. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, Ö.; Manikhas, G.; Lordick, F.; Rusyn, A.; Vynnychenko, I.; Dudov, A.; Bazin, I.; Bondarenko, I.; Melichar, B.; et al. FAST: A Randomised Phase II Study of Zolbetuximab (IMAB362) plus EOX versus EOX Alone for First-Line Treatment of Advanced CLDN18.2-Positive Gastric and Gastro-Oesophageal Adenocarcinoma. Ann. Oncol. 2021, 32, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Hagen, S.J.; Ang, L.-H.; Zheng, Y.; Karahan, S.N.; Wu, J.; Wang, Y.E.; Caron, T.J.; Gad, A.P.; Muthupalani, S.; Fox, J.G. Loss of Tight Junction Protein Claudin 18 Promotes Progressive Neoplasia Development in Mouse Stomach. Gastroenterology 2018, 155, 1852–1867. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Z.; Jiang, L.; Zhang, M.; Zhang, C.; Shen, L. Claudin-18.2 Mediated Interaction of Gastric Cancer Cells and Cancer-Associated Fibroblasts Drives Tumor Progression. Cell Commun. Signal 2024, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Guan, B.; Li, Z.; Jiao, M.; Zhou, C.; Li, H. Correlation of Claudin18.2 Expression with Clinicopathological Characteristics and Prognosis in Gastric Cancer. Pathol.—Res. Pract. 2023, 248, 154699. [Google Scholar] [CrossRef]
- Nakayama, I.; Qi, C.; Chen, Y.; Nakamura, Y.; Shen, L.; Shitara, K. Claudin 18.2 as a Novel Therapeutic Target. Nat. Rev. Clin. Oncol. 2024, 21, 354–369. [Google Scholar] [CrossRef]
- Cao, W.; Xing, H.; Li, Y.; Tian, W.; Song, Y.; Jiang, Z.; Yu, J. Claudin18.2 Is a Novel Molecular Biomarker for Tumor-Targeted Immunotherapy. Biomark. Res. 2022, 10, 38. [Google Scholar] [CrossRef]
- Ungureanu, B.S.; Lungulescu, C.-V.; Pirici, D.; Turcu-Stiolica, A.; Gheonea, D.I.; Sacerdotianu, V.M.; Liliac, I.M.; Moraru, E.; Bende, F.; Saftoiu, A. Clinicopathologic Relevance of Claudin 18.2 Expression in Gastric Cancer: A Meta-Analysis. Front. Oncol. 2021, 11, 643872. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Riquelme, I.; Sagredo, E.A.; Rosa, L.; García, P.; Bizama, C.; Apud-Bell, M.; Leal, P.; Weber, H.; Benavente, F.; et al. Mucin 5B, Carbonic Anhydrase 9 and Claudin 18 Are Potential Theranostic Markers of Gallbladder Carcinoma. Histopathology 2019, 74, 597–607. [Google Scholar] [CrossRef]
- Wöll, S.; Schlitter, A.M.; Dhaene, K.; Roller, M.; Esposito, I.; Sahin, U.; Türeci, Ö. Claudin 18.2 Is a Target for IMAB362 Antibody in Pancreatic Neoplasms. Int. J. Cancer 2014, 134, 731–739. [Google Scholar] [CrossRef]
- Lee, J.H.; KIm, K.S.; Kim, T.-J.; Sung, P.H.; Song, S.Y.; Chung, J.B.; Park, S.W. Immunohistochemical Analysis of Claudin Expression in Pancreatic Cystic Tumors. Oncol. Rep. 2011, 25, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Zhou, L.; Zhang, M.; Liang, Z. Investigation of Clinical Application of Claudin 18 Isoform 2 in Pancreatic Ductal Adenocarcinoma: A Retrospective Analysis of 302 Chinese Patients. Histol. Histopathol. 2022, 37, 1031–1040. [Google Scholar] [CrossRef]
- Lyu, S.I.; Fretter, C.; Simon, A.G.; Spielmann, S.-M.; Damanakis, A.I.; Zhao, Y.; Bruns, C.J.; Schmidt, T.; Popp, F.C.; Waldschmidt, D.; et al. Extent and Clinical Significance of the Therapy-Relevant Tight Junction Protein Claudin 18.2 in Pancreatic Ductal Adenocarcinoma—Real-World Evidence. Transl. Oncol. 2024, 47, 102044. [Google Scholar] [CrossRef]
- Arseneau, R.J.; Kempster, E.; Bekkers, C.; Samson, T.; Gala-Lopez, B.L.; Ramjeesingh, R.; Boudreau, J.E.; Arnason, T. Claudin 18 (43–14A Clone) Expression in Pancreatic Ductal Adenocarcinoma: Assessment of a Potential Clinical Biomarker for Zolbetuximab Therapy. Transl. Oncol. 2025, 55, 102362. [Google Scholar] [CrossRef] [PubMed]
- Angerilli, V.; Sacchi, D.; Rizzato, M.; Gasparello, J.; Ceccon, C.; Sabbadin, M.; Niero, M.; Bergamo, F.; Cillo, U.; Franzina, C.; et al. Claudin 18.2: A Promising Actionable Target in Biliary Tract Cancers. ESMO Open 2025, 10, 105049. [Google Scholar] [CrossRef] [PubMed]
- Kinzler, M.N.; Gretser, S.; Schulze, F.; Bankov, K.; Abedin, N.; Bechstein, W.O.; Finkelmeier, F.; Zeuzem, S.; Reis, H.; Wild, P.J.; et al. Expression of Claudin-18.2 in Cholangiocarcinoma: A Comprehensive Immunohistochemical Analysis from a German Tertiary Centre. Histopathology 2025, 86, 640–646. [Google Scholar] [CrossRef]
- Takasawa, K.; Takasawa, A.; Osanai, M.; Aoyama, T.; Ono, Y.; Kono, T.; Hirohashi, Y.; Murata, M.; Sawada, N. Claudin-18 Coupled with EGFR/ERK Signaling Contributes to the Malignant Potentials of Bile Duct Cancer. Cancer Lett. 2017, 403, 66–73. [Google Scholar] [CrossRef]
