The Clinical Relevance of Tumor Biomarkers in Prostate Cancer—A Review
Simple Summary
Abstract
1. Introduction
2. Methods
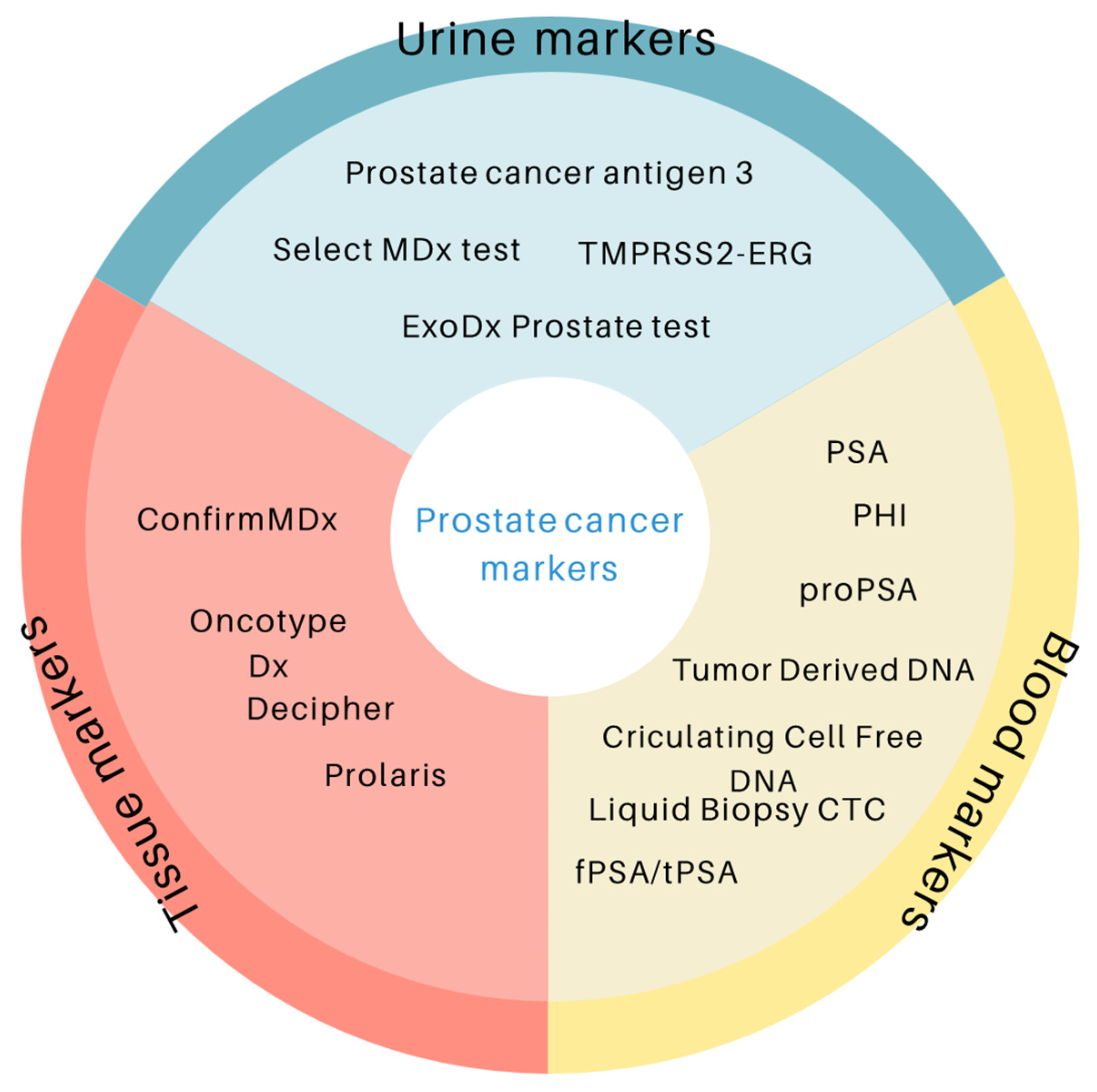
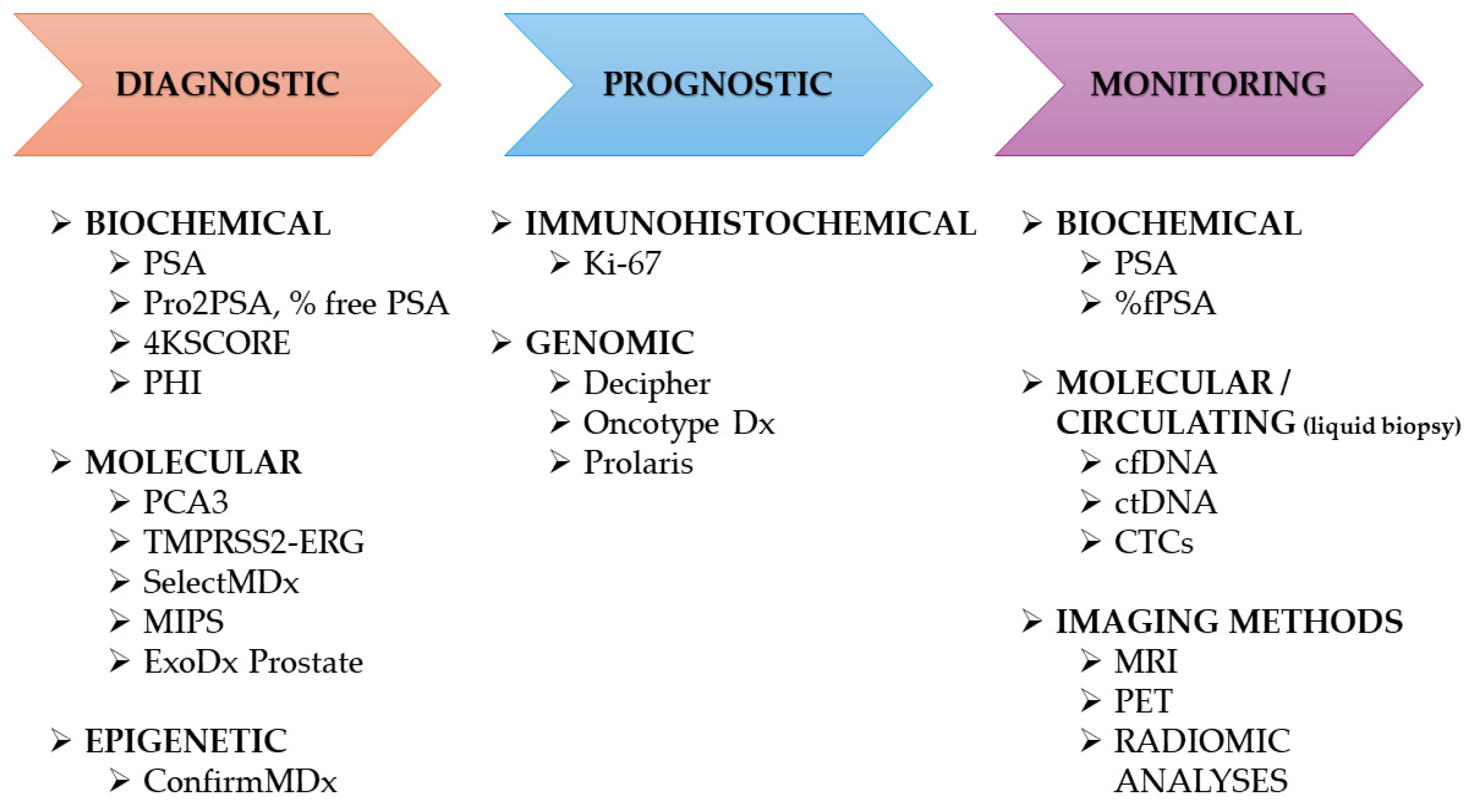
3. Prostate Cancer Markers
3.1. Blood Markers
3.1.1. PSA and Its Derivatives
3.1.2. Liquid Biopsy (Circulating Tumor Cell (CTC), Tumor Derived (ct) DNA, Circulating Cell Free (cf) DNA)
3.2. Urine Markers
3.2.1. Prostate Cancer Antigen 3 (PCA3)
3.2.2. Select MDx Test
3.2.3. TMPRSS2-ERG
3.2.4. ExoDx Prostate Test
3.3. Tissue Markers
3.3.1. ConfirmMDx
3.3.2. Prolaris
3.3.3. Decipher
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Wasim, S.; Lee, S.-Y.; Kim, J. Complexities of Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 14257. [Google Scholar] [CrossRef]
- Benafif, S.; Kote-Jarai, Z.; Eeles, R.A. A review of prostate cancer genome wide association studies (GWAS). Cancer Epidemiol. Biomark. Prev. 2018, 27, 845–857. [Google Scholar] [CrossRef]
- Berry, R.; Schaid, D.J.; Smith, J.R.; French, A.J.; Schroeder, J.J.; McDonnell, S.K.; Peterson, B.J.; Wang, Z.Y.; Carpten, J.D.; Roberts, S.G.; et al. Linkage analyses at the chromosome 1 loci 1q24-25 (HPC1), 1q42.2-43 (PCAP), and 1p36 (CAPB) in families with hereditary prostate cancer. Am. J. Hum. Genet. 2000, 66, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.W.; Mosquera, J.M.; Garofalo, A.; Oh, C.; Baco, M.; Amin-Mansour, A.; Rabasha, B.; Bahl, S.; Mullane, S.A.; Robinson, B.D.; et al. Exome sequencing of African-American prostate cancer reveals loss-of-function ERF mutations. Cancer Discov. 2017, 7, 973–983. [Google Scholar] [CrossRef]
