Colorectal Cancer Hepatic Metastasis Modeling by Advanced 3D Bioprinting Allows Demonstration of Oncolytic Viral Chemotherapeutic Delivery
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Collection and Production
2.2. Bioprinting Procedures
2.3. Viral Characteristics and Chemotherapeutic Testing
2.4. Viability Analysis
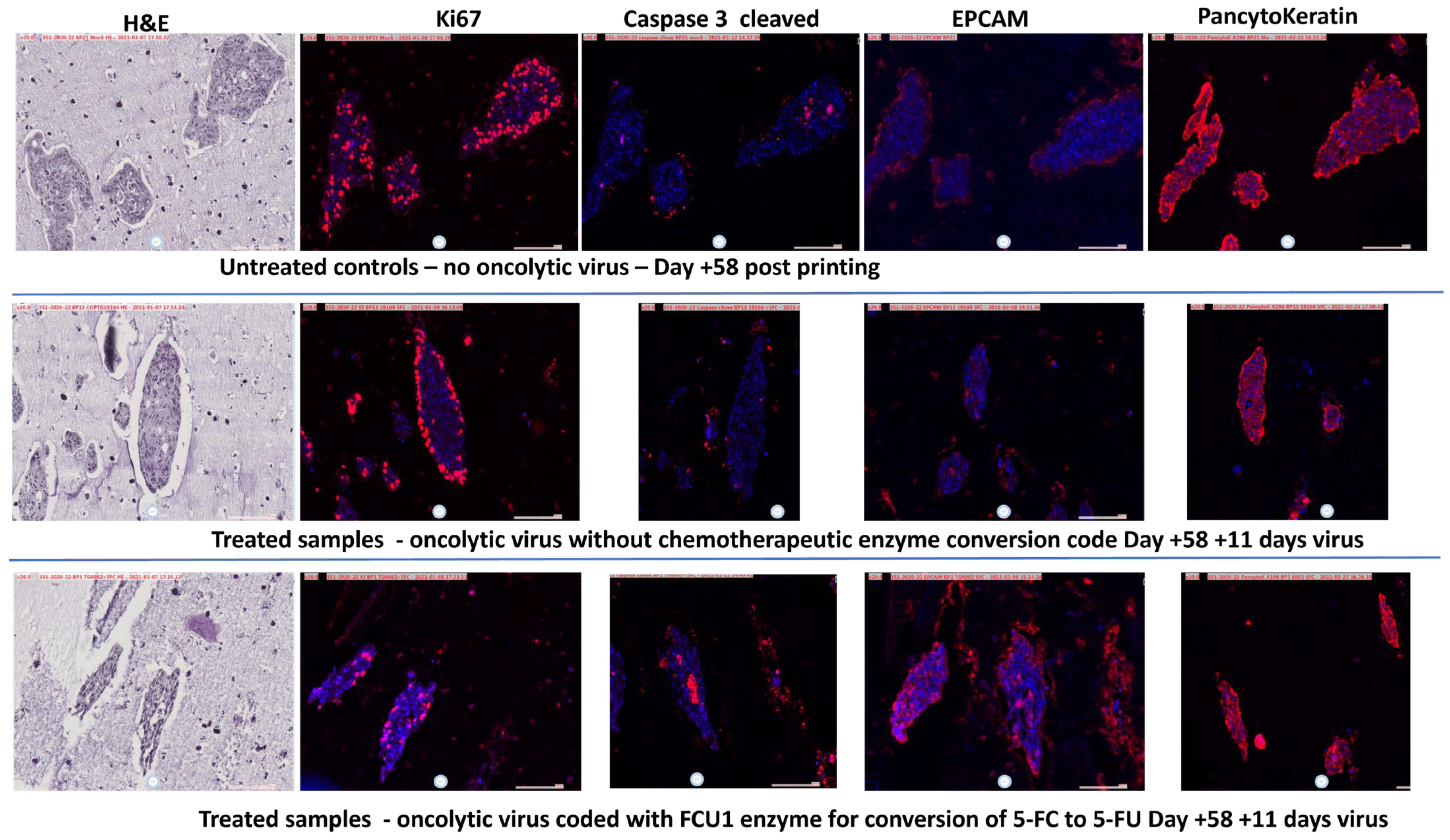
2.5. Histological Analysis
3. Results
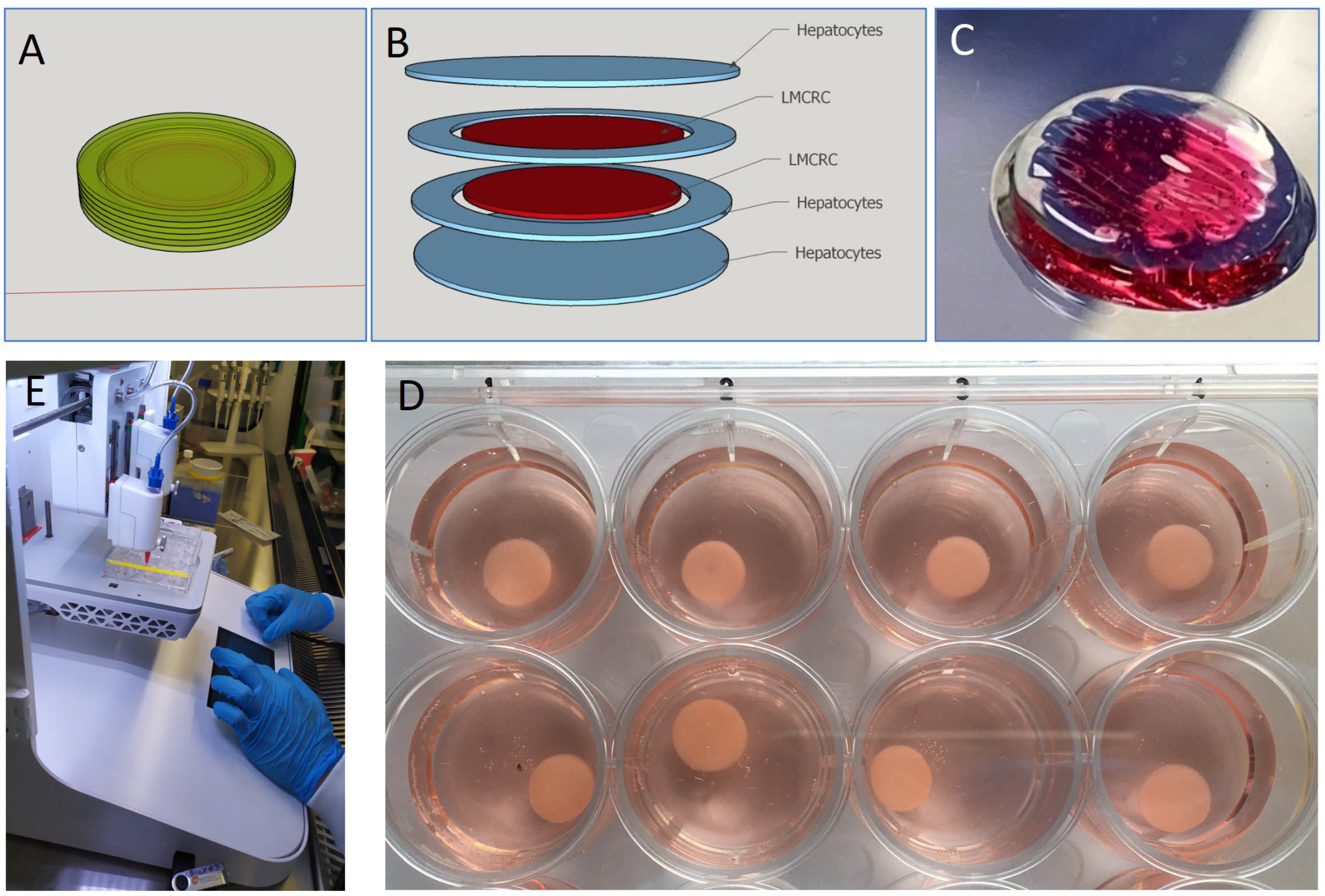
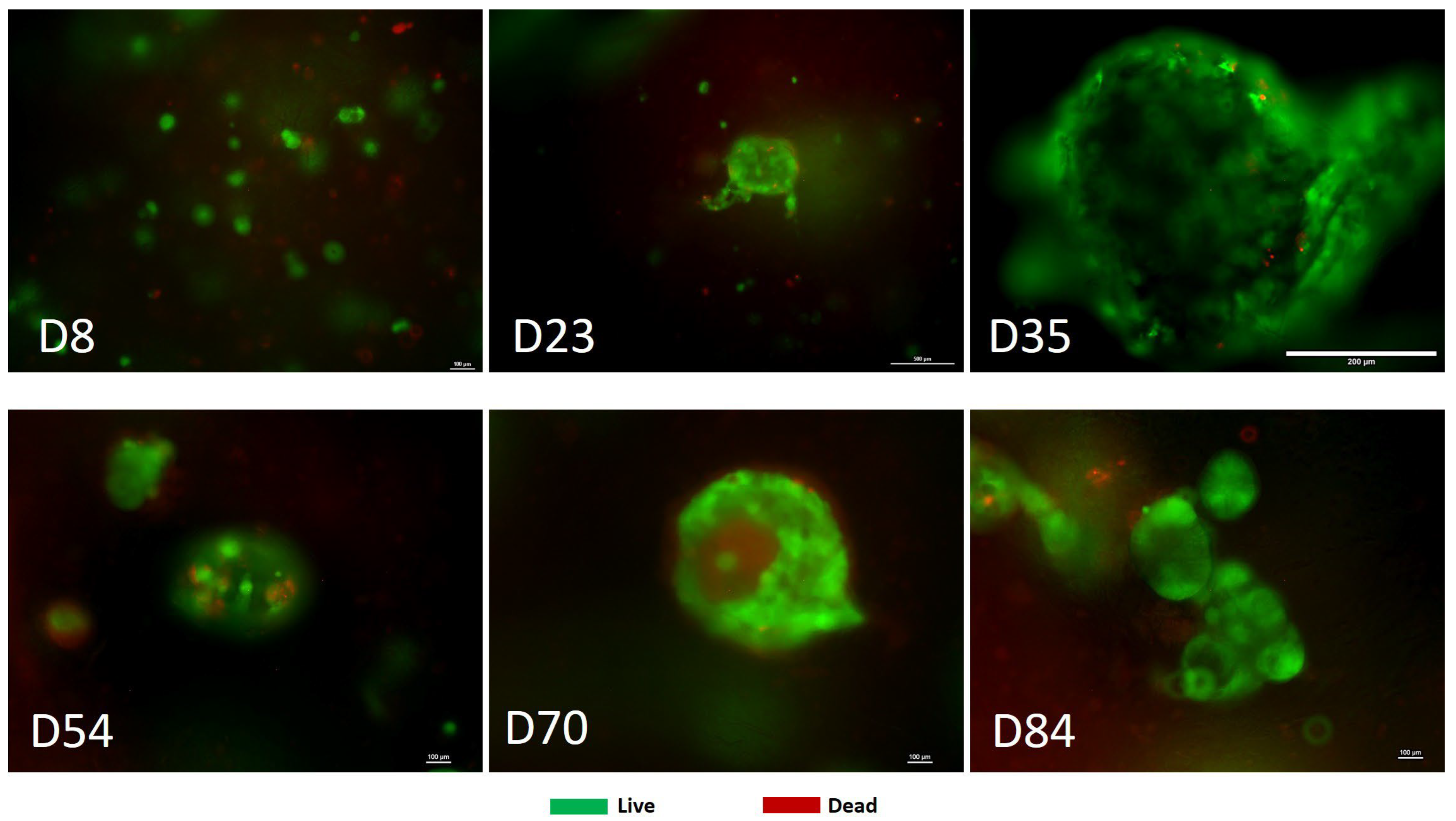
3.1. Reproducibility Can Be Achieved in Creating Tumor Models by 3D Bioprinting for Colorectal Cancer
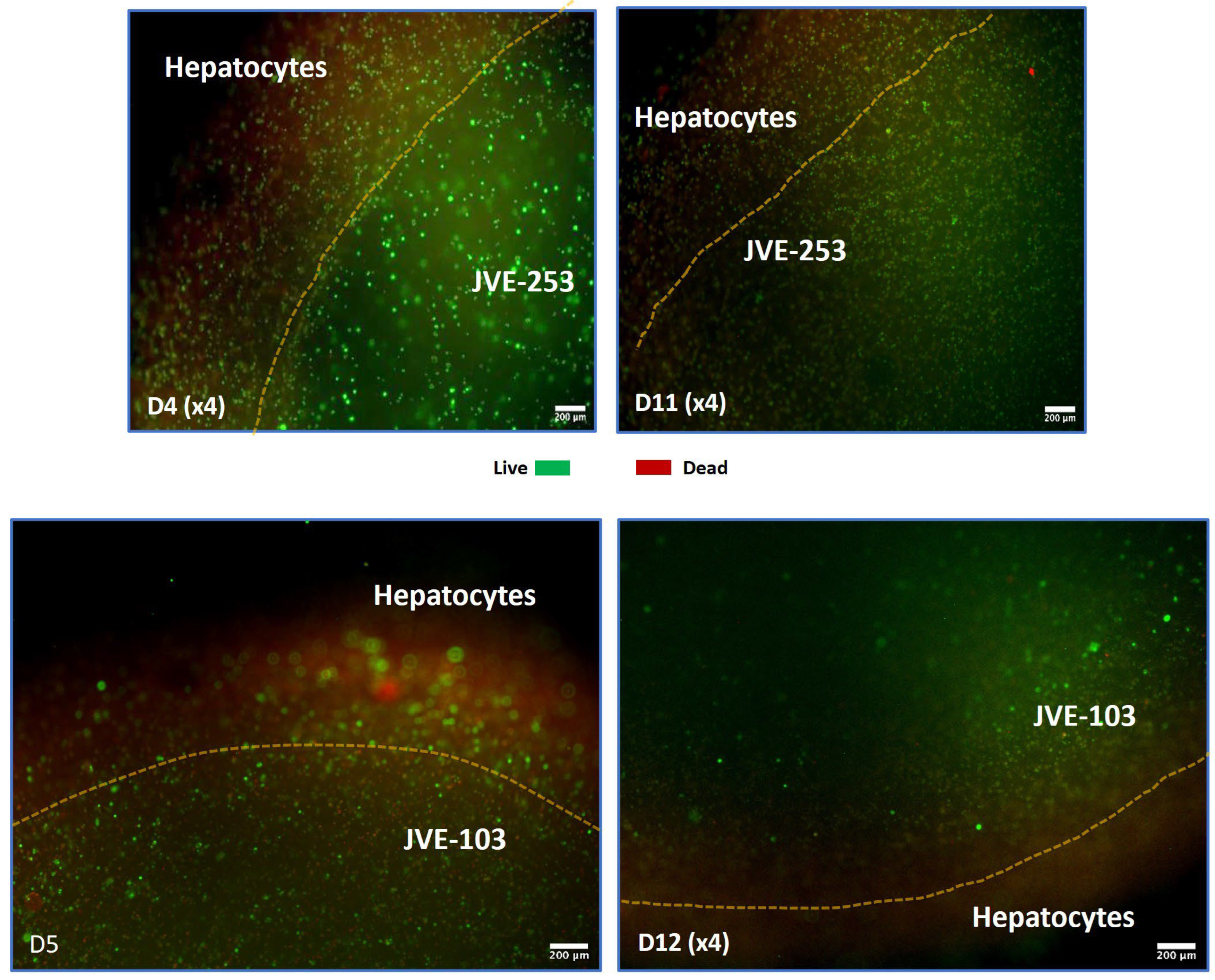
3.2. Co-Printing of Human Hepatocytes with Colorectal Cancer Cells, Models the Formation of Metastatic Cancer
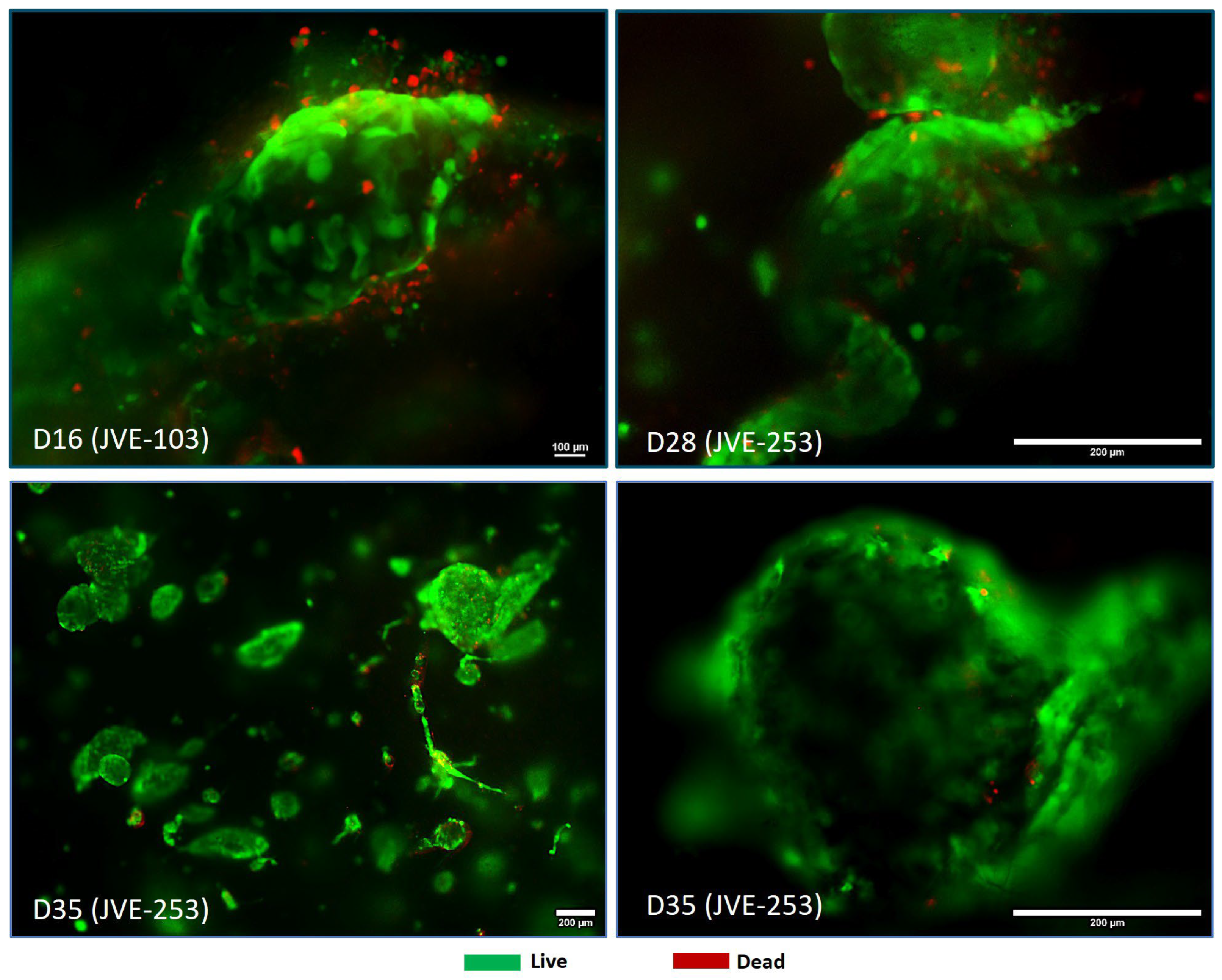
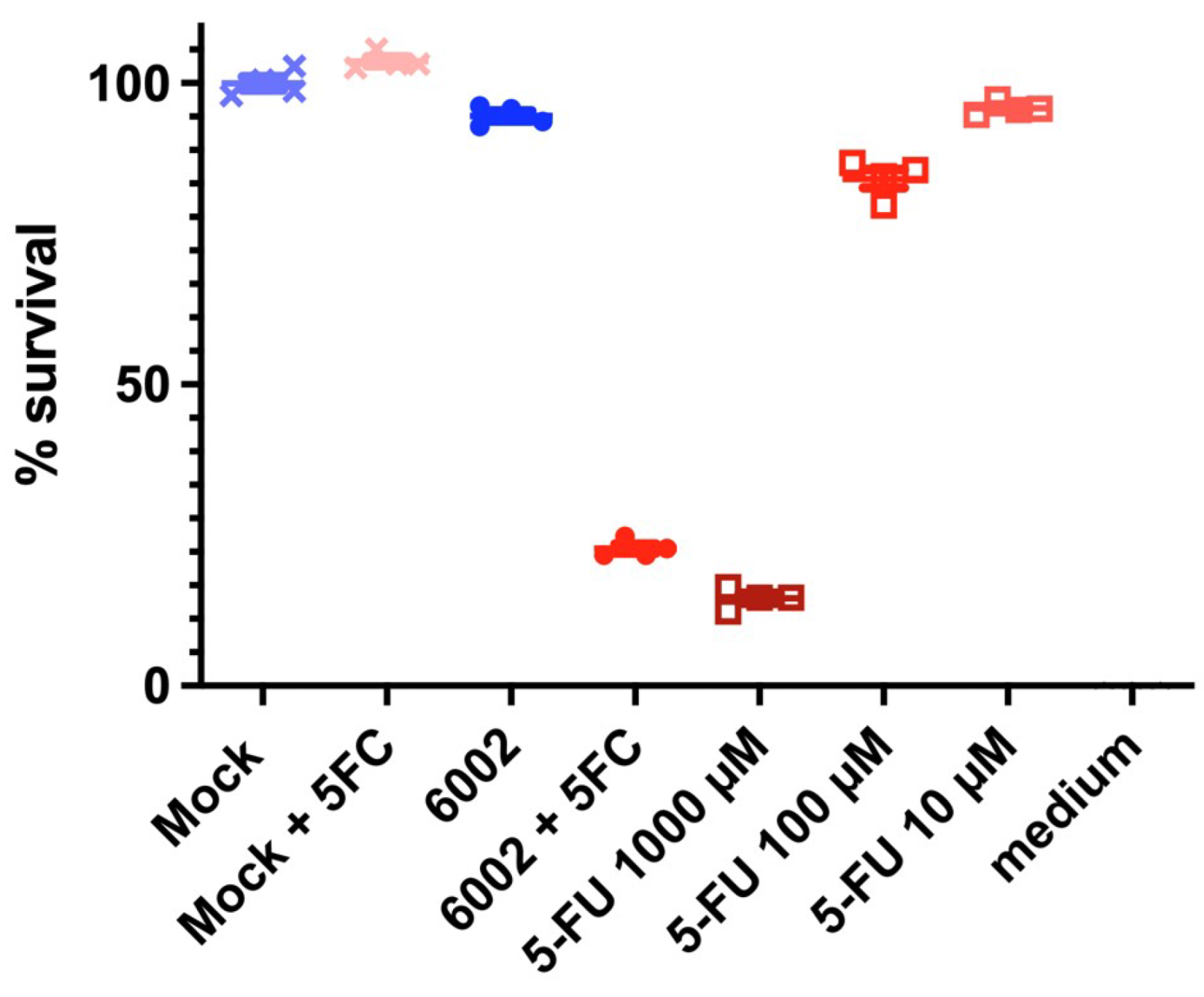
3.3. Treatment with Oncolytic Viral Loaded Chemotherapeutics Promotes the Stable and Effective Destruction of Colorectal 3D Tumors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | Colorectal Cancer |
| 3D | 3-dimensional |
| 5-FU | 5 Fluorouracil |
| 5-FC | Flucytosine |
| FCU1 | Bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene |
| HEP | Hepatocyte |
| DAPI | 4′,6-diamidino-2-phenylindole |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, F.; Siegel, R.L.; Laversanne, M.; Soergomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Crooke, H.; Kobayashi, M.; Mitchell, B.; Nwokeji, E.; Laurie, M.; Kamble, S.; McKenna, M.; Masood, A.; Korytowsky, B. Estimating 1- and 5-year relative survival trends in colorectal cancer (CRC) in the United States: 2004 to 2014. J. Clin. Oncol. 2018, 36, 587. [Google Scholar] [CrossRef]