- Tojjari, A.; Idrissi, Y.A.; Saeed, A. Emerging Targets in Gastric and Pancreatic Cancer: Focus on Claudin 18.2. Cancer Lett. 2025, 611, 217362. [Google Scholar] [CrossRef]
- Sanada, Y.; Oue, N.; Mitani, Y.; Yoshida, K.; Nakayama, H.; Yasui, W. Down-Regulation of the Claudin-18 Gene, Identified through Serial Analysis of Gene Expression Data Analysis, in Gastric Cancer with an Intestinal Phenotype. J. Pathol. 2006, 208, 633–642. [Google Scholar] [CrossRef]
- Fan, L.; Chong, X.; Zhao, M.; Jia, F.; Wang, Z.; Zhou, Y.; Lu, X.; Huang, Q.; Li, P.; Yang, Y.; et al. Ultrasensitive Gastric Cancer Circulating Tumor Cellular CLDN18.2 RNA Detection Based on a Molecular Beacon. Anal. Chem. 2021, 93, 665–670. [Google Scholar] [CrossRef]
- Angerilli, V.; Ghelardi, F.; Nappo, F.; Grillo, F.; Parente, P.; Lonardi, S.; Luchini, C.; Pietrantonio, F.; Ugolini, C.; Vanoli, A.; et al. Claudin-18.2 Testing and Its Impact in the Therapeutic Management of Patients with Gastric and Gastroesophageal Adenocarcinomas: A Literature Review with Expert Opinion. Pathol.—Res. Pract. 2024, 254, 155145. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kwak, Y.; Nam, S.K.; Han, D.; Oh, D.Y.; Im, S.A.; Lee, H.S. Clinicopathological Analysis of Claudin 18.2 Focusing on Intratumoral Heterogeneity and Survival in Patients with Metastatic or Unresectable Gastric Cancer. ESMO Open 2024, 9, 104000. [Google Scholar] [CrossRef]
- Wang, C.; Wu, N.; Pei, B.; Ma, X.; Yang, W. Claudin and Pancreatic Cancer. Front. Oncol. 2023, 13, 1136227. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.-S.; Dong, X.-Y.; Hu, Y.; Duan, B.-J.; Bai, J.; Wu, Y.-Y.; Fan, L.; Liao, X.-H.; Kang, Y.; et al. Claudin 18.2 Is a Potential Therapeutic Target for Zolbetuximab in Pancreatic Ductal Adenocarcinoma. World J. Gastrointest. Oncol. 2022, 14, 1252–1264. [Google Scholar] [CrossRef]
- Choi, E.; Shin, J.; Ryu, M.-H.; Kim, H.-D.; Park, Y.S. Heterogeneity of Claudin 18.2 Expression in Metastatic Gastric Cancer. Sci. Rep. 2024, 14, 17648. [Google Scholar] [CrossRef]
- Son, S.-M.; Woo, C.G.; Lee, O.-J.; Lee, S.K.; Cho, M.; Lee, Y.-P.; Kim, H.; Kim, H.K.; Yang, Y.; Kwon, J.; et al. Discordance in Claudin 18.2 Expression Between Primary and Metastatic Lesions in Patients with Gastric Cancer. J. Gastric Cancer 2025, 25, 303–317. [Google Scholar] [CrossRef]
- Bang, K.; Cheon, J.; Park, Y.S.; Kim, H.D.; Ryu, M.H.; Park, Y.; Moon, M.; Lee, H.; Kang, Y.K. Association between HER2 Heterogeneity and Clinical Outcomes of HER2-Positive Gastric Cancer Patients Treated with Trastuzumab. Gastric Cancer 2022, 25, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Valenza, C.; Guidi, L.; Battaiotto, E.; Trapani, D.; Sartore Bianchi, A.; Siena, S.; Curigliano, G. Targeting HER2 Heterogeneity in Breast and Gastrointestinal Cancers. Trends Cancer 2024, 10, 113–123. [Google Scholar] [CrossRef]
- Türeci, O.; Sahin, U.; Schulze-Bergkamen, H.; Zvirbule, Z.; Lordick, F.; Koeberle, D.; Thuss-Patience, P.; Ettrich, T.; Arnold, D.; Bassermann, F.; et al. A Multicentre, Phase IIa Study of Zolbetuximab as a Single Agent in Patients with Recurrent or Refractory Advanced Adenocarcinoma of the Stomach or Lower Oesophagus: The MONO Study. Ann. Oncol. 2019, 30, 1487–1495. [Google Scholar] [CrossRef]
- Klempner, S.J.; Lee, K.-W.; Shitara, K.; Metges, J.-P.; Lonardi, S.; Ilson, D.H.; Fazio, N.; Kim, T.Y.; Bai, L.-Y.; Moran, D.; et al. ILUSTRO: Phase II Multicohort Trial of Zolbetuximab in Patients with Advanced or Metastatic Claudin 18.2-Positive Gastric or Gastroesophageal Junction Adenocarcinoma. Clin. Cancer Res. 2023, 29, 3882–3891. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, D.; Li, N.; Guo, W.; Liu, T.; Li, H.; Li, J.; Bai, Y.; Deng, Y.; Zhuang, Z.; et al. Osemitamab in Combination with Capecitabine and Oxaliplatin (CAPOX) as a First Line Treatment of Advanced G/GEJ Cancer: Updated Data of Cohort C from a Phase I/IIa, Multi-Center Study (TranStar102/TST001-1002). J. Clin. Oncol. 2023, 41, 4046. [Google Scholar] [CrossRef]
- Gong, J.; Liu, D.; Guo, Z.; Zhang, J.; Guo, W.; Sun, M.; Xu, N.; Qi, C.; Zhang, L.; Xia, Z.; et al. First-Line Osemitamab (TST001) plus Nivolumab and CAPOX for Advanced G/GEJ Cancer (TranStar102): Updated Results of Cohort G from a Phase I/IIa Study. J. Clin. Oncol. 2025, 43, 4032. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, J.; Wang, J.; Xu, H.; Ji, Y.; Han, L.; Chen, J.; Lu, J.; Shen, L. Second-Line ASKB589 plus Chemotherapy for Advanced Gastric or Gastroesophageal Cancers: Results from Cohort 5 of a Phase I/II Study. J. Clin. Oncol. 2025, 43, 4044. [Google Scholar] [CrossRef]