- Bhindi, B.; Kulkarni, G.S.; Finelli, A.; Alibhai, S.M.; Hamilton, R.J.; Toi, A.; van der Kwast, T.H.; Evans, A.; Hersey, K.; Jewett, M.A.; et al. Obesity is associated with risk of progression for low-risk prostate cancers managed expectantly. Eur. Urol. 2014, 66, 841–848. [Google Scholar] [CrossRef]
- Schatten, H. Brief Overview of Prostate Cancer Statistics, Grading, Diagnosis and Treatment Strategies. In Cell and Molecular Biology of Prostate Cancer: Updates, Insights and New Frontiers; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1095, pp. 1–14. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.S. Metastatic prostate cancer. N. Engl. J. Med. 2018, 378, 645–657. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W., Jr.; et al. Screening for prostate cancer: US Preventive services task force recommendation statement. JAMA 2018, 319, 1901–1913. [Google Scholar] [CrossRef]
- Richardson, T.D.; Oesterling, J.E. Age-specific reference ranges for serum prostate-specific antigen. Urol. N. Am. 1997, 24, 339–351. [Google Scholar] [CrossRef]
- Filella, X.; Foj, L.; Augé, J.M.; Molina, R.; Alcover, J. Clinical utility of %p2PSA and prostate health index in the detection of prostate cancer. Clin. Chem. Lab. Med. 2014, 52, 1347–1355. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. Guidelines on Prostate Cancer. European Association of Urology. 2013. Available online: http://www.uroweb.org (accessed on 7 October 2025).