- Inamura, K. Colorectal Cancers: An Update on Their Molecular Pathology. Cancers 2018, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Loomans-Kropp, H.A.; Umar, A. Increasing Incidence of Colorectal Cancer in Young Adults. J. Cancer Epidemiol. 2019, 11, 9841295. [Google Scholar] [CrossRef]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunne, P.D.; McArt, D.G.; Bradley, C.A.; O’Reilly, P.G.; Barrett, H.L.; Cummins, R.; O’Grady, T.; Arthur, K.; Loughrey, M.B.; Allen, W.L.; et al. Challenging the Cancer Molecular Stratification Dogma: Intratumoral Heterogeneity Undermines Consensus Molecular Subtypes and Potential Diagnostic Value in Colorectal Cancer. Clin. Cancer Res. 2016, 22, 4095–4104. [Google Scholar] [CrossRef] [PubMed]
- Boutin, A.T.; Liao, W.-T.; Wang, W.; Hwang, S.S.; Karpinets, T.V.; Cheung, H.; Chu, G.C.; Jiang, S.; Hu, J.; Chang, K.; et al. Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev. 2017, 31, 370–382. [Google Scholar] [CrossRef]
- Creasy, J.M.; Sadot, E.; Koerkamp, B.G.; Chou, J.F.; Gonen, M.; Kemeny, N.E.; Balachandran, V.P.; Kingham, T.P.; DeMatteo, R.P.; Allen, P.J.; et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: What factors preclude cure? Surgery 2018, 163, 1238–1244. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Klemm, F.; Joyce, J.A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015, 25, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Carmical, J.R.; Ives, K.L.; Ives, K.L.; Wood, T.G.; Aronson, J.F.; Gomez, G.A.; Djukom, C.D.; Hellmich, M.R. CD133+ colon cancer cells are more interactive with the tumor microenvironment than CD133- cells. Lab. Investig. 2012, 92, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 2015, 15, 250. [Google Scholar] [CrossRef]
- Reidy, E.; Leonard, N.A.; Treacy, O.; Ryan, A.E. A 3D View of Colorectal Cancer Models in Predicting Therapeutic Responses and Resistance. Cancers 2021, 13, 227. [Google Scholar] [CrossRef]