- Peng, Z.; Zhu, H.; Wang, J.; Han, L.; Chen, J.; Lu, J.; Shen, L. Updated Efficacy Results of ASKB589 Combined with CAPOX and PD-1 Inhibitor as First-Line Treatment for Metastatic Gastric/Gastro-Esophageal Junction (G/GEJ) Adenocarcinoma from a Phase Ib/II Study. J. Clin. Oncol. 2025, 43, 454. [Google Scholar] [CrossRef]
- Gong, J.; Liu, F.; Jin, Z.; Zhang, M.; Zhang, S.; Zhang, Y.; Liang, X.; Li, Y.; Yang, Y.; Zhu, L.; et al. FG-M108 plus Capecitabine and Oxaliplatin (CAPOX) for First-Line Treatment of CLDN18.2+/HER2- Advanced Gastric/Gastroesophageal Junction Adenocarcinoma (GC): Update Results of a Phase I/II Trial to Determine RP2D. J. Clin. Oncol. 2025, 43, 431. [Google Scholar] [CrossRef]
- Liu, F.; Gong, J.; Zhang, S.; Zhang, M.; Liang, X.; Wang, J.; Li, Y.; Yang, Y.; Tian, C.; Jin, Z.; et al. Preliminary Result of a Phase Ib Study: Efficacy and Safety of FG-M108 plus Gemcitabine and Nab-Paclitaxel in Patients with Claudin18.2-Positive, Locally Advanced, Unresectable, or Metastatic Pancreatic Cancer. J. Clin. Oncol. 2025, 43, 4188. [Google Scholar] [CrossRef]
- Inamoto, R.; Takahashi, N.; Yamada, Y. Claudin18.2 in Advanced Gastric Cancer. Cancers 2023, 15, 5742. [Google Scholar] [CrossRef]
- Teng, F.; Gu, Y.; Chai, H.; Guo, H.; Li, H.; Wu, X.; Yao, X.; Xu, F.; Shi, L.; Yan, Z.; et al. Abstract 5183: The Preclinical Characterization of TST001, a Novel Humanized Anti-Claudin18.2 mAb with Enhanced Binding Affinity and Anti-Tumor Activity. Cancer Res. 2020, 80, 5183. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Sun, W.; Rocha Lima, C.M.S.P.; Shah, S.; Scott, A.J.; Monga, D.K.; Kundranda, M.N.; Sher, A.F.; Gold, P.J.; Berlin, J.; et al. A Multi-Cohort Phase I/IIa Clinical Trial to Evaluate the Safety, Tolerability, and Pharmacokinetics of TST001 Administered as a Monotherapy, with Nivolumab or Standard of Care in Patients with Locally Advanced or Metastatic Solid Tumors: TransStar101. J. Clin. Oncol. 2023, 41, TPS4176. [Google Scholar] [CrossRef]
- Janjigian, Y.; Tolcher, A.; Mehta, R.; Cecchini, M.; Van Tine, B.; Kundranda, M.; Olatunji, A.; Patel, M.R.; Berlin, J.; Rocha-Lima, C.M.S.P.; et al. Abstract CT132: A Phase I/IIa Clinical Trial (TranStar101) to Evaluate the Safety, Tolerability and Pharmacokinetics of OSEMITAMAB Administered as Monotherapy or in Combination with Nivolumab or Standard of Care in Patients with Locally Advanced or Metastatic Solid Tumors. Cancer Res. 2024, 84, CT132. [Google Scholar] [CrossRef]
- Shen, L.; Liu, D.; Li, N.; Guo, W.; Liu, T.; Zhang, L.; Zhu, X.; Qi, C.; Xu, L.; Qian, X.; et al. 1524P First-Line TST001 plus Capecitabine and Oxaliplatin (CAPOX) for Advanced G/GEJ Cancer with CLDN18.2 Positive Overall Survival Data from Study Transtar102-Cohort C. Ann. Oncol. 2023, 34, S858–S859. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, J.; Wang, J.; Shi, J.; Zhu, H.; Wang, Y.; Chen, Y.; Wang, F.; Qu, X.; Yu, J.; et al. A Phase I/II Study of ASKB589 (Anti-Claudin 18.2 [CLDN18.2] Monoclonal Antibody) in Patients with Solid Tumors. J. Clin. Oncol. 2023, 41, 397. [Google Scholar] [CrossRef]
- Ruan, D.-Y.; Liu, F.-R.; Wei, X.-L.; Luo, S.-X.; Zhuang, Z.-X.; Wang, Z.-N.; Liu, F.-N.; Zhang, Y.-Q.; Yang, J.-W.; Chen, Z.-D.; et al. Claudin 18.2-Targeting Antibody–Drug Conjugate CMG901 in Patients with Advanced Gastric or Gastro-Oesophageal Junction Cancer (KYM901): A Multicentre, Open-Label, Single-Arm, Phase 1 Trial. Lancet Oncol. 2025, 26, 227–238. [Google Scholar] [CrossRef]
- Raufi, A.G.; Goyal, L.; Smyth, E.; Szekeres, P.; Petrone, M.; Hobson, R.; Thress, K.; Origuchi, M.; Nehra, J.; Brown, J.S.; et al. CLARITY-PanTumor01: A Phase 2 Trial of the Claudin 18.2-Specific Antibody-Drug Conjugate AZD0901 (CMG901) in Patients with CLDN18.2-Expressing Advanced Solid Tumors. J. Clin. Oncol. 2024, 42, TPS3163. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Elimova, E.; Zhang, J.; Chen, M.-H.; Smyth, E.C.; Lee, J.; Miao, R.; Liu, S.; Holmblad, M.; et al. CLARITY-Gastric 01: A Randomized Phase 3 Study of AZD0901, a Claudin18.2 (CLDN18.2)-Targeted Antibody-Drug Conjugate, in Second- or Later-Line (2L+) Advanced Gastric or Gastroesophageal Junction Cancer (GC/GEJC). J. Clin. Oncol. 2025, 43, TPS507. [Google Scholar] [CrossRef]
- Shen, L.; Shitara, K.; Chen, J.-S.; Oh, D.-Y.; Jiang, A.; Liu, S.; Dong, Z.; Zhu, Q.; Kumar, R.; Rha, S.Y. GEMINI-Gastric: A Phase 2 Study of Novel Treatment Combinations in Patients with Locally Advanced Unresectable or Metastatic Gastric Cancers. J. Clin. Oncol. 2024, 42, TPS4182. [Google Scholar] [CrossRef]