- Aminsharifi, A.; Howard, L.; Wu, Y.; De Hoedt, A.; Bailey, C.; Freedland, S.J.; Polascik, T.J. Prostate Specific Antigen Density as a Predictor of Clinically Significant Prostate Cancer When the Prostate Specific Antigen is in the Diagnostic Gray Zone: Defining the Optimum Cutoff Point Stratified by Race and Body Mass Index. J. Urol. 2018, 200, 758–766. [Google Scholar] [CrossRef]
- Falagario, U.G.; Jambor, I.; Lantz, A.; Ettala, O.; Stabile, A.; Taimen, P.; Aronen, H.J.; Knaapila, J.; Perez, I.M.; Gandaglia, G.; et al. Combined use of prostate-specific antigen density and magnetic resonance imaging for prostate biopsy decision planning: A retrospective multi-institutional study using the Prostate Magnetic Resonance Imaging Outcome Database (PROMOD). Eur. Urol. Oncol. 2020, 4, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Catalona, W.J.; Partin, A.W.; Slawin, K.M.; Brawer, M.K.; Flanigan, R.C.; Patel, A.; Richie, J.P.; deKernion, J.B.; Walsh, P.C.; Scardino, P.T.; et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: A prospective multicenter clinical trial. JAMA 1998, 279, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Z.Z.; Huang, Y.L.; Song, H.J.; Wang, Y.J. Value of free/total prostate-specific antigen (f/t PSA) ratios for prostate cancer detection in patients with total serum prostate-specific antigen between 4 and 10 ng/mL: A meta-analysis. Medicine 2018, 97, e0249. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Miyakubo, M.; Sekine, Y.; Koike, H.; Matsui, H.; Shibata, Y.; Suzuki, K. Diagnostic significance of [−2]pro-PSA, prostate dimension-adjusted PSA-related indices in men with total PSAin the 20–100 ng/mLrange. World J. Urol. 2013, 31, 305–311. [Google Scholar] [CrossRef]
- Guazzoni, G.; Nava, L.; Lazzeri, M.; Scattoni, V.; Lughezzani, G.; Maccagnano, C.; Dorigatti, F.; Ceriotti, F.; Pontillo, M.; Bini, V.; et al. Prostate-specific antigen (PSA) isoform p2PSA significantly improves the prediction of prostate cancer at initial extended prostate biopsies in patients with total PSA between 2.0 and 10 ng/mL: Results of a prospective study in a clinical setting. Eur. Urol. 2011, 60, 214–222. [Google Scholar] [CrossRef]
- Loeb, S.; Sanda, M.G.; Broyles, D.L.; Shin, S.S.; Bangma, C.H.; Wei, J.T.; Partin, A.W.; Klee, G.G.; Slawin, K.M.; Marks, L.S.; et al. The prostate health index selectively identifies clinically significant prostate cancer. J. Urol. 2015, 193, 1163–1169. [Google Scholar] [CrossRef]
- Friedersdorff, F.; Gross, B.; Maxeiner, A.; Jung, K.; Miller, K.; Stephan, C.; Busch, J.; Kilic, E. Does the prostate health index depend on tumor volume?—A study on 196 patients after radical prostatectomy. Int. J. Mol. Sci. 2017, 18, E488. [Google Scholar] [CrossRef]
- Maxeiner, A.; Kilic, E.; Matalon, J.; Friedersdorff, F.; Miller, K.; Jung, K.; Stephan, C.; Busch, J. The Prostate Health Index (PHI) predicts oncological outcome and biochemical recurrence after radical prostatectomy—Analysis in 437 patients. Oncotarget 2017, 8, 79279–79288. [Google Scholar] [CrossRef]
- Füzéry, A.K.; Levin, J.; Chan, M.M.; Chan, D.W. Translation of proteomic biomarkers into FDA approved cancer diagnostics: Issues and challenges. Clin. Proteom. 2013, 10, 13. [Google Scholar] [CrossRef]
- Millner, L.M.; Linder, M.W.; Valdes, R., Jr. Circulating tumor cells: A review of present methods and the need to identify heterogeneous phenotypes. Ann. Clin. Lab. Sci. 2013, 43, 295–304. [Google Scholar] [PubMed]
- Lepor, A.; Catalona, W.J.; Loeb, S. The prostate health index: Its utility in prostate cancer detection. Urol. Clin. N. Am. 2016, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.E.G.; Matos, A.D.R.; Ferreira, L.B.; Gimba, E.R.P. The long non-coding RNA PCA3: An update of its functions and clinical applications as a biomarker in prostate cancer. Oncotarget 2019, 10, 6589–6603. [Google Scholar] [CrossRef] [PubMed]
- Ravi, N.; John, N.A.; Taranikanti, M. Serum and urine biomarkers for prostate cancer: A mini review. Mymensingh Med. J. 2025, 34, 598–603. [Google Scholar]
- Tosoian, J.J.; Druskin, S.C.; Andreas, D.; Mullane, P.; Chappidi, M.; Joo, S.; Ghabili, K.; Mamawala, M.; Agostino, J.; Carter, H.B.; et al. Prostate Health Index Density improves detection of clinically significant prostate cancer. BJU Int. 2017, 120, 793–798. [Google Scholar] [CrossRef]
- Nordstrom, T.; Vickers, A.; Assel, M.; Lilja, H.; Gronberg, H.; Eklund, M. Comparison between the four-kallikrein panel and prostate health index for predicting prostate cancer. Eur. Urol. 2015, 68, 139–146. [Google Scholar] [CrossRef]