- Rios de la Rosa, J.M.; Wubetu, J.; Tirelli, N.; Tirella, A. Colorectal tumor 3D in vitro models: Advantages of biofabrication for the recapitulation of early stages of tumour development. Biomed. Phys. Eng. Express 2018, 4, 045010. [Google Scholar] [CrossRef]
- Cascinu, S.; Berardi, R.; Salvagni, S.; Beretta, G.D.; Catalano, V.; Pucci, F.; Sobrero, A.; Tagliaferri, P.; Labianca, R.; Scartozzi, M.; et al. A combination of gefitinib and FOLFOX-4 as first-line treatment in advanced colorectal cancer patients. A GISCAD multicentre phase II study including a biological analysis of EGFR overexpression, amplification and NF-κB activation. Br. J. Cancer 2008, 98, 71–76. [Google Scholar] [CrossRef]
- Shah, M.A.; Schwartz, G.K. Cell cycle-mediated drug resistance: An emerging concept in cancer therapy. Clin. Cancer Res. 2001, 7, 2168–2181. [Google Scholar]
- Scartozzi, M.; Bearzi, I.; Pierantoni, C.; Mandolesi, A.; Loupakis, F.; Zaniboni, A.; Catalano, V.; Quadri, A.; Zorzi, F.; Berardi, R.; et al. Nuclear factor-kB tumor expression predicts response and survival in irinotecan-refractory metastatic colorectal cancer treated with cetuximab-irinotecan therapy. J. Clin. Oncol. 2007, 25, 3930–3935. [Google Scholar] [CrossRef]
- Van der Jeught, K.; Xu, H.C.; Li, Y.J.; Lu, X.-B.; Ji, G. Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef]
- Seyhan, A.A. Lost in translation: The valley of death across preclinical and clinical divide—Identification of problems and overcoming obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef]