- Bai, C.; Xue, J.; Zheng, Y.; Sun, M.; Ying, J.; Zhou, F.; Yu, Y.; Sun, Y.; Xing, L.; Zhang, Y.; et al. A Phase 1/2 Study of LM-302, an Anti-Claudin 18.2 (CLDN18.2) Antibody-Drug Conjugate in Patients with Advanced Gastric/Gastroesophageal Junction Cancer. J. Clin. Oncol. 2024, 42, 3028. [Google Scholar] [CrossRef]
- Jiang, H.; Huang, M.; Wan, L.; Markman, B.; Pan, H.; Bai, C.; Zhou, A.; Mou, Y.; Yuan, X.; Dou, L.; et al. Efficacy and Safety of LM-302 (Anti-Claudin 18.2 ADC) in Combination with Anti-PD-1 Therapy for Advanced Gastric, Gastroesophageal Junction Cancer and Esophageal Adenocarcinoma: Early-Phase Study Results. J. Clin. Oncol. 2025, 43, 4039. [Google Scholar] [CrossRef]
- Fan, J.; Zhou, J.; Shi, G.; Huang, X.; Gao, Q.; Liang, F.; Ren, N.; Shi, Y.; Yi, Y.; Wang, Z.; et al. Two Stage, Multi-Center Trial of Cadonilimab and LM-302 for Patients with CLDN18.2+ Biliary Tract Cancer (BTC) That Failed Chemotherapy and PD-(L)1 Antibody (ZSAB-Calm). J. Clin. Oncol. 2024, 42, e16152. [Google Scholar] [CrossRef]
- Ruan, D.-Y.; Wu, H.-X.; Luo, S.-X.; Huang, W.-W.; Liang, X.-J.; Niu, Z.-X.; Dang, Q.; Li, H.-L.; Pan, Z.-Y.; Lu, H.-X.; et al. The Antibody–Drug Conjugate SHR-A1904 for Targeting CLDN18.2 in Advanced Gastric or Gastroesophageal Junction Cancer: A Phase 1 Trial. Nat. Med. 2025, 31, 3037–3046. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Sun, Y.; Gong, J.; Yue, J.; Pan, Y.; Sun, M.; Song, R.; Xiao, X.; Tazbirkova, A.; et al. CLDN18.2–Targeting Antibody–Drug Conjugate IBI343 in Advanced Gastric or Gastroesophageal Junction Adenocarcinoma: A Phase 1 Trial. Nat. Med. 2025, 31, 3028–3036. [Google Scholar] [CrossRef]
- Liu, T.; Yu, Y.; Ni, S.; Liu, B.; Li, N.; Zhu, J.; Liang, X.; Xiao, M.; Xu, N.; Li, W.; et al. 1456P First in Human Phase I/II Trial of Claudin 18.2 ADC RC118 in Patients with Advanced Gastric/Gastroesophageal Junction Cancer. Ann. Oncol. 2024, 35, S903. [Google Scholar] [CrossRef]
- Cheng, X.; Song, Z.; Li, N.; Han, L.; Zhang, Y.; Zheng, T.; Suo, A.; Zhang, Z.; Li, H.; Du, X.; et al. First in Human Phase I Study of TQB2103, a Claudin18.2 (CLDN18.2) Targeted Antibody-Drug Conjugate (ADC), in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2025, 43, 3026. [Google Scholar] [CrossRef]
- Klempner, S.J.; Dayyani, F.; Kratz, J.; Kim, S.; Xu, C.; Ahlers, C.M.; Liu, X.; Chung, J.-K.; Sabbatini, P.; Dennis, P.A.; et al. 388MO Preliminary Safety and Efficacy of Givastomig, a Novel Claudin 18.2/4-1BB Bispecific Antibody, in Combination with Nivolumab and mFOLFOX in Metastatic Gastroesophageal Carcinoma (mGEC). Ann. Oncol. 2025, 36, S151–S152. [Google Scholar] [CrossRef]
- Shitara, K.; Choi, H.J.; Dayyani, F.; Kazmi, S.; Korakis, I.; Moy, R.H.; O’Reilly, E.M.; Picozzi, V.J.; Simonelli, M.; Smolenschi, C.; et al. 2137P ASP2138 Monotherapy in Patients (Pts) with Claudin 18 Isoform 2 (CLDN18.2)+, Advanced Solid Tumors: Phase I/Ib Trial. Ann. Oncol. 2025, 36, S1202–S1203. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, J.; Zhang, M.; Sun, Y.; Yang, S.; Lv, J.; Cao, Y.; Zhang, Y.; Cui, J.; Zhang, J.; et al. Safety and Efficacy of QLS31905 in Patients with Advanced Solid Tumors: Updated Data from Phase 1 Study. J. Clin. Oncol. 2025, 43, 2527. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, L.; Li, Y.; Wen, J.; Xue, J.; Wang, Z.; Li, P.; Zhao, W.; Liu, J.; Rao, X.; et al. First-in-Human Phase I/II Safety and Preliminary Efficacy of PM1032, a Bispecific Antibody Targeting CLDN18.2 and 4-1BB, in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2024, 42, 2662. [Google Scholar] [CrossRef]
- Zheng, L.; Ruihong, D.; Jieer, Y.; Xu, Q.; Guo, Z.; Hu, C.; Sun, Y.; Niu, Z.; Hao, J.; Zhang, M.; et al. Safety and Preliminary Efficacy Results of IBI389, an Anti-CLDN18.2/CD3 Bispecific Antibody, in Patients with Solid Tumors and Gastric or Gastro-Esophageal Tumors: A Phase 1 Dose Escalation and Expansion Study. J. Clin. Oncol. 2024, 42, 2519. [Google Scholar] [CrossRef]
- Croft, M. The Role of TNF Superfamily Members in T-Cell Function and Diseases. Nat. Rev. Immunol. 2009, 9, 271–285. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Z.; Jiang, W.; Zhang, Y.; Meng, Z.; Niu, Y.; Sheng, Z.; Chen, C.; Liu, X.; Chen, X.; et al. CLDN18.2 and 4-1BB Bispecific Antibody Givastomig Exerts Antitumor Activity through CLDN18.2-Expressing Tumor-Directed T-Cell Activation. J. Immunother. Cancer 2023, 11, e006704. [Google Scholar] [CrossRef]