- Russo, G.I.; Regis, F.; Castelli, T.; Favilla, V.; Privitera, S.; Giardina, R.; Cimino, S.; Morgia, G. A systematic review and meta-analysis of the diagnostic accuracy of Prostate Health Index and 4-Kallikrein Panel score in predicting overall and high-grade prostate cancer. Clin. Genitourin. Cancer 2017, 15, 429–439. [Google Scholar] [CrossRef]
- Heidrich, I.; Ačkar, L.; Mohammadi, P.M.; Pantel, K. Liquid biopsies: Potential and challenges. Int. J. Cancer 2021, 148, 528–545. [Google Scholar] [CrossRef]
- Joosse, S.A.; Beyer, B.; Gasch, C.; Nastały, P.; Kuske, A.; Isbarn, H.; Horst, L.J.; Hille, C.; Gorges, T.M.; Cayrefourcq, L.; et al. Tumor-associated release of prostatic cells into the blood after transrectal ultrasound-guided biopsy in patients with histologically confirmed prostate cancer. Clin. Chem. 2020, 66, 161–168. [Google Scholar] [CrossRef]
- Gourdin, T.; Sonpavde, G. Utility of cell-free nucleic acid and circulating tumor cell analyses in prostate cancer. Asian J. Androl. 2018, 20, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Riethdorf, S.; Fritsche, H.; Muller, V.; Rau, T.; Schindlbeck, C.; Rack, B.; Janni, W.; Coith, C.; Beck, K.; Jänicke, F.; et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch system. Clin. Cancer Res. 2007, 13, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Maccaferri, M.; Percesepe, A.; Tomasi, A.; Ozben, T. Liquid biopsy with cell free DNA: New horizons for prostate cancer. Crit. Rev. Clin. Lab. Sci. 2021, 58, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Brighi, N.; Conteduca, D.; Bleve, S.; Gianni, C.; Schepisi, G.; Iaia, M.L.; Gurioli, G.; Lolli, C.; De Giorgi, U. An update on our ability to monitor castration-resistant prostate cancer dynamics with cell-free DNA. Expert Rev. Mol. Diagn. 2021, 21, 631–640. [Google Scholar] [CrossRef]
- Haese, A.; de la Taille, A.; van Poppel, H.; Marberger, M.; Stenzl, A.; Mulders, P.F.; Huland, H.; Abbou, C.C.; Remzi, M.; Tinzl, M.; et al. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur. Urol. 2008, 54, 1081–1088. [Google Scholar] [CrossRef]
- Kohaar, I.; Petrovics, G.; Srivastava, S. A rich array of prostate cancer molecular biomarkers: Opportunities and challenges. Int. J. Mol. Sci. 2019, 20, 1813. [Google Scholar] [CrossRef]
- Marks, L.S.; Fradet, Y.; Deras, I.L.; Blase, A.; Mathis, J.; Aubin, S.M.; Cancio, A.T.; Desaulniers, M.; Ellis, W.J.; Rittenhouse, H.; et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology 2007, 69, 532–535. [Google Scholar] [CrossRef]
- Leyten, G.H.J.M.; Hessels, D.; Jannink, S.A.; Smit, F.P.; de Jong, H.; Cornel, E.B.; de Reijke, T.M.; Vergunst, H.; Kil, P.; Knipscheer, B.C.; et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur. Urol. 2014, 65, 534–542. [Google Scholar] [CrossRef]
- Wei, J.T.; Feng, Z.; Partin, A.W.; Brown, E.; Thompson, I.; Sokoll, L.; Chan, D.W.; Lotan, Y.; Kibel, A.S.; Busby, J.E.; et al. Can urinary PCA3 supplement PSA in the early detection of prostate cancer? J. Clin. Oncol. 2014, 32, 4066. [Google Scholar] [CrossRef]
- Soleimani, M.; Masoumi, N.; Jouzi, A.; Darzi, M.M. Potential value of Prostate Cancer Antigen 3 score in prediction of final cancer pathology parameters in radical prostatectomy patients. Urologia 2025, 92, 259–266. [Google Scholar] [CrossRef]
- Schmid, M.; Hansen, J.; Chun, F.K. Urinary Prostate Cancer Antigen 3 as a tumour marker: Biochemical and clinical aspects. Adv. Exp. Med. Biol. 2015, 867, 277–289. [Google Scholar] [CrossRef]
- Deras, I.L.; Aubin, S.M.; Blase, A.; Day, J.R.; Koo, S.; Partin, A.W.; Ellis, W.J.; Marks, L.S.; Fradet, Y.; Rittenhouse, H.; et al. PCA3: A molecular urine assay for predicting prostate biopsy outcome. J. Urol. 2008, 179, 1587–1592. [Google Scholar] [CrossRef]
- Hendriks, R.J.; van der Leest, M.M.G.; Dijkstra, S.; Barentsz, J.O.; Van Criekinge, W.; Hulsbergen-van de Kaa, C.A.; Schalken, J.A.; Mulders, P.F.A.; van Oort, I.M. A urinary biomarker-based risk score correlates with multiparametric MRI for prostate cancer detection. Prostate 2017, 77, 1401–1407. [Google Scholar] [CrossRef]