- Kim, D.S.; Min, K.; Lee, S.K. Cell Cycle Dysregulation Is Associated With 5-Fluorouracil Resistance in Gastric Cancer Cells. Anticancer Res. 2020, 40, 3247–3254. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Z.; Zhu, Y.; Pan, Q.; Lui, Y.; Qi, X.; Jin, L.; Jin, J.; Ma, X.; Hua, D. Inhibition of transient receptor potential channel 5 reverses 5-Fluorouracil resistance in human colorectal cancer cells. J. Biol. Chem. 2015, 290, 448–456. [Google Scholar] [CrossRef]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 2019, 20, 273–286. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, J.; Tong, Y. Gene signatures of drug resistance predict patient survival in colorectal cancer. Pharmacogenom. J. 2015, 15, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef]
- Hoare, O.; Fraunhoffer, N.; Elkaoutari, A.; Gayet, O.; Bigonnet, M.; Roques, J.; Nicolle, R.; McGuckin, C.; Forraz, N.; Sohier, E.; et al. Exploring the Complementarity of Pancreatic Ductal Adenocarcinoma Preclinical Models. Cancers 2021, 13, 2473. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bit, A. 3D bioprinting in tissue engineering and regenerative medicine. Cell Tissue Bank. 2022, 23, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Jurga, M.; Dainiak, M.B.; Sarnowska, A.; Jablonska, A.; Tripathi, A.; Plieva, F.M.; Savina, I.N.; Strojek, L.; Lungvid, H.; Kumar, A.; et al. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials 2011, 32, 3423–3434. [Google Scholar] [CrossRef]
- McGuckin, C.P.; Jurga, M.; Miller, A.M.; Sarnowska, A.; Wiedner, M.; Boyle, N.T.; Lynch, M.A.; Jablonska, A.; Drela, K.; Lukomska, B.; et al. Ischemic brain injury: A consortium analysis of key factors involved in mesenchymal stem cell-mediated inflammatory reduction. Arch. Biochem. Biophys. 2013, 534, 88–97. [Google Scholar] [CrossRef]
- Sarnowska, A.; Jablonska, A.; Jurga, M.; Dainiak, M.; Strojek, L.; Drela, K.; Wright, K.; Tripathi, A.; Kumar, A.; Jungvid, H.; et al. Encapsulation of mesenchymal stem cells by bioscaffolds protects cell survival and attenuates neuroinflammatory reaction in injured brain tissue after transplantation. Cell Transplant. 2013, 1, S67–S82. [Google Scholar] [CrossRef]
