Individualized Triplet Chemotherapy Decision-Making in Metastatic Colorectal Cancer: A Machine-Learning-Driven Study †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Demographic data (gender, age at diagnosis),
- Comorbidities (diabetes, hypertension, coronary artery disease, hypothyroidism),
- Lifestyle information (smoking status),
- Family history (presence of cancer in first-degree relatives),
- Tumor characteristics (primary localization, histological subtype—mucinous; molecular markers—MSI, RAS, RAF),
- Sites of metastatic involvement (liver, peritoneum, lung, bone),
- Need for emergency surgery, primary tumor resection status,
- Comprehensive laboratory parameters (CEA, CA19-9, LDH, thyroid function tests, lipid panel, complete blood count, liver and kidney function tests, vitamin D, B12, folate, ferritin, zinc),
- Performance status (ECOG),
- Anthropometric measurements (height, weight),
- Local treatment information (surgery or radiofrequency ablation).
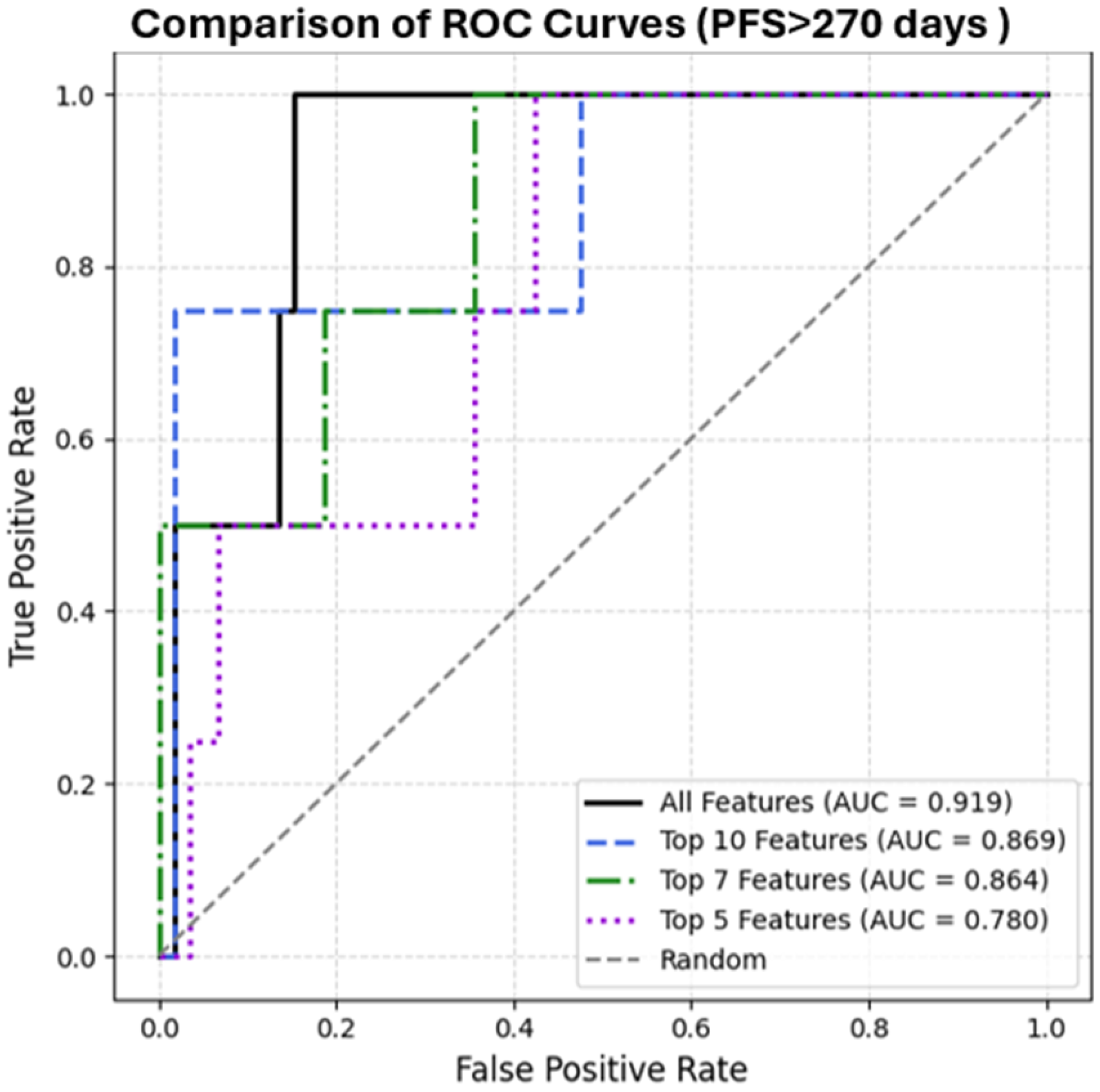
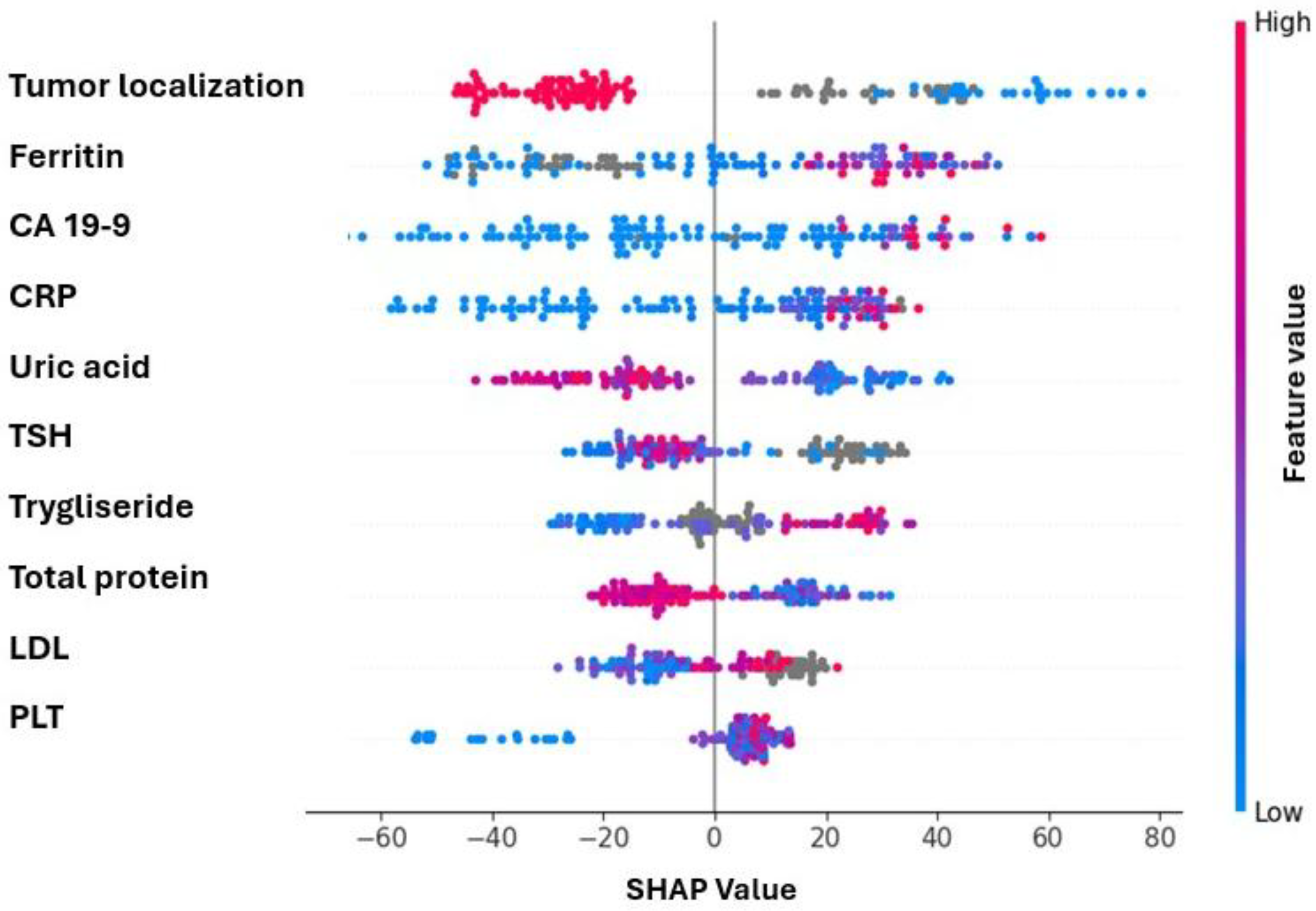
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ITE | Individual Treatment Effect |
| LOOCV | Leave-One-Out Cross-Validation |
| SHAP | SHapley Additive exPlanations |
References
- Kayaalp, M.; Akkus, E.; Karaoglan, B.B.; Utkan, G. Individualised triplet chemotherapy decision-making in metastatic colorectal cancer: A machine learning–driven study. In Proceedings of the ESMO Congress 2025, Berlin, Germany, 17–21 October 2025. [Google Scholar]
- Falcone, A.; Ricci, S.; Brunetti, I.; Pfanner, E.; Allegrini, G.; Barbara, C.; Crinò, L.; Benedetti, G.; Evangelista, W.; Fanchini, L.; et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 2007, 25, 1670–1676. [Google Scholar] [CrossRef]
- Rossini, D.; Antoniotti, C.; Lonardi, S.; Pietrantonio, F.; Moretto, R.; Antonuzzo, L.; Boccaccino, A.; Morano, F.; Brugia, M.; Pozzo, C.; et al. Upfront Modified Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan Plus Panitumumab Versus Fluorouracil, Leucovorin, and Oxaliplatin Plus Panitumumab for Patients with RAS/BRAF Wild-Type Metastatic Colorectal Cancer: The Phase III TRIPLETE Study by GONO. J. Clin. Oncol. 2022, 40, 2878–2888. [Google Scholar] [CrossRef] [PubMed]