- Van Neste, L.; Hendriks, R.J.; Dijkstra, S.; Trooskens, G.; Cornel, E.B.; Jannink, S.A.; de Jong, H.; Hessels, D.; Smit, F.P.; Melchers, W.J.; et al. Detection of high-grade prostate cancer using a urinary molecular biomarker-based risk score. Eur. Urol. 2016, 70, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Rocco, B.; Maggi, M.; Lucarelli, G.; Falagario, U.G.; Del Giudice, F.; Crocetto, F.; Barone, B.; La Civita, E.; Lasorsa, F.; et al. Beyond blood biomarkers: The role of SelectMDX in clinically significant prostate cancer identification. Expert Rev. Mol. Diagn. 2023, 23, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Lendínez-Cano, G.; Ojeda-Claro, A.V.; Gómez-Gómez, E.; Morales Jimenez, P.; Flores Martin, J.; Dominguez, J.F.; Amores, J.; Cozar, J.M.; Bachiller, J.; Juárez, A.; et al. Prospective study of diagnostic accuracy in the detection of high-grade prostate cancer in biopsy-naïve patients with clinical suspicion of prostate cancer who underwent the Select MDx test. Prostate 2021, 81, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef]
- Eryilmaz, I.E.; Kordan, Y.; Vuruskan, B.A.; Kaygısız, O.; Tunca, B.; Cecener, G. T2E (TMPRSS2-ERG) fusion transcripts are associated with higher levels of AMACR mRNA and a subsequent prostate cancer diagnosis in patients with atypical small acinar proliferation. Gene 2018, 645, 69–75. [Google Scholar] [CrossRef]
- Font-Tello, A.; Juanpere, N.; de Muga, S.; Lorenzo, M.; Lorente, J.A.; Fumado, L.; Serrano, L.; Serrano, S.; Lloreta, J.; Hernández, S. Association of ERG and TMPRSS2-ERG with grade, stage, and prognosis of prostate cancer is dependent on their expression levels. Prostate 2015, 75, 1216–1226. [Google Scholar] [CrossRef]
- Knuuttila, M.; Mehmood, A.; Mäki-Jouppila, J.; Ryberg, H.; Taimen, P.; Knaapila, J.; Ettala, O.; Boström, P.J.; Ohlsson, C.; Venäläinen, M.S.; et al. Intratumoral androgen levels are linked to TMPRSS2-ERG fusion in prostate cancer. Endocr. Relat. Cancer 2018, 25, 807–819. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, K.W.; Lee, H.J.; Kim, H.S. Systematic analysis of cellular signaling pathways and therapeutic targets for SLC45A3:ERG fusion-positive prostate cancer. J. Pers. Med. 2022, 12, 1818. [Google Scholar] [CrossRef] [PubMed]
- Blee, A.M.; Liu, S.; Wang, L.; Huang, H. BET bromodomain-mediated interaction between ERG and BRD4 promotes prostate cancer cell invasion. Oncotarget 2016, 7, 38319–38332. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, F.; Chellini, L.; Riccioni, V.; Paronetto, M.P. Oncogenic fusions harboring ETS genes: Exploring novel targetable opportunities in prostate cancer. Cancers 2025, 17, 1657. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, A.; Kajau, H.; Margolis, E.; Tutrone, R.; Grimm, T.; Trottmann, M.; Stief, C.; Stoll, G.; Fischer, C.A.; Flinspach, C.; et al. Validation of a CE-IVD, urine exosomal RNA expression assay for risk assessment of prostate cancer prior to biopsy. Sci. Rep. 2022, 12, 4777. [Google Scholar] [CrossRef]
- McKiernan, J.; Noerholm, M.; Tadigotla, V.; Kumar, S.; Torkler, P.; Sant, G.; Alter, J.; Donovan, M.J.; Skog, J. A urine-based exosomal gene expression test stratifies risk of high-grade prostate cancer in men with prior negative prostate biopsy undergoing repeat biopsy. BMC Urol. 2020, 20, 138. [Google Scholar] [CrossRef]
- Tutrone, R.; Donovan, M.J.; Torkler, P.; Tadigotla, V.; McLain, T.; Noerholm, M.; Skog, J.; McKiernan, J. Clinical utility of the exosome-based ExoDx Prostate (IntelliScore) EPI test in men presenting for initial biopsy with a PSA 2–10 ng/mL. Prostate Cancer Prostatic Dis. 2020, 23, 607–614. [Google Scholar] [CrossRef]
- Margolis, E.; Brown, G.; Partin, A.; Carter, B.; McKiernan, J.; Tutrone, R.; Torkler, P.; Fischer, C.; Tadigotla, V.; Noerholm, M.; et al. Predicting high-grade prostate cancer at initial biopsy: Clinical performance of the ExoDx (EPI) Prostate Intelliscore test in three independent prospective studies. Prostate Cancer Prostatic Dis. 2021, 25, 296–301. [Google Scholar] [CrossRef]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.K.; Enderle, D.; Noerholm, M.; Breakefield, X.O.; Skog, J.K. Exosome-based liquid biopsies in cancer: Opportunities and challenges. Ann. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef]
- Kretschmer, A.; Tutrone, R.; Alter, J.; Berg, E.; Fischer, C.; Kumar, S.; Torkler, P.; Tadigotla, V.; Donovan, M.; Sant, G.; et al. Pre-diagnosis urine exosomal RNA (ExoDx EPI score) is associated with post-prostatectomy pathology outcome. World J. Urol. 2022, 40, 983–989. [Google Scholar] [CrossRef]
- Boehm, B.E.; York, M.E.; Petrovics, G.; Kohaar, I.; Chesnut, G.T. Biomarkers of aggressive prostate cancer at diagnosis. Int. J. Mol. Sci. 2023, 24, 2185. [Google Scholar] [CrossRef]