- Mueller, A.A.; Forraz, N.; Gueven, S.; Atzeni, G.; Degoul, O.; Pagnon-Minot, A.; Hartmann, D.; Martin, I.; Scherberich, A.; McGuckin, C. Osteoblastic differentiation of Wharton jelly biopsy specimens and their mesenchymal stromal cells after serum-free culture. Plast. Reconstr. Surg. 2014, 134, 59e–69e. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, Y.; Wang, X.; Wang, J.; Shi, X.; Tan, W.; Tan, Z. 3D printed in vitro tumor tissue model of colorectal cancer. Theranostics 2020, 10, 12127–12143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef] [PubMed]
- Grolman, J.M.; Zhang, D.; Smith, A.M.; Moore, J.S.; Kilian, K.A. Rapid 3D Extrusion of Synthetic Tumor Microenvironments. Adv. Mater. 2015, 27, 5512–5517. [Google Scholar] [CrossRef] [PubMed]
- Maloney, E.; Clark, C.; Sivakumar, H.; Yoo, K.; Aleman, J.; Rajan, S.A.P.; Forsythe, S.; Mazzocchi, A.; Laxton, A.W.; Tatter, S.B. Immersion Bioprinting of Tumor Organoids in Multi-Well Plates for Increasing Chemotherapy Screening Throughput. Micromachines 2020, 11, 208. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, W.; Nowicki, M.; Miao, S.; Cui, H.; Holmes, B.; Glazer, R.I.; Zhang, L.G. 3D Bioprinting a Cell-Laden Bone Matrix for Breast Cancer Metastasis Study. ACS Appl. Mater. Interfaces 2016, 8, 30017–30026. [Google Scholar] [CrossRef]
- Dai, X.; Ma, C.; Lan, Q.; Xu, T. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 2016, 8, 045005. [Google Scholar] [CrossRef]
- Swaminathan, S.; Hamid, Q.; Sun, W.; Clyne, A.M. Bioprinting of 3D breast epithelial spheroids for human cancer models. Biofabrication 2019, 11, 025003. [Google Scholar] [CrossRef]