- Nakazawa, T.; Tanaka, H.; Kikuchi, A.; Rashid, R.; Avery, K.N.; Qi, J.; Nisthal, A.; Shimazaki, M.; Shirasuna, K. Abstract 2962: ASP2138, a Novel 2+1 Format, Claudin 18.2 x CD3 Bispecific Antibody, Demonstrates Selectivity and Activity in Preclinical Cancer Models. Cancer Res. 2023, 83, 2962. [Google Scholar] [CrossRef]
- Zugasti, I.; Espinosa-Aroca, L.; Fidyt, K.; Mulens-Arias, V.; Diaz-Beya, M.; Juan, M.; Urbano-Ispizua, Á.; Esteve, J.; Velasco-Hernandez, T.; Menéndez, P. CAR-T Cell Therapy for Cancer: Current Challenges and Future Directions. Signal Transduct. Target. Ther. 2025, 10, 210. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, B.; Li, Z.; Li, J.; Wang, H.; Chen, L.; Jiang, H.; Wu, M.; Xiao, J.; Peng, X.; et al. Phase I Trial of Claudin 18.2-Specific Chimeric Antigen Receptor T Cells for Advanced Gastric and Pancreatic Adenocarcinoma. J. Clin. Oncol. 2019, 37, 2509. [Google Scholar] [CrossRef]
- Qi, C.; Liu, C.; Gong, J.; Liu, D.; Wang, X.; Zhang, P.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; et al. Claudin18.2-Specific CAR T Cells in Gastrointestinal Cancers: Phase 1 Trial Final Results. Nat. Med. 2024, 30, 2224–2234. [Google Scholar] [CrossRef]
- Botta, G.P.; Kelly, R.J.; Jin, Z.; Ma, H.; Ku, G.Y.; Zhao, D.; Mehta, R.; Carnevale, J.C.; Sierra, G.; JIA, J.; et al. CLDN18.2 Chimeric Antigen Receptor T Cell Therapy for Patients with Advanced Gastric and Pancreatic Adenocarcinoma: Results of ELIMYN18.2 Phase 1b Clinical Trial. J. Clin. Oncol. 2024, 42, 356. [Google Scholar] [CrossRef]
- Qi, C.; Zhang, P.; Liu, C.; Zhang, J.; Zhou, J.; Yuan, J.; Liu, D.; Zhang, M.; Gong, J.; Wang, X.; et al. Safety and Efficacy of CT041 in Patients With Refractory Metastatic Pancreatic Cancer: A Pooled Analysis of Two Early-Phase Trials. J. Clin. Oncol. 2024, 42, 2565–2577. [Google Scholar] [CrossRef]
- Dumbrava, E.E.; Iqbal, S.; Turcotte, S.; Botta, G.P.; Schlechter, B.L.; Ku, G.Y.; Hosein, P.J.; Saibil, S.; Gavriliuc, M.; Apostolopoulou, M.; et al. A Phase 1/2 Study Evaluating the Safety and Efficacy of Autologous TAC T Cells in Subjects with Claudin 18.2+ Advanced Solid Tumors. J. Clin. Oncol. 2025, 43, 828. [Google Scholar] [CrossRef]
- Qi, C.; Liu, C.; Gong, J.; Li, J.; Liu, D.; Wang, X.; Zhang, P.; Qin, Y.; Zhang, M.; Peng, Z.; et al. Claudin18.2-Targeted Chimeric Antigen Receptor T Cell-Therapy for Patients with Gastrointestinal Cancers: Final Results of CT041-CG4006 Phase 1 Trial. J. Clin. Oncol. 2024, 42, 2501. [Google Scholar] [CrossRef]
- Qi, C.; Liu, C.; Peng, Z.; Zhang, Y.; Wei, J.; Qiu, W.; Zhang, X.; Pan, H.; Niu, Z.; Qiu, M.; et al. Claudin18.2-Specific CAR T Cells (Satri-Cel) versus Treatment of Physician’s Choice (TPC) for Previously Treated Advanced Gastric or Gastroesophageal Junction Cancer (G/GEJC): Primary Results from a Randomized, Open-Label, Phase II Trial (CT041-ST-01). J. Clin. Oncol. 2025, 43, 4003. [Google Scholar] [CrossRef]
- Qi, C.; Liu, C.; Peng, Z.; Zhang, Y.; Wei, J.; Qiu, W.; Zhang, X.; Pan, H.; Niu, Z.; Qiu, M.; et al. Claudin-18 Isoform 2-Specific CAR T-Cell Therapy (Satri-Cel) versus Treatment of Physician’s Choice for Previously Treated Advanced Gastric or Gastro-Oesophageal Junction Cancer (CT041-ST-01): A Randomised, Open-Label, Phase 2 Trial. Lancet 2025, 405, 2049–2060. [Google Scholar] [CrossRef]
- Xu, S.X.; Wang, L.; Ip, P.; Randhawa, R.R.; Benatar, T.; Prosser, S.L.; Lal, P.; Khan, A.N.; Nitya-Nootan, T.; Thakor, G.; et al. Preclinical Development of T Cells Engineered to Express a T-Cell Antigen Coupler Targeting Claudin 18.2–Positive Solid Tumors. Cancer Immunol. Res. 2025, 13, 35–46. [Google Scholar] [CrossRef]
- Jia, K.; Chen, Y.; Sun, Y.; Hu, Y.; Jiao, L.; Ma, J.; Yuan, J.; Qi, C.; Li, Y.; Gong, J.; et al. Multiplex Immunohistochemistry Defines the Tumor Immune Microenvironment and Immunotherapeutic Outcome in CLDN18.2-Positive Gastric Cancer. BMC Med. 2022, 20, 223. [Google Scholar] [CrossRef]
- Korpan, M.; Puhr, H.C.; Berger, J.M.; Friedrich, A.; Prager, G.W.; Preusser, M.; Ilhan-Mutlu, A. Current Landscape of Molecular Biomarkers in Gastroesophageal Tumors and Potential Strategies for Co-Expression Patterns. Cancers 2025, 17, 340. [Google Scholar] [CrossRef]
- Xu, X.; Yu, T.; Wang, Z. Combining Antibody-Drug Conjugates with Immune Checkpoint Inhibitors: A New Paradigm for Breast Cancer Therapy. Cancer Treat. Rev. 2025, 140, 103012. [Google Scholar] [CrossRef] [PubMed]