- Carroll, P.R.; Parsons, J.K.; Andriole, G.; Bahnson, R.R.; Castle, E.P.; Catalona, W.J.; Dahl, D.M.; Davis, J.W.; Epstein, J.I.; Etzioni, R.B.; et al. NCCN Guidelines Insights: Prostate Cancer Early Detection, Version 2. 2016. J. Natl. Compr. Cancer Netw. 2016, 14, 509–519. [Google Scholar] [CrossRef]
- Stewart, G.D.; Van Neste, L.; Delvenne, P.; Delrée, P.; Delga, A.; McNeill, S.A.; O’Donnell, M.; Clark, J.; Van Criekinge, W.; Bigley, J.; et al. Clinical utility of an epigenetic assay to detect occult prostate cancer in histopathologically negative biopsies: Results of the MATLOC study. J. Urol. 2013, 189, 1110–1116. [Google Scholar] [CrossRef]
- Partin, A.W.; Van Neste, L.; Klein, E.A.; Marks, L.S.; Gee, J.R.; Troyer, D.A.; Rieger-Christ, K.; Jones, J.S.; Magi-Galluzzi, C.; Mangold, L.A.; et al. Clinical validation of an epigenetic assay to predict negative histopathological results in repeat prostate biopsies. J. Urol. 2014, 192, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Wojno, K.J.; Costa, F.J.; Cornell, R.J.; Small, J.D.; Pasin, E.; Van Criekinge, W.; Bigley, J.W.; Van Neste, L. Reduced rate of repeated prostate biopsies observed in ConfirmMDx clinical utility field study. Am. Health Drug Benefits 2014, 7, 129–134. [Google Scholar] [PubMed]
- Waterhouse, R.L.; Van Neste, L.; Moses, K.A.; Barnswell, C.; Silberstein, J.L.; Jalkut, M.; Tutrone, R.; Sylora, J.; Anglade, R.; Murdock, M.; et al. Evaluation of an epigenetic assay for predicting repeat prostate biopsy outcome in African American men. Urology 2019, 128, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Belkacemi, Y.; Debbi, K.; Coraggio, G.; Bendavid, J.; Nourieh, M.; To, N.H.; Cherif, M.A.; Saldana, C.; Ingels, A.; De La Taille, A.; et al. Genomic Prostate Score: A New Tool to Assess Prognosis and Optimize Radiation Therapy Volumes and ADT in Intermediate-Risk Prostate Cancer. Cancers 2023, 15, 945. [Google Scholar] [CrossRef]
- Duffy, M.J. Biomarkers for prostate cancer: Prostate-specific antigen and beyond. Clin. Chem. Lab. Med. 2020, 58, 326–339. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K.; Lu, R.; Zhang, N.; Quesenberry, C.P.; Shan, J.; Han, J.S.; Tsiatis, A.C.; Leimpeter, A.D.; Lawrence, H.J.; Febbo, P.G.; et al. A Biopsy-Based 17-Gene Genomic Prostate Score as a Predictor of Metastases and Prostate Cancer Death in Surgically Treated Men with Clinically Localized Disease. Eur. Urol. 2018, 73, 129–138. [Google Scholar] [CrossRef]
- Wei, L.; Wang, J.; Lampert, E.; Schlanger, S.; DePriest, A.D.; Hu, Q.; Gomez, E.C.; Murakam, M.; Glenn, S.T.; Conroy, J.; et al. Intratumoral and intertumoral genomic heterogeneity of multifocal localized prostate cancer impacts molecular classifications and genomic prognosticators. Eur. Urol. 2017, 71, 183–192. [Google Scholar] [CrossRef]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef]
- Health Quality Ontario. Prolaris Cell Cycle Progression Test for Localized Prostate Cancer: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2017, 17, 1–75. [Google Scholar]
- Cuzick, J.; Swanson, G.P.; Fisher, G.; Brothman, A.R.; Berney, D.M.; Reid, J.E.; Mesher, D.; Speights, V.O.; Stankiewicz, E.; Foster, C.S.; et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: A retrospective study. Lancet Oncol. 2011, 12, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Jairath, N.K.; Dal Pra, A.; Vince, R.; Dess, R.T.; Jackson, W.C.; Tosoian, J.J.; McBride, S.M.; Zhao, S.G.; Berlin, A.; Mahal, B.A.; et al. A systematic review of the evidence for the Decipher genomic classifier in prostate cancer. Eur. Urol. 2021, 79, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Erho, N.; Crisan, A.; Vergara, I.A.; Mitra, A.P.; Ghadessi, M.; Buerki, C.; Bergstralh, E.J.; Kollmeyer, T.; Fink, S.; Haddad, Z.; et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE 2013, 8, e66855. [Google Scholar] [CrossRef] [PubMed]
- Dolgetta, G.G.; King, T.S.; Behers, B.J.; Carey, M.S.; Ghazli, K.; Carey, R.I. Correlation of post-prostatectomy Decipher genomic classification to clinical outcomes in prostate cancer over 8 year follow-up. J. Urol. 2023, 209 (Suppl. 4), e746. [Google Scholar] [CrossRef]
- Treacy, P.J.; Falagario, U.G.; Magniez, F.; Ratnani, P.; Wajswol, E.; Martini, A.; Jambor, I.; Wiklund, P.; Bentellis, I.; Barthe, F.; et al. Decipher score predicts prostate specific antigen persistence after prostatectomy. Minerva Urol. Nephrol. 2023, 75, 583–590. [Google Scholar] [CrossRef]