- Sbirkov, Y.; Molander, D.; Milet, C.; Bodurov, I.; Atanasov, B.; Penkov, R.; Belev, N.; Forraz, N.; McGuckin, C.; Sarafian, V. A Colorectal Cancer 3D Bioprinting Workflow as a Platform for Disease Modeling and Chemotherapeutic Screening. Front. Bioeng. Biotechnol. 2021, 18, 755563. [Google Scholar] [CrossRef]
- McGuckin, C.; Forraz, N.; Milet, C.; Lacroix, M.; Sbirkov, Y.; Sarafian, V.; Ebel, C.; Spindler, A.; Koerper, V.; Balloul, J.-M.; et al. World’s First Long-Term Colorectal Cancer Model by 3D Bioprinting as a Mechanism for Screening Oncolytic Viruses. Cancers 2023, 15, 4724. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, C.; Forraz, N.; Milet, C.; Lacroix, M.; Sbirkov, Y.; Sarafian, V.; Ebel, C.; Spindler, A.; Koerper, V.; Quéméneur, E.; et al. Stable Biotherapeutic Penetration Screening in Critically Small Colorectal Metastatic Cancer Biopsies Allows for Oncolytic Virus-Delivered Chemotherapeutic Response Assessment through 3D Bioprinted Organoid Expansion. Adv. Ther. 2025, 8, 2400221. [Google Scholar] [CrossRef]
- Guo, Z.S.; Lu, B.; Guo, Z.; Giehl, E.; Feist, M.; Dai, E.; Liu, W.; Storkus, W.J.; He, Y.; Liu, Z.; et al. Vaccinia virus-mediated cancer immunotherapy: Cancer vaccines and oncolytics. J. Immunother. Cancer 2019, 7, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Vermes, A.; Guchelaar, H.J.; Dankert, J. Flucytosine: A review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 2000, 46, 171–179. [Google Scholar] [CrossRef]
- Erbs, P.; Regulier, E.; Kintz, J.; Leroy, P.; Poitevin, Y.; Exinger, F.; Jund, R.; Mehtali, M. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000, 14, 3813–3822. [Google Scholar] [PubMed]
- Foloppe, J.; Kintz, J.; Futin, N.; Findeli, A.; Cordier, P.; Schlesinger, Y.; Hoffmann, C.; Tosch, C.; Balloul, J.M.; Erbs, P. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 2008, 20, 1361–1371. [Google Scholar] [CrossRef]