- Souglakos, J.; Androulakis, N.; Syrigos, K.; Polyzos, A.; Ziras, N.; Athanasiadis, A.; Kakolyris, S.; Tsousis, S.; Kouroussis, C.; Vamvakas, L.; et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): A multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). Br. J. Cancer 2006, 94, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Antoniotti, C.; Rossini, D.; Lonardi, S.; Loupakis, F.; Pietrantonio, F.; Bordonaro, R.; Latiano, T.P.; Tamburini, E.; Santini, D.; et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Antoniotti, C.; Marmorino, F.; Rossini, D.; Schmoll, H.J.; Lonardi, S.; Tonini, G.; Borelli, B.; Cupini, S.; Stein, A.; Moretto, R.; et al. FOLFOXIRI/bevacizumab versus doublets/bevacizumab as initial therapy of unresectable liver-only, right-sided and/or RAS or BRAF mutated metastatic colorectal cancer: An individual patient data-based pooled analysis of randomized trials. Eur. J. Cancer 2025, 228, 115713. [Google Scholar] [CrossRef]
- Bachet, J.B.; Lucidarme, O.; Levache, C.B.; Barbier, E.; Raoul, J.L.; Lecomte, T.; Desauw, C.; Brocard, F.; Pernot, S.; Breysacher, G.; et al. FOLFIRINOX as induction treatment in rectal cancer patients with synchronous metastases: Results of the FFCD 1102 phase II trial. Eur. J. Cancer 2018, 104, 108–116. [Google Scholar] [CrossRef]
- Pearl, J. An introduction to causal inference. Int. J. Biostat. 2010, 6, 7. [Google Scholar] [CrossRef]
- Shalit, U.; Johansson, F.D.; Sontag, D. Estimating individual treatment effect: Generalization bounds and algorithms. In Proceedings of the International Conference on Machine Learning, Sydney, Australia, 6–11 August 2017; pp. 3076–3085. [Google Scholar]
- Thao, L.T.P.; Nguyen, T.L.; Singh, J.; Byars, S.G.; Bick, A.G.; Ford, L.; Gibbs, P.; McNeil, J.J.; Murray, A.M.; Orchard, S.G.; et al. Low-Dose Aspirin for Individualized Cancer Prevention in Older Adults: A Secondary Analysis of the ASPREE Randomized Clinical Trial. JAMA Oncol. 2025. [Google Scholar] [CrossRef]
- Künzel, S.R.; Sekhon, J.S.; Bickel, P.J.; Yu, B. Metalearners for estimating heterogeneous treatment effects using machine learning. Proc. Natl. Acad. Sci. USA 2019, 116, 4156–4165. [Google Scholar] [CrossRef]
- Msaouel, P.; Lee, J.; Karam, J.A.; Thall, P.F. A Causal Framework for Making Individualized Treatment Decisions in Oncology. Cancers 2022, 14, 3923. [Google Scholar] [CrossRef]
- Hamaya, R.; Hara, K.; Manson, J.E.; Rimm, E.B.; Sacks, F.M.; Xue, Q.; Qi, L.; Cook, N.R. Machine-learning approaches to predict individualized treatment effect using a randomized controlled trial. Eur. J. Epidemiol. 2025, 40, 151–166. [Google Scholar] [CrossRef]