- Gore, J.L.; du Plessis, M.; Santiago-Jiménez, M.; Yousefi, K.; Thompson, D.J.S.; Karsh, L.; Lane, B.R.; Franks, M.; Chen, D.Y.T.; Bandyk, M.; et al. Decipher test impacts decision making among patients considering adjuvant and salvage treatment after radical prostatectomy: Interim results from the multicenter prospective PRO-IMPACT study. Cancer 2017, 123, 2850–2859. [Google Scholar] [CrossRef]
- Huang, J.; Liu, D.; Li, J.; Xu, J.; Dong, S.; Zhang, H. A 12-gene panel in estimating hormone-treatment responses of castration-resistant prostate cancer patients generated using a combined analysis of bulk and single-cell sequencing data. Ann. Med. 2023, 55, 2260387. [Google Scholar] [CrossRef]
- Merriel, S.W.D.; Pocock, L.; Gilbert, E.; Creavin, S.; Walter, F.M.; Spencer, A.; Hamilton, W. Systematic Review and Meta-Analysis of the Diagnostic Accuracy of Prostate-Specific Antigen (PSA) for the Detection of Prostate Cancer in Symptomatic Patients. BMC Med. 2022, 20, 54. [Google Scholar] [CrossRef]
- Ilic, D.; Djulbegovic, M.; Jung, J.H.; Hwang, E.C.; Zhou, Q.; Cleves, A.; Agoritsas, T.; Dahm, P. Prostate Cancer Screening with Prostate-Specific Antigen (PSA) Test: A Systematic Review and Meta-Analysis. BMJ 2018, 362, k3519. [Google Scholar] [CrossRef]
- Agnello, L.; Vidali, M.; Giglio, R.V.; Gambino, C.M.; Ciaccio, A.M.; Lo Sasso, B.; Ciaccio, M. Prostate Health Index (PHI) as a Reliable Biomarker for Prostate Cancer: A Systematic Review and Meta-Analysis. Clin. Chem. Lab. Med. 2022, 60, 1261–1277. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Hou, C.M.; Vo, T.T.T.; Hong, J.H.; Huang, C.Y.; Wang, Y.L.; Chen, Y.L.; Chiang, C.H. Optimizing Prostate Cancer Care: Clinical Utility of the Prostate Health Index. Prostate 2025, 85, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Zappala, S.M.; Scardino, P.T.; Okrongly, D.; Linder, V.; Dong, Y. Clinical Performance of the 4Kscore Test to Predict High-Grade Prostate Cancer at Biopsy: A Meta-Analysis of U.S. and European Clinical Validation Study Results. Rev. Urol. 2017, 19, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Mi, C.; Bai, L.; Yang, Y.; Duan, J.; Gao, L. 4Kscore Diagnostic Value in Patients with High-Grade Prostate Cancer Using Cutoff Values of 7.5% to 10%: A Meta-Analysis. Urol. Oncol. 2021, 39, 366.e1–366.e10. [Google Scholar] [CrossRef]
- Rodríguez, S.V.M.; García-Perdomo, H.A. Diagnostic Accuracy of Prostate Cancer Antigen 3 (PCA3) Prior to First Prostate Biopsy: A Systematic Review and Meta-Analysis. Can. Urol. Assoc. J. 2020, 14, E214–E219. [Google Scholar] [CrossRef]
- Lee, D.; Shim, S.R.; Ahn, S.T.; Oh, M.M.; Moon, D.G.; Park, H.S.; Cheon, J.; Kim, J.W. Diagnostic Performance of the Prostate Cancer Antigen 3 Test in Prostate Cancer: Systematic Review and Meta-Analysis. Clin. Genitourin. Cancer 2020, 18, 402–408.e5. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Aubin, S.M.; Siddiqui, J.; Lonigro, R.J.; Sefton-Miller, L.; Miick, S.; Williamsen, S.; Hodge, P.; Meinke, J.; Blase, A.; et al. Urine TMPRSS2:ERG Fusion Transcript Stratifies Prostate Cancer Risk in Men with Elevated Serum PSA. Sci. Transl. Med. 2011, 3, 94ra72. [Google Scholar] [CrossRef]
- Salami, S.S.; Schmidt, F.; Laxman, B.; Regan, M.M.; Rickman, D.S.; Scherr, D.; Bueti, G.; Siddiqui, J.; Tomlins, S.A.; Wei, J.T.; et al. Combining Urinary Detection of TMPRSS2:ERG and PCA3 with Serum PSA to Predict Diagnosis of Prostate Cancer. Urol. Oncol. 2013, 31, 566–571. [Google Scholar] [CrossRef]
- Hendriks, R.J.; van der Leest, M.M.G.; Israël, B.; Hannink, G.; Yanti Setiasti, A.; Cornel, E.B.; Hulsbergen-van de Kaa, C.A.; Klaver, O.S.; Sedelaar, J.P.M.; Van Criekinge, W.; et al. Clinical Use of the SelectMDx Urinary-Biomarker Test with or without mpMRI in Prostate Cancer Diagnosis: A Prospective, Multicenter Study in Biopsy-Naïve Men. Prostate Cancer Prostatic Dis. 2021, 24, 1110–1119. [Google Scholar] [CrossRef]
- Wu, H.; Wu, Y.; He, P.; Liang, J.; Xu, X.; Ji, C. A Meta-Analysis for the Diagnostic Accuracy of SelectMDx in Prostate Cancer. PLoS ONE 2024, 19, e0285745. [Google Scholar] [CrossRef]