- Foloppe, J.; Kempf, J.; Futin, N.; Kintz, J.; Cordier, P.; Pichon, C.; Findeli, A.; Vorburger, F.; Quemeneur, E.; Erbs, P. The Enhanced Tumor Specificity of TG6002, an Armed Oncolytic Vaccinia Virus Deleted in Two Genes Involved in Nucleotide Metabolism. Mol. Ther. Oncolytics 2019, 14, 1–14. [Google Scholar] [CrossRef]
- West, E.J.; Sadoun, A.; Bendjama, K.; Erbs, P.; Smolenschi, C.; Cassier, P.A.; de Baere, T.; Sainte-Croix, S.; Brandely, M.; Melcher, A.A.; et al. A Phase I Clinical Trial of Intrahepatic Artery Delivery of TG6002 in Combination with Oral 5-Fluorocytosine in Patients with Liver-Dominant Metastatic Colorectal Cancer. Clin. Cancer Res. 2025, 31, 1243–1256. [Google Scholar] [CrossRef]
- Kamal, R.; Paul, P.; Diksha; Awasthi, A. Exploring Gene Therapy: The Next Generation of Colorectal Cancer Treatment. Curr. Gene Ther. 2025, 25, 195–198. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Li, D.; Gou, M. Comprehensive Analysis and Experimental Validation of HEPACAM2 as a Potential Prognosis Biomarker and Immunotherapy Target in Colorectal Cancer. Curr. Gene Ther. 2025, 25, 518–531. [Google Scholar] [CrossRef]
- Suleman, M.; Khan, S.U.; Ali, S.; Alghamdi, A.; Alissa, M.; Mushtaq, R.Y.; Crovella, S. Probing the Depths of Molecular Complexity: STAT3 as a Key Architect in Colorectal Cancer Pathogenesis. Curr. Gene Ther. 2025, 25, 433–452. [Google Scholar] [CrossRef]
- Tian, S.; Chen, M.; Jing, W.; Meng, Q.; Wu, J. miR-1204 Positioning in 8q24.21 Involved in the Tumorigenesis of Colorectal Cancer by Targeting MASPIN. Protein Pept. Lett. 2024, 31, 544–558. [Google Scholar] [CrossRef]
| Category | JVE-103 | JVE-253 | HEP1 | HEP2 |
|---|---|---|---|---|
| Sex | Human Male | Human Male | Human Male | Human Male |