- Lee, K.H.; Choi, G.H.; Yun, J.; Choi, J.; Goh, M.J.; Sinn, D.H.; Jin, Y.J.; Kim, M.A.; Yu, S.J.; Jang, S.; et al. Machine learning-based clinical decision support system for treatment recommendation and overall survival prediction of hepatocellular carcinoma: A multi-center study. npj Digit. Med. 2024, 7, 2. [Google Scholar] [CrossRef]
- Chalkidis, G.; McPherson, J.; Beck, A.; Newman, M.; Yui, S.; Staes, C. Development of a Machine Learning Model Using Limited Features to Predict 6-Month Mortality at Treatment Decision Points for Patients with Advanced Solid Tumors. JCO Clin. Cancer Inf. 2022, 6, e2100163. [Google Scholar] [CrossRef]
- Wang, J.W.; Meng, M.; Dai, M.W.; Liang, P.; Hou, J. Correlation does not equal causation: The imperative of causal inference in machine learning models for immunotherapy. Front. Immunol. 2025, 16, 1630781. [Google Scholar] [CrossRef]
- Thomsen, M.; Skovlund, E.; Sorbye, H.; Bolstad, N.; Nustad, K.J.; Glimelius, B.; Pfeiffer, P.; Kure, E.H.; Johansen, J.S.; Tveit, K.M.; et al. Prognostic role of carcinoembryonic antigen and carbohydrate antigen 19-9 in metastatic colorectal cancer: A BRAF-mutant subset with high CA 19-9 level and poor outcome. Br. J. Cancer 2018, 118, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Taniguchi, H.; Komori, A.; Nitta, S.; Yamaguchi, K.; Kondo, C.; Nomura, M.; Kadowaki, S.; Takahari, D.; Ura, T.; et al. CA19-9 level as a prognostic and predictive factor of bevacizumab efficacy in metastatic colorectal cancer patients undergoing oxaliplatin-based chemotherapy. Cancer Chemother. Pharmacol. 2014, 73, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Xu, X.H.; Wang, X.L.; Xu, L.; Chen, Z.; Li, Y.Q. Relationship between serum uric acid and metastatic and nonmetastatic rectal cancer patients with undergoing no chemotherapy. Medicine 2016, 95, e5463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Q.H.; Yang, Y.; Lin, J.X.; Li, Y.C.; Zhong, T.Y.; Chen, J.; Wu, S.Q.; Chen, X.H.; Zhou, R.S.; et al. Baseline serum uric acid level is associated with progression-free survival, disease control rate, and safety in postoperative patients with colorectal cancer treated by FOLFOX, FOLFIRI, or XELOX. Front. Oncol. 2022, 12, 918088. [Google Scholar] [CrossRef]
- Li, W.; Liu, T.; Siyin, S.T.; Zhang, Q.; Wang, Y.; Cao, L.; Qu, J. The relationship between serum uric acid and colorectal cancer: A prospective cohort study. Sci. Rep. 2022, 12, 16677. [Google Scholar] [CrossRef]