- McKiernan, J.; Donovan, M.J.; Margolis, E.; Partin, A.; Carter, B.; Brown, G.; Torkler, P.; Noerholm, M.; Skog, J.; Shore, N.; et al. A Prospective Adaptive Utility Trial to Validate Performance of a Novel Urine Exosome Gene Expression Assay to Predict High-Grade Prostate Cancer in Patients with Prostate-Specific Antigen 2–10 ng/mL at Initial Biopsy. Eur. Urol. 2018, 74, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.E.; Zhang, J.; Fishbane, N.; Hoedt, A.M.; Klaassen, Z.; Spratt, D.E.; Vidal, A.C.; Lin, D.; Hitchins, M.P.; You, S.; et al. Validation of a Genomic Classifier for Prediction of Metastasis and Prostate Cancer-Specific Mortality in African-American Men Following Radical Prostatectomy in an Equal Access Healthcare Setting. Prostate Cancer Prostatic Dis. 2020, 23, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Stone, S.; Fisher, G.; Yang, Z.H.; North, B.V.; Berney, D.M.; Beltran, L.; Greenberg, D.; Møller, H.; Reid, J.E.; et al. Validation of an RNA Cell Cycle Progression Score for Predicting Death from Prostate Cancer in a Conservatively Managed Needle Biopsy Cohort. Br. J. Cancer 2015, 113, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Scholz, M.C.; Kar, A.J.; Fegan, J.E.; Haregewoin, A.; Kaldate, R.R.; Brawer, M.K. Cell Cycle Progression Score and Treatment Decisions in Prostate Cancer: Results from an Ongoing Registry. Curr. Med. Res. Opin. 2014, 30, 1025–1031. [Google Scholar] [CrossRef]
- Carlsson, S.V.; Roobol, M.J. Improving the Evaluation and Diagnosis of Clinically Significant Prostate Cancer in 2017. Curr. Opin. Urol. 2017, 27, 198–204. [Google Scholar] [CrossRef]
- Filippi, L.; Urso, L.; Bianconi, F.; Palumbo, B.; Marzola, M.C.; Evangelista, L.; Schillaci, O. Radiomics and Theranostics with Molecular and Metabolic Probes in Prostate Cancer: Toward a Personalized Approach. Expert Rev. Mol. Diagn. 2023, 23, 243–255. [Google Scholar] [CrossRef]

| Test | Sample Type | Clinical Indication | Year of FDA Approval | References |
|---|---|---|---|---|
| Total PSA | Blood | Screening and diagnosis | 1986 | [23] |
| Free PSA | Blood | Screening and diagnosis | 1997 | [23] |
| CellSearchTM system | Blood | Monitoring patients with metastatic prostate cancer | 2004 | [24] |
| Pro2PSA | Blood | Screening and diagnosis | 2012 | [23] |
| PHI | Blood | Diagnostic accuracy improvement | 2012 | [25] |
| PCA3 | Urine | Re-biopsy decision | 2012 | [26] |
| 4Kscore | Blood | Help in deciding on a prostate biopsy | 2021 | [27] |
| Biomarker | Sample Type | Mechanism | References |
|---|---|---|---|
| PSA and derivatives (f/t PSA, PHI, PHID, 4Kscore) | Serum | Kallikrein-related proteases | [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] |
| Liquid biopsy (CTC, ctDNA, cfDNA) | Blood | Circulating tumor material | [22,27,28,29,30,31] |
| PCA3 | Urine | Non-coding RNA | [32,33,34,35,36,37,38,39] |
| SelectMDx | Urine | DLX1, HOXC6 mRNA | [40,41,42,43] |
| TMPRSS2-ERG | Urine/tissue | Gene fusion | [44,45,46,47,48,49,50] |
| ConfirmMDx | Tissue | Gene methylation (APC, GSTP1, RASSF1) | [51,52,53,54,55,56] |
| OncotypeDx GPS | Tissue | 17-gene RT-PCR panel | [57,58,59,60,61,62,63,64,65,66,67] |
| Prolaris (CCP) | Tissue | 31 cell cycle genes | [68,69] |
| Decipher | Tissue | 22 RNA biomarkers | [70,71,72,73,74,75] |
| Biomarker | Sensitivity | Specificity | AUC | Ref. |
|---|---|---|---|---|
| PSA | ~72–86% | ~30–47% | 0.55–0.70 | [81,82] |
| PHI (Prostate Health Index) | ~90% | ~31–66% | 0.70–0.78 | [83,84] |
| 4Kscore | ~86–97% | ~61–72% | 0.82–0.90 | [85,86] |
| PCA3 | ~55–69% | ~74–83% | 0.66–0.75 | [87,88] |
| TMPRSS2-ERG fusion | ~24–93% | ~93–97% | 0.63–0.74 | [89,90] |
| SelectMDx | ~76–91% | ~49–73% | 0.76–0.87 | [91,92] |
| ExoDx Prostate (EPI) | ~92% | ~34–47% | 0.70–0.72 | [58,93] |
| Decipher | – | – | 0.75–0.83 | [75,94] |
| Prolaris (CCR score) | – | – | 0.70–0.78 | [95,96] |
| ConfirmMDx | ~62–68% | ~64–71% | 0.70–0.80 | [66,97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewska, Z.; Zajkowska, M.; Pączek, S.; Nowiński, A.R.; Sokólska, W.; Gryko, M.; Orywal, K. The Clinical Relevance of Tumor Biomarkers in Prostate Cancer—A Review. Cancers 2025, 17, 3742. https://doi.org/10.3390/cancers17233742
Majewska Z, Zajkowska M, Pączek S, Nowiński AR, Sokólska W, Gryko M, Orywal K. The Clinical Relevance of Tumor Biomarkers in Prostate Cancer—A Review. Cancers. 2025; 17(23):3742. https://doi.org/10.3390/cancers17233742
Chicago/Turabian StyleMajewska, Zuzanna, Monika Zajkowska, Sara Pączek, Adam Rafał Nowiński, Weronika Sokólska, Mariusz Gryko, and Karolina Orywal. 2025. "The Clinical Relevance of Tumor Biomarkers in Prostate Cancer—A Review" Cancers 17, no. 23: 3742. https://doi.org/10.3390/cancers17233742
APA StyleMajewska, Z., Zajkowska, M., Pączek, S., Nowiński, A. R., Sokólska, W., Gryko, M., & Orywal, K. (2025). The Clinical Relevance of Tumor Biomarkers in Prostate Cancer—A Review. Cancers, 17(23), 3742. https://doi.org/10.3390/cancers17233742