| Age | 51 | 48 | 29 | 74 |
| Diagnosis | Primary colorectal adenocarcinoma | Metastatic adenocarcinoma | Sarcoma | Hepatic metastasis |
| Extent | Metastatic | Metastatic | - | Metastatic |
| Tumor Stage | Duke Stage D | Duke Stage D | n/a | n/a |
| Million Cells per Model | 15 | 15 | 10 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGuckin, C.; Forraz, N.; Milet, C.; Lacroix, M.; Sbirkov, Y.; Sarafian, V.; Ebel, C.; Spindler, A.; Koerper, V.; Balloul, J.-M.; et al. Colorectal Cancer Hepatic Metastasis Modeling by Advanced 3D Bioprinting Allows Demonstration of Oncolytic Viral Chemotherapeutic Delivery. Cancers 2025, 17, 3705. https://doi.org/10.3390/cancers17223705
McGuckin C, Forraz N, Milet C, Lacroix M, Sbirkov Y, Sarafian V, Ebel C, Spindler A, Koerper V, Balloul J-M, et al. Colorectal Cancer Hepatic Metastasis Modeling by Advanced 3D Bioprinting Allows Demonstration of Oncolytic Viral Chemotherapeutic Delivery. Cancers. 2025; 17(22):3705. https://doi.org/10.3390/cancers17223705
Chicago/Turabian StyleMcGuckin, Colin, Nico Forraz, Clément Milet, Mathieu Lacroix, Yordan Sbirkov, Victoria Sarafian, Caroline Ebel, Anita Spindler, Véronique Koerper, Jean-Marc Balloul, and et al. 2025. "Colorectal Cancer Hepatic Metastasis Modeling by Advanced 3D Bioprinting Allows Demonstration of Oncolytic Viral Chemotherapeutic Delivery" Cancers 17, no. 22: 3705. https://doi.org/10.3390/cancers17223705
APA StyleMcGuckin, C., Forraz, N., Milet, C., Lacroix, M., Sbirkov, Y., Sarafian, V., Ebel, C., Spindler, A., Koerper, V., Balloul, J.-M., & Erbs, P. (2025). Colorectal Cancer Hepatic Metastasis Modeling by Advanced 3D Bioprinting Allows Demonstration of Oncolytic Viral Chemotherapeutic Delivery. Cancers, 17(22), 3705. https://doi.org/10.3390/cancers17223705