- Alkan, A.; Doğaner, G.İ.; Tanrıverdi, Ö. Serum Uric Acid Level May Be a Predictive Factor for BRAF V600E Mutation in Older Patients with Metastatic Colorectal Cancer: An Exploratory Analysis. Oncology 2024, 102, 952–959. [Google Scholar] [CrossRef]
- Ramzy, G.M.; Meister, I.; Rudaz, S.; Boccard, J.; Nowak-Sliwinska, P. Identification of Lipid Species Signatures in FOLFOXIRI-Resistant Colorectal Cancer Cells. Int. J. Mol. Sci. 2025, 26, 1169. [Google Scholar] [CrossRef]
- L’Heureux, A.; Wieland, D.R.; Weng, C.-H.; Chen, Y.-H.; Lin, C.-H.; Lin, T.-H.; Weng, C.-H. Association between thyroid disorders and colorectal cancer risk in adult patients in Taiwan. JAMA Netw. Open 2019, 2, e193755. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Szallasi, A. Thrombocytosis Portends Adverse Prognosis in Colorectal Cancer: A Meta-Analysis of 5,619 Patients in 16 Individual Studies. Anticancer Res. 2017, 37, 4717–4726. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Lin, G.; Ye, M.; Tong, D.; Zhao, J.; Zhu, D.; Yu, Q.; Zhang, W.; Li, W. Decreased mean platelet volume predicts poor prognosis in metastatic colorectal cancer patients treated with first-line chemotherapy: Results from mCRC biomarker study. BMC Cancer 2019, 19, 15. [Google Scholar] [CrossRef]
- Matsuda, A.; Yamada, T.; Matsumoto, S.; Shinji, S.; Ohta, R.; Sonoda, H.; Shinozuka, E.; Sekiguchi, K.; Suzuki, H.; Yoshida, H. Prognostic Role of the Platelet-to-Lymphocyte Ratio for Patients with Metastatic Colorectal Cancer Treated with Aflibercept. In Vivo 2020, 34, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Clayton, E.A.; Matyunina, L.V.; McDonald, L.D.; Benigno, B.B.; Vannberg, F.; McDonald, J.F. Machine learning predicts individual cancer patient responses to therapeutic drugs with high accuracy. Sci. Rep. 2018, 8, 16444. [Google Scholar] [CrossRef]
- Santos, C.S.; Amorim-Lopes, M. Externally validated and clinically useful machine learning algorithms to support patient-related decision-making in oncology: A scoping review. BMC Med. Res. Methodol. 2025, 25, 45. [Google Scholar] [CrossRef]
- Lee, S.; Song, A.; Eo, W. Serum Ferritin as a Prognostic Biomarker for Survival in Relapsed or Refractory Metastatic Colorectal Cancer. J. Cancer 2016, 7, 957–964. [Google Scholar] [CrossRef]