- Lop Gros, J.; Santiago Díaz, P.; Larrubia Loring, M.; Patriarca, M.E.; Lloveras, B.; Iglesias, M. Claudin 18.2 Immunohistochemistry Expression in Gastric Cancer: A Systematic Review. Appl. Immunohistochem. Mol. Morphol. AIMM 2025, 33, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Moran, D.; Maurus, D.; Rohde, C.; Arozullah, A. Prevalence of CLDN18.2, HER2 and PD-L1 in Gastric Cancer Samples. Ann. Oncol. 2018, 29, viii32. [Google Scholar] [CrossRef]
- Ogawa, H.; Abe, H.; Yagi, K.; Seto, Y.; Ushiku, T. Claudin-18 Status and Its Correlation with HER2 and PD-L1 Expression in Gastric Cancer with Peritoneal Dissemination. Gastric Cancer 2024, 27, 802–810. [Google Scholar] [CrossRef]
- Yue, J.; Shao, S.; Zhou, J.; Luo, W.; Xu, Y.; Zhang, Q.; Jiang, J.; Zhu, M.M. A Bispecific Antibody Targeting HER2 and CLDN18.2 Eliminates Gastric Cancer Cells Expressing Dual Antigens by Enhancing the Immune Effector Function. Investig. New Drugs 2024, 42, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Deng, J.; Wu, H.; Huang, Z. HER2-Positive Gastric Cancer: From Targeted Therapy to CAR-T Cell Therapy. Front. Immunol. 2025, 16, 1560280. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kuwata, T.; An, M.; Hong, J.Y.; Kim, S.T.; Matsubara, Y.; Shitara, K.; Lee, J. Dynamic Modulation of Claudin18.2 Expression and Remodeling of the Tumor Microenvironment in Gastric Cancer during Chemo-Immunotherapy. J. Immunother. Cancer 2025, 13, e012683. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, H.-W. Antigen Loss after Targeted Immunotherapy in Hematological Malignancies. Clin. Lab. Med. 2021, 41, 341–357. [Google Scholar] [CrossRef]
- Ito, T.; Kojima, T.; Yamaguchi, H.; Kyuno, D.; Kimura, Y.; Imamura, M.; Takasawa, A.; Murata, M.; Tanaka, S.; Hirata, K.; et al. Transcriptional Regulation of Claudin-18 via Specific Protein Kinase C Signaling Pathways and Modification of DNA Methylation in Human Pancreatic Cancer Cells. J. Cell Biochem. 2011, 112, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Sun, J.; Liu, Z.; Ouyang, S.; Zhang, Z.; Zeng, Z.; Li, J.; Kang, W. The Immune Microenvironment in Gastric Cancer: Prognostic Prediction. Front. Oncol. 2022, 12, 836389. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Nakayama, I.; Shitara, K. Cell-Based Therapies in GI Cancers: Current Landscape and Future Directions. Am. Soc. Clin. Oncol. Educ. Book 2024, 45, e471716. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Ohuchida, K.; Yamada, Y.; Shimada, Y.; Imamura, M.; Son, K.; Mochida, Y.; Katayama, N.; Iwamoto, C.; Torata, N.; et al. Claudin18.2-Positive Gastric Cancer-Specific Changes in Neoadjuvant Chemotherapy-Driven Immunosuppressive Tumor Microenvironment: Cellular and Molecular Biology. Br. J. Cancer 2025, 132, 793–804. [Google Scholar] [CrossRef]
- Hu, Z.I.; O’Reilly, E.M. Therapeutic Developments in Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 7–24. [Google Scholar] [CrossRef]
- Principe, D.R.; Timbers, K.E.; Atia, L.G.; Koch, R.M.; Rana, A. TGFβ Signaling in the Pancreatic Tumor Microenvironment. Cancers 2021, 13, 5086. [Google Scholar] [CrossRef] [PubMed]
- Zavros, Y.; Merchant, J.L. The Immune Microenvironment in Gastric Adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Jarahian, M.; et al. CAR T Cells in Solid Tumors: Challenges and Opportunities. Stem Cell Res. Ther. 2021, 12, 81. [Google Scholar] [CrossRef]
- Barrett, A.M.; Britton, Z.T.; Carrasco, R.A.; Breen, S.; Broggi, M.A.S.; Hatke, A.; Clark, B.; Yang, C.; Phipps, S.; Ortiz, L.; et al. Preclinical Evaluation of AZD6422, an Armored Chimeric Antigen Receptor T Cell Targeting CLDN18.2 in Gastric, Pancreatic, and Esophageal Cancer. Clin. Cancer Res. 2024, 30, 5413–5429. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Wang, P.; Li, S.; Dong, Y.; Zhou, M.; Shi, B.; Jiang, H.; Sun, R.; Li, Z. FAP-Targeted CAR-T Suppresses MDSCs Recruitment to Improve the Antitumor Efficacy of Claudin18.2-Targeted CAR-T against Pancreatic Cancer. J. Transl. Med. 2023, 21, 255. [Google Scholar] [CrossRef]
- Grosser, R.; Cherkassky, L.; Chintala, N.; Adusumilli, P.S. Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer Cell 2019, 36, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, S.; Ma, L.; Kuang, Z.; Mu, C.; Yang, J.; Liu, Y.; Li, Z.; Li, Q. Targeted Radionuclide Therapy of CLDN18.2-Positive Gastric Cancer with [131I]I-Zolbetuximab: An In Vitro and In Vivo Study. Mol. Pharm. 2025, 22, 2535–2544. [Google Scholar] [CrossRef]
- Bordeau, B.M.; Balthasar, J.P. Strategies to Enhance Monoclonal Antibody Uptake and Distribution in Solid Tumors. Cancer Biol. Med. 2021, 18, 649. [Google Scholar] [CrossRef] [PubMed]