- Zhu, E.; Wang, J.; Shi, W.; Jing, Q.; Ai, P.; Shan, D.; Ai, Z. Optimizing adjuvant treatment options for patients with glioblastoma. Front. Neurol. 2024, 15, 1326591. [Google Scholar] [CrossRef]
- Pan, H.; Wang, J.; Shi, W.; Xu, Z.; Zhu, E. Quantified treatment effect at the individual level is more indicative for personalized radical prostatectomy recommendation: Implications for prostate cancer treatment using deep learning. J. Cancer Res. Clin. Oncol. 2024, 150, 67. [Google Scholar] [CrossRef]
- Zhu, E.; Wang, J.; Shi, W.; Chen, Z.; Zhu, M.; Xu, Z.; Li, L.; Shan, D. Utilizing machine learning to tailor radiotherapy and chemoradiotherapy for low-grade glioma patients. PLoS ONE 2024, 19, e0306711. [Google Scholar] [CrossRef] [PubMed]
| Gender n (%) | Male 84 (61.8) Female 52 (38.2) |
|---|---|
| meanAge(d) | 59.27 (10.71) |
| Comorbidity n (%) | Overall 84 (61.8) Diabetes 25 (18.4) Hypertension 42 (30.9) Hypothyroidism 9 (6.6) Coronary artery disease 13 (9.6) |
| Smoking Status n (%) | Never 70 (51.5) Ex-smoker 39 (28.6) Current 27 (19.8) |
| Primary Site n (%) | Right 27 (19.9) Left 78 (57.4) Not determined 31 (22.8) |
| MetastaticSite n (%) | Liver 120 (82) Peritoneal 18 (13.2) Lung 35 (25.7) Bone 6 (4.4) |
| Mutation | RAS Mutant 55 (40.4) RAF Mutant 3 (2.2) |
| Emergent Surgery n (%) | Performed 21 (15.4) |
| Chemotherapy n (%) | Single 2 (1.5) Doublet 128 (94.1) Triplet 6 (4.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayaalp, M.; Akkuş, E.; Karaoğlan, B.B.; Utkan, G. Individualized Triplet Chemotherapy Decision-Making in Metastatic Colorectal Cancer: A Machine-Learning-Driven Study. Cancers 2025, 17, 3704. https://doi.org/10.3390/cancers17223704
Kayaalp M, Akkuş E, Karaoğlan BB, Utkan G. Individualized Triplet Chemotherapy Decision-Making in Metastatic Colorectal Cancer: A Machine-Learning-Driven Study. Cancers. 2025; 17(22):3704. https://doi.org/10.3390/cancers17223704
Chicago/Turabian StyleKayaalp, Mehmet, Erman Akkuş, Beliz Bahar Karaoğlan, and Güngör Utkan. 2025. "Individualized Triplet Chemotherapy Decision-Making in Metastatic Colorectal Cancer: A Machine-Learning-Driven Study" Cancers 17, no. 22: 3704. https://doi.org/10.3390/cancers17223704
APA StyleKayaalp, M., Akkuş, E., Karaoğlan, B. B., & Utkan, G. (2025). Individualized Triplet Chemotherapy Decision-Making in Metastatic Colorectal Cancer: A Machine-Learning-Driven Study. Cancers, 17(22), 3704. https://doi.org/10.3390/cancers17223704