- Abken, H. CAR T Cell Therapies in Gastrointestinal Cancers: Current Clinical Trials and Strategies to Overcome Challenges. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Jiang, K.; Jiang, H.; Zhao, Y.; Lai, C.H.; Aicher, A.; Li, Z.; Heeschen, C. CLDN18.2 CAR-Derived Extracellular Vesicle Immunotherapy Improves Outcome in Murine Pancreatic Cancer. Adv. Healthc. Mater. 2025, 14, 2500546. [Google Scholar] [CrossRef] [PubMed]
| Agent | NCT | Phase | Enrollment | Subjects | Combination(s) | Outcomes † | Status |
|---|---|---|---|---|---|---|---|
| Zolbetuximab | NCT01197885 | II | 54 | GC/GEJC | monotherapy | ORR: 9% [50] | Completed |
| Zolbetuximab | NCT01630083 | II | 252 | GC/GEJC, esophageal | + chemotherapy | PFS: 7.5 mo, HR (0.44, 95% CI 0.29–0.67) [23] | Completed |
| Zolbetuximab | NCT03505320 | II | 143 | GC/GEJC | +/− chemotherapy or ICI | monotherapy: ORR 0% + Chemo: ORR 71.4%, mPFS 17.8 mo + ICI: ORR 0% [51] | Active, not recruiting |
| Zolbetuximab | NCT03816163 | II | 393 | PC | + chemotherapy | --- | Active, not recruiting |
| Zolbetuximab | NCT03653507 | III | 507 | GC/GEJC | + chemotherapy | PFS 8.2 mo (HR 0.771) [9] | Active, not recruiting |
| Zolbetuximab | NCT03504397 | III | 565 | GC/GEJC | + chemotherapy | PFS 10.6 mo (HR 0.75) [8] | Active, not recruiting |
| Zolbetuximab | NCT06901531 | III | 500 * | GC/GEJC | + chemotherapy + ICI | --- | Recruiting |
| TST001 | NCT04396821 | I/II | 150 * | solid tumors | +/− chemotherapy +/− ICI | --- | Active, not recruiting |
| TST001 | NCT04495296 | I/II | 320 * | solid tumors | +/− chemotherapy +/− ICI | + Chemo: ORR 65.4% [52] + Chemo + ICI: ORR 55.7% [53] | Recruiting |
| TST001 | NCT05190575 | II | 8 | BTC | monotherapy | --- | Completed |
| TST001 | NCT06093425 | III | 950 * | GC/GEJC | + chemotherapy + ICI | --- | Not yet recruiting |
| ASKB589 | NCT04632108 | I/II | 214 * | solid tumors | + chemotherapy | ORR 31.1%, DCR 71.4% [54] | Recruiting |
| ASKB589 | NCT05632939 | I/II | 57 * | GC/GEJC | + chemotherapy + ICI | ORR 73.5% [55] | Recruiting |
| ASKB589 | NCT06206733 | III | 780 * | GC/GEJC | + chemotherapy + ICI | --- | Recruiting |
| FG-M108 | NCT04894825 | I/II | 152 * | solid tumors | + chemotherapy | ORR: 78% in GC [56], ORR: 53% in PC [57] | Recruiting |
| FG-M108 | NCT06177041 | III | 486 * | GC/GEJC | + chemotherapy | --- | Recruiting |
| Agent | NCT | Phase | Enrollment | Subjects | Combination(s) | Outcomes † | Status |
|---|---|---|---|---|---|---|---|
| AZD0901 | NCT04805307 | I | 176 | solid tumors | monotherapy | ORR 28%, DCR 63%, mPFS 3.7 mo [64] | Completed |
| AZD0901 | NCT06219941 | II | 190 * | solid tumors | +/− chemotherapy | --- | Recruiting |
| AZD0901 | NCT05702229 | II | 240 * | GC/GEJC | + chemotherapy + ICI | --- | Recruiting |
| AZD0901 | NCT06346392 | III | 572 * | GC/GEJC | monotherapy | --- | Recruiting |
| LM-302 | NCT05994001 | I/II | 96 * | BTC | + ICI | --- | Recruiting |
| LM-302 | NCT05161390 | I/II | 206 * | solid tumors | monotherapy | ORR 30.6%, DCR 75% [68] | Active, not recruiting |
| LM-302 | NCT05934331, NCT05188664 | I/II | 276 * | solid tumors | + ICI | ORR 65.9%, DCR 85.4% [69] | Recruiting |
| LM-302 | NCT06587425 | II | 50 * | GC/GEJC | + chemotherapy + ICI | --- | Recruiting |
| LM-302 | NCT06351020 | III | 375 * | GC/GEJC | monotherapy | --- | Recruiting |
| SHR-A1904 | NCT04877717 | I | 107 | solid tumors | monotherapy | ORR 24.2% [71] | Active, not recruiting |
| SHR-A1904 | NCT06350006 | I/III | 924 * | solid tumors | + chemotherapy + ICI | --- | Recruiting |
| SHR-A1904 | NCT06649292 | III | 524 * | GC/GEJC | monotherapy | --- | Recruiting |
| IBI343 | NCT05458219 | I | 127 | GC/GEJC | monotherapy | ORR 29%, mPFS 5.5 mo [72] | Recruiting |
| IBI343 | NCT07025889 | I/II | 55 * | GC/GEJC | + ICI | --- | Recruiting |
| IBI343 | NCT06770439 | II | 64 * | PC | + chemotherapy | --- | Not yet recruiting |
| IBI343 | NCT06238843 | III | 450 * | GC/GEJC | monotherapy | --- | Enroll by invitation |
| IBI343 | NCT07066098 | III | 201 * | PC | monotherapy | --- | Not yet recruiting |
| RC118 | NCT05205850 | I/II | 135 * | solid tumors | monotherapy | ORR 47.1% [73] | Recruiting |
| TQB2103 | NCT05867563 | I | 71 * | solid tumors | monotherapy | ORR 20%, DCR 76.7% [74] | Unknown status |
| ATG-022 | NCT05718895 | I | 156 * | solid tumors | monotherapy | --- | Recruiting |
| Agent | NCT | Phase | Enrollment | Subjects | Combination(s) | Outcomes † | Status |
|---|---|---|---|---|---|---|---|
| Givastomig | NCT04900818 | I | 168 * | GC/GEJC, EAC | + chemotherapy + ICI | ORR 71% [75] | Recruiting |
| ASP2138 | NCT05365581 | I | 378 * | GC/GEJC, PC | monotherapy | DCR 41.7% [76] | Recruiting |
| QLS31905 | NCT05278832 | I | 104 * | solid tumors | monotherapy | ORR 18.2%, DCR 87.9% [77] | Unknown status |
| QLS31905 | NCT06041035 | I/II | 115 * | solid tumors | + chemotherapy | --- | Not yet recruiting |
| QLS31905 | NCT07079228 | III | 602 * | PC | + chemotherapy | --- | Not yet recruiting |
| Q-1802 | NCT04856150 | I | 66 * | solid tumors | monotherapy | --- | Unknown status |
| Q-1802 | NCT05964543 | I/II | 72 * | GC/GEJC | + chemotherapy | --- | Recruiting |
| SG1906 | NCT05857332 | I | 60 * | solid tumors | monotherapy | --- | Recruiting |
| PT886 | NCT05482893 | I/II | 258 * | GC/GEJC, PC, BTC | +/− chemotherapy +/− ICI | --- | Recruiting |
| AZD5863 | NCT06005493 | I/II | 240 * | solid tumors | monotherapy | --- | Recruiting |
| PM1032 | NCT05839106 | I/II | 200 * | solid tumors | monotherapy | ORR 20% [78] | Recruiting |
| LB4330 | NCT06468358 | I/II | 194 * | solid tumors | monotherapy | --- | Recruiting |
| IBI389 | NCT05164458 | I | 320 * | solid tumors | monotherapy | ORR 30.8%, DCR 73.1% [79] | Recruiting |
| Agent Name | NCT | Phase | Enrollment | Subjects | Combination(s) | Outcomes † | Study Status |
|---|---|---|---|---|---|---|---|
| CT041 | NCT03159819 | I | 12 | GC, PC | monotherapy | ORR 33.3%, mPFS 130 days [84] | Unknown status |
| CT041 | NCT03874897 | I | 98 | solid tumors | monotherapy +/- ICI | ORR 38.8%, DCR 91.8%, mPFS 4.4 mo, mOS 8.8 mo [85] | Completed |
| CT041 | NCT04404595 | I/II | 110 * | GC/GEJC, PC | monotherapy | ORR 26.3%, DOR 3.7 mo, mPFS 3.3 mo, mOS 8.9 mo [86] | Active, not recruiting |
| CT041 | NCT04581473 NCT03874897 | I/II | 192 * | PC | monotherapy | ORR 16.7%, DCR 70.8%, mPFS 3.3 mo, mOS 10.0 mo [87] | Active, not recruiting |
| IMC002 | NCT05472857 | I | 30 * | solid tumors | monotherapy | --- | Recruiting |
| IMC002 | NCT05946226 | I | 18 * | GI tumors | monotherapy | --- | Recruiting |
| IMC008 | NCT05837299 | I | 18 * | solid tumors | monotherapy | --- | Recruiting |
| KD-496 | NCT05583201 | I | 18 * | solid tumors | monotherapy | --- | Recruiting |
| IBI345 | NCT05199519 | I | 7 | solid tumors | monotherapy | --- | Completed |
| LB1908 | NCT05539430 | I | 56 * | GC/GEJC, PC | monotherapy | --- | Recruiting |
| TAC01-CLDN18.2 | NCT05862324 | I/II | 113 * | solid tumors | monotherapy | DCR 100% [88] | Active, not recruiting |
| AZD6422 | NCT05981235 | I | 8 | solid tumors | monotherapy | --- | Completed |
| CT048 | NCT05393986 | I | 63 * | solid tumors | monotherapy | --- | Unknown status |
| Modalities | Most Common TRAEs | Mechanistic Rationale | Mitigation Strategies |
|---|---|---|---|
| mAbs | Nausea, vomiting, decreased appetite | On-target binding in gastric mucosa (CLDN18.2 in normal mucosa) | Prophylactic antiemetics; slow infusion rate; hold/reduce dose per protocol |
| BsAbs | Nausea and vomiting; infusion reactions (less common) | Similar on-target gastric effects; potential T-cell engagement component | Antiemetics; premedication for infusion reactions; infusion rate control |
| ADCs | Hematologic AEs; GI AEs (generally lower grade vs. mAbs) | Payload-mediated marrow toxicity; some on-target/off-tumor effects | CBC monitoring; infection risk reduction; antiemetics; infusion adjustments |
| CAR T | CRS (grade 1–2); hematologic cytopenias (very common) | Immune activation and lymphodepletion; less on-target gastric effects | CRS support +/− steroids, tocilizumab; antimicrobial prophylaxis; CBC monitoring |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez Wiscovitch, A.; Sanchez Mendez, R.J.; Chuy, J. CLDN18.2-Targeted Therapy in Gastrointestinal Cancers. Cancers 2025, 17, 3764. https://doi.org/10.3390/cancers17233764
Dominguez Wiscovitch A, Sanchez Mendez RJ, Chuy J. CLDN18.2-Targeted Therapy in Gastrointestinal Cancers. Cancers. 2025; 17(23):3764. https://doi.org/10.3390/cancers17233764
Chicago/Turabian StyleDominguez Wiscovitch, Andrea, Ricardo J. Sanchez Mendez, and Jennifer Chuy. 2025. "CLDN18.2-Targeted Therapy in Gastrointestinal Cancers" Cancers 17, no. 23: 3764. https://doi.org/10.3390/cancers17233764
APA StyleDominguez Wiscovitch, A., Sanchez Mendez, R. J., & Chuy, J. (2025). CLDN18.2-Targeted Therapy in Gastrointestinal Cancers. Cancers, 17(23), 3764. https://doi.org/10.3390/cancers17233764







